Abstract
Inhibitors of histone deacetylase (HDAC) have anti-inflammatory and antifibrotic effects in several organs and tissues, but their effect on the progression of renal disease is unknown. Here, we studied the effect of valproic acid in adriamycin-induced nephropathy in mice. Administration of valproic acid before kidney injury prevented the development of proteinuria and the onset of glomerulosclerosis. Even after postponing treatment until the peak of adriamycin-induced proteinuria, valproic acid rapidly decreased the quantity of proteinuria and attenuated the progression of renal disease. Valproic acid abrogated the decrease in glomerular acetylation observed during adriamycin-induced nephropathy. Furthermore, valproic acid attenuated the significant upregulation of profibrotic and proinflammatory genes, the deposition of collagen, and the infiltration of macrophages into the kidney. Valproic acid decreased glomerular apoptosis and proliferation induced by adriamycin. Ultrastructural studies further supported the protective effect of valproic acid on podocytes in this model. Taken together, these data suggest that HDACs contribute to the pathogenesis of renal disease and that HDAC inhibitors may have therapeutic potential in CKD.
Focal segmental glomerulosclerosis (FSGS) is the final common pathway of glomerular injury, where podocyte loss is a critical event in the initiation. FSGS is characterized by the disappearance of cellular elements from the glomerular tuft, increased accumulation of amorphous material, and collapse of the capillary lumen. Associated with progressive glomerulosclerosis are tubular atrophy/dilation and eventually infiltration of inflammatory cells and accumulation of myofibroblasts in the interstitium.1 Experimental FSGS can nonimmunologically be induced in rodents by intravenous administration of adriamycin (ADR-doxorubicin).2–4 ADR nephropathy is a nondiabetic podocyte injury model classified as the classic variant of FSGS in humans, leading to chronic proteinuria and renal failure.5
Previously, we demonstrated the beneficial effects of trichostatin A (TSA) and valproic acid (VPA), both histone deacetylase (HDAC) inhibitors, in a known model of liver fibrosis, that is, culture-induced hepatic stellate cell activation.6–9 Both inhibitors have been shown to have anti-inflammatory and antifibrotic effects in several other organs and tissues,10,11 for example, in renal models of tubulointerstitial fibrosis and lupus.12–17 The effect of TSA or VPA on renal cells is mainly studied in vitro.12,13,15,18–21 All these studies have focused on the effect of HDAC inhibitors in the renal tubulointerstitial space and their inhibition of EMT (epithelial-to-mesenchymal transition). In this study, we examine the in vivo effect of VPA in an experimental model of glomerulosclerosis.
Among the growing list of HDAC inhibitors, the short-chain fatty acid VPA (2-propylpentanoic acid) is a well-tolerated anticonvulsive drug, which has been extensively studied as an antineoplastic agent and is considered primarily a class I HDAC inhibitor.22,23 The family of HDACs comprises 18 genes, which are grouped into classes I through IV. Class I (HDACs 1, 2, 3, and 8), class II (HDACs 4, 5, 6, 7, 9, and 10), and class IV (HDAC 11) isoforms are the “classical” HDACs, which are dependent on zinc for their enzymatic activity, whereas the 7 class III members are called sirtuins 1 through 7, which are nicotinamide adenine dinucleotide+-dependent enzymes.24 HDACs have an opposing function to histone acetyltransferases (HATs); they maintain the deacetylated state of histones, resulting in a tightly wrapped DNA and transcriptional repression.25 Recently, a rapidly growing number of nonhistone proteins have also been found to be targets for HDACs.26 HDAC inhibitors interfere with the function of HDACs, which are known as modulators of gene transcription important for cell function, proliferation, and differentiation. In this study, we demonstrate that chronic administration of VPA has beneficial effects on proteinuria, glomerulosclerosis, and renal inflammation in the experimental mouse ADR nephropathy model.
RESULTS
Valproic Acid Prevents Proteinuria and Kidney Injury in Adriamycin Nephropathy
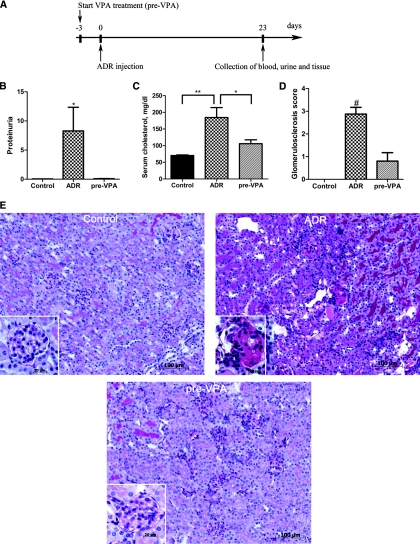
To assess the possible antifibrotic or renoprotective effect of the HDAC inhibitor VPA in the murine ADR nephropathy model, we supplemented drinking water with VPA 3 days before the intravenous ADR injection (pre-VPA group) as shown in Figure 1A. Animals injected with ADR (ADR group) developed severe proteinuria in contrast to the pre-VPA group where no significant increase in proteinuria was observed compared with controls (Figure 1B). In a comparison of the clinical blood parameters, no significant decrease in serum albumin was found in the ADR group (2.16 ± 0.09 g/dl) nor in the pre-VPA group (2.05 ± 0.09 g/dl) versus controls (2.20 ± 0.03 g/dl). Serum cholesterol was significantly elevated in the ADR group and not in the pre-VPA group (Figure 1C).
Figure 1.
Valproic acid prevents proteinuria and kidney injury in adriamycin nephropathy. (A) Schematic of the experimental design for pre-VPA treatment. (B) Proteinuria expressed as total urinary protein over creatinine is prevented when VPA treatment was started 3 days prior (pre-VPA, n = 5) to ADR injection. (C) In the ADR group (n = 9) serum cholesterol (mg/dl) is elevated significantly versus controls (n = 5) and versus the pre-VPA group. (D) Blinded quantitative histologic evaluation of glomerulosclerosis was scored by a pathologist. (E) Representative cortical fields demonstrate kidney injury at the end of the experiment in the different groups of mice as indicated. Kidney sections were subjected to PAS staining. (#P < 0.001; **P < 0.01; *P < 0.05)
Quantitative evaluation of the FSGS lesions demonstrated a glomerulosclerosis score of 2.9 ± 0.3 in the ADR group, 0.8 ± 0.4 in the pre-VPA group, and 0.0 ± 0.1 in the control group (Figure 1D). Histopathologic analysis revealed ADR-induced abnormalities typical for FSGS. Glomeruli of the ADR group showed segmental to global hyaline deposits, with collapse of the associated glomerular tuft and mesangial expansion. The glomerulosclerosis was accompanied by prominent tubular dilation, intraluminal protein casts, and reabsorption droplets. Furthermore, interstitial fibrosis, tubular atrophy, and accumulation of mononuclear cells were observed. In contrast, the pre-VPA group showed only few glomerular lesions and no tubulointerstitial fibrosis (Figure 1E).
Valproic Acid Corrects an Established Proteinuria and Limits Kidney Injury in Adriamycin Nephropathy
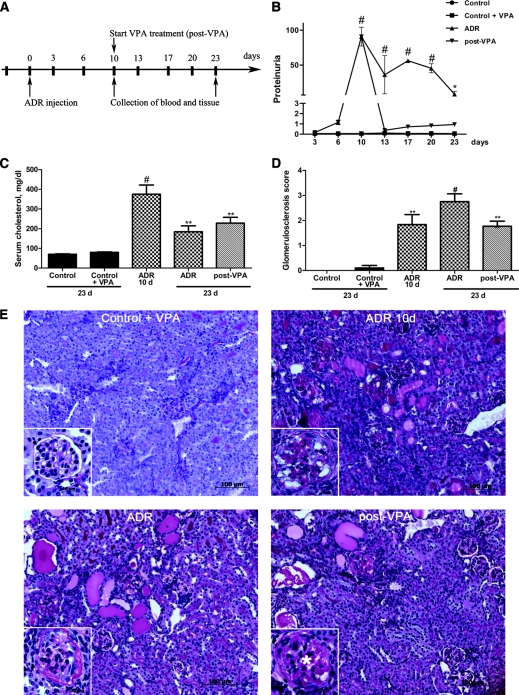
To investigate whether VPA can limit or reverse ADR nephropathy in mice with established proteinuria, the start of VPA treatment was postponed (post-VPA group) until a significant peak in proteinuria was observed as shown in Figure 2A. Post-VPA animals therefore received VPA-supplemented drinking water from day 10 after ADR injection. Proteinuria in these animals dropped significantly already after 3 days of VPA treatment and remained significantly lower than that in the ADR group at all following time points. During the course of the experiment, no proteinuria was observed in control animals receiving VPA treatment and in untreated controls (Figure 2B). Overt proteinuria in the ADR-10d group was accompanied by hypoalbuminemia (1.60 ± 0.03 g/dl) and hypercholesterolemia. No significant reduction in serum albumin was observed in the post-VPA group (2.14 ± 0.08 g/dl). The post-VPA group as well as the ADR group at the end of the experiment retained a high serum cholesterol level, which was however significantly decreased compared with the ADR-10d group (Figure 2C). Importantly, even when proteinuria is fully established, VPA was able to hamper renal disease progression in the murine ADR nephropathy model. Renal histopathologic evaluation of post-VPA animals showed only moderate lesions of glomerulosclerosis (1.7 ± 0.2) with hyaline deposits, mesangial expansion, and weak tubulointerstitial fibrosis, comparable with the injury in the ADR-10d group (1.8 ± 0.4) and significantly less than that in the ADR group at the end of the experiment (2.9 ± 0.3). In addition, renal histology of control animals was not affected by VPA treatment (Figure 2, D and E).
Figure 2.
Valproic acid corrects an established proteinuria and limits kidney injury in adriamycin nephropathy. (A) Schematic of the experimental design for post-VPA treatment. (B) Proteinuria expressed as total urinary protein over creatinine was detected during the course of the experiment until 23 days after ADR injection. When VPA treatment was postponed 10 days after ADR injection (post-VPA group, n = 14), proteinuria drops already 3 days after the start of the treatment and stays significantly lower compared with the ADR group (n = 9). (C) Hypercholesterolemia (mg/dl) is observed in the ADR-10d group (n = 6), in the ADR group at the end of the experiment, and in the post-VPA group. (D) Blinded quantitative histologic evaluation of glomerulosclerotic lesions were scored by a pathologist. (E) Representative cortical fields demonstrate kidney injury in the different groups of mice as indicated (PAS staining). Note: In glomeruli of post-VPA–treated animals, a clear swelling of the glomerulus with mesangial expansion (asterisk) is found comparable with the ADR-10d group. This glomerulosclerotic stage precedes the one seen in the ADR group at the end of the experiment, where glomeruli collapse and are surrounded by a fibrotic Bowman's capsule. (#P < 0.001; **P < 0.01; *P < 0.05)
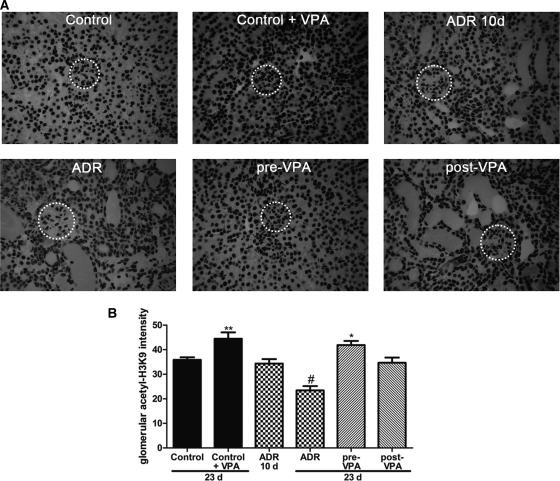
Our results suggest that VPA has beneficial effects in ADR nephropathy and we hypothesize that this is due to the HDAC inhibitory capacity of VPA. Thus, we used the acetylation status of H3K9 as a measure for VPA activity in the kidney.15,16,27 Immunohistochemistry revealed that acetyl-H3K9 is readily detected under normal conditions in the kidney cortex (Figure 3A). Quantification showed that in all VPA-treated animals glomerular acetyl-H3K9 is increased when compared with the corresponding untreated group. Interestingly, in untreated ADR-injected animals, acetyl-H3K9 is diminished significantly toward the end of the experiment. This decrease in glomerular acetyl-H3K9 staining was inhibited in both pre-VPA and post-VPA groups (Figure 3B).
Figure 3.
Valproic acid increases glomerular acetylation. (A) Representative cortical fields are shown for the acetyl-H3K9 staining (dotted circle surrounds a glomerulus). (B) Glomerular acetylation in 20 glomeruli of at least three animals of each group was quantified by using ImageJ software. (#P < 0.001; **P < 0.01; *P < 0.05)
Valproic Acid Hampers Renal Fibrosis
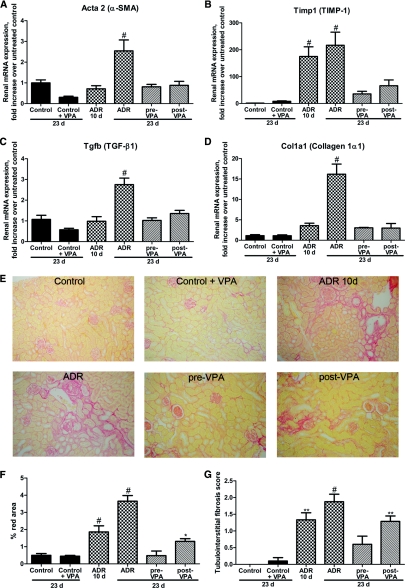
The well-known fibrotic markers28 α-SMA, TIMP-1, collagen type-1α1, and the fibrotic cytokine TGF-β1 were assessed by quantitative PCR (qPCR). The renal mRNA expression of α-SMA, TGF-β1, and collagen type-1α1 was not yet elevated significantly 10 days after ADR injection, whereas TIMP-1 expression was augmented compared with control animals. At the end of the experiment mRNA expression of the fibrotic markers was significantly induced in the ADR group compared with controls. Pre-VPA and even post-VPA treatment of ADR injected mice abrogated the α-SMA, TIMP-1, collagen type-1α1, and TGF-β1 induction (Figure 4, A through D).
Figure 4.
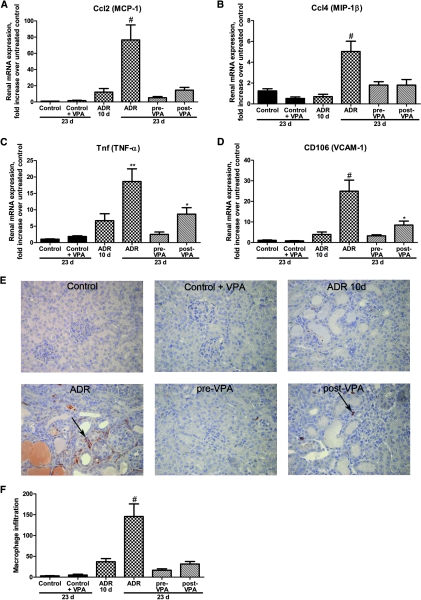
Valproic acid hampers renal fibrosis. (A through D) Graphic presentation shows the relative renal mRNA levels of α-SMA, TIMP-1, collagen type-1α1, and TGF-β1 determined by qPCR in the different groups. (E) Picrosirius Red staining was performed to visualize the collagen deposition in all groups. (F) ImageJ software was used for the quantification of the Picrosirius Red area. (G) Tubulointerstitial fibrosis score was assessed by a pathologist on Masson trichrome-stained sections. (#P < 0.001; **P < 0.01; *P < 0.05)
The antifibrotic effect of VPA was further confirmed by Picrosirius Red staining of kidney sections for collagen deposition, and through blinded scoring of the tubulointerstitial fibrosis. Collagen deposition in the untreated and VPA-treated control animals was similar. In the ADR-10d group a fourfold induction in collagen deposition was seen, which increased to eightfold in the ADR group at the end of the experiment. Picrosirius Red staining revealed that pre-VPA treatment inhibited collagen deposition in the ADR nephropathy model. In the post-VPA group, the collagen deposition was similar to that in the ADR-10d group, indicating that the induction of fibrosis was hampered by VPA treatment (Figure 4, E and F). The tendency that VPA attenuates fibrosis in experimental glomerulosclerosis was also seen when the tubulointerstitial fibrosis was quantified (Figure 4G).
Valproic Acid Hampers Renal Inflammation
In ADR nephropathy, chemokines are released by injured resident kidney cells, including MCP-1 and MIP-1β. These chemotactic cytokines promote the recruitment of both macrophages and T cells in the diseased kidney.28–30 To examine whether VPA could reduce inflammation, real-time qPCR for Ccl2 (MCP-1), Ccl4 (MIP-1β), TNF-α, and CD106 (VCAM-1) was performed. All these proinflammatory genes were significantly induced toward the end of the experiment. This was in contrast to the pre-VPA group in which no upregulation of proinflammatory genes could be observed. The assessed genes were significantly downregulated in the post-VPA group to levels comparable with those of the ADR-10d group, although TNF-α and CD106 levels were higher than controls (Figure 5, A through D).
Figure 5.
Valproic acid hampers renal inflammation. (A through D) Graphic presentation shows the relative renal mRNA levels of MCP-1, MIP-1β, TNF-α, and VCAM-1 determined by qPCR in the different groups. (E) Representative cortical fields show the interstitial macrophage infiltration of ER-HR3+ macrophages/monocytes (arrows) for all groups. (F) ER-HR3+ cells were counted in 100 cortical fields for each animal to quantify the degree of inflammation. (#P < 0.001; **P < 0.01; *P < 0.05)
To confirm the anti-inflammatory effect of VPA in the ADR nephropathy model, we assessed the interstitial macrophage infiltration (ER-HR3+ cells). Only a limited number of macrophages were observed in the tubulointerstitial space of the ADR-10d group, whereas at the end of the experiment a more prominent macrophage infiltration was observed, as also reported by Wang et al. and Vielhauer et al.4,29 Pre-VPA treatment of ADR mice blocked the interstitial macrophage infiltration, whereas post-VPA treatment prevented a further increase of macrophage infiltration between day 10 and the end of the experiment (Figure 5, E and F).
Valproic Acid Reduces Glomerular Injury in Adriamycin Nephropathy
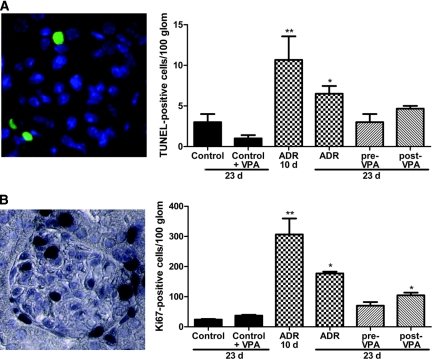
Cell depletion by apoptosis is a typical phenomenon in renal disorders, as is seen in the ADR nephropathy model by Marshall et al.31 The TUNEL assay was performed to determine whether VPA could modulate apoptosis in the ADR model (Figure 6A and Supplemental Figure S1). The number of TUNEL-positive cells in 100 glomeruli in the ADR-10d group was higher than those at the end of the experiment. In both ADR groups the number of TUNEL-positive cells was higher than those in untreated or VPA-treated controls, whereas the pre-VPA and post-VPA groups showed no significant increase in apoptosis compared with controls.
Figure 6.
Valproic acid reduces glomerular injury in adriamycin nephropathy. (A) To quantify the rate of apoptosis in the glomeruli, a TUNEL staining was done and positive cells in 100 glomeruli of at least three animals of each group were counted. (B) Proliferation was quantified by counting the Ki67+ cells in 100 glomeruli of at least three animals of each group. (**P < 0.01; *P < 0.05)
To analyze whether VPA treatment affects glomerular proliferation, a Ki67 staining was performed. As was seen for proteinuria and apoptosis, a peak in glomerular proliferating cells was found 10 days after the ADR injection. The number of Ki67+ cells in 100 glomeruli was higher in both ADR groups than in untreated or VPA-treated controls. Moreover, the pre-VPA group showed no significant increase in proliferation compared with controls, whereas proliferation in the post-VPA group was significantly decreased compared with that in the untreated ADR animals, but was elevated compared with controls (Figure 6B and Supplemental Figure S2).
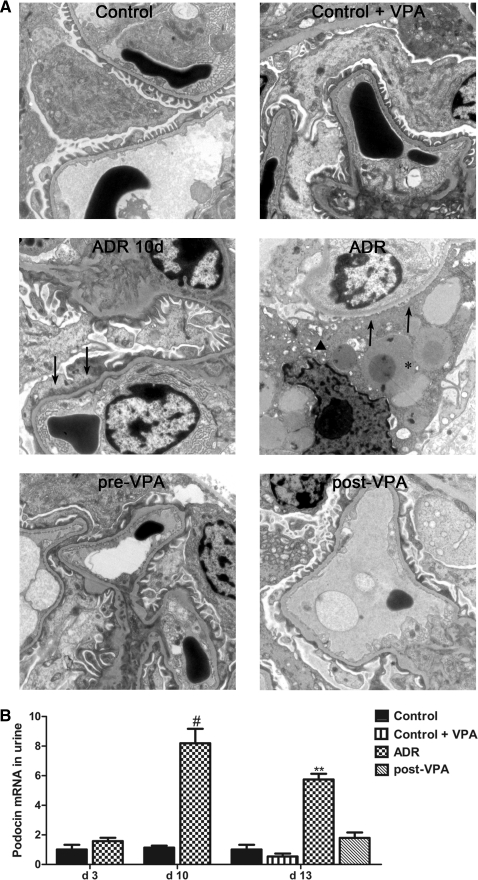
Transmission electron microscopy imaging was performed to study the ultrastructure of the podocytes. In glomeruli of ADR animals, widespread fusion and effacement of the epithelial foot processes was observed and some podocytes showed cytoplasmic vacuoles. Besides these vacuoles, which were surrounded by thin epithelial cytoplasm, amorphous electron-dense material was seen, which corresponded to hyalinosis observed under light microscopy. In contrast, only minimal effacement of the epithelial foot processes was seen in the pre-VPA group. Podocytes of post-VPA animals showed a swollen cytoplasm and segmental fusion of epithelial foot processes. Control and VPA-treated control animals revealed normal ultrastructural findings (Figure 7A).
Figure 7.
Valproic acid reduces podocyte detachment and injury in adriamycin nephropathy. (A) Transmission electron microscopy was performed to study the ultrastructure of the glomeruli. Podocytes of ADR animals revealed complete effacement of the epithelial foot processes (arrows), vacuolization (arrowhead), and amorphous electron-dense material (asterisk). Original magnification, ×6200. (B) Urinary podocin mRNA was detected at key time points in the post-VPA treatment setup by qPCR. At day 10 the urinary podocin mRNA level reaches a peak, coinciding with the proteinuria peak. After 3 days of VPA treatment the urinary podocin mRNA level has already dropped significantly compared with that of the ADR group. (#P < 0.001; **P < 0.01)
We also determined the podocyte-specific gene podocin in the urine to investigate whether VPA treatment could significantly decrease the number of podocytes lost in urine through detachment.32 Ten days after ADR injection, a peak in podocin mRNA in the urine coinciding with the peaks in proteinuria, apoptosis, and proliferation was observed. After only 3 days of VPA treatment urinary podocin mRNA was significantly decreased in the post-VPA group (Figure 7B). After the initial ADR insult, urinary podocin mRNA returned to control levels at the end of the experiment in all groups (data not shown).
DISCUSSION
The experimental model of FSGS induced by ADR is a well-established and reproducible nephropathy model, which can be useful in the development of new therapeutic strategies. In this study, we evaluate the HDAC inhibitor VPA as a possible novel therapeutic agent for chronic renal diseases. Our previous work shows that similarities exist in the fibrotic changes of both liver and kidney33 and that in vivo amelioration of liver fibrosis induced by CCl4 is seen in mice treated with VPA.6 Therefore, we further investigated whether VPA could improve experimental glomerulosclerosis in the mouse ADR nephropathy model.
Studies on HDAC inhibitors and kidney have mainly focused on the tubulointerstitial compartment. However, Noh et al. demonstrated in the streptozotocin-induced diabetic rat model of nephropathy that TSA reduces proteinuria and prevents extracellular matrix accumulation.15 In the ADR nephropathy model, we confirm the reduction of proteinuria by another HDAC inhibitor VPA. Moreover, we find a complete inhibition of proteinuria when this HDAC inhibitor is given before the ADR insult. Postponing the VPA treatment, until a significant peak in proteinuria is observed, results in a drop of proteinuria to control levels within 3 days after the start of the treatment. These data suggest that VPA treatment in an early phase of renal disease can halt and even prevent the development of proteinuria and the progression of kidney damage.
Furthermore, we find that VPA abrogates the decreased glomerular acetylation of H3K9 during ADR nephropathy. Recently, hypoacetylation in the kidney was also found in Wistar-Kyoto rats in the hemorrhagic shock model27 and in obese db/db mice with early glomerulosclerosis.16 In contrast, however, advanced glomerulosclerosis in accelerated uninephrectomized obese db/db mice is associated with renal histone H3K9 and H3K23 acetylation, H3K4 dimethylation, and H3 phosphorylation at serine 10.16 This suggests that when HDAC inhibitors are given at the end stage of renal disease no beneficial effect will be observed. Timing of HDAC inhibitor administration might therefore be crucial for successful treatment of renal diseases. Our data confirm the increasing evidence that epigenetic modulations can attenuate fibrotic changes not only in the tubulointerstitial compartment12,13,15,18,21 but also in the glomerular compartment. In the ADR nephropathy model, fibrosis is accompanied by inflammation.28,29 We show a reduction in inflammatory chemokines/cytokines and interstitial infiltrated macrophages in the ADR nephropathy model after VPA treatment. Less inflammation was also observed in the obstructive nephropathy model12,13 and the autoimmune lupus model17 after HDAC inhibition therapy.
Furthermore, we confirm that ADR-induced damaged DNA leads to apoptosis, as was recently described by Marshall et al.31 HDAC inhibition by VPA in the ADR nephropathy model results in less glomerular apoptosis. Less apoptosis was also shown for both VPA and SAHA (Suberoylanilide Hydroxamic Acid) in the hemorrhagic shock model.27 The ADR nephropathy model is considered as a toxin-mediated podocyte injury model in rodents,5 but Jeansson et al. found involvement of the glomerular endothelium in proteinuria34 in a less susceptible mouse strain35 requiring a high ADR dose. Besides glomerular apoptosis, podocytes can be lost in the urine because of detachment.32,36,37 We show that the podocyte-specific gene podocin can be found in the urine after ADR insult and that VPA can reduce this loss. Moreover, we see that, after the initial insult by ADR, urinary podocin mRNA levels return to control levels at the end of the experiment in all groups, suggesting that the primary loss of podocytes sustains the renal pathologic process. We find that in the mouse ADR nephropathy model glomerular proliferation is highly induced, as was observed in the rat puromycin aminonucleoside nephrosis model.38 Our data show that VPA can diminish glomerular proliferation.
In response to injury, podocytes may undergo several cell fates; besides apoptosis and proliferation, dedifferentiation can also occur.31,39,40 HDAC inhibitors are found to inhibit EMT of renal proximal tubular epithelial cells.12,13,15,18–21 Specifically, VPA is found to prevent EMT in NRK-52E cells, along with the selective class I HDAC inhibitor SK-7041, which indicates that class I HDACs play a pivotal role in EMT.15 Additional results show that VPA inhibits the induction of EMT-specific markers, such as vimentin, FSP1, and Snail (see Supplemental Figure S3). In vitro data suggest that besides renal proximal tubular epithelial cells also endothelial cells (endoMT)41 and podocytes (podoMT) can undergo EMT after ADR insult.42 This implies that VPA might inhibit the podoMT process in vivo in the ADR nephropathy model. Performing in vitro studies on podocyte cell lines43 would greatly facilitate mechanistic studies that are necessary to elucidate the role of epigenetic modulation44 of histones and nonhistone protein acetylation in renal disease.
VPA is mainly known for its HDAC inhibitory effect. Its antiepileptic activity however has been attributed to its ability to increase the level of the inhibitory neurotransmitter γ-aminobutyric acid (GABA) by inhibiting GABA transaminase. Moreover, VPA inhibits T-type calcium channels, voltage-dependent sodium channels,45 and also signaling pathways of TNF-α, NF-κB, and IL-6.22,46 It would also be worthwhile to investigate the effect of VPA on the renal progenitor cell population. Thus far, TSA and 4-phenylthiobutanoic acid were shown to expand the renal progenitor cell population.20,21 Furthermore, VPA exhibits a wide range of effects upon various tissues and pathologies. It can alter the expression of >20% of the transcriptome in a tissue-dependent manner.10,26,47
In conclusion, we demonstrate that VPA has a beneficial effect on the development of proteinuria and the progression of glomerulosclerosis in the experimental ADR nephropathy model. We show that VPA halts glomerulosclerosis through inhibition of podocyte detachment, EMT, apoptosis, and proliferation. However, currently it is very difficult to anticipate all of the effects of VPA; therefore, further studies should be done to evaluate whether VPA treatment might be useful as a possible novel therapeutic approach for patients with early glomerulosclerosis, either in monotherapy or combination therapy with, for example, antioxidants or corticosteroids.
CONCISE METHODS
Murine ADR Nephropathy Model of Experimental FSGS
All animal studies were conducted under a protocol approved by the committee for the care and use of laboratory animals of the “Vrije Universiteit Brussel”. Adult female Balb/c mice (Harlan, Horst, The Netherlands), weighing 20 g (8 weeks old), were divided at random into six groups. After 1 week of acclimatization, two groups of mice were injected with saline solution (control, n = 5; VPA-treated control, n = 9) in the tail vein. The other four groups were administrated ADR (adriamycin or doxorubicin, Pharmacia, Brussels, Belgium) through a single intravenous tail vein injection (10 mg/kg).2,4,48,49 Two groups of ADR-injected animals received 0.4% VPA in their drinking water50 respectively 3 days prior to (pre-VPA group, n = 5) and 10 days after (post-VPA group, n = 14) ADR administration. All animals were allowed ad libitum drinking water and free access to standard chow (A04, UAR, Epinay, France). One group of untreated ADR animals was sacrificed at day 10 (ADR-10d group, n = 6) as the reference point for the start of VPA treatment in the post-VPA group. Another ADR group (n = 9) was sacrificed 23 days after ADR injection together with all other animal groups (control, control + VPA, pre- and post-VPA). During the course of the experiment, mice were housed in metabolic cages at different time points for the collection of 24-hour urine samples. Proteinuria was expressed as total urinary protein over creatinine. The mice were anesthetized with natriumpentobarbital (CEVA, Brussels, Belgium) before sacrifice and blood was collected from the inferior vena cava. The kidneys were harvested and processed for (immuno)histologic evaluation. Serum albumin and urinary creatinine and total protein were analyzed by the Kodak Ektachem method (Kodak Eastman, Rochester, New York), whereas serum cholesterol was determined by an enzymatic method.
Histologic Examination
Transversal slices of kidneys were fixed in 4% buffered formaldehyde at 4°C for 24 hours and embedded in paraffin. Five-micrometer sections were cut and stained with periodic acid–Schiff and analyzed by a pathologist in a blind fashion by light microscopy. Glomerulosclerosis was graded on a scale of 0 to 4, with 0 indicating normal, 1 indicating 1% to 10% of glomeruli with sclerotic lesions, 2 indicating 11% to 25% of glomeruli with sclerotic lesions, 3 indicating 26% to 50%, and 4 indicating >50% of sclerotic glomeruli.51 In a similar manner, renal interstitial fibrosis was scored for all conditions on Masson trichrome-stained sections.
Picrosirius Red Staining
Five-micrometer paraffin sections were dewaxed, rehydrated, and fixed with SUSA fixative for 1 hour and stained for 45 minutes with 0.1% Sirius Red F3BA in a saturated picric acid solution. For each condition, 12 pictures were made using an Axioskop light microscope (Carl Zeiss, Zaventem, Belgium) and the pictures were recorded using an Axiom digital camera. Red staining was quantified in the tubulointerstitial space using NIH ImageJ software (http://rsb.info.nih.gov/ij/).
Immunohistochemistry
Five-micrometer paraffin sections were cut and staining was performed after dewaxing and antigen retrieval (low pH) using DakoCytomation Target Retrieval Solution Citrate (Dako, Heverlee, Belgium) in a 98°C water bath. After blocking, sections were subjected to ER-HR3 (rat monoclonal; Abcam, Cambridge, UK, 1/50) or Ki67 (rabbit monoclonal, ThermoFisher Scientific, Cheshire, UK, 1/100) or acetylated-H3K9 (rabbit polyclonal; Abcam, Cambridge, UK, 1/700) overnight at 4°C. Anti-rat (rabbit polyclonal) antibody was used only for the ER-HR3 antibody for 30 minutes before the secondary antibody; anti-rabbit (goat polyclonal) was used subsequently for 30 minutes. The staining was visualized with a hydrogen peroxide substrate and 3,3′-diaminobenzidine tetrahydrochloride chromogen. Tissues were counterstained with Harris hematoxylin (1/8) for 30 seconds and mounted with Faramount (DAKO, Heverlee, Belgium). The number of positive cells in the tubulointerstitial space in 100 cortical fields for each animal were counted for statistical analysis of inflammation. For statistical analysis of proliferation, the number of proliferating cells in 100 glomeruli was counted for each animal. Counterstaining was omitted for the acetylated-H3K9 antibody and quantification of glomerular intensity for each condition was performed using NIH ImageJ software (http://rsb.info.nih.gov/ij/) in 20 glomeruli of at least three animals per group.
Transmission Electron Microscopy
Kidney cortex was fixed in 2% glutaraldehyde in cacodylate buffer at 4°C, postfixed in 1% osmium-tetroxide, and stained with 2% uranyl acetate. The samples were dehydrated and embedded in Poly/bed 812 Araldite resin (Polysciences Inc., Eppelheim, Germany). Ultrathin sections (50 to 100 nm) were cut with an ultramicrotome (Ultracut; Reichert-Jung, Depew, New York), mounted on copper grids, and examined in a Tecnai 10 (Philips, Eindhoven, The Netherlands). Digital images were taken using a megaview G2 CCD camera (SIS-company, Münster, Germany) at ×6200.
Messenger RNA Analysis
With use of the liquid nitrogen disruption method with mortar and pestle and the QIAshredder spin column followed by the RNeasy kit (Qiagen, Hilden, Germany), total RNA was extracted from renal cortical tissue. The RNA was reverse-transcribed using the RevertAid Premium Reverse Transcriptase kit (Fermentas, St. Leon-Rot, Germany). Gene-specific primers and a Universal Probe Library probe were determined using the Probe Finder software of Roche (https://www.roche-applied-science.com/sis/rtpcr/upl/index.jsp?id=uplct_030000). For real-time PCR (RT-PCR), 2x Maxima Probe qPCR Master Mix was used (Fermentas), subjected to qPCR in an ABI 7500 Real Time PCR System, and analyzed using System SDS software (Applied Biosystems), using 18S ribosomal RNA for normalization. The fold change differences were determined using the comparative threshold cycle method.
For the urinary mRNA analysis,32 we centrifuged the 24-hour urine samples at 3200g for 15 minutes at 4°C. Urine was removed and the remaining pellet was resuspended in 1.5 ml of sterile PBS and then centrifuged at 13,000g for 5 minutes at 4°C. The pellet was resuspended in 1 ml of RLT buffer plus β-mercaptoethanol and stored at −80°C until use. Total RNA was isolated using the protocol of the RNeasy kit (Qiagen). The RNA was reverse-transcribed using the method described above.
Apoptosis Detection
With use of the nonradioactive TdT-mediated fluorescein-dUTP nick end labeling (TUNEL) staining technique, apoptotic cells in the glomeruli were visualized by immunofluorescence following manufacturer's recommendations. Briefly, the TUNEL staining was performed on 5-μm deparaffinized tissue sections after antigen retrieval in DakoCytomation Target Retrieval Solution Citrate buffer (pH 6) by microwave irradiation (350 W for 5 minutes). Slides were stained using the In Situ Cell Death Detection Kit (Roche, Vilvoorde, Belgium) following manufacturer's recommendations and mounted using VectaShield plus DAPI (Vector Laboratories, Brussels, Belgium). Double-positive nuclei (TUNEL and DAPI) in 100 glomeruli were counted for statistical analysis.
Statistical Analysis
Values are presented as mean ± SEM and were compared using the one-way ANOVA and post hoc Fisher protected least significant difference test. P < 0.05 was considered significant.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
We thank Dr. Vet. E. Wyffels for his technical assistance and his skillful assistance in handling experimental animals and M. Berghmans for her technical assistance with transmission electron microscopy. This work was supported by the Research Council of the Vrije Universiteit Brussel (OZR 1428 and OZR 1796 to K.V.B. and C.V.d.B.; GOA48 and GOA78 to L.v.G.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/.
REFERENCES
- 1. Kriz W, Gretz N, Lemley KV: Progression of glomerular diseases: Is the podocyte the culprit? Kidney Int 54: 687–697, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Chen A, Sheu LF, Ho YS, Lin YF, Chou WY, Chou TC, Lee WH: Experimental focal segmental glomerulosclerosis in mice. Nephron 78: 440–452, 1998 [DOI] [PubMed] [Google Scholar]
- 3. Okuda S, Oh Y, Tsuruda H, Onoyama K, Fujimi S, Fujishima M: Adriamycin-induced nephropathy as a model of chronic progressive glomerular disease. Kidney Int 29: 502–510, 1986 [DOI] [PubMed] [Google Scholar]
- 4. Wang Y, Wang YP, Tay YC, Harris DC: Progressive adriamycin nephropathy in mice: Sequence of histologic and immunohistochemical events. Kidney Int 58: 1797–1804, 2000 [DOI] [PubMed] [Google Scholar]
- 5. Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 6. Mannaerts I, Nuytten NR, Rogiers V, Vanderkerken K, van Grunsven LA, Geerts A: Chronic administration of valproic acid inhibits activation of mouse hepatic stellate cells in vitro and in vivo. Hepatology 51: 603–614, 2010 [DOI] [PubMed] [Google Scholar]
- 7. Niki T, Rombouts K, De Bleser P, De Smet K, Rogiers V, Schuppan D, Yoshida M, Gabbiani G, Geerts A: A histone deacetylase inhibitor, trichostatin A, suppresses myofibroblastic differentiation of rat hepatic stellate cells in primary culture. Hepatology 29: 858–867, 1999 [DOI] [PubMed] [Google Scholar]
- 8. Rombouts K, Knittel T, Machesky L, Braet F, Wielant A, Hellemans K, De Bleser P, Gelman I, Ramadori G, Geerts A: Actin filament formation, reorganization and migration are impaired in hepatic stellate cells under influence of trichostatin A, a histone deacetylase inhibitor. J Hepatol 37: 788–796, 2002 [DOI] [PubMed] [Google Scholar]
- 9. Rombouts K, Niki T, Wielant A, Hellemans K, Geerts A: Trichostatin A, lead compound for development of antifibrogenic drugs. Acta Gastroenterol Belg 64: 239–246, 2001 [PubMed] [Google Scholar]
- 10. Bush EW, McKinsey TA: Protein acetylation in the cardiorenal axis: The promise of histone deacetylase inhibitors. Circ Res 106: 272–284, 2010 [DOI] [PubMed] [Google Scholar]
- 11. Pang M, Zhuang S: Histone deacetylase: A potential therapeutic target for fibrotic disorders. J Pharmacol Exp Ther 335: 266–272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Marumo T, Hishikawa K, Yoshikawa M, Hirahashi J, Kawachi S, Fujita T: Histone deacetylase modulates the proinflammatory and -fibrotic changes in tubulointerstitial injury. Am J Physiol Renal Physiol 298: F133–F141, 2010 [DOI] [PubMed] [Google Scholar]
- 13. Pang M, Kothapally J, Mao H, Tolbert E, Ponnusamy M, Chin YE, Zhuang S: Inhibition of histone deacetylase activity attenuates renal fibroblast activation and interstitial fibrosis in obstructive nephropathy. Am J Physiol Renal Physiol 297: F996–F1005, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cao Y, Semanchik N, Lee SH, Somlo S, Barbano PE, Coifman R, Sun Z: Chemical modifier screen identifies HDAC inhibitors as suppressors of PKD models. Proc Natl Acad Sci U S A 106: 21819–21824, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noh H, Oh EY, Seo JY, Yu MR, Kim YO, Ha H, Lee HB: Histone deacetylase-2 is a key regulator of diabetes- and transforming growth factor-beta1-induced renal injury. Am J Physiol Renal Physiol 297: F729–F739, 2009 [DOI] [PubMed] [Google Scholar]
- 16. Sayyed SG, Gaikwad AB, Lichtnekert J, Kulkarni O, Eulberg D, Klussmann S, Tikoo K, Anders HJ: Progressive glomerulosclerosis in type 2 diabetes is associated with renal histone H3K9 and H3K23 acetylation, H3K4 dimethylation and phosphorylation at serine 10. Nephrol Dial Transplant 25: 1811–1817, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS: Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest 111: 539–552, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yoshikawa M, Hishikawa K, Marumo T, Fujita T: Inhibition of histone deacetylase activity suppresses epithelial-to-mesenchymal transition induced by TGF-beta1 in human renal epithelial cells. J Am Soc Nephrol 18: 58–65, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Freidkin I, Herman M, Tobar A, Chagnac A, Ori Y, Korzets A, Gafter U: Effects of histone deacetylase inhibitors on rat mesangial cells. Am J Physiol Renal Physiol 298: F426–F434, 2010 [DOI] [PubMed] [Google Scholar]
- 20. de Groh ED, Swanhart LM, Cosentino CC, Jackson RL, Dai W, Kitchens CA, Day BW, Smithgall TE, Hukriede NA: Inhibition of histone deacetylase expands the renal progenitor cell population. J Am Soc Nephrol 21: 794–802, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imai N, Hishikawa K, Marumo T, Hirahashi J, Inowa T, Matsuzaki Y, Okano H, Kitamura T, Salant D, Fujita T: Inhibition of histone deacetylase activates side population cells in kidney and partially reverses chronic renal injury. Stem Cells 25: 2469–2475, 2007 [DOI] [PubMed] [Google Scholar]
- 22. Blaheta RA, Michaelis M, Driever PH, Cinatl J, Jr.: Evolving anticancer drug valproic acid: Insights into the mechanism and clinical studies. Med Res Rev 25: 383–397, 2005 [DOI] [PubMed] [Google Scholar]
- 23. Gottlicher M, Minucci S, Zhu P, Kramer OH, Schimpf A, Giavara S, Sleeman JP, Lo Coco F, Nervi C, Pelicci PG, Heinzel T: Valproic acid defines a novel class of HDAC inhibitors inducing differentiation of transformed cells. EMBO J 20: 6969–6978, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Khan N, Jeffers M, Kumar S, Hackett C, Boldog F, Khramtsov N, Qian X, Mills E, Berghs SC, Carey N, Finn PW, Collins LS, Tumber A, Ritchie JW, Jensen PB, Lichenstein HS, Sehested M: Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J 409: 581–589, 2008 [DOI] [PubMed] [Google Scholar]
- 25. Johnstone RW: Histone-deacetylase inhibitors: novel drugs for the treatment of cancer. Nat Rev Drug Discov 1: 287–299, 2002 [DOI] [PubMed] [Google Scholar]
- 26. Choudhary C, Kumar C, Gnad F, Nielsen ML, Rehman M, Walther TC, Olsen JV, Mann M: Lysine acetylation targets protein complexes and co-regulates major cellular functions. Science 325: 834–840, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Zacharias N, Sailhamer EA, Li Y, Liu B, Butt MU, Shuja F, Velmahos GC, de Moya M, Alam HB: Histone deacetylase inhibitors prevent apoptosis following lethal hemorrhagic shock in rodent kidney cells. Resuscitation 82: 105–109, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Rangan GK, Wang Y, Tay YC, Harris DC: Cytokine gene expression in Adriamycin nephropathy: Effects of antioxidant nuclear factor kappaB inhibitors in established disease. Nephron 86: 482–490, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Vielhauer V, Berning E, Eis V, Kretzler M, Segerer S, Strutz F, Horuk R, Grone HJ, Schlondorff D, Anders HJ: CCR1 blockade reduces interstitial inflammation and fibrosis in mice with glomerulosclerosis and nephrotic syndrome. Kidney Int 66: 2264–2278, 2004 [DOI] [PubMed] [Google Scholar]
- 30. Wu H, Wang Y, Tay YC, Zheng G, Zhang C, Alexander SI, Harris DC: DNA vaccination with naked DNA encoding MCP-1 and RANTES protects against renal injury in adriamycin nephropathy. Kidney Int 67: 2178–2186, 2005 [DOI] [PubMed] [Google Scholar]
- 31. Marshall CB, Krofft RD, Pippin JW, Shankland SJ: The CDK-inhibitor p21 is Pro-survival in adriamycin(R)-induced podocyte injury, in vitro and in vivo. Am J Physiol Renal Physiol 298: F1140–F1151, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sato Y, Wharram BL, Lee SK, Wickman L, Goyal M, Venkatareddy M, Chang JW, Wiggins JE, Lienczewski C, Kretzler M, Wiggins RC: Urine podocyte mRNAs mark progression of renal disease. J Am Soc Nephrol 20: 1041–1052, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Van Beneden K, van Grunsven LA, Geers C, Pauwels M, Desmouliere A, Verbeelen D, Geerts A, Van den Branden C: CRBP-I in the renal tubulointerstitial compartment of healthy rats and rats with renal fibrosis. Nephrol Dial Transplant 23: 3464–3471, 2008 [DOI] [PubMed] [Google Scholar]
- 34. Jeansson M, Bjorck K, Tenstad O, Haraldsson B: Adriamycin alters glomerular endothelium to induce proteinuria. J Am Soc Nephrol 20: 114–122, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zheng Z, Schmidt-Ott KM, Chua S, Foster KA, Frankel RZ, Pavlidis P, Barasch J, D'Agati VD, Gharavi AG: A Mendelian locus on chromosome 16 determines susceptibility to doxorubicin nephropathy in the mouse. Proc Natl Acad Sci U S A 102: 2502–2507, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mundel P, Reiser J: Proteinuria: An enzymatic disease of the podocyte? Kidney Int 77: 571–580, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shankland SJ: The podocyte's response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Shiiki H, Sasaki Y, Nishino T, Kimura T, Kurioka H, Fujimoto S, Dohi K: Cell proliferation and apoptosis of the glomerular epithelial cells in rats with puromycin aminonucleoside nephrosis. Pathobiology 66: 221–229, 1998 [DOI] [PubMed] [Google Scholar]
- 39. Griffin SV, Hiromura K, Pippin J, Petermann AT, Blonski MJ, Krofft R, Takahashi S, Kulkarni AB, Shankland SJ: Cyclin-dependent kinase 5 is a regulator of podocyte differentiation, proliferation, and morphology. Am J Pathol 165: 1175–1185, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Liu Y: New insights into epithelial-mesenchymal transition in kidney fibrosis. J Am Soc Nephrol 21: 212–222, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zeisberg EM, Potenta SE, Sugimoto H, Zeisberg M, Kalluri R: Fibroblasts in kidney fibrosis emerge via endothelial-to-mesenchymal transition. J Am Soc Nephrol 19: 2282–2287, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kang YS, Li Y, Dai C, Kiss LP, Wu C, Liu Y: Inhibition of integrin-linked kinase blocks podocyte epithelial-mesenchymal transition and ameliorates proteinuria. Kidney Int 78: 363–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Shankland SJ, Pippin JW, Reiser J, Mundel P: Podocytes in culture: Past, present, and future. Kidney Int 72: 26–36, 2007 [DOI] [PubMed] [Google Scholar]
- 44. Sun G, Reddy MA, Yuan H, Lanting L, Kato M, Natarajan R: Epigenetic histone methylation modulates fibrotic gene expression. J Am Soc Nephrol 21: 2069–2080, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Johannessen CU: Mechanisms of action of valproate: A commentatory. Neurochem Int 37: 103–110, 2000 [DOI] [PubMed] [Google Scholar]
- 46. Chateauvieux S, Morceau F, Dicato M, Diederich M: Molecular and therapeutic potential and toxicity of valproic acid. J Biomed Biotechnol 2010. doi: 10.1155/2010/479364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Bannister AJ, Kouzarides T: Regulation of chromatin by histone modifications. Cell Res 21: 381–395, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Deman A, Ceyssens B, Pauwels M, Zhang J, Houte KV, Verbeelen D, Van den Branden C: Altered antioxidant defence in a mouse adriamycin model of glomerulosclerosis. Nephrol Dial Transplant 16: 147–150, 2001 [DOI] [PubMed] [Google Scholar]
- 49. Van den Branden C, Deman A, Ceyssens B, Pauwels M, Empsen C, Verbeelen D: Vitamin E protects renal antioxidant enzymes and attenuates glomerulosclerosis in adriamycin-treated rats. Nephron 91: 129–133, 2002 [DOI] [PubMed] [Google Scholar]
- 50. Xia Q, Sung J, Chowdhury W, Chen CL, Hoti N, Shabbeer S, Carducci M, Rodriguez R: Chronic administration of valproic acid inhibits prostate cancer cell growth in vitro and in vivo. Cancer Res 66: 7237–7244, 2006 [DOI] [PubMed] [Google Scholar]
- 51. Lea WB, Kwak ES, Luther JM, Fowler SM, Wang Z, Ma J, Fogo AB, Brown NJ: Aldosterone antagonism or synthase inhibition reduces end-organ damage induced by treatment with angiotensin and high salt. Kidney Int 75: 936–944, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.