Abstract
Background:
Endovascular embolization represents the method of choice for the treatment of carotid-cavernous fistulas (CCFs).
Methods:
We report our experience using the endovascular technique in 24 patients harboring 25 CCFs treated between October 1994 and April 2010, with an emphasis on the role of detachable balloons for the treatment of direct CCFs.
Results:
Of the 16 patients who presented with direct CCFs (Barrow Type A CCFs) (age range, 7–62 years; mean age, 34.3 years), 14 were caused by traumatic injury and 2 by a ruptured internal carotid artery (ICA) aneurysm. Eight patients (age range, 32–71 years; mean age, 46.5 years) presented with nine indirect CCFs (Barrow Types B, C, and D). The clinical follow-up after endovascular treatment ranged from 2 to 108 months (mean, 35.2 months). In two cases (8%), the endovascular approach failed. Symptomatic complications related to the procedure occurred in three patients (12.5%): transient cranial nerve palsy in two patients and a permanent neurological deficit in one patient. Detachable balloons were used in 13 out of 16 (81.3%) direct CCFs and were associated with a cure rate of 92.3%. Overall, the angiographic cure rate was obtained in 22 out of 25 (88%) fistulas. Patients presenting with III nerve palsy improved gradually between 1 day and 6 months after treatment. Good clinical outcomes [modified Rankin scale (mRS) ≤ 2] were observed in 22 out of 24 (91.6%) patients at last follow-up.
Conclusions:
Endovascular treatment using detachable balloons still constitutes a safe and effective method to treat direct carotid-cavernous fistulas.
Keywords: Carotid-cavernous fistula, endovascular embolization, gold valve detachable balloon
INTRODUCTION
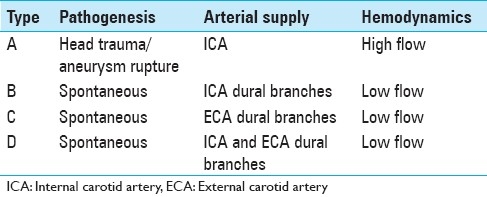
Carotid-cavernous fistulas (CCFs) are abnormal arteriovenous shunts in the cavernous sinus (CS). According to Barrow et al.,[2] CCFs are classified into two categories: direct fistulas (Type A) and indirect fistulas (Types B, C, and D) [Table 1]. Direct CCFs are high-flow shunts between the cavernous segment of the internal carotid artery (ICA) and the CS, usually caused by traumatic injury or a ruptured aneurysm at this level.[14,21] Indirect CCFs or dural shunts usually occur spontaneously and are supplied either by dural branches of the ICA (Type B) or dural branches of the external carotid artery (Type C) or both (Type D). Endovascular embolization represents the first therapeutic modality for CCFs because it is associated with high occlusion and low complication rates. The endovascular treatment of CCFs involves the use of different embolic materials including detachable balloons, silk, coils, n-butyl cyanoacrylate (n-BCA), covered stents, and recently, Onyx. In this study, we report our experience with the use of different embolic agents to treat 24 patients with a total of 25 CCFs. Also, we emphasize the role of detachable balloons in the treatment of direct CCFs.
Table 1.
Barrow's classification of carotid-cavernous fistulas
MATERIALS AND METHODS
Patients
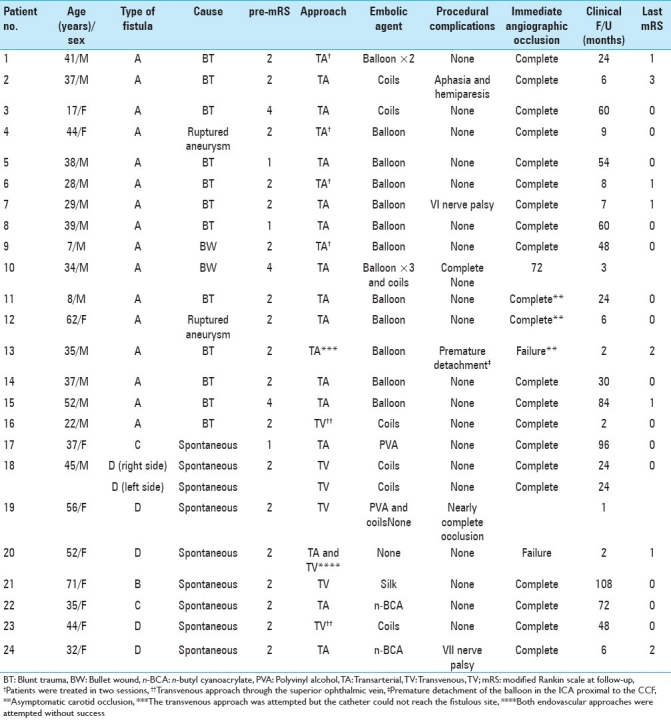
We analyzed the clinical and radiological data of 24 patients harboring 25 CCFs (16 direct and 9 indirect) treated between October 1994 and April 2010. Endovascular embolization using the transarterial and/or transvenous approach was performed. Patient's age and sex, type of fistula, endovascular approach, complications, clinical and angiographic evaluation were recorded [Tables 2 and 3].
Table 2.
Symptoms and signs pre- and post-embolization in 24 patients with carotid-cavernous fistulas
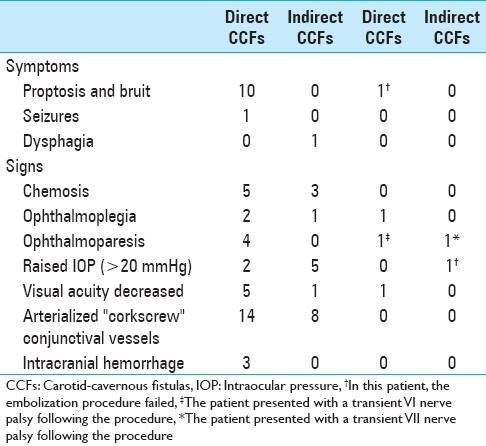
Table 3.
Demographic and treatment outcomes of 24 patients with direct and indirect carotid-cavernous fistulas
Methods
Endovascular technique
All the procedures were performed under general anesthesia and using standard interventional neuroradiology techniques. Anticoagulation was performed with heparin IV administered as a bolus and then by hourly maintenance doses to maintain the anticoagulation time (ACT) at twice the baseline. Anticoagulation was initiated after groin puncture in all patients except in those who presented with intracranial hemorrhage. Endovascular treatment with detachable balloons was chosen as the first-line therapeutic modality for the treatment of direct CCFs. Manual or balloon test occlusion of the ICA at the time of contralateral ICA or vertebral artery contrast injection was performed to locate the fistulous site prior to the embolization of CCFs Type A. For the treatment of CCFs Type A, Fast-Tracker-10 and Fast-Tracker-18 microcatheters (Boston Scientific Co., Boston, Mass, USA ) were utilized for the detachment of balloons (gold valve balloon #9, #12, #16, and # 17; Nycomed Ingenor, Paris, France) and platinum coils, respectively. The detachable balloons were mounted on the tip of the microcatheter with the support of microguidewires (Dasher 10; Boston Scientific Co., Fremont, CA, USA). Under road mapping technique, the balloon was navigated across the fistulous gap to reach the involved compartment of the CS. Once at the fistulous site, either balloons or coils were inflated or deployed, respectively, until the fistula showed complete obliteration. Occasionally, more than one balloon was used to occlude large compartments of the CS.
In CCFs Types C and D, polyvinyl alcohol (PVA) particles (250–350 μm, Ivalon, Nycomed Ingenor) or n-BCA (Histoacryl, Braun, Melsungen, Germany) mixed with lipiodol were injected via external carotid artery (ECA) dural feeders as first line of treatment. The transvenous approach was mainly used for CCFs Types B and D. A transvenous access via the inferior petrosal sinus (IPS) was attempted in four cases. Direct surgical exposure of the superior ophthalmic vein (SOV) was performed using a 16-gauge needle catheter in two cases. Then, with coaxial technique, the fistulous site at the CS was approached via a microcatheter for the deployment of platinum coils (Boston Scientific Co., USA) until occlusion was obtained.
Clinical and angiographic follow-up
Immediate clinical and angiographic follow-up was available in all cases. Long-term clinical outcomes were evaluated by reviewing the patient's clinical notes and by phone interviews. Initial and last clinical status were graded according to the modified Rankin scale (mRS) defined as follows: Grade 0, no symptoms at all; Grade 1, no significant disability despite symptoms being present, but able to carry out all usual activities; Grade 2, slight disability: unable to carry out all previous activities but able to look after own affairs without assistance; Grade 3, moderate disability: requiring some help but able to walk without assistance; Grade 4, moderately severe disability: unable to walk and attend to own bodily needs without assistance; Grade 5, severe disability: bedridden, incontinent, and requiring constant nursing care and attention; and Grade 6, death. An mRS of less than or equal to 2 was considered a good outcome. Immediate and long-term imaging follow-up was performed using digital subtraction angiography (DSA) and magnetic resonance imaging, respectively.
RESULTS
General characteristics
There were 14 men and 10 women, aged from 7 to 71 years (mean age, 37.5 years). The elapsed time between onset of symptoms and treatment ranged between 6 weeks and 4 years. Of the 25 CCFs treated, 16 were direct CCFs (Barrow Type A) and 9 were indirect CCFs (Barrow Types B, C, and D). Direct CCFs were treated as follows: detachable balloons alone (n = 12), coils alone (n = 3), and combined detachable balloons and coils (n = 1). Indirect CCFs were treated as follows: coils alone (n = 3), n-BCA (n = 2), PVA alone (n = 1), combined coils and PVA (n = 1), silk (n = 1); and in one patient the procedure failed. After treatment, ocular signs and symptoms resolved in 22 out of 24 patients (91.6%). Clinical symptoms at presentation and following treatment are presented in Table 2. Representative cases are shown in Figures 1–3.
Figure 1.
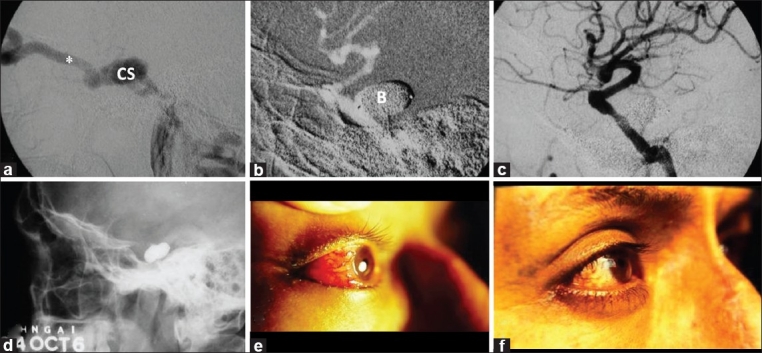
A 41-year-old man with red eye, proptosis, chemosis, bruit and visual loss of the right eye (Case #1). (a) Cerebral angiography of the right internal carotid artery (ICA) confirmed high-flow direct carotid cavernous fistula with “vascular steal” phenomenon. The cavernous sinus and superior ophthalmic vein (*) showed marked dilatation. (b) Under road mapping technique, two gold-valve balloons (B) were detached. (c) Immediate angiography after balloon embolization showed complete obliteration of the fistula, preserving the ICA lumen. (d) Cranial X-ray shows contrast-filled balloons. Patient's eye (e) pre-embolization and (f) 1 week post-embolization. The patient experienced marked visual improvement
Figure 3.
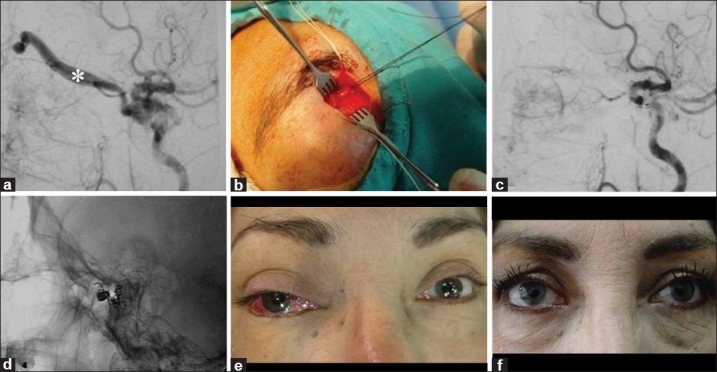
A 44-year-old female with red eye, bruit, glaucoma, and diplopia of the right eye (Case #23). (a) Cerebral angiography of the right internal carotid artery showed a CCF Type D with exclusive drainage into the superior ophthalmic vein (*). (b) A surgical exposure of the SOV was performed in order to place a microcatheter at the fistulous site of the carotid-cavernous fistula. (c) Cerebral angiography post-embolization showed complete obliteration of the fistula. (d) Cranial X-ray showed a coiled mass packed into the cavernous sinus. Patient's eye (e) pre-embolization and (f) 3 months after the endovascular procedure
Direct carotid-cavernous fistulae
Of the 16 patients with CCFs Type A, 15 were cured (93.7%). In three patients (12%) who presented with CCFs Type A, the ICA lumen could not be preserved. Of these three patients, two (Case #11 and #12) showed complete occlusion of the fistula and in one patient the procedure failed (Case #13). Four patients (Case #1, #4, #6, and #9) required a second session of endovascular treatment using detachable balloons because of symptoms of recurrence. In this group of patients, the intervention was performed during the first week. The transarterial endovascular approach was utilized in 15 patients and the transvenous approach through the SOV in 1 patient. Detachable latex gold valve balloons alone were utilized in 12 patients, coils alone in 3 patients, and combined balloons with coils in 1 patient. Procedural complications occurred in three patients: two patients were symptomatic and one patient was asymptomatic. One patient (Case #7) presented with transient VI nerve palsy, most probably caused by compression due to the balloon, and showed a good clinical grade (mRS = 1). A permanent neurologic complication occurred in one patient (Case #2) due to air embolism during the endovascular procedure and resulted in aphasia with right hemiparesis (mRS = 3). In one patient (Case #13), a prolonged manipulation of the balloon through a tortuous right ICA led to its premature detachment proximal to the fistulous site occluding the petrous segment of the ICA without occluding the fistulous site, and the patient remained neurologically stable (mRS = 2). In this case, we attempted to catheterize the IPS, but the catheter could not reach the fistulous site.
All ocular signs and symptoms resolved after closure of the fistula except in three patients: in one patient, the embolization procedure failed; in another patient, the ophthalmoplegia did not resolve; and one patient presented with VI nerve palsy probably caused by compression due to the balloon [Table 2].
Indirect carotid-cavernous fistulas
Eight patients with nine indirect CCFs were treated by endovascular means. The transarterial approach alone was utilized in two patients: one patient (Case #17) was treated with PVA and the other one (Case #22) with n-BCA. In both patients, good clinical grades were achieved (mRS = 0). The transvenous route alone was utilized in three patients harboring four fistulas: two fistulas were treated with coils (Case #18), one fistula was treated with PVA and coils [Figure 2] (Case #19), and one fistula was treated with silk fragments (Case #21). All patients had good clinical grades at the last follow-up (mRS = 0). Transarterial and transvenous endovascular approaches were attempted in one patient (Case #20) without success. In one patient, the fistula was treated with n-BCA using the stylomastoid artery (Case #24), resulting in a good clinical grade (mRS = 2).
Figure 2.
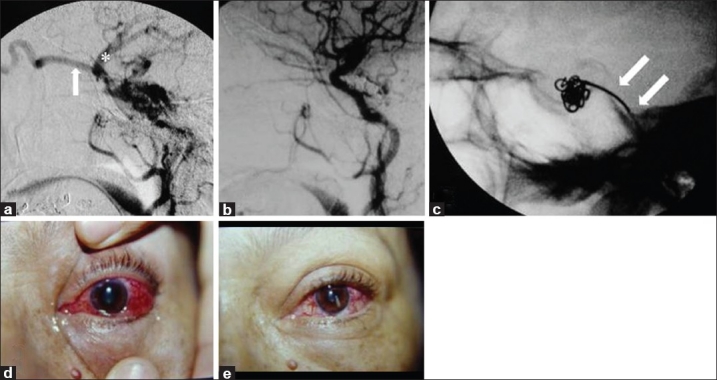
A 56-year-old female with red eye, chemosis, bruit, glaucoma, diplopia, and visual loss of the left eye (Case #19). (a) Cerebral angiography of the left internal carotid artery confirmed an indirect CCF Type D with drainage into the SOV (arrow) and the sphenoparietal sinus (FNx01). (b) Post-embolization angiography. (c) Platinum coils were delivered transvenously through the inferior petrous sinus (double arrow). Patient's eye (d) pre-embolization and (e) 1 month after the procedure
In one case (Case #19), a near-complete occlusion (90% angiographic occlusion) of the fistula was achieved using the transvenous route alone and was associated with symptomatic improvement (mRS = 1). In another patient [Figure 3] (Case #23), the transvenous route through the SOV was utilized for the deployment of platinum coils and resulted in a good clinical grade (mRS = 0). Of the nine patients with indirect CCFs, seven were angiographically cured (77.8%). In all cases, angiographic obliteration of indirect fistulae was associated with remission of the clinical picture. A transient neurological complication related to the procedure occurred in one patient (Case #24). In this case, the transvenous route was attempted without success, making the transarterial embolization through the stylomastoid artery with n-BCA necessary since the main drainage of the fistula was directed toward the basal vein of Rosenthal, anticipating an aggressive clinical course if it had not been treated. However, 6 hours after the procedure, the patient presented with cranial nerve palsy because of the inadvertent embolization of one of the branches of the stylomastoid artery supplying the facial nerve. At 6 months of follow-up, the patient showed complete resolution of her ocular signs and symptoms related to the fistula with some improvement of the iatrogenic facial nerve palsy (mRS = 2).
Overall results in carotid-cavernous fistulas
The overall analysis of the 25 CCFs treated by endovascular means shows that 22 (88%) fistulas were cured. One indirect CCF had near-complete occlusion (90% angiographic occlusion) and was associated with symptomatic improvement. In two cases (8%), the endovascular procedure failed. Complications related to the procedure occurred in four patients: three symptomatic and one asymptomatic. The symptomatic neurologic complications were as follows: a transient VI cranial nerve palsy in one patient, a transient VII cranial nerve palsy in one patient, and aphasia with hemiparesis in one patient. In one patient, the manipulation of the balloon in a tortuous ICA led to its premature detachment occluding the ICA proximal to the fistula, and the patient remained asymptomatic. Twenty-two out of 24 patients (91.6%) showed good clinical outcomes (mRS ≤ 2) at the last follow-up.
DISCUSSION
The clinical manifestations and course of CCFs can be benign, intermediate, or severe, depending on their angioarchitectural and hemodynamic characteristics.[10] Symptoms of direct CCFs are often abrupt in onset and progress rapidly. On the other hand, indirect fistulas generally present with a gradual onset and milder clinical manifestations. In general, the most common clinical manifestations of CCFs are proptosis, chemosis, exophthalmos, conjunctival injection, cranial nerve palsy, visual deficits, headache, and tinnitus or bruit.[19] In cases with aggressive clinical behavior accompanied by severe orbital venous hypertension and/or leptomeningeal venous drainage, occlusion of the fistula is mandatory in order to decrease the risk of visual loss, severe epistaxis, progressive focal neurological deficits, dementia, venous infarction, intracranial hemorrhage, and ultimately death.[31,41] Due to its relative rarity and surgical difficulty, embolization of CCFs represented a reliable procedure to evaluate the feasibility of introducing endovascular therapy in Peru. In the early 1990s, endovascular therapy in a country with a broken economy brought additional challenges such as a scarce set of latex balloons and microcatheters due to problems with the import of these expensive materials and the necessity to succeed completely in embolizing a given CCF to justify the costs involved, not to mention the difficulties imposed by the intervention per se with a neurointerventionalist beginning his learning curve. At the end of the 1990s, both balloons and Guglielmi detachable coils (GDC) became available in Peru, but due to acceptable results achieved by us and mainly for economical reasons, latex balloons still represent our first choice to manage Type A CCFs. This balloon technology also applies to the endovascular treatment of very large and giant cerebral aneurysms in our practice. Controlled-release detachable coil embolization is much more expensive, especially in large lesions. Training of specialists was a difficult task due to the scarcity of both materials and endovascular interventions.
Endovascular treatment of direct CCFs
Although the use of stent grafts and liquid embolic materials is gaining popularity, transarterial embolization using detachable balloons has been considered the first therapeutic modality for the treatment of direct CCFs.[5,6,12,33,36] Serbinenko and later Debrun developed the balloon technology and techniques for the endovascular obliteration of direct CCFs, preserving the lumen of the ICA.[5–7,33] The majority of patients who are successfully treated using this technology show postoperative ICA patency ranging from 59 to 88%.[6,17,21,22] In some cases, occlusion of the ICA lumen is necessary sometimes to obliterate the fistula completely.[4] The accumulated experience has ascertained the efficacy of this treatment modality and has become the first-line therapy for high-flow direct CCFs. In our practice, endovascular treatment with detachable balloons is chosen as the first-line therapeutic modality for direct CCFs. We utilized detachable balloons in 81.3% of patients with direct CCFs, resulting in a cure rate of 92.3%, with ICA lumen preservation in 80% of patients. From our point of view, detachable balloons still constitute a safe, economic, and effective method for the treatment of direct CCFs with preservation of the ICA. However, despite the advantages of this procedure, there are some technical limitations if the preservation of the parent artery is desired, such as: 1) a small fistula in the CS may not allow the passage and inflation of the balloon; 2) the balloon may rupture if it comes into contact with sharp osseous fragments; and 3) a premature detachment and migration of the balloon into the ICA can lead to stroke. Also, once deployed, an inflated balloon may cause serious neurologic damage by two different mechanisms:[32,40] 1) venous drainage may redirect either to the orbit or to the pial veins, worsening the symptoms and increasing the probability of visual loss and cerebral hemorrhage and 2) an overinflated balloon may exert direct compression on the cranial nerves. In our series, we reported two technical complications associated with the use of detachable balloons: a patient (Case #13) in whom the premature detachment of the balloon resulted in asymptomatic internal carotid artery occlusion proximal to the fistula and a patient (Case #7) with reversible VI nerve palsy probably caused by compression of the balloon. Three out of 16 patients with direct CCFs (18.8%) had ICA occlusion. In these cases, detachable balloons were employed. Occlusion of the carotid artery did not result in neurological deficits in any of the patients. In two patients (Cases # 11 and #12), the carotid artery occlusion was intentional, while in the last patient (Case #13), it was accidental. None of the patients had previous balloon test occlusion. However, contralateral ICA and/or vertebral angiogram performed immediately before balloon detachment demonstrated a patent circle of Willis with good perfusion of the carotid tree in the side of the lesion; otherwise, the detachment of the balloons could not have been attempted and coil embolization would have proceeded. Other embolic agents for the treatment of direct CCFs include coils, n-BCA, Onyx, and silk. Occlusion of a direct CCF by transvenous approach usually involves the IPS up into the shunt involving the CS.[19] If the IPS is occluded or absent, access into the CS can be obtained through the SOV, as seen in Case #16.
Endovascular treatment of indirect CCFs
Since spontaneous remissions occur in 9.4–50% of indirect CCFs, some authors recommend expectant or palliative treatment for benign cases.[2,7,13,15,30] However, besides the apparent efficacy of the expectant management of indirect CCFs, it may be a bias to select low-flow fistulas with benign course and significant tendency to spontaneous thrombosis. Additionally, these patients would need a close follow-up for early detection of visual loss and neurological symptoms. Thus, endovascular embolization should be the primary treatment for indirect CCFs.[15,24] Transarterial embolization of indirect CCFs generally is cumbersome because of the small size, tortuous anatomy, and multiplicity of arterial feeders. In patients with indirect CCFs, especially the ones supplied predominantly by meningeal branches of the ECA (Types C and D), the microcatheter must be placed as close as possible to the fistulous site for the injection of proper embolic agents under fluoroscopic control with the goal of occluding the fistulous connection and penetrating the CS.[7,20] The transarterial embolization technique in these types of fistulas may cure CCFs Type C and markedly decrease the blood flow in CCFs Type D to such an extent that the fistulous compartment of the CS may eventually lead to spontaneous thrombosis. Since embolization of small branches coming off the ICA in indirect CCFs may carry a higher risk of cerebral infarction, the transvenous embolization with coils via the IPS or SOV through surgical exposure has gained acceptance and has been demonstrated to be effective in this type of fistulas. The most commonly used agents for transarterial embolization of indirect CCFs include n-BCA,[27] Onyx,[3,28] and PVA.[38,39] One of the earliest and more relevant experiences with the transarterial embolization technique of indirect CCFs was reported by Vinuela et al.,[38] using PVA particles and isobutyl cyanoacrylate (IBCA). The risk of transarterial embolization of indirect CCFs lies in the anastomotic communication between dural arteries supplying the fistula and meningeal and pial vessels supplying cranial nerves and brain tissue, increasing the risk of cranial nerve palsy and stroke, respectively. In our series, we report one patient with an indirect CCF whose embolization through the stylomastoid artery with n-BCA resulted in transient VII nerve palsy. The cure rate for indirect CCFs using the transarterial approach ranges between 70 and 78%.[38] In our series, we embolized three indirect CCFs using the transarterial approach, achieving cure in two patients (66.7%).
The transvenous approach has become the treatment modality of choice for symptomatic indirect CCFs due to its better results compared to other therapeutic modalities.[19,24] The high long-term success rate of this technique lies in its simplicity compared to transarterial methods and the ability to cure the fistula often in a single session. The most common embolic agents include coils, PVA, n-BCA, and Onyx, either alone or in combination. The advantage of using coils lies in its radiopacity, ease of use, controlled deployment, and ability to be removed if the initial placement is not optimal.[10] Also, coils adopt the shape of the fistulous compartment and rarely migrate or experience deformation. In our series, we mainly used coils for the treatment of indirect CCFs [Figure 2]. In CCFs Types B and D, transvenous embolization with coils has demonstrated to be effective and represents the treatment of choice.[13] Onyx (eV3 Neurovascular Inc., Irvine, CA, USA) is a liquid embolic agent that is effective for the treatment of direct and indirect CCFs. It can be used alone[23,43] or in combination with coils[16,35] and stents.[34,42] However, the use of Onyx is not exempt from complications such as transient compressive neuropathies or cranial nerve ischemia/infarction caused by post-embolization CS thrombosis and penetration within arterial collaterals, respectively.[9] There is a need for larger series and follow-up data to determine if Onyx can be considered a definitive treatment for this type of lesion.
The largest reported series using the transvenous approach for indirect CCFs comes from Kirsch et al.,[18] with 141 patients who underwent 161 treatment sessions achieving complete obliteration of the fistula in 81% of patients. Meyers et al.[24] reported the largest series of indirect CCF treated by endovascular means with 133 patients and a mean follow-up of 56 months. They reported a cure rate of 90%, with good clinical outcomes in 97% of the patients and a procedure-related morbidity of 2.3%. Similarly, in our series, we reported eight patients with indirect CCFs with a mean follow-up of 42.7 months. The reported cure rate was 77.8% and good clinical outcomes were obtained in all patients. The procedure-related morbidity was 12.5%.
The transvenous approach is frequently achieved via the IPS; however, the facial vein or angular vein can also be used for catheterization, with the approach involving the angular vein being the most technically difficult. If transvenous jugular route fails, a retrograde catheterization with surgical exposure of the SOV at the level of the eyelid could be performed.[11,19,26,30,37] Complications associated with different venous approaches are injury of the cranial nerves due to the dense packing of the CS with coils as well as vascular dissections and perforations.[29] The reported complications associated with the SOV approach include hemorrhage, visual loss, IV nerve palsy, and infection.[1,8,25] Despite the fear of causing these complications, the SOV approach is still considered a direct, safe, and effective technique in the treatment of CCFs. In our series, we utilized the SOV approach in one patient with an indirect CCF (Case #23) and in another patient with a direct CCF (Case #16), both resulting in complete obliteration of the fistula.
Training in the maneuvers to handle latex balloons is critical because proximal or distal displacement of the microcatheter tip from the balloon after subsequent attempts to reach the fistulous site may occur and this may lead to occlusion of the parent vessel as seen in our Case #13. Postoperative bed rest, analgesia, prophylactic antiemetics, stool softeners, sedatives, and keeping a low blood pressure in the first 5–7 days postoperatively represent the essential measures to avoid displacement of the deployed balloons and consequent reopening of the fistula. In our practice, embolization of direct CCFs using detachable balloons represents the treatment of choice since this technology is still the gold standard of treatment for this type of lesion, and also due to economical reasons. In our experience, transarterial embolization using detachable balloons has demonstrated acceptable rates of cure and complications.
CONCLUSIONS
Endovascular embolization represents the first-line therapy for the management of CCFs. The main goal of the endovascular treatment is to achieve complete obliteration of the fistula and at the same time preserve the patency of the ICA. Endovascular embolization with detachable balloons still represents a safe, economic, and effective method to occlude direct carotid-cavernous fistulas, and should be performed by experienced interventionalists.
ACKNOWLEDGMENT
We would like to express our deepest gratitude to Dr. Fernando Vinuela (UCLA Medical Center) for his training, teaching, and invaluable support. Without his permanent assistance, none of our patients could have been treated.
Footnotes
Available FREE in open access from: http://www.surgicalneurologyint.com/text.asp?2012/3/1/5/92167
Contributor Information
Andres R. Plasencia, Email: andresplasencia2000@yahoo.com.
Alejandro Santillan, Email: santillanmd@yahoo.com.
REFERENCES
- 1.Aihara N, Mase M, Yamada K, Banno T, Watanabe K, Kamiya K, et al. Deterioration of ocular motor dysfunction after transvenous embolization of dural arteriovenous fistula involving the cavernous sinus. Acta Neurochir (Wien) 1999;141:707–9. doi: 10.1007/s007010050365. discussion 709-10. [DOI] [PubMed] [Google Scholar]
- 2.Barrow DL, Spector RH, Braun IF, Landman JA, Tindall SC, Tindall GT. Classification and treatment of spontaneous carotid-cavernous sinus fistulas. J Neurosurg. 1985;62:248–56. doi: 10.3171/jns.1985.62.2.0248. [DOI] [PubMed] [Google Scholar]
- 3.Cognard C, Januel AC, Silva NA, Jr, Tall P. Endovascular treatment of intracranial dural arteriovenous fistulas with cortical venous drainage: New management using Onyx. AJNR Am J Neuroradiol. 2008;29:235–41. doi: 10.3174/ajnr.A0817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coley SC, Pandya H, Hodgson TJ, Jeffree MA, Deasy NP. Endovascular trapping of traumatic carotid-cavernous fistulae. AJNR Am J Neuroradiol. 2003;24:1785–8. [PMC free article] [PubMed] [Google Scholar]
- 5.Debrun G, Lacour P, Caron JP, Hurth M, Comoy J, Keravel Y. Detachable balloon and calibrated-leak balloon techniques in the treatment of cerebral vascular lesions. J Neurosurg. 1978;49:635–49. doi: 10.3171/jns.1978.49.5.0635. [DOI] [PubMed] [Google Scholar]
- 6.Debrun G, Lacour P, Vinuela F, Fox A, Drake CG, Caron JP. Treatment of 54 traumatic carotid-cavernous fistulas. J Neurosurg. 1981;55:678–92. doi: 10.3171/jns.1981.55.5.0678. [DOI] [PubMed] [Google Scholar]
- 7.Debrun GM, Vinuela F, Fox AJ, Davis KR, Ahn HS. Indications for treatment and classification of 132 carotid-cavernous fistulas. Neurosurgery. 1988;22:285–9. doi: 10.1227/00006123-198802000-00001. [DOI] [PubMed] [Google Scholar]
- 8.Devoto MH, Egbert JE, Tomsick TA, Kulwin DR. Acute exophthalmos during treatment of a cavernous sinus-dural fistula through the superior ophthalmic vein. Arch Ophthalmol. 1997;115:823–4. doi: 10.1001/archopht.1997.01100150825035. [DOI] [PubMed] [Google Scholar]
- 9.Elhammady MS, Wolfe SQ, Farhat H, Moftakhar R, Aziz-Sultan MA. Onyx embolization of carotid-cavernous fistulas. J Neurosurg. 2010;112:589–94. doi: 10.3171/2009.6.JNS09132. [DOI] [PubMed] [Google Scholar]
- 10.Gemmete JJ, Chaudhary N, Pandey A, Ansari S. Treatment of carotid cavernous fistulas. Curr Treat Options Neurol. 2010;12:43–53. doi: 10.1007/s11940-009-0051-3. [DOI] [PubMed] [Google Scholar]
- 11.Goldberg RA, Goldey SH, Duckwiler G, Vinuela F. Management of cavernous sinus-dural fistulas.Indications and techniques for primary embolization via the superior ophthalmic vein. Arch Ophthalmol. 1996;114:707–14. doi: 10.1001/archopht.1996.01100130699011. [DOI] [PubMed] [Google Scholar]
- 12.Graeb DA, Robertson WD, Lapointe JS, Nugent RA. Avoiding intraarterial balloon detachment in the treatment of posttraumatic carotid-cavernous fistulae with detachable balloons. AJNR Am J Neuroradiol. 1985;6:602–5. [PMC free article] [PubMed] [Google Scholar]
- 13.Halbach VV, Hieshima GB, Higashida RT, Reicher M. Carotid cavernous fistulae: Indications for urgent treatment. AJR Am J Roentgenol. 1987;149:587–93. doi: 10.2214/ajr.149.3.587. [DOI] [PubMed] [Google Scholar]
- 14.Halbach VV, Higashida RT, Hieshima GB, Hardin CW, Yang PJ. Transvenous embolization of direct carotid cavernous fistulas. AJNR Am J Neuroradiol. 1988;9:741–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Halbach VV, Higashida RT, Hieshima GB, Reicher M, Norman D, Newton TH. Dural fistulas involving the cavernous sinus: Results of treatment in 30 patients. Radiology. 1987;163:437–42. doi: 10.1148/radiology.163.2.3562823. [DOI] [PubMed] [Google Scholar]
- 16.He HW, Jiang CH, Wu ZX, Li YX, Lu XL, Wang ZC. Transvenous embolization with a combination of detachable coils and Onyx for a complicated cavernous dural arteriovenous fistula. Chin Med J (Engl) 2008;121:1651–5. [PubMed] [Google Scholar]
- 17.Higashida RT, Halbach VV, Tsai FY, Norman D, Pribram HF, Mehringer CM, et al. Interventional neurovascular treatment of traumatic carotid and vertebral artery lesions: Results in 234 cases. AJR Am J Roentgenol. 1989;153:577–82. doi: 10.2214/ajr.153.3.577. [DOI] [PubMed] [Google Scholar]
- 18.Kirsch M, Henkes H, Liebig T, Weber W, Esser J, Golik S, et al. Endovascular management of dural carotid-cavernous sinus fistulas in 141 patients. Neuroradiology. 2006;48:486–90. doi: 10.1007/s00234-006-0089-9. [DOI] [PubMed] [Google Scholar]
- 19.Klisch J, Huppertz HJ, Spetzger U, Hetzel A, Seeger W, Schumacher M. Transvenous treatment of carotid cavernous and dural arteriovenous fistulae: Results for 31 patients and review of the literature. Neurosurgery. 2003;53:836. doi: 10.1227/01.neu.0000083551.26295.ab. discussion 856-7. [DOI] [PubMed] [Google Scholar]
- 20.Kupersmith MJ, Berenstein A, Choi IS, Warren F, Flamm E. Management of nontraumatic vascular shunts involving the cavernous sinus. Ophthalmology. 1988;95:121–30. doi: 10.1016/s0161-6420(88)33221-5. [DOI] [PubMed] [Google Scholar]
- 21.Lewis AI, Tomsick TA, Tew JM., Jr Management of 100 consecutive direct carotid-cavernous fistulas: Results of treatment with detachable balloons. Neurosurgery. 1995;36:239–44. doi: 10.1227/00006123-199502000-00001. discussion 244-5. [DOI] [PubMed] [Google Scholar]
- 22.Luo CB, Teng MM, Yen DH, Chang FC, Lirng JF, Chang CY. Endovascular embolization of recurrent traumatic carotid-cavernous fistulas managed previously with detachable balloons. J Trauma. 2004;56:1214–20. doi: 10.1097/01.ta.0000131213.93205.57. [DOI] [PubMed] [Google Scholar]
- 23.Lv X, Jiang C, Li Y, Wu Z. Results and complications of transarterial embolization of intracranial dural arteriovenous fistulas using Onyx-18. J Neurosurg. 2008;109:1083–90. doi: 10.3171/JNS.2008.109.12.1083. [DOI] [PubMed] [Google Scholar]
- 24.Meyers PM, Halbach VV, Dowd CF, Lempert TE, Malek AM, Phatouros CC, et al. Dural carotid cavernous fistula: Definitive endovascular management and long-term follow-up. Am J Ophthalmol. 2002;134:85–92. doi: 10.1016/s0002-9394(02)01515-5. [DOI] [PubMed] [Google Scholar]
- 25.Miller NR. Severe vision loss and neovascular glaucoma complicating superior ophthalmic vein approach to carotid-cavernous sinus fistula. Am J Ophthalmol. 1998;125:883–4. [PubMed] [Google Scholar]
- 26.Miller NR, Monsein LH, Debrun GM, Tamargo RJ, Nauta HJ. Treatment of carotid-cavernous sinus fistulas using a superior ophthalmic vein approach. J Neurosurg. 1995;83:838–42. doi: 10.3171/jns.1995.83.5.0838. [DOI] [PubMed] [Google Scholar]
- 27.Nelson PK, Russell SM, Woo HH, Alastra AJ, Vidovich DV. Use of a wedged microcatheter for curative transarterial embolization of complex intracranial dural arteriovenous fistulas: Indications, endovascular technique, and outcome in 21 patients. J Neurosurg. 2003;98:498–506. doi: 10.3171/jns.2003.98.3.0498. [DOI] [PubMed] [Google Scholar]
- 28.Nogueira RG, Dabus G, Rabinov JD, Eskey CJ, Ogilvy CS, Hirsch JA, et al. Preliminary experience with onyx embolization for the treatment of intracranial dural arteriovenous fistulas. AJNR Am J Neuroradiol. 2008;29:91–7. doi: 10.3174/ajnr.A0768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oishi H, Arai H, Sato K, Iizuka Y. Complications associated with transvenous embolisation of cavernous dural arteriovenous fistula. Acta Neurochir (Wien) 1999;141:1265–71. doi: 10.1007/s007010050429. [DOI] [PubMed] [Google Scholar]
- 30.Quinones D, Duckwiler G, Gobin PY, Goldberg RA, Vinuela F. Embolization of dural cavernous fistulas via superior ophthalmic vein approach. AJNR Am J Neuroradiol. 1997;18:921–8. [PMC free article] [PubMed] [Google Scholar]
- 31.Sanders MD, Hoyt WF. Hypoxic ocular sequelae of carotid-cavernous fistulae.Study of the caues of visual failure before and after neurosurgical treatment in a series of 25 cases. Br J Ophthalmol. 1969;53:82–97. doi: 10.1136/bjo.53.2.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selky AK, Purvin VA. Isolated trochlear nerve palsy secondary to dural carotid-cavernous sinus fistula. J Neuroophthalmol. 1994;14:52–4. [PubMed] [Google Scholar]
- 33.Serbinenko FA. Balloon catheterization and occlusion of major cerebral vessels. J Neurosurg. 1974;41:125–45. doi: 10.3171/jns.1974.41.2.0125. [DOI] [PubMed] [Google Scholar]
- 34.Shi ZS, Qi TW, Gonzalez NR, Ziegler J, Huang ZS. Combined covered stent and onyx treatment for complex dural arteriovenous fistula involving the clivus and cavernous sinus. Surg Neurol. 2009;72:169–74. doi: 10.1016/j.surneu.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki S, Lee DW, Jahan R, Duckwiler GR, Vinuela F. Transvenous treatment of spontaneous dural carotid-cavernous fistulas using a combination of detachable coils and Onyx. AJNR Am J Neuroradiol. 2006;27:1346–9. [PMC free article] [PubMed] [Google Scholar]
- 36.Tsai FY, Hieshima GB, Mehringer CM, Grinnell V, Pribram HW. Delayed effects in the treatment of carotid-cavernous fistulas. AJNR Am J Neuroradiol. 1983;4:357–61. [PMC free article] [PubMed] [Google Scholar]
- 37.Uflacker R, Lima S, Ribas GC, Piske RL. Carotid-cavernous fistulas: embolization through the superior ophthalmic vein approach. Radiology. 1986;159:175–9. doi: 10.1148/radiology.159.1.3952304. [DOI] [PubMed] [Google Scholar]
- 38.Vinuela F, Fox AJ, Debrun GM, Peerless SJ, Drake CG. Spontaneous carotid-cavernous fistulas: Clinical, radiological, and therapeutic considerations. Experience with 20 cases. J Neurosurg. 1984;60:976–84. doi: 10.3171/jns.1984.60.5.0976. [DOI] [PubMed] [Google Scholar]
- 39.Wakhloo AK, Perlow A, Linfante I, Sandhu JS, Cameron J, Troffkin N, et al. Transvenous n-butyl-cyanoacrylate infusion for complex dural carotid cavernous fistulas: Technical considerations and clinical outcome. AJNR Am J Neuroradiol. 2005;26:1888–97. [PMC free article] [PubMed] [Google Scholar]
- 40.Wilms G, Peene P, Herpels V, van Laer L, Baert AL. Balloon embolisation of carotid-cavernous fistula.Fatal cavernous sinus and brain stem venous thrombosis by balloon migration. Rofo. 1992;156:393–5. doi: 10.1055/s-2008-1032908. [DOI] [PubMed] [Google Scholar]
- 41.Wilson CB, Markesbery W. Traumatic carotid-cavernous fistula with fatal epistaxis.Report of a case. J Neurosurg. 1966;24:111–3. doi: 10.3171/jns.1966.24.1.0111. [DOI] [PubMed] [Google Scholar]
- 42.Zaidat OO, Lazzaro MA, Niu T, Hong SH, Fitzsimmons BF, Lynch JR, et al. Multimodal endovascular therapy of traumatic and spontaneous carotid cavernous fistula using coils, n-BCA, Onyx and stent graft. J Neurointerv Surg. 2011;3:255–62. doi: 10.1136/jnis.2010.003103. [DOI] [PubMed] [Google Scholar]
- 43.Zenteno M, Santos-Franco J, Rodriguez-Parra V, Balderrama J, Aburto-Murrieta Y, Vega-Montesinos S, et al. Management of direct carotid-cavernous sinus fistulas with the use of ethylene-vinyl alcohol (Onyx) only: Preliminary results. J Neurosurg. 2010;112:595–602. doi: 10.3171/2009.6.JNS09440. [DOI] [PubMed] [Google Scholar]


