Abstract
Recent proteomic data have uncovered an interdependence of PI3K and STAT3. In PI3K-tranformed murine cells, STAT3 is phosphorylated on Y705 and activated in a PI3K-dependent manner. Dominant negative STAT3 interferes with PI3K-induced oncogenic transformation. Phosphorylation of STAT3 in PI3K-transformed murine cells is mediated by the TEC kinase BMX. Observations on glioblastoma stem cells reveal similar critical roles for STAT3 and BMX. The new data document an important role of STAT3 in PI3K-driven oncogenic transformation and mark BMX as a promising therapeutic target that could enhance the effectiveness of PI3K inhibitors.
Keywords: SILAC, TEC kinase, TOR, PH domain, BMX
Introduction
In cellular signaling networks, diverse inputs trigger a fixed sequence of events that includes branching points, crosstalk with other signaling networks and feedback loops. All of these combine to induce characteristic effects on cellular functions. For such systems, it is possible to draw a map of interactions and connections that depicts the core pathway as well as branches and feedback loops. Although such maps commonly include a distinct number of proteins, they are open to additions and modifications. This review will focus on a modification of an established signaling system, viz. the essential contribution of STAT3 to the PI3K-TOR pathway in cancer.
We will first summarize the consensus features of the PI3K-TOR signaling and control system and of STAT3 activation and signaling. Against this background, we will consider highlights from new proteomic data obtained with cells that are transformed into cancer cells by PI3K. Some of these data are not explicable by the known effects of upregulated PI3K and suggest the involvement of STAT3. Evidence supporting this suggestion will be examined, and the relevance of these findings for human cancer and for cancer therapy will be explored.
Canonical PI3K and STAT3 signaling
PI3K is a lipid kinase that controls a core signaling and regulatory network in the cell. This network responds to multiple inputs including growth signals as well as metabolic and nutritional cues (1–3). PI3K controls cell growth, proliferation and survival, anabolic and autophagic activities and cytoskeletal organization. The oncogenic signal originating from hyperactive PI3K proceeds through AKT via the TSC complex and RHEB to activate TOR in a multiprotein complex referred to as TORC1. TORC1 stimulates protein synthesis and inhibits autophagy. Activation of AKT and TORC1 are necessary but probably not sufficient for oncogenic cellular transformation. Other essential elements of the PI3K-generated oncogenic signal, possibly involving the TORC2 complex, remain to be identified (4).
STAT3 belongs to a family of transcriptional regulators. They are mobilized in response to interferon and initiate the transcription of interferon-induced genes (5, 6). STAT3 is activated by phosphorylation on residue Y705 which induces homodimerization and heterodimerization with other STAT proteins and results in nuclear translocation and activation of the STAT3 transcriptional regulator function. The activating phosphorylation of STAT3 can be triggered by cytokines such as IL6 and also by receptor and non-receptor tyrosine kinases such as EGFR (7, 8) and SRC (9). Activation by IL6 is mediated by members of the JAK kinase family; the tyrosine kinases EGFR and SRC can directly phosphorylate STAT3.
The consensus interaction networks of PI3K-TOR and of STAT3 do not include established or default lines of communication (Fig. 1). But an interdependence of PI3K and of STAT3 signaling in cancer is now emerging from basic and from clinical studies.
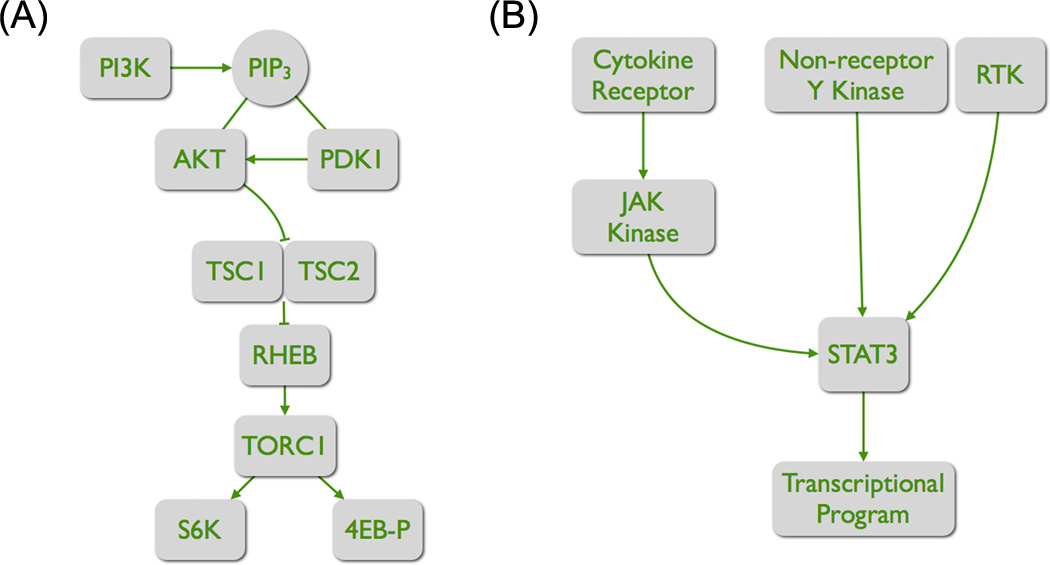
Figure 1. The core elements of two distinct signaling pathways.
(A) The PI3K-TORC1 pathway. The product of PI3K, PIP3, recruits the two serine-threonine kinases AKT and PDK1. PDK1 phosphorylates and thereby activates AKT. AKT has numerous targets. By phosphorylating TSC2, it inactivates the GTPase activity of the TSC complex. As a consequence, the GTP-bound form of the RAS-like protein RHEB increases, and RHEB activates TORC1. Important targets of TORC1 are S6K and 4EB-P. Phosphorylation of these targets increases the rate of protein synthesis.
(B) The activation of STAT3. Two principal routes lead to the phosphorylation and activation of STAT3. Binding of a cytokine to its cognate receptor leads to receptor dimerization and transphosphorylation of the associated JAK kinases. These kinases then phosphorylate STAT3. The phosphorylation results in dimerization, nuclear translocation and transcriptional regulator activity. Alternatively, STAT3 can be directly phosphorylated by RTKs and by non-receptor tyrosine kinases.
STAT3 is activated in murine cells transformed by PI3K
Methods for the analysis of the global proteome are now available and are increasingly applied to cancer cells to identify changes that are specific for oncogenic transformation (10). Such studies require comparison with a normal progenitor cell that, in its genetic and epigenetic make-up, is identical to the cancer cell, except for the differences that are the cause of the oncogenic transformation. Stable transfection with an activated oncogene can generate such an isogenic pair; a more stringent method is to knock in the activated oncogene into the genome of the normal progenitor to effect oncogenic transformation (11, 12).
A recent study has used SILAC (stable isotope labeling with amino acids in cell culture) in conjunction with tandem mass spectroscopy to identify and analyze proteomic differences between the murine embryonic fibroblast line C3H 10T1/2 and its isogenic, PI3K-transformed descendant (13). The transformed cells carry the H1047R mutation of PIK3CA as an actively expressed and stably integrated transgene. PIK3CA encodes the catalytic subunit p110α of PI3K, and H1047R is a highly oncogenic mutant of this gene (14, 15). The transformed C3H 10T1/2 cells are derived from a single clone; unlike their progenitors, they are capable of anchorage-independent proliferation and display characteristic features of cancer cells: rounded cell shape, reduced adhesion and multilayered growth. They also show elevated expression of p110α and constitutive phosphorylation of the downstream targets AKT and ribosomal protein S6. In this isogenic pair of cell lines, SILAC identified about 50,000 peptides, revealing the relative abundance of over 4,000 proteins. In the transformed cells, about 100 proteins are upregulated, and nearly 50 proteins are downregulated by a factor of >2 (p<0.05). Notable among the upregulated proteins are several whose expression is controlled by STAT transcription factors. The corresponding genes contain interferon-stimulated response elements or interferon γ activation sites. These sites bind transcriptional regulator complexes that include STAT proteins. The SILAC data therefore suggest elevated STAT activity, and this suggestion is confirmed by Western blots for phosphorylated STAT proteins (16). Both STAT3 and STAT6 show enhanced tyrosine phosphorylation in the transformed cells. Because of the prominent role of STAT3 in cancer, initial investigations have focused on the connection between this member of the STAT family and PI3K-induced oncogenic transformation.
A possible link between PI3K and the activation of STAT6 remains to be investigated. STAT6 is primarily active in the transcriptional regulation of lymphocytes (17) and is involved in hematopoietic malignancies (18). STAT6 is strongly activated by IL4 and IL13 via JAK kinases but can also be activated by the receptor tyrosine kinase PDGFR. STAT6 is involved in the regulation of allergic responses and inflammation in asthma and ulcerative colitis, playing a key role in immune cells and in the epithelium. In solid tumors, STAT6 can have pro-survival functions but can also inhibit the growth of tumor cells (19–22). The experiments described below for the characterization of the PI3K-STAT3 connection should also be performed with STAT6. They could be particularly informative on cells derived from leukemias and lymphomas.
Upon phosphorylation, STAT proteins homodimerize, but can also heterodimerize with other members of the STAT family. Because the transcriptional target spectra of different STAT proteins are similar but not identical (23), STAT heterodimers can act in complex ways. An example for this complexity is the interaction between STAT1 and STAT3 (24). STAT1 and STAT3 mutually antagonize each other. When they are incorporated into heterodimers, activated STAT1 downregulates STAT3 targets and STAT3 downregulates STAT1 targets. The balance of these effects depends on the levels of STAT expression and phosphorylation.
Interdependence of PI3K and STAT3
The activation of STAT3 in the H1047R-transformed cells could reflect a generic property of the oncogenic phenotype without specific relevance to the PI3K-generated transforming signal, or it could play a specific role in the oncogenicity of PI3K. The answer to this question comes from dominant negative STAT3. Expression of dominant negative STAT3DB in chicken embryo fibroblasts induces resistance to oncogenic transformation by the p110α mutants H1047R and E545K, by myristylated p110α, and by the wild type versions of p110β, p110γ, and p110δ. The activation of STAT3 is therefore not a dispensable feature of the PI3K-transformed C3H 10T1/2 cells but plays an important role in the oncogenic process. Conversely, inhibition of PI3K by the small molecule inhibitor GDC-0941 reduces the phosphorylation of STAT3. In this system PI3K and STAT3 are linked by a mutual dependence.
TEC kinases (tyrosine kinase expressed in hepatocellular carcinoma) connect PI3K to STAT3 (25)
PI3K signaling from p110 to TOR does not involve a tyrosine kinase. The PI3K-initiated activation of STAT3 by phosphorylation on Y705 must therefore be carried out by a PI3K-activable kinase that operates outside this consensus pathway. The TEC kinases TEC, BMX, BTK, ITK and TXK meet this criterion (26). They are a family of non-receptor tyrosine kinases with diverse roles in the transmission of signals initiated by G-protein-coupled receptors, antigen and integrin receptors and receptors of growth factors and cytokines (27–30). They contain a PH domain that mediates binding solely to the PI3K product PIP3 with subsequent kinase activation by nearby RTKs, nonreceptor tyrosine kinases or by autophosphorylation (31, 32). Most TEC kinases are preferentially expressed in hematopoietic cells, but BMX (also known as Etk) and TEC kinase itself occur in several non-hematopoietic tissues. Of these, only BMX is phosphorylated and hence activated in H1047R-transformed cells; it is therefore the most likely candidate for mediating the phosphorylation of STAT3. BMX has previously been identified as an activator of STAT3, and BMX can phosphorylate STAT3 in vitro (33, 34).
The involvement of TEC kinases in the activation of STAT3 is documented by the effect of the TEC kinase inhibitor LFM-A13. At concentrations that affect only TEC kinases, this inhibitor greatly reduces the phosphorylation of STAT3 at Y705. The JAK kinase inhibitor AG490, the SRC inhibitor Src1 and rapamycin have no effect on the phosphorylation of STAT3 in H1047R-transformed cells. The new small-molecule, ATP-competitive inhibitors of TOR still require testing. Interestingly, treatment of H1047R-transformed cells with LFM-A13 leads to elevated phosphorylation of AKT, suggesting that TEC kinases can function as negative regulators of PI3K. In assays for oncogenic transformation conducted with chicken embryo fibroblasts, LFM-A13 strongly interferes with focus formation by a retroviral H1047R expression vector but does not affect transformation induced by SRC (16).
STAT3 can also be phosphorylated by TOR at the S727 target residue, and this phosphorylation enhances STAT3 activity (35–37). However, the level of this phosphorylation is not affected by PI3K-induced transformation. This observation together with the failure of rapamycin to affect STAT3 phosphorylation at Y705 suggests that TOR does not play an immediate role in the PI3K-STAT3 connection.
H1047R-transformed cells release a STAT3-activating factor
Since activation of STAT3 by phosphorylation is part of the cellular response to interferon, it is important to examine a possible role of interferon in the PI3K-STAT3 connection. Transfection of non-transformed C3H 10T1/2 cells with poly-I:C induces the production and release of interferon. Conditioned medium from these cells, placed on fresh C3H 10T1/2 cells, triggers an interferon response in these recipients including a large increase in ISG15, but it does not change the phosphorylation of STAT3. In contrast, conditioned medium from untreated, H1047R-transformed C3H 10T1/2 strongly enhances the phosphorylation of STAT3 but has no effect on the abundance of ISG15. These data suggest that interferon is not involved in the link between PI3K and STAT3 but that H1047R-transformed cells release a factor capable of activating STAT3 in recipient cells. The identity of this factor and its mechanism of operation remain to be determined. IL6 is a likely candidate for such a factor as it is known to activate STAT3 (38). Although in preliminary tests IL6 antibody has had no detectable effect on the activity of the factor released by H1047R-transformed cells, the possibility that IL6 is identical with this factor remains open. It also remains an unanswered question whether this factor functions in an autocrine fashion and is involved in the activation of STAT3 in transformed cells. In other systems, IL-6 can readily form a self-stimulatory autocrine loop (39–41). Its paracrine activity, documented on normal C3H 10T1/2 cells, could be an important factor in the remodeling of the tumor stroma by cancer cells (42–44).
The PI3K-STAT3 connection in human cancer cell lines
For any given cell line, there is a simple test that can reveal an active link between PI3K and STAT3: the phosphorylation of STAT3 should be sensitive to the inhibition of PI3K and to the inhibition of TEC kinases. Many human cancer cells show phosphorylation of STAT3, and the level of this phosphorylation varies within wide boundaries (45, 46). In a small sample of such cell lines tested, about half show the diagnostic sensitivity of STAT3 phosphorylation to PI3K and to TEC kinase inhibition (16). However, the level of STAT3 phosphorylation is not correlated with a gain of function in PI3K. Cells carrying oncogenic mutations in p110α may or may not show elevated STAT3 phosphorylation, and the link between PI3K and STAT3 can be demonstrable in cells with low levels of STAT3 phosphorylation. Much larger numbers of cancer cell lines and of primary tumors will have to be examined to allow generalizations on the prevalence and relevance of the PI3K-STAT3 connection in human cancer.
BMX activates STAT3 in glioblastoma stem cells
Recent investigations on glioblastoma complement the studies in cell culture and document the activation of STAT3 by BMX (33). Glioblastomas contain a minority population of cells, referred to as glioblastoma stem cells, which self-renew and generate identical tumors upon transplantation (47). They are characterized by specific progenitor cell markers, including CD133, OLIG2 and SOX2. These cells are also distinguished from the rest of the cell population in the glioblastoma by the expression of BMX. Further, the expression of BMX is an important differentiator between normal neural progenitor cells and glioblastoma stem cells. In glioblastoma stem cells, BMX mediates the activation of STAT3. Active STAT3 is essential for the maintenance of self-renewal and of tumorigenicity (33, 48). These properties of cancer stem cells depend on IL6 signaling (49). IL6 can activate PI3K, and this in turn mediates the autophosphorylation of BMX (27). Additionally, glioblastomas frequently carry inactivating mutations of PTEN and activating mutations of the PI3K regulatory subunit p85 that result in enhanced PI3K activity (50–52). It appears therefore likely that the PI3K-STAT3 connection is active in glioblastoma stem cells and forms the backbone of a regulatory pathway that determines and sustains stemness. The IL6-STAT3 axis is not confined to glioblastoma; it is also well established in pancreatic and colon cancer and may be of universal significance in oncogenesis (53–55).
Is the alliance between PI3K and STAT3 universal?
It is possible that the full oncogenic activity of PI3K always depends on the phosphorylation of STAT3. This requirement would mark the proteins of the PI3K to STAT3 signaling chain as potential therapeutic targets in PI3K-driven tumors. Currently available data appear not to support such a view. The human cancer cell lines HCC-1954 and T-47D both carry the H1047R mutation of p110α, but STAT3 phosphorylation in these cells is not affected by a PI3K inhibitor or by a TEC kinase inhibitor. In these instances, the H1047R mutation could function independently of STAT3 or it could be irrelevant for the oncogenic phenotype. Alternatively, STAT3 phosphorylation may be independent of the PI3K pathway and mediated by other activation mechanisms such as JAK- or RTK-induced phosphorylation. But there is an alternative interpretation of these observations, suggested by the work on glioblastoma. As in glioblastoma and in other cancers, the tumor-initiating cells in the cell lines probably constitute only a small fraction of the cell population (56). However, Western blots of culture lysates used to determine the sensitivity of STAT3 phosphorylation to PI3K and TEC kinase inhibitors reflect the average in protein levels of the entire cell population and are therefore not suitable for the characterization of minority cell types. The tumor-initiating cells have to be isolated from the heterogeneous population, and only with these cells would it be possible to decide the question of the universality of the PI3K-STAT3 alliance. In the meantime, it is possible to explore the PI3K to STAT3 signaling chain for “druggability”. In preliminary tests, the TEC kinase inhibitor LFM-A13 has greatly enhanced the cytostatic and cell-killing effectiveness of PI3K inhibitors. This observation suggests that targeting TEC kinases, and specifically BMX, in conjunction with inhibition of PI3K could result in therapeutic benefit. The combination with PI3K inhibitors would be important because of the possibility that TEC kinase inhibitors activate AKT. BMX is an attractive drug target for two additional reasons (33): It is not expressed in neural progenitor cells that share several properties with glioblastoma stem cells. On a general scale, mice in which BMX is genetically inactivated are largely normal (57). Therefore, BMX does not have essential functions in development, and a specific BMX inhibitor is unlikely to cause significant side effects.
The fusion of two networks
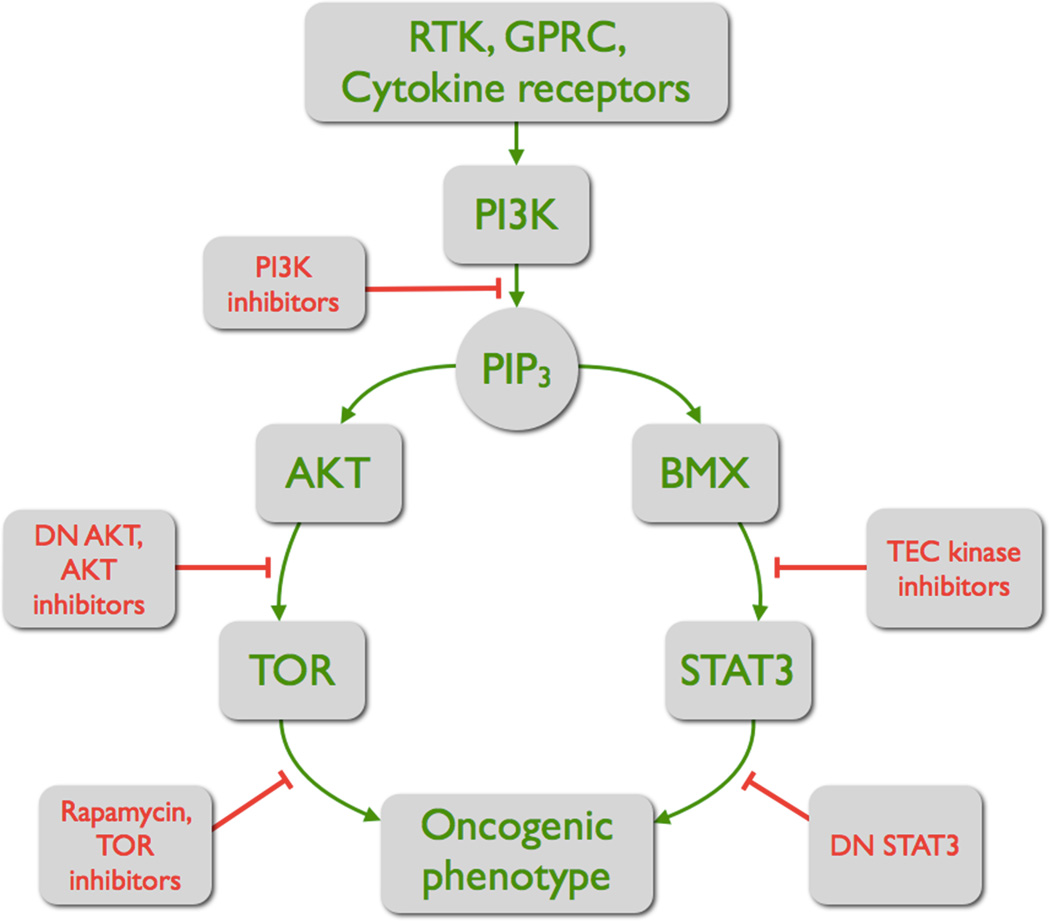
The oncogenic signals originating in PI3K are schematically presented in Fig. 2. The product of PI3K, PIP3, serves as a joint platform and as the branching point for two pathways that both contribute to the oncogenic cellular phenotype. PIP3 recruits AKT and its activating kinase PDK1 (58), and it recruits BMX (31, 59). The recruitment of these kinases is mediated by PH domains. The binding of different PH domains to PIP3 is characterized by dissociation constants that depend on the amino acid sequence of the PH domain. It is therefore conceivable that such differences in affinity mediate a compartmentalization, facilitating the AKT-PDK1 and the BMX-BMX interactions. The signaling pathway connecting AKT to TOR has been studied in great detail. The BMX to STAT3 pathway has not been analyzed to the same extent. Signaling components that lie between PI3K and BMX and STAT3 have not been identified. Alternative pathways of PI3K-dependent activation of Stat3 also deserve exploration. These include possible non-catalytic functions of PI3K that could lead to activation of a tyrosine kinase and to phosphorylation of Stat3 (60, 61).
Figure 2.
The two branches of the oncogenic signal initiated by PI3K. PIP3 functions as starting platform and as branching point of two pathways, the AKT-TORC1 pathway and the BMX-STAT3 pathway. Both are required for full oncogenic transformation. Individual steps in these pathways can be repressed by specific inhibitors or dominant-negative constructs. The AKT-TORC1 pathway stimulates cell growth and survival; the BMX-STAT3 pathway includes inflammation-related targets. (DN = dominant negative).
The relevant target proteins that induce the oncogenic phenotype downstream of TOR and of STAT3 are not completely known. The TORC1 complex activates protein translation, but this activity is probably not the complete spectrum of oncogenic signals that originate in TOR. Similarly, the transcriptional targets of STAT3 that are essential for oncogenic transformation remain to be identified. STAT3 activates the anti-apoptotic genes BCL-XL, MCL1 and Survivin (53), cyclin D1 (62) and NFκB (63). STAT3 collaborates with MYC and with NFκB in transcriptional activation (64, 65). STAT3 is also connected to IL6 in an autocrine loop that can function as an important determinant of oncogenicity (66, 67).
At PIP3, the PI3K signal bisects. The branch proceeding through AKT carries signals that primarily stimulate cell growth and survival. The branch that is transmitted by BMX and STAT3 involves signals related to inflammation, including IL6 and NFκB. Recent studies have documented the importance of the link between promotion of cell growth and inflammation in oncogenesis (65, 68). With the connection to STAT3, the PI3K signal integrates growth promoting and inflammatory functions.
Statement of significance.
The PI3K-TOR pathway and STAT3 signaling represent two distinct regulatory networks. The discovery of a functional link between these two is significant for our understanding of PI3K- and STAT3-driven oncogenic mechanisms. It also identifies the TEC kinase BMX as a new cancer target.
Acknowledgments
This is manuscript 21406 of The Scripps Research Institute.
Grant support: Work of the authors is supported by NIH grant R01 CA078230.
Abbreviations
- AKT
cellular homolog of murine thymoma virus akt8 oncoprotein
- BCL-XL
B cell lymphoma like X
- BMX
Bone Marrow tyrosine kinase gene in chromosome X
- CD133
cluster of differentiation 133, prominin 1
- EGFR
epidermal growth factor receptor
- IL4/IL6/IL13
interleukin 4/6/13
- ISG15
interferon-induced protein 15
- JAK
Janus kinase
- MCL1
myeloid cell leukemia sequence 1
- NF-kB
nuclear factor of kappa light polypeptide gene enhancer in B-cells 1
- OLIG2
oligodendrocyte transcription factor 2
- PDGFR
platelet-derived growth factor receptor
- PDK1
3-phosphoinositide dependent protein kinase-1
- PI3K
phosphatidylinositol 3-kinase
- PIP3
phosphatidylinositol 3,4,5-trisphosphate
- PH domain
pleckstrin homology domain
- PTEN
Phosphatase and tensin homolog
- RHEB
Ras homolog enriched in brain
- RTK
receptor tyrosine kinase
- SILAC
stable isotope labeling with amino acids in cell culture
- SOX2
SRY (sex determining region Y)-box 2
- SRC
cellular homolog of the Src oncoprotein of Rous sarcoma virus
- STAT1/STAT3/STAT6
signal transducer and activator of transcription 1/3/6
- TEC
tyrosine kinase expressed in hepatocellular carcinoma
- TOR
target of rapamycin
- TSC
tuberous sclerosis complex
Footnotes
Conflict of interest: The authors declare no conflict of interest.
References
- 1.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7:606–619. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- 3.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 4.Garcia-Martinez JM, Alessi DR. mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Biochem J. 2008;416:375–385. doi: 10.1042/BJ20081668. [DOI] [PubMed] [Google Scholar]
- 5.Mertens C, Darnell JE., Jr SnapShot: JAK-STAT signaling. Cell. 2007;131:612. doi: 10.1016/j.cell.2007.10.033. [DOI] [PubMed] [Google Scholar]
- 6.Levy DE, Darnell JE., Jr Stats: transcriptional control and biological impact. Nat Rev Mol Cell Biol. 2002;3:651–662. doi: 10.1038/nrm909. [DOI] [PubMed] [Google Scholar]
- 7.Zhou W, Grandis JR, Wells A. STAT3 is required but not sufficient for EGF receptor-mediated migration and invasion of human prostate carcinoma cell lines. Br J Cancer. 2006;95:164–171. doi: 10.1038/sj.bjc.6603234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grandis JR, Drenning SD, Chakraborty A, Zhou MY, Zeng Q, Pitt AS, et al. Requirement of Stat3 but not Stat1 activation for epidermal growth factor receptor- mediated cell growth In vitro. J Clin Invest. 1998;102:1385–1392. doi: 10.1172/JCI3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cao X, Tay A, Guy GR, Tan YH. Activation and association of Stat3 with Src in v-Src-transformed cell lines. Mol Cell Biol. 1996;16:1595–1603. doi: 10.1128/mcb.16.4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mann M. Functional and quantitative proteomics using SILAC. Nat Rev Mol Cell Biol. 2006;7:952–958. doi: 10.1038/nrm2067. [DOI] [PubMed] [Google Scholar]
- 11.Gustin JP, Karakas B, Weiss MB, Abukhdeir AM, Lauring J, Garay JP, et al. Knockin of mutant PIK3CA activates multiple oncogenic pathways. Proc Natl Acad Sci U S A. 2009;106:2835–2840. doi: 10.1073/pnas.0813351106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Konishi H, Karakas B, Abukhdeir AM, Lauring J, Gustin JP, Garay JP, et al. Knock-in of mutant K-ras in nontumorigenic human epithelial cells as a new model for studying K-ras mediated transformation. Cancer Res. 2007;67:8460–8467. doi: 10.1158/0008-5472.CAN-07-0108. [DOI] [PubMed] [Google Scholar]
- 13.Hart JR, Liao L, Ueno L, Yates JR, 3rd, Vogt PK. Protein expression profiles of C3H 10T1/2 murine fibroblasts and of isogenic cells transformed by the H1047R mutant of phosphoinositide 3-kinase (PI3K) Cell Cycle. 2011;10:971–976. doi: 10.4161/cc.10.6.15077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 15.Bader AG, Kang S, Vogt PK. Cancer-specific mutations in PIK3CA are oncogenic in vivo. Proc Natl Acad Sci U S A. 2006;103:1475–1479. doi: 10.1073/pnas.0510857103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hart JR, Liao L, Yates JR, 3rd, Vogt PK. Essential role of Stat3 in PI3K-induced oncogenic transformation. Proc Natl Acad Sci U S A. 2011 doi: 10.1073/pnas.1110486108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wurster AL, Tanaka T, Grusby MJ. The biology of Stat4 and Stat6. Oncogene. 2000;19:2577–2584. doi: 10.1038/sj.onc.1203485. [DOI] [PubMed] [Google Scholar]
- 18.Bruns HA, Kaplan MH. The role of constitutively active Stat6 in leukemia and lymphoma. Crit Rev Oncol Hematol. 2006;57:245–253. doi: 10.1016/j.critrevonc.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 19.Das S, Roth CP, Wasson LM, Vishwanatha JK. Signal transducer and activator of transcription-6 (STAT6) is a constitutively expressed survival factor in human prostate cancer. Prostate. 2007;67:1550–1564. doi: 10.1002/pros.20640. [DOI] [PubMed] [Google Scholar]
- 20.Gooch JL, Christy B, Yee D. STAT6 mediates interleukin-4 growth inhibition in human breast cancer cells. Neoplasia. 2002;4:324–331. doi: 10.1038/sj.neo.7900248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanai A, Suzuki K, Tanimoto K, Mizushima-Sugano J, Suzuki Y, Sugano S. Characterization of STAT6 Target Genes in Human B Cells and Lung Epithelial Cells. DNA Res. 2011 doi: 10.1093/dnares/dsr025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsukura S, Stellato C, Georas SN, Casolaro V, Plitt JR, Miura K, et al. Interleukin-13 upregulates eotaxin expression in airway epithelial cells by a STAT6-dependent mechanism. Am J Respir Cell Mol Biol. 2001;24:755–761. doi: 10.1165/ajrcmb.24.6.4351. [DOI] [PubMed] [Google Scholar]
- 23.Ehret GB, Reichenbach P, Schindler U, Horvath CM, Fritz S, Nabholz M, et al. DNA binding specificity of different STAT proteins. Comparison of in vitro specificity with natural target sites. J Biol Chem. 2001;276:6675–6688. doi: 10.1074/jbc.M001748200. [DOI] [PubMed] [Google Scholar]
- 24.Ho HH, Ivashkiv LB. Role of STAT3 in type I interferon responses. Negative regulation of STAT1-dependent inflammatory gene activation. J Biol Chem. 2006;281:14111–14118. doi: 10.1074/jbc.M511797200. [DOI] [PubMed] [Google Scholar]
- 25.Mano H, Ishikawa F, Nishida J, Hirai H, Takaku F. A novel protein-tyrosine kinase, tec, is preferentially expressed in liver. Oncogene. 1990;5:1781–1786. [PubMed] [Google Scholar]
- 26.Scharenberg AM, El-Hillal O, Fruman DA, Beitz LO, Li Z, Lin S, et al. Phosphatidylinositol-3,4,5-trisphosphate (PtdIns-3,4,5-P3)/Tec kinase-dependent calcium signaling pathway: a target for SHIP-mediated inhibitory signals. Embo J. 1998;17:1961–1972. doi: 10.1093/emboj/17.7.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Qiu Y, Kung HJ. Signaling network of the Btk family kinases. Oncogene. 2000;19:5651–5661. doi: 10.1038/sj.onc.1203958. [DOI] [PubMed] [Google Scholar]
- 28.Hussain A, Yu L, Faryal R, Mohammad DK, Mohamed AJ, Smith CI. TEC family kinases in health and disease - loss-of-function of BTK and ITK and the gain-of-function fusions ITK-SYK and BTK-SYK. Febs J. 2011 doi: 10.1111/j.1742-4658.2011.08134.x. [DOI] [PubMed] [Google Scholar]
- 29.Koprulu AD, Ellmeier W. The role of Tec family kinases in mononuclear phagocytes. Crit Rev Immunol. 2009;29:317–333. doi: 10.1615/critrevimmunol.v29.i4.30. [DOI] [PubMed] [Google Scholar]
- 30.Finkelstein LD, Schwartzberg PL. Tec kinases: shaping T-cell activation through actin. Trends Cell Biol. 2004;14:443–451. doi: 10.1016/j.tcb.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 31.Jiang X, Borgesi RA, McKnight NC, Kaur R, Carpenter CL, Balk SP. Activation of nonreceptor tyrosine kinase Bmx/Etk mediated by phosphoinositide 3-kinase, epidermal growth factor receptor, and ErbB3 in prostate cancer cells. J Biol Chem. 2007;282:32689–32698. doi: 10.1074/jbc.M703412200. [DOI] [PubMed] [Google Scholar]
- 32.August A, Sadra A, Dupont B, Hanafusa H. Src-induced activation of inducible T cell kinase (ITK) requires phosphatidylinositol 3-kinase activity and the Pleckstrin homology domain of inducible T cell kinase. Proc Natl Acad Sci U S A. 1997;94:11227–11232. doi: 10.1073/pnas.94.21.11227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Guryanova OA, Wu Q, Cheng L, Lathia JD, Huang Z, Yang J, et al. Nonreceptor tyrosine kinase BMX maintains self-renewal and tumorigenic potential of glioblastoma stem cells by activating STAT3. Cancer Cell. 2011;19:498–511. doi: 10.1016/j.ccr.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsai YT, Su YH, Fang SS, Huang TN, Qiu Y, Jou YS, et al. Etk, a Btk family tyrosine kinase, mediates cellular transformation by linking Src to STAT3 activation. Mol Cell Biol. 2000;20:2043–2054. doi: 10.1128/mcb.20.6.2043-2054.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Decker T, Kovarik P. Serine phosphorylation of STATs. Oncogene. 2000;19:2628–2637. doi: 10.1038/sj.onc.1203481. [DOI] [PubMed] [Google Scholar]
- 36.Kristof AS, Marks-Konczalik J, Billings E, Moss J. Stimulation of signal transducer and activator of transcription-1 (STAT1)-dependent gene transcription by lipopolysaccharide and interferon-gamma is regulated by mammalian target of rapamycin. J Biol Chem. 2003;278:33637–33644. doi: 10.1074/jbc.M301053200. [DOI] [PubMed] [Google Scholar]
- 37.Yokogami K, Wakisaka S, Avruch J, Reeves SA. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr Biol. 2000;10:47–50. doi: 10.1016/s0960-9822(99)00268-7. [DOI] [PubMed] [Google Scholar]
- 38.Wegenka UM, Buschmann J, Lutticken C, Heinrich PC, Horn F. Acute-phase response factor, a nuclear factor binding to acute-phase response elements, is rapidly activated by interleukin-6 at the posttranslational level. Mol Cell Biol. 1993;13:276–288. doi: 10.1128/mcb.13.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hartman ZC, Yang XY, Glass O, Lei G, Osada T, Dave SS, et al. HER2 overexpression elicits a proinflammatory IL-6 autocrine signaling loop that is critical for tumorigenesis. Cancer Res. 2011;71:4380–4391. doi: 10.1158/0008-5472.CAN-11-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leslie K, Gao SP, Berishaj M, Podsypanina K, Ho H, Ivashkiv L, et al. Differential interleukin-6/Stat3 signaling as a function of cellular context mediates Ras-induced transformation. Breast Cancer Res. 2010;12:R80. doi: 10.1186/bcr2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer. 2005;41:2502–2512. doi: 10.1016/j.ejca.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 42.Li N, Grivennikov SI, Karin M. The unholy trinity: inflammation, cytokines, and STAT3 shape the cancer microenvironment. Cancer Cell. 2011;19:429–431. doi: 10.1016/j.ccr.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Groner B, Lucks P, Borghouts C. The function of Stat3 in tumor cells and their microenvironment. Semin Cell Dev Biol. 2008;19:341–350. doi: 10.1016/j.semcdb.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat Rev Immunol. 2007;7:41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 45.Walker SR, Nelson EA, Zou L, Chaudhury M, Signoretti S, Richardson A, et al. Reciprocal effects of STAT5 and STAT3 in breast cancer. Mol Cancer Res. 2009;7:966–976. doi: 10.1158/1541-7786.MCR-08-0238. [DOI] [PubMed] [Google Scholar]
- 46.Berishaj M, Gao SP, Ahmed S, Leslie K, Al-Ahmadie H, Gerald WL, et al. Stat3 is tyrosine-phosphorylated through the interleukin-6/glycoprotein 130/Janus kinase pathway in breast cancer. Breast Cancer Res. 2007;9:R32. doi: 10.1186/bcr1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park DM, Rich JN. Biology of glioma cancer stem cells. Mol Cells. 2009;28:7–12. doi: 10.1007/s10059-009-0111-2. [DOI] [PubMed] [Google Scholar]
- 48.Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–2392. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang H, Lathia JD, Wu Q, Wang J, Li Z, Heddleston JM, et al. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells. 2009;27:2393–2404. doi: 10.1002/stem.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun M, Hillmann P, Hofmann BT, Hart JR, Vogt PK. Cancer-derived mutations in the regulatory subunit p85alpha of phosphoinositide 3-kinase function through the catalytic subunit p110alpha. Proc Natl Acad Sci U S A. 2010;107:15547–15552. doi: 10.1073/pnas.1009652107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jaiswal BS, Janakiraman V, Kljavin NM, Chaudhuri S, Stern HM, Wang W, et al. Somatic mutations in p85alpha promote tumorigenesis through class IA PI3K activation. Cancer Cell. 2009;16:463–474. doi: 10.1016/j.ccr.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Parsons DW, Jones S, Zhang X, Lin JC, Leary RJ, Angenendt P, et al. An integrated genomic analysis of human glioblastoma multiforme. Science. 2008;321:1807–1812. doi: 10.1126/science.1164382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lesina M, Kurkowski MU, Ludes K, Rose-John S, Treiber M, Kloppel G, et al. Stat3/Socs3 activation by IL-6 transsignaling promotes progression of pancreatic intraepithelial neoplasia and development of pancreatic cancer. Cancer Cell. 2011;19:456–469. doi: 10.1016/j.ccr.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 54.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell. 2009;15:91–102. doi: 10.1016/j.ccr.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 56.Hirsch HA, Iliopoulos D, Tsichlis PN, Struhl K. Metformin selectively targets cancer stem cells, and acts together with chemotherapy to block tumor growth and prolong remission. Cancer Res. 2009;69:7507–7511. doi: 10.1158/0008-5472.CAN-09-2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rajantie I, Ekman N, Iljin K, Arighi E, Gunji Y, Kaukonen J, et al. Bmx tyrosine kinase has a redundant function downstream of angiopoietin and vascular endothelial growth factor receptors in arterial endothelium. Mol Cell Biol. 2001;21:4647–4655. doi: 10.1128/MCB.21.14.4647-4655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mora A, Komander D, van Aalten DM, Alessi DR. PDK1, the master regulator of AGC kinase signal transduction. Semin Cell Dev Biol. 2004;15:161–170. doi: 10.1016/j.semcdb.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 59.Kojima T, Fukuda M, Watanabe Y, Hamazato F, Mikoshiba K. Characterization of the pleckstrin homology domain of Btk as an inositol polyphosphate and phosphoinositide binding domain. Biochem Biophys Res Commun. 1997;236:333–339. doi: 10.1006/bbrc.1997.6947. [DOI] [PubMed] [Google Scholar]
- 60.Patrucco E, Notte A, Barberis L, Selvetella G, Maffei A, Brancaccio M, et al. PI3Kgamma modulates the cardiac response to chronic pressure overload by distinct kinase-dependent and -independent effects. Cell. 2004;118:375–387. doi: 10.1016/j.cell.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 61.Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, et al. Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature. 2008;454:776–779. doi: 10.1038/nature07091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Masuda M, Suzui M, Yasumatu R, Nakashima T, Kuratomi Y, Azuma K, et al. Constitutive activation of signal transducers and activators of transcription 3 correlates with cyclin D1 overexpression and may provide a novel prognostic marker in head and neck squamous cell carcinoma. Cancer Res. 2002;62:3351–3355. [PubMed] [Google Scholar]
- 63.Lee H, Herrmann A, Deng JH, Kujawski M, Niu G, Li Z, et al. Persistently activated Stat3 maintains constitutive NF-kappaB activity in tumors. Cancer Cell. 2009;15:283–293. doi: 10.1016/j.ccr.2009.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barre B, Vigneron A, Coqueret O. The STAT3 transcription factor is a target for the Myc and riboblastoma proteins on the Cdc25A promoter. J Biol Chem. 2005;280:15673–15681. doi: 10.1074/jbc.M413203200. [DOI] [PubMed] [Google Scholar]
- 65.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iliopoulos D, Hirsch HA, Wang G, Struhl K. Inducible formation of breast cancer stem cells and their dynamic equilibrium with non-stem cancer cells via IL6 secretion. Proc Natl Acad Sci U S A. 2011;108:1397–1402. doi: 10.1073/pnas.1018898108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell. 2009;15:79–80. doi: 10.1016/j.ccr.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]