Abstract
Higher levels of albuminuria associate with increased risk for adverse outcomes independent of estimated GFR (eGFR), but whether albuminuria also associates with concurrent complications specific to chronic kidney disease (CKD) is unknown. Here, we assessed the association of spot albumin-to-creatinine ratio with anemia, acidosis, hyperphosphatemia, hypoalbuminemia, hyperparathyroidism, and hypertension among 30,528 adult participants in NHANES 1988–1994 and 1999–2006. After multivariable adjustment including eGFR, higher albumin-to-creatinine ratios associated with anemia, acidosis, hypoalbuminemia, hyperparathyroidism, and hypertension but only weakly associated with acidosis and anemia. Furthermore, the associations between albumin-to-creatinine ratio and both anemia and acidosis were not consistent across eGFR strata. Higher albumin-to-creatinine ratio levels did not associate with hyperphosphatemia. Lower eGFR associated with higher prevalence ratios for all complications, and these associations were stronger than those observed for the albumin-to-creatinine ratio; after multivariable adjustment, however, the associations between eGFR and both hypoalbuminemia and hypertension were NS. In conclusion, albuminuria and eGFR differentially associate with complications of CKD.
Chronic kidney disease (CKD) is a worldwide major public health problem with an increasing prevalence of kidney failure and poor outcomes.1 It is estimated that 11.5% of U.S. adults have CKD,2 and multiple studies have demonstrated its relationship to an increased risk for mortality, cardiovascular disease, and kidney failure.3–5
CKD is defined by the presence of reduced estimated GFR (eGFR) or markers of kidney damage, generally determined by elevated albuminuria, and it is staged for severity primarily by level of eGFR.6,7 Recent data have demonstrated that albuminuria is a strong and independent predictor of risk for mortality, cardiovascular disease, end-stage renal disease, acute kidney injury, and CKD progression, and it has been suggested that incorporation of albuminuria into the CKD staging system will facilitate clinical decision making regarding patients' prognosis.8–12 However, there is little data on whether albuminuria is also associated with concurrent complications of CKD, which is also relevant for physicians' decisions regarding evaluation and management at a particular patient encounter.
Data from the U.S.-based National Health and Nutrition Examination Surveys (NHANES) have been used to document the prevalence of CKD complications by level of eGFR in the general population.7,13–15 Similar published data on the relation between albuminuria and CKD complications are limited. We pooled data from the NHANES III, conducted in 1988–1994, and NHANES 1999–2006 to determine the relationship between level of albuminuria and six conditions considered to be complications of CKD: anemia, acidosis, hyperphosphatemia, hypoalbuminemia, hyperparathyroidism, and hypertension. The association of albuminuria and eGFR with these complications was evaluated separately and in combination.
RESULTS
The majority of the population had albumin-to-creatinine ratio (ACR) <10 mg/g (72.2%), and 18.2%, 7.5%, and 2.1% of the population had ACR of 10 to 29, 30 to 299, and ≥300 mg/g, respectively (Table 1). Similarly, most people had eGFR ≥60 ml/min per 1.73 m2, with only 3.9%, 1.3%, and 0.3% of the population having eGFR levels of 45 to 59, 30 to 44, and <30 ml/min per 1.73 m2, respectively (Supplemental Table 1). Those with higher levels of ACR and lower eGFR were older, were more likely to be non-Hispanic black, had a higher body mass index, and had diabetes mellitus (Table 1 and Supplemental Table 1). Additionally, eGFR levels <90 ml/min per 1.73 m2 were more common at higher ACR levels.
Table 1.
Characteristics of study participants by level of albuminuria
| Albuminuria, mg/g |
||||||
|---|---|---|---|---|---|---|
| Overall (n = 30,528) | <10 (n = 20,416) | 10 to 29 (n = 6115) | 30 to 299 (n = 3044) | >300 (n = 953) | P Trend | |
| % of population | 100.0 | 72.2 | 18.2 | 7.5 | 2.1 | |
| Age in years, mean (SD) | 45.5 (0.3) | 43.1 (0.2) | 50.4 (0.5) | 54.6 (0.5) | 55.6 (1.2) | <0.001 |
| Men, % | 49.2 | 52.8 | 37.8 | 42.1 | 50.5 | <0.001 |
| Race | ||||||
| non-Hispanic whites, % | 74.7 | 75.1 | 75.5 | 69.6 | 70.9 | Reference |
| non-Hispanic blacks, % | 10.2 | 10.0 | 9.4 | 12.7 | 14.9 | <0.001 |
| Hispanic, % | 9.0 | 8.9 | 8.8 | 10.4 | 8.2 | 0.060 |
| other, % | 6.1 | 6.0 | 6.2 | 7.3 | 6.0 | 0.140 |
| Current smoker, % | 24.6 | 25.0 | 23.2 | 24.1 | 22.3 | 0.108 |
| Body mass index, kg/m2 | 27.4 (0.1) | 27.2 (0.1) | 27.6 (0.2) | 28.4 (0.2) | 28.6 (0.4) | <0.001 |
| Diabetes mellitus, % | 6.5 | 3.5 | 9.7 | 22.6 | 25.3 | <0.001 |
| C-reactive protein, mg/L | ||||||
| <3.0, % | 67.4 | 70.7 | 61.4 | 53.0 | 56.4 | Reference |
| 3.0 to 9.9, % | 24.2 | 22.7 | 27.2 | 31.0 | 26.6 | <0.001 |
| ≥10.0, % | 8.5 | 6.7 | 11.4 | 16.0 | 17.1 | <0.001 |
| eGFR in ml/min per 1.73 m2 | ||||||
| mean (SD) | 96.2 (0.3) | 98.0 (0.3) | 93.9 (0.5) | 87.9 (0.7) | 81.7 (1.6) | <0.001 |
| ≥120, % | 12.4 | 12.8 | 11.9 | 10.2 | 9.0 | 0.168 |
| 90 to 119, % | 51.5 | 54.4 | 47.2 | 39.0 | 34.0 | Reference |
| 60 to 89, % | 30.6 | 29.5 | 33.3 | 35.5 | 30.0 | <0.001 |
| 45 to 59, % | 3.9 | 2.6 | 5.6 | 9.5 | 12.0 | <0.001 |
| 30 to 44, % | 1.3 | 0.6 | 1.7 | 4.5 | 9.7 | <0.001 |
| <30, % | 0.3 | 0.1 | 0.4 | 1.2 | 5.3 | <0.001 |
| Hemoglobin, g/dl mean (SD) | 14.5 (0.1) | 14.6 (0.1) | 14.3 (0.1) | 14.4 (0.1) | 14.2 (0.1) | <0.001 |
| Bicarbonate, mEq/L mean (SD) | 24.7 (0.1) | 24.7 (0.1) | 24.8 (0.1) | 24.6 (0.2) | 24.5 (0.3) | 0.316 |
| Phosphorus, mg/dl mean (SD) | 3.82 (0.01) | 3.82 (0.01) | 3.82 (0.01) | 3.80 (0.02) | 3.85 (0.02) | 0.469 |
| Albumin, mg/dl mean (SD) | 4.21 (0.01) | 4.23 (0.01) | 4.20 (0.01) | 4.14 (0.01) | 4.01 (0.03) | <0.001 |
| iPTH, pg/ml median | 37.8 | 36.3 | 39.8 | 42.2 | 51.6 | |
| (25th to 75th percentile) | (28.1, 50.1) | (27.1, 48.2) | (30.2, 53.1) | (31.0, 59.1) | (33.1, 76.0) | <0.001 |
| Systolic BP, mmHg mean (SD) | 122.9 (0.2) | 120.1 (0.2) | 127.9 (0.4) | 134.9 (0.7) | 136.6 (1.4) | <0.001 |
| Diastolic BP, mmHg mean (SD) | 73.1 (0.1) | 72.8 (0.2) | 73.5 (0.3) | 75.1 (0.4) | 75.5 (0.6) | <0.001 |
% of population represents the distribution of albuminuria weighted to represent the U.S. population. eGFR, estimated GFR (via the Chronic Kidney Disease Epidemiology Collaboration equation); iPTH, intact parathyroid hormone; BP, blood pressure.
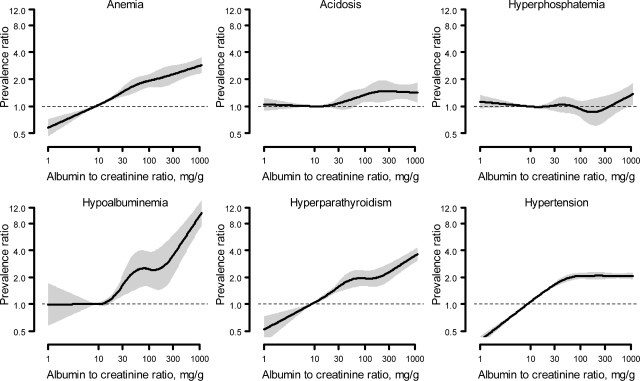
Higher levels of ACR were associated with anemia, acidosis, hypoalbuminemia, and hyperparathyroidism but not hyperphosphatemia (Figure 1). For hypertension, the prevalence ratio increased progressively through approximately ACR of 100 mg/g and thereafter remained stable. For acidosis, there was an increase in the prevalence ratio at approximately ACR of 30 mg/g. Table 2 shows the prevalence and adjusted prevalence ratios for the six complications by level of ACR. At ACR levels <10 mg/g, <10% of the population had anemia, acidosis, hyperphosphatemia, or hyperparathyroidism whereas 20.3% of the population had hypertension. The prevalence of anemia, acidosis, hypoalbuminemia, hyperparathyroidism, and hypertension was higher with higher ACR, whereas the prevalence of hyperphosphatemia was not. Overall, 13.5%, 16.0%, 10.6%, 28.9%, and 55.5% of those with ACR ≥300 mg/g had anemia, acidosis, hypoalbuminemia, hyperparathyroidism, and hypertension, respectively. After multivariable adjustment, there was a graded association between higher levels of ACR and higher prevalence ratios for anemia, acidosis, hypoalbuminemia, and hyperparathyroidism, but there was a substantial attenuation of the relationship between ACR and hypertension, such that the prevalence ratios were similar for all ACR levels >10 mg/g (Table 2). There appeared to be a threshold for hypoalbuminemia with a large increase in the prevalence ratio at ACR ≥300 mg/g compared with ACR of 30 to 299 mg/g. These results were similar in sensitivity analyses, where we modeled urine albumin concentration instead of ACR.
Figure 1.
Unadjusted prevalence ratios for anemia, acidosis, hyperphosphatemia, hypoalbuminemia, hyperparathyroidism, and hypertension associated with level of urine albumin-to-creatinine ratio.
Table 2.
Prevalence rates and age-adjusted and multivariable-adjusted prevalence ratios for anemia, acidosis, hyperphosphatemia, hypoalbuminemia, hyperparathyroidism, and hypertension associated with level of albuminuria
| Albuminuria | Anemia | Acidosis | Hyperphosphatemia | Hypoalbuminemia | Hyperparathyroidism | Hypertension |
|---|---|---|---|---|---|---|
| Unadjusted prevalence rates | ||||||
| <10 | 4.4% | 10.5% | 7.5% | 1.0% | 7.1% | 20.3% |
| 10 to 29 | 6.4% | 10.6% | 7.9% | 1.6% | 10.6% | 39.0% |
| 30 to 299 | 8.7% | 13.0% | 7.2% | 2.4% | 15.5% | 53.0% |
| ≥300 | 13.5.% | 16.0% | 8.6% | 10.6% | 28.9% | 55.5% |
| P trend | <0.001 | <0.001 | 0.295 | <0.001 | <0.001 | <0.001 |
| Age-adjusted prevalence ratios | ||||||
| <10 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 10 to 29 | 1.2 (1.1 to 1.5) | 1.1 (1.0 to 1.3) | 1.1 (0.9 to 1.3) | 1.4 (1.0 to 1.9) | 1.3 (1.1 to 1.6) | 1.3 (1.3 to 1.4) |
| 30 to 299 | 1.6 (1.3 to 1.9) | 1.4 (1.2 to 1.71) | 1.0 (0.9 to 1.3) | 1.8 (1.2 to 2.7) | 1.7 (1.4 to 2.1) | 1.5 (1.4 to 1.6) |
| ≥300 | 2.4 (1.9 to 3.0) | 1.5 (1.16 to 1.92) | 1.3 (1.0 to 1.8) | 7.1 (4.9 to 10.03) | 2.9 (2.1 to 4.0) | 1.5 (1.3 to 1.6) |
| P trend | <0.001 | <0.001 | 0.026 | <0.001 | <0.001 | <0.001 |
| Adjusted prevalence ratios: model 1a | ||||||
| <10 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 10 to 29 | 1.2 (1.0 to 1.4) | 1.0 (0.9 to 1.2) | 1.1 (0.9 to 1.3) | 1.2 (0.9 to 1.7) | 1.3 (1.1 to 1.6) | 1.3 (1.3 to 1.4) |
| 30 to 299 | 1.4 (1.2 to 1.6) | 1.3 (1.1 to 1.6) | 1.0 (0.8 to 1.2) | 1.6 (1.1 to 2.4) | 1.5 (1.2 to 1.9) | 1.5 (1.4 to 1.6) |
| ≥300 | 1.9 (1.5 to 2.4) | 1.4 (1.1 to 1.8) | 1.2 (0.9 to 1.6) | 6.1 (4.2 to 8.7) | 1.6 (1.2 to 2.2) | 1.4 (1.3 to 1.5) |
| P trend | <0.001 | <0.001 | 0.322 | <0.001 | 0.021 | <0.001 |
| Adjusted prevalence ratios: model 2b | ||||||
| <10 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 10 to 29 | 1.2 (1.0 to 1.4) | 1.0 (0.9 to 1.2) | 1.2 (0.9 to 1.2) | 1.1 (0.8 to 1.6) | 1.3 (1.0 to 1.7) | 1.3 (1.2 to 1.4) |
| 30 to 299 | 1.4 (1.2 to 1.7) | 1.3 (1.1 to 1.5) | 1.0 (0.8 to 1.2) | 1.1 (0.8 to 1.8) | 1.5 (1.2 to 1.9) | 1.4 (1.3 to 1.5) |
| ≥300 | 1.9 (1.5 to 2.4) | 1.4 (1.1 to 1.8) | 1.1 (0.8 to 1.5) | 4.4 (3.1 to 6.1) | 1.7 (1.2 to 2.5) | 1.3 (1.2 to 1.4) |
| P trend | <0.001 | <0.001 | 0.675 | <0.001 | 0.012 | <0.001 |
All prevalence ratios include adjustment for NHANES phase (1988–1994, 1999–2000, 2001–2002, 2003–2004, or 2005–2006).
aThe adjusted prevalence ratio model 1 includes age, race/ethnicity, gender, and estimated GFR.
bThe adjusted prevalence ratio model 2 includes variables in model 1 and cigarette smoking, body mass index, hypertension, diabetes, and C-reactive protein except in the model for hypertension, where hypertension was not adjusted.
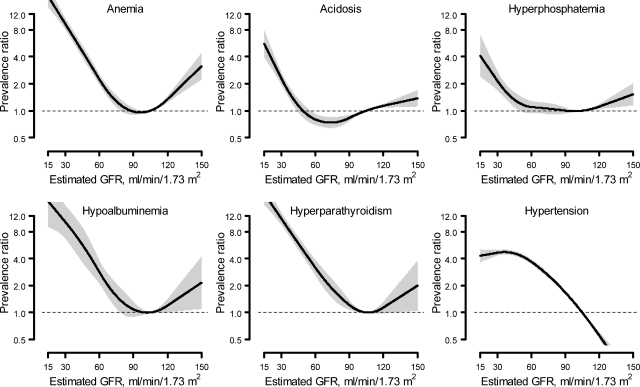
There was an increase in the prevalence of anemia, acidosis, hyperphosphatemia, hypoalbuminemia, and hyperparathyroidism with decreasing levels of eGFR, with the increase in prevalence beginning at approximately eGFR of 60 ml/min per 1.73 m2 for acidosis and hyperphosphatemia and at eGFR of 90 ml/min per 1.73 m2 for the remaining three complications. Other than for hypertension, there was also an increase in the prevalence ratio at eGFR levels ≥120 ml/min per 1.73 m2, with the most pronounced effects for anemia, hypoalbuminemia, and hyperparathyroidism (Figure 2). Table 3 shows the prevalence and prevalence ratios for complications by level of eGFR. In contrast to albuminuria, there was a significantly higher prevalence of each complication at lower eGFR. Other than hypertension, <10% of the population with eGFR ≥ 90 ml/min per 1.73 m2 had any complication, whereas for those with eGFR <30 ml/min per 1.73 m2 the prevalence of anemia, hyperphosphatemia, acidosis, hypoalbuminemia, hyperparathyroidism, and hypertension was 51.5%, 31.5%, 23.0%, 7.5%, 72.5%, and 82.1%, respectively. After age and multivariable adjustment, an association remained between lower eGFR levels and higher prevalence ratios for anemia, acidosis, hyperphosphatemia, and hyperparathyroidism, but not for hypoalbuminemia or hypertension (Table 3).
Figure 2.
Unadjusted prevalence ratios for anemia, acidosis, hyperphosphatemia, hypoalbuminemia, hyperparathyroidism, and hypertension associated with level of estimated GFR.
Table 3.
Prevalence rates and age-adjusted and multivariable-adjusted prevalence ratios for anemia, acidosis, hyperphosphatemia, hypoalbuminemia, hyperparathyroidism, and hypertension associated with level of eGFR
| eGFR | Anemia | Acidosis | Hyperphosphatemia | Hypoalbuminemia | Hyperparathyroidism | Hypertension |
|---|---|---|---|---|---|---|
| Unadjusted prevalence rates | ||||||
| ≥120 | 6.6% | 14.5% | 8.3% | 1.5% | 7.1% | 7.1% |
| 90 to 119 | 4.0% | 11.2% | 7.2% | 1.0% | 5.5% | 18.3% |
| 60 to 89 | 4.7% | 8.4% | 7.4% | 1.3% | 9.4% | 41.0% |
| 45 to 59 | 12.3% | 9.4% | 9.2% | 2.8% | 23.0% | 71.8% |
| 30 to 44 | 22.7% | 18.1% | 9.3% | 9.0% | 44.0% | 78.3% |
| <30 | 51.5% | 31.5% | 23.0% | 7.5%a | 72.5% | 82.1% |
| P trend | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 |
| Age-adjusted prevalence ratios | ||||||
| ≥120 | 2.4 (2.0 to 3.0) | 1.0 (0.9 to 1.2) | 0.9 (0.8 to 1.0) | 2.5 (1.7 to 3.8) | 1.5 (1.0 to 2.2) | 0.8 (0.7 to 0.9) |
| 90 to 119 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 60 to 89 | 0.7 (0.6 to 0.9) | 1.0 (1.0 to 1.2) | 1.3 (1.1 to 1.4) | 0.7 (0.5 to 0.9) | 1.5 (1.2 to 1.9) | 1.1 (1.0 to 1.1) |
| 45 to 59 | 1.4 (1.1 to 1.6) | 1.4 (1.1 to 1.8) | 2.0 (1.7 to 2.4) | 0.8 (0.5 to 1.5) | 3.2 (2.3 to 4.4) | 1.1 (1.1 to 1.1) |
| 30 to 44 | 2.3 (1.8 to 2.9) | 2.7 (2.2 to 3.4) | 2.1 (1.5 to 3.1) | 2.5 (1.3 to 4.8) | 5.8 (4.2 to 7.9) | 1.0 (1.0 to 1.1) |
| <30 | 5.1 (4.1 to 6.5) | 4.9 (3.5 to 6.8) | 5.1 (3.4 to 7.6) | 2.0 (0.9 to 4.7) | 9.6 (7.0 to 13.3) | 1.1 (0.9 to 1.2) |
| P trend | <0.001 | <0.001 | <0.001 | 0.096 | <0.001 | <0.001 |
| Adjusted prevalence ratios: model 1b | ||||||
| ≥120 | 1.6 (1.3 to 1.9) | 0.9 (0.8 to 1.1) | 0.9 (0.7 to 1.0) | 1.6 (1.1 to 2.3) | 1.2 (0.8 to 1.7) | 0.7 (0.6 to 0.7) |
| 90 to 119 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 60 to 89 | 0.9 (0.7 to 1.0) | 1.1 (0.9 to 1.2) | 1.3 (1.2 to 1.5) | 0.8 (0.6 to 1.1) | 1.7 (1.3 to 2.1) | 1.1 (1.0 to 1.2) |
| 45 to 59 | 1.5 (1.3 to 1.8) | 1.4 (1.1 to 1.8) | 2.0 (1.7 to 2.4) | 0.7 (0.5 to 1.5) | 3.4 (2.5 to 4.6) | 1.1 (1.0 to 1.1) |
| 30 to 44 | 2.4 (1.9 to 3.0) | 2.6 (2.1 to 3.3) | 2.2 (1.5 to 3.2) | 1.9 (1.3 to 3.9) | 5.7 (4.3 to 7.6) | 1.0 (0.9 to 1.0) |
| <30 | 4.4 (3.4 to 5.8) | 4.7 (3.3 to 6.7) | 5.33 (3.6 to 7.8) | 1.2 (0.5 to 2.8) | 8.2 (5.7 to 11.8) | 0.8 (0.7 to 1.0) |
| P trend | <0.001 | <0.001 | <0.001 | 0.151 | <0.001 | 0.236 |
| Adjusted prevalence ratios: model 2c | ||||||
| ≥120 | 1.5 (1.3 to 1.8) | 0.7 (0.9 to 1.1) | 0.9 (0.8 to 1.1) | 1.6 (1.0 to 2.4) | 1.1 (0.8 to 1.7) | 0.7 (0.6 to 0.8) |
| 90 to 119 | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) | 1 (reference) |
| 60 to 89 | 0.9 (0.7 to 1.0) | 1.1 (0.9 to 1.2) | 1.3 (1.2 to 1.5) | 0.9 (0.6 to 1.1) | 1.6 (1.2 to 2.0) | 1.1 (1.1 to 1.2) |
| 45 to 59 | 1.5 (1.3 to 1.8) | 1.4 (1.1 to 1.8) | 2.1 (1.7 to 2.5) | 0.8 (0.5 to 1.4) | 3.1 (2.2 to 4.4) | 1.0 (1.0 to 1.1) |
| 30 to 44 | 2.3 (1.8 to 2.9) | 2.8 (2.1 to 3.6) | 2.2 (1.5 to 3.3) | 1.8 (1.0 to 3.3) | 5.0 (3.6 to 6.9) | 1.0 (0.9 to 1.0) |
| <30 | 4.2 (3.1 to 5.7) | 4.9 (3.4 to 7.1) | 4.7 (3.2 to 6.9) | 1.3 (0.5 to 3.0) | 8.2 (5.7 to 11.5) | 0.8 (0.7 to 1.0) |
| P trend | <0.001 | <0.001 | <0.001 | 0.343 | <0.001 | 0.866 |
All prevalence ratios include adjustment for NHANES phase (1988–1994, 1999–2000, 2001–2002, 2003–2004, or 2005–2006).
aEstimate may be unreliable due to limited number of participants with low serum albumin in this cell.
bThe adjusted prevalence ratio model 1 includes age, race/ethnicity, gender, and log albuminuria.
cThe adjusted prevalence ratio model 2 includes variables in model 1 and cigarette smoking, body mass index, hypertension, diabetes, and C-reactive protein except in the model for hypertension, where hypertension is not adjusted.
In stratified analyses, the association between ACR and anemia and acidosis was present for certain eGFR strata (40 to 59 and ≥120 ml/min per 1.73 m2 for anemia and 90 to 119 ml/min per 1.73 m2 for acidosis, P values for the interaction of <0.001 and 0.036, respectively), whereas the association between ACR and hypoalbuminemia and hypertension was significant for multiple eGFR strata (P values for the interaction of 0.059 and 0.075, respectively; Table 4 with the number of participants in each ACR/eGFR stratum provided in Supplemental Table 2). Higher ACR was not associated with an increased age-, race/ethnicity-, and sex-adjusted prevalence for hyperphosphatemia within any eGFR strata. Indeed, for eGFR levels 30 to 89 ml/min per 1.73 m2, the prevalence of hyperphosphatemia was lower at higher ACR categories. Within most ACR strata, the adjusted prevalence of anemia, acidosis, hyperphosphatemia, and hypertension was higher at lower levels of eGFR.
Table 4.
Age-, race/ethnicity-, and gender-adjusted prevalence of anemia, acidosis, hyperphosphatemia, hypoalbuminemia, and hypertension by cross-tabulation of albuminuria and estimated GFR
| eGFR Category | ACR Category (%) |
|||||
|---|---|---|---|---|---|---|
| Overall | <10 | 10 to 29 | 30 to 299 | ≥300 | P Trend | |
| Anemia | ||||||
| overall | 6.3 | 7.5 | 8.7 | 12.8 | <0.001 | |
| ≥120 | 9.5 | 8.1 | 12.9 | 12.1 | 10.4 | 0.041 |
| 90 to 119 | 6.2 | 5.9 | 6.5 | 7.6 | 5.9 | 0.278 |
| 60 to 89 | 5.4 | 5.1 | 6.0 | 5.8 | 5.5 | 0.201 |
| 45 to 59 | 10.1 | 9.7 | 7.5 | 12.3 | 16.8 | 0.049 |
| 30 to 44 | 17.5 | 17.2 | 17.7 | 15.0 | 23.5 | 0.730 |
| <30 | 41.5 | 36.8 | 31.5 | 35.1 | 56.9 | 0.082 |
| P trend | <0.001 | 0.001 | 0.040 | <0.001 | <0.001 | P interaction <0.001 |
| Acidosis | ||||||
| overall | 8.5 | 8.6 | 11.5 | 15.1 | <0.001 | |
| ≥120 | 9.0 | 8.6 | 7.1 | 13.3 | 13.5 | 0.187 |
| 90 to 119 | 8.6 | 8.1 | 7.9 | 11.0 | 12.8 | 0.007 |
| 60 to 89 | 8.0 | 7.5 | 8.2 | 9.3 | 9.7 | 0.067 |
| 45 to 59 | 10.7 | 9.6 | 13.2 | 10.5 | 7.4 | 0.767 |
| 30 to 44 | 20.9 | 17.6 | 21.1 | 18.7 | 30.0 | 0.442 |
| <30 | 36.3 | 9.9 | 44.6 | 22.8 | 50.8 | 0.056 |
| P trend | <0.001 | 0.939 | <0.001 | 0.010 | 0.001 | P interaction = 0.036 |
| Hyperphosphatemia | ||||||
| overall | 6.7 | 7.2 | 6.7 | 8.4 | 0.234 | |
| ≥120 | 4.7 | 4.6 | 4.5 | 4.1 | 11.7 | 0.071 |
| 90 to 119 | 5.3 | 5.0 | 5.9 | 5.0 | 5.2 | 0.595 |
| 60 to 89 | 7.0 | 7.0 | 7.2 | 7.0 | 4.7 | 0.559 |
| 45 to 59 | 10.8 | 11.6 | 11.0 | 9.8 | 5.0 | 0.182 |
| 30 to 44 | 11.5 | 14.1 | 8.8 | 9.6 | 12.5 | 0.881 |
| <30 | 27.5 | 22.8 | 27.9 | 18.7 | 35.3 | 0.186 |
| P trend | <0.001 | <0.001 | 0.035 | 0.011 | 0.099 | P interaction = 0.226 |
| Hypoalbuminemia | ||||||
| overall | 1.5 | 1.7 | 2.3 | 10.3 | <0.001 | |
| ≥120 | 3.4 | 2.7 | 2.3 | 5.1 | 16.7 | 0.004 |
| 90 to 119 | 1.9 | 1.5 | 2.3 | 1.5 | 9.4 | <0.001 |
| 60 to 89 | 1.5 | 1.3 | 0.8 | 1.7 | 9.4 | <0.001 |
| 45 to 59 | 1.7 | 0.6 | 1.8 | 2.7 | 6.3 | <0.001 |
| 30 to 44 | 5.3 | 3.3 | 4.3 | 5.3 | 14.1 | 0.070 |
| <30 | 4.2 | 4.9 | 0.5 | 0.3 | 10.2 | 0.147 |
| P trend | 0.098 | 0.346 | 0.694 | 0.079 | 0.095 | P interaction = 0.059 |
| Hypertension | ||||||
| overall | 39.2 | 52.9 | 63.2 | 63.9 | <0.001 | |
| ≥120 | 36.3 | 28.7 | 40.3 | 51.6 | 65.6 | <0.001 |
| 90 to 119 | 42.7 | 35.9 | 50.9 | 62.6 | 57.6 | <0.001 |
| 60 to 89 | 45.6 | 40.2 | 54.8 | 61.3 | 58.0 | <0.001 |
| 45 to 59 | 56.0 | 51.4 | 56.1 | 71.0 | 75.2 | <0.001 |
| 30 to 44 | 58.6 | 47.6 | 58.5 | 68.8 | 77.1 | 0.102 |
| <30 | 63.4 | 41.9 | 41.2 | 77.4 | 83.4 | 0.034 |
| P trend | <0.001 | 0.012 | 0.039 | 0.019 | <0.001 | P interaction <0.075 |
Prevalence values are adjusted to age 60 years, 50% men and 50% women, and 75% non-Hispanic whites, 10% non-Hispanic blacks, 10% Hispanic, and 5% other race/ethnicities.
ACR, urine albumin-to-creatinine ratio (mg/g); eGFR, estimated GFR (ml/min per 1.73 m2 via the Chronic Kidney Disease Epidemiology Collaboration equation).
DISCUSSION
GFR is traditionally considered the best marker for the overall function of the kidney and is therefore used to classify CKD into stages. Recent publications have emphasized the role of albuminuria, a marker of kidney damage to the glomerular permselectivity barrier, in predicting outcomes.8–12,16 Clinical encounters also focus on detection, evaluation, and management of current conditions, as well as future risk of outcomes, and there are fewer data available on the association between ACR and concurrent complications of CKD. The present analysis of a general population sample of U.S. adults showed a minimal association between higher levels of ACR and an increased prevalence of anemia, hypoalbuminemia, acidosis, hypertension, and hyperparathyroidism, and no association with hyperphosphatemia. In contrast, lower eGFR was strongly associated with all six of these complications, although hypertension and hypoalbuminemia were attenuated after adjustment for demographic factors and comorbid conditions. These findings have implications for clinical practice, research, and the classification of CKD.
Several studies have previously documented the strong association of GFR to complications of CKD.7,14,15,17–19 Consistent with these prior studies, in the current analysis, a strong relationship was observed between lower eGFR and a higher prevalence for all six concurrent complications. Associations remained strong for anemia, acidosis, hyperphosphatemia, and hyperparathyroidism, even after adjustment for demographic factors, cigarette smoking, body mass index, diabetes mellitus, hypertension, and ACR. Associations with hypertension were substantially attenuated after adjustment for age, which is likely secondary to the increasing prevalence of hypertension with older age regardless of level of eGFR. Although associations with hypoalbuminemia were nonlinear at the lowest levels of eGFR, this may be secondary to small numbers of people with hypoalbuminemia and severe reductions in eGFR. For hyperphosphatemia, hyperparathyroidism, and hypertension, a significant association was observed at eGFR levels as high as 90 ml/min per 1.73 m2, whereas the significant association for anemia and acidosis started at eGFR of 60 ml/min per 1.73 m2. A higher prevalence of complications was observed at eGFR >120 ml/min per 1.73 m2 for all complications, other than hypertension.
A graded relationship between ACR and anemia, acidosis, hypoalbuminemia, and hyperparathyroidism was observed. Compared with ACR <10 mg/g, people with ACR ≥300 mg/g had 2.4-, 1.5-, 7.1-, and 2.9-fold increases in the age-adjusted prevalence ratio for anemia, acidosis, hypoalbuminemia, and hyperparathyroidism, respectively. The prevalence of anemia and hyperparathyroidism began to increase at levels of ACR of 10 to 29 mg/g and between 30 and 299 mg/g for acidosis and hypoalbuminemia, and these associations were robust to adjustment for age, sex, race/ethnicity, eGFR, cigarette smoking, diabetes, hypertension, and body mass index. Nevertheless, the overall association between ACR and acidosis was weak and, for both acidosis and anemia, the association was not consistently present within different strata of eGFR. For all complications, the associations were not as strong as was observed for eGFR. No association was present between higher ACR levels and the prevalence of hyperphosphatemia, and even in the analysis stratified by eGFR and ACR, the association was NS. As with eGFR, the relationship with hypertension was substantially attenuated after adjustment, again likely demonstrating the collinearity with age. Little data is available on ACR and each of these complications in the general population. However, in patients with diabetes, it has been previously demonstrated that there is a relationship between higher levels of proteinuria and lower hemoglobin levels.20,21
Recently, there have been several publications that have demonstrated a strong association between albuminuria and proteinuria with long-term clinical outcomes. These associations are independent of eGFR, consistent across outcomes, and comparable in magnitude to the excess risk associated with lower eGFR.8–12,16 Taken together, data from multiple sources now demonstrate that albuminuria is a strong risk factor for long-term outcomes and is moderately associated with some but not all complications of CKD. These divergent findings likely reflect the relationship of concurrent complications with different functions of the kidney, the heterogeneous nature of CKD, and other nonrenal causes for these abnormalities. One possible explanation is that excretions of both hydrogen ions and phosphate are specifically regulated by the renal tubule and that these excretory functions are more closely related to GFR. A second possible explanation is that albuminuria may reflect systemic disorders that result in inflammation and generalized endothelial damage, and that anemia, hypertension, and hyperparathyroidism are secondary to these systemic disorders as well as decreased GFR. This is more expected with anemia and hypertension than with hyperparathyroidism, but there is some preliminary supporting evidence for this hypothesis. Prior studies have described the association of low vitamin D levels to higher C-reactive protein.22 Treatment with vitamin D in animal studies and humans has decreased albuminuria.23–25 Hypoalbuminemia may relate in part to urinary protein loss, or to malnutrition, but may also be related to systemic inflammation and endothelial dysfunction.
In many prior analyses, a J-shaped relationship has been observed between eGFR and long-term and concurrent complications of CKD, reflecting low creatinine generation and overestimation of measured GFR in people with muscle wasting.3,26 In the current analyses, the J-shaped relationship was observed with all complications other than hypertension but was most pronounced with anemia, hypoalbuminemia, and hyperparathyroidism. Lower levels of hemoglobin, bicarbonate, and serum albumin at higher eGFR levels have been observed previously. However, most prior analyses have focused on hyperparathyroidism at lower levels of GFR, and it has not been reported at higher eGFR levels.13–14,27 Presumably, the J-shaped relationship identifies a group of people with chronic disease leading to muscle wasting. If anemia, hypoalbuminemia, and hyperparathyroidism do reflect systemic disease, as was hypothesized above, then this may provide a possible explanation as to why these conditions were most strongly associated with the J-shaped curve.
Currently, there are proposals to include albuminuria in the staging system for CKD given its predictive power for the occurrence of long-term outcomes.28 These proposals assume that its inclusion will facilitate patient care. For long-term outcomes, the strong associations may mean that knowing a patient's albuminuria level will help clinicians develop prognoses for patients, and as such may possibly facilitate decisions regarding treatment. More studies are required before specific strategies can be implemented. In the current study, ACR was associated with only some of the complications that were evaluated, and apart from hypoalbuminemia, the prevalence of complications at higher levels of ACR among individuals with eGFR >60 ml/min per 1.73 m2 was low. As such, ACR does not appear to contribute much additional information for identification of patients who should be tested for most CKD complications or who may require specialized care for these complications. Recommendations for the evaluation and management of decreased GFR and elevated albuminuria should differ depending upon the specific outcome and complication in question.
Strengths of the analysis include a large ethnically diverse representative sample, measurement of multiple CKD complications, and rigorously collected data using standardized protocols and laboratory procedures. In addition, there are several limitations of this analysis. First, we evaluated only selected complications. Nevertheless, we purposely selected complications that reflect different biologic processes. Second, ACR was used to assess albuminuria and, consistent with most large epidemiology studies, a 24-hour urine collection was not feasible. Only a single ACR measurement was available. It is estimated that approximately 50% of individuals with ACR ≥30 mg/g on a single measurement will not have persistent albuminuria.29 Furthermore, the ACR may be high due to extremely low levels of creatinine as well as high levels of albuminuria; however, results were consistent when considering urinary albumin concentration rather than ACR. Third, different assays were used across the NHANES for some biochemical measures (e.g., bicarbonate). Fourth, despite a large sample size, there were some cells with small numbers. In particular, there were few people with lower levels of eGFR and higher albuminuria levels. Hypoalbuminemia is uncommon and associations for this complication had wide confidence intervals, and the patterns observed may be secondary to these small numbers. Fifth, immunoreactive parathyroid hormone (iPTH) was only available on a subset of participants, and we were not able to perform an analysis stratified by ACR and eGFR for this complication. Finally, although we adjusted for C-reactive protein, we are not able to state with certainty that associations between albuminuria or eGFR with concurrent complications are mediated by inflammation because only a low-sensitivity C-reactive protein assay was available in NHANES III.
In conclusion, ACR was significantly associated with anemia, acidosis hypoalbuminemia, hyperparathyroidism, and hypertension but not with hyperphosphatemia. However, the associations for anemia and acidosis were not consistent within eGFR strata, and relationships were substantially weaker than those observed between eGFR and these same complications. Revisions to the CKD classification system should consider implications for all aspects of the clinical action plan including these complications.
CONCISE METHODS
Study Population
The NHANES are cross-sectional, multistage, stratified, clustered probability samples of the U.S. civilian noninstitutionalized population conducted by the National Center for Health Statistics. The NHANES cycles included in the current analysis were conducted from 1988 through 1994 in two phases (1988–1991 and 1991–1994) and from 1999 through 2006 in four phases (1999–2000, 2001–2002, 2003–2004, and 2005–2006).30 The total number of adults enrolled included in these six phases was 39,136. The study population was limited to participants who completed a medical evaluation in the NHANES mobile examination center and were aged 20 years or older. Those who were pregnant or were missing measurements of urinary albumin or creatinine excretion, serum creatinine, phosphorous, hemoglobin, bicarbonate, serum albumin, or BP, or who had eGFR <15 ml/min per 1.73 m2 were excluded from the current analyses. After these exclusions, data for 30,528 participants were available for the analyses of anemia, acidosis, hyperphosphatemia, hypoalbuminemia, and hypertension. Data were available for 8,242 NHANES 2003–2004 and 2005–2006 participants for the analysis of iPTH.
Age, sex, race/ethnicity, and cigarette smoking were self-reported. Height and weight were measured, and body mass index was calculated. Diabetes mellitus was defined as a self-report of a previous diagnosis, not during pregnancy, with concurrent use of insulin or oral hypoglycemic medication or a glucose ≥126 mg/dl among participants who fasted ≥9 hours before their study visit or ≥200 mg/dl among nonfasting participants.
Measures of Kidney Function
Serum creatinine was recalibrated to standardized creatinine measurements obtained at the Cleveland Clinic Research Laboratory.31 eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation.2 eGFR categories included ≥120, 90 to 119, 60 to 89, 45 to 59, 30 to 44, and 15 to 29 ml/min per 1.73 m2.28 Urine albumin and creatinine concentrations were obtained from a random spot urine sample from participants examined at the mobile examination center using a clean-catch technique and sterile containers. Urinary ACR was computed and is reported in milligrams per gram (mg/g; 1 mg/g = 0.131 mg/mmol). Albuminuria categories included normal, high-normal, high, and very high defined as ACR <10, 10 to 29, 30 to 299, and ≥300 mg/g, respectively. In a sensitivity analysis, we repeated the analyses using urine albumin instead of ACR, with categories of <12, 12 to 31, 32 to 99, and >100 mg/dl.
Assessment of CKD Complications
Complications that reflect different biologic mechanisms were included. Anemia was defined by the World Health Organization as hemoglobin levels <12 g/dl for women and <13.5 g/dl for men. Acidosis was defined as serum bicarbonate <21 mEq/L. Hyperphosphatemia was defined as serum phosphate ≥4.5 mg/dl. Hypoalbuminemia was defined as serum albumin <3.5 g/dl. Hyperparathyroidism was defined as iPTH levels ≥70 pg/ml. To standardize the laboratory values across all surveys, the age-, sex-, and race/ethnicity-adjusted difference in the mean level for hemoglobin, bicarbonate, phosphate, serum albumin, and iPTH for participants 20 to 39 years old without diabetes and hypertension, with eGFR >60 ml/min per 1.73 m2, and with ACR <10 for each survey was calculated. Differences from the value for NHANES 2005–2006 was then added/subtracted for values for the other NHANES phases. BP was measured six times in NHANES III and three times in NHANES 1999–2006. With use of the average of all available BP measurements, hypertension was defined as a systolic BP ≥140 mmHg, diastolic BP ≥90 mmHg, or self-reported use of antihypertensive medication.
Statistical Analysis
The unadjusted prevalence ratios for each CKD complication associated with ACR and eGFR, modeled separately as continuous variables, were calculated using restricted quadratic splines with knots at ACR levels of 10, 30, 100, and 300 mg/g and eGFR levels of 30, 60, 90, and 120 ml/min per 1.73 m2. ACR was log-transformed for the spline analyses. Prevalence ratios are recommended for use in cross-sectional studies with common outcomes32; therefore, with ACR <10 mg/g as the reference group, log-linear generalized estimating equations were used to calculate the prevalence ratios for anemia, acidosis, hyperphosphatemia, hyperparathyroidism, hypoalbuminemia, and hypertension associated with higher levels of ACR. Prevalence ratios were adjusted initially for age and NHANES phase and subsequently for age, sex, race/ethnicity, and eGFR (or ACR for the associations of eGFR with complications), and NHANES phase. A final model included additional adjustment for cigarette smoking, body mass index, diabetes mellitus, and hypertension, except in the final model with hypertension as the outcome that included cigarette smoking, body mass index, and diabetes mellitus. The prevalence ratios for these complications associated with eGFR categories, with levels of 90 to 119 ml/min per 1.73 m2 as the referent, were also calculated. In sensitivity analyses, we modeled urine albumin instead of ACR, with categories of <12, 12 to 31, 32 to 99, and >100 mg/dl.
The age-, race/ethnicity-, and sex-adjusted prevalence of anemia, acidosis, hyperphosphatemia, hypoalbuminemia, and hypertension were calculated by the cross-categorization of ACR and eGFR. The prevalence estimates were adjusted to age 60 years and to a sex-race distribution similar to the U.S. population (50% women, 75% non-Hispanic white, 10% non-Hispanic black, 10% Hispanic, and 5% other race/ethnicity). The number of participants with iPTH levels was not sufficient to achieve reliable estimates for the cross-categorization analysis.
Analyses were performed incorporating the sampling weights to obtain unbiased estimates using SUDAAN version 10 (Research Triangle Institute, Research Triangle Park, NC). Sampling weights were combined across all survey phases from NHANES 1988–2006.
DISCLOSURES
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. James MT, Hemmelgarn BR, Tonelli M: Early recognition and prevention of chronic kidney disease. Lancet 375: 1296–1309, 2010 [DOI] [PubMed] [Google Scholar]
- 2. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J: A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW: Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 4. Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 5. Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 6. Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, Zeeuw D, Hostetter TH, Lameire N, Eknoyan G: Definition and classification of chronic kidney disease: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 67: 2089–2100, 2005 [DOI] [PubMed] [Google Scholar]
- 7. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002 [PubMed] [Google Scholar]
- 8. Hemmelgarn BR, Manns BJ, Lloyd A, James MT, Klarenbach S, Quinn RR, Wiebe N, Tonelli M: Relation between kidney function, proteinuria, and adverse outcomes. JAMA 303: 423–429, 2010 [DOI] [PubMed] [Google Scholar]
- 9. Chronic Kidney Disease Prognosis Consortium, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT: Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT; Chronic Kidney Disease Prognosis Consortium: Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 11. Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium: Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J; Chronic Kidney Disease Prognosis Consortium: Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Astor B, Muntner P, Levin A, Eustace J, Coresh J: Association of kidney function with anemia: The Third National Health and Nutrition Examination Survey (1988–1994). Arch Intern Med 162: 1401–1408, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Eustace JA, Astor B, Muntner PM, Ikizler TA, Coresh J: Prevalence of acidosis and inflammation and their association with low serum albumin in chronic kidney disease. Kidney Int 65: 1031–1040, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Muntner P, Jones TM, Hyre AD, Melamed ML, Alper A, Raggi P, Leonard MB: Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin J Am Soc Nephrol 4: 186–194, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Astor BC, Hallan SI, Miller ER, 3rd, Yeung E, Coresh J: Glomerular filtration rate, albuminuria, and risk of cardiovascular and all-cause mortality in the US population. Am J Epidemiol 167: 1226–1234, 2008 [DOI] [PubMed] [Google Scholar]
- 17. Hsu C-J, McCulloch C, Curhan G: Epidemiology of anemia associated with chronic renal insufficiency among adults in the United States: Results from the Third National Health and Nutrition Examination Survey. J Am Soc Nephrol 13: 504–510, 2002 [DOI] [PubMed] [Google Scholar]
- 18. Hsu CY, Chertow GM: Elevations of serum phosphorus and potassium in mild to moderate chronic renal insufficiency. Nephrol Dial Transplant 17: 1419–1425, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Astor BC, Arnett DK, Brown A, Coresh J: Association of kidney function and hemoglobin with left ventricular morphology among African Americans: The Atherosclerosis Risk in Communities (ARIC) study. Am J Kidney Dis 43: 836–845, 2004 [DOI] [PubMed] [Google Scholar]
- 20. Wolf G, Muller N, Hunger-Battefeld W, Kloos C, Muller UA: Hemoglobin concentrations are closely linked to renal function in patients with type 1 or 2 diabetes mellitus. Kidney Blood Press Res 31: 313–321, 2008 [DOI] [PubMed] [Google Scholar]
- 21. Sonmez A, Yilmaz MI, Saglam M, Kilic S, Eyileten T, Uckaya G, Caglar K, Oguz Y, Vural A, Yenicesu M, Kutlu M, Kinalp C, Zoccali C: The relationship between hemoglobin levels and endothelial functions in diabetes mellitus. Clin J Am Soc Nephrol 5: 45–50, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kendrick J, Targher G, Smits G, Chonchol M: 25-Hydroxyvitamin D deficiency and inflammation and their association with hemoglobin levels in chronic kidney disease. Am J Nephrol 30: 64–72, 2009 [DOI] [PubMed] [Google Scholar]
- 23. Agarwal R, Acharya M, Tian J, Hippensteel RL, Melnick JZ, Qiu P, Williams L, Batlle D: Antiproteinuric effect of oral paricalcitol in chronic kidney disease. Kidney Int 68: 2823–2828, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Kuhlmann A, Haas CS, Gross ML, Reulbach U, Holzinger M, Schwarz U, Ritz E, Amann K: 1,25-Dihydroxyvitamin D3 decreases podocyte loss and podocyte hypertrophy in the subtotally nephrectomized rat. Am J Physiol 286: F526–F533, 2004 [DOI] [PubMed] [Google Scholar]
- 25. Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E: Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 53: 1696–1705, 1998 [DOI] [PubMed] [Google Scholar]
- 26. Manjunath G, Tighiouart H, Coresh J, Macleod B, Salem DN, Griffith JL, Levey AS, Sarnak MJ: Level of kidney function as a risk factor for cardiovascular outcomes in the elderly. Kidney Int 63: 1121–1129, 2003 [DOI] [PubMed] [Google Scholar]
- 27. National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39: S1–S266, 2002. (Available from: http://www.kidney.org/professionals/kdoqi/guidelines_ckd/toc.htm) [PubMed] [Google Scholar]
- 28. Levey A, de Jong P, Coresh J, El Nahas M, Astor B, Matsushita K, Gansevoort R, Kasiske B, Eckardt K: The Definition, Classification, and Prognosis of Chronic Kidney Disease: A KDIGO Controversies Conference Report. Kidney Int 80: 17–28, 2011 [DOI] [PubMed] [Google Scholar]
- 29. Coresh J, Astor BC, Greene T, Eknoyan G, Levey AS: Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am J Kidney Dis 41: 1–12, 2003 [DOI] [PubMed] [Google Scholar]
- 30. National Center for Health Statistics Centers for Disease Control and Prevention: National Health and Nutrition Examination Survey. Available from: http://www.cdc.gov/nchs/nhanes.htm Accessed July 21, 2011
- 31. Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, Coresh J: Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999–2004. Am J Kidney Dis 50: 918–926, 2007 [DOI] [PubMed] [Google Scholar]
- 32. Barros AJ, Hirakata VN: Alternatives for logistic regression in cross-sectional studies: An empirical comparison of models that directly estimate the prevalence ratio. BMC Med Res Methodol 3: 21, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.