Abstract
Stage has been defined as the major prognostic factor in resected non-small cell lung cancer (NSCLC). However, there is some evidence that indicates season of operation could play a role in the survival of patients. Between January 1995 and June 2008, 698 (621 men and 77 women) patients who had undergone pulmonary resection for NSCLC were evaluated. Patients were analysed according to surgical–pathological stages and month of the year in which they were operated. Vitamin D receptor (VDR) polymorphism was also analysed in 62 patients. The median survival time in all patients was 60 ± 6 months (95% confidence interval (CI): 44–81 months). The survival of patients who underwent resection in winter was statistically significantly shorter than those operated in summer (P = 0.03). When patients were analysed according to T, N and season, resection time of the year was calculated to be an independent determinant of survival (P = 0.04). A VDR genotype was also associated with better prognosis (P = 0.04). Season of the operation, VDR polymorphism and N status seemed to have independent effects on survival of operated NSCLC patients.
Keywords: Season, Lung cancer, Prognosis, Surgical resection, Vitamin D receptor, Polymorphism
INTRODUCTION
Despite a better new staging system having been developed for non-small cell lung cancer (NSCLC), patients with earliest lung cancer (IA) have a 73% of 5-year survival [1]. Patients with clinical IA disease have a 50% 5-year survival [1]. For this reason, it is plausible to suggest that, additional factors such as different tumor, nodes and metastasis (TNM) factors, anti-tumour status of patients and environmental factors could play roles as prognostic elements in this disease.
Several epidemiological studies have shown an association between the season in which certain cancers are diagnosed and subsequent survival, with diagnosis in the summer and autumn months being associated with better survival in most of these studies [2–4]. It has been suggested by the authors of these studies that exposure to sunlight and the associated higher levels of cutaneous vitamin D production at the time of diagnosis and/or treatment might be the underlying biological basis for the improved survival of patients diagnosed in the summer and autumn months [4, 5]. Geographical regions with lower ultraviolet B radiation have been noted to have increased incidence and mortality rates of a wide range of cancers [6, 7].
In this study, we attempted to find the possible effect of the season of surgery along with vitamin D receptor (VDR) polymorphism.
Patients and methods
This study consisted of two parts. We retrospectively analysed and prospectively recorded data of patients who underwent resectional surgery for NSCLC. Blood samples from 62 resected and 75 healthy subjects were collected in order to analyse the role of VDR polymorphism. Institutional Review Board Approval has been obtained.
Between January 1995 and June 2008, 698 consecutive patients with histological evidence of NSCLC were enrolled in the study after complete pulmonary resection and lymph node dissection. Routine blood tests included haemoglobin, alkaline phosphatase and serum calcium estimations. All patients underwent posteroanterior and lateral chest radiographs and bronchoscopy. Computerized tomography scans of the thorax, abdomen (or abdominal ultrasonography) and cranium, all body bone scintigraphies were done in all patients for pretreatment staging. Mediastinoscopy were carried out in almost all patients. The following patients were excluded: (i) patients who underwent partial resection or segmentectomy; (ii) patients with multiple lung tumours; (iii) patients with low-grade malignancy, such as bronchial carcinoid; (iv) patients who were found to have mediastinal nodal tumour involvement and underwent neoadjuvant therapy and (v) patients who had metastatic carcinoma. Fifteen patients underwent bilobectomy. All patients underwent a uniform staging protocol (UICC's, TNM classification revised in 1997) [8] in construction of a final surgical–pathological stage (pTNM) using information obtained at thoracotomy and supplemented by pathological examination.
Seasons were defined as Winter (December–February); Spring (March–May); Summer (June–August) and Autumn (September–November). Hazard ratios for the different seasons were calculated after adjustment for age and period of surgery.
Determination of vitamin D receptor polymorphism: DNA isolation and genotyping
Blood specimens were collected in tubes containing ethylene diamine tetra-acidic acid, and DNA samples were extracted from whole blood by a salting out procedure [9].
Two polymorphic restriction fragment length polymorphisms were genotyped; the FokI polymorphism in the initiation codon at the 5′ end and TaqI polymorphism in the 3′ region of VDR [9]. For TaqI polymorphism, the following primers (MBI Fermentas, Lithuania) were used to amplify the VDR gene; 5′ –CAG AGC ATG GAC AGG GAG CAA G-3′; 5′- GCA ACT CCT CAT GGG CTG AGG TCT CA-3′.
The TaqI Genotypes were determined as TT (490 245 bp), Tt (490,290,245,205 bp) or tt (290,245,205 bp) as described earlier [9]. For FokI polymorphism, the following primers (MBI Fermentas, Lithuania) were used to amplify the VDR gene; 5′ –GAT GCC AGC TGG CCC TGG CAC TG -3′;5′–ATG GAA ACA CCT TGC TTC TTC TCC CTC-3′. They were determined as described elsewhere [9]. The FF genotype (homozygote of common allele) lacks a FokI site and shows only one band of 272 bp. The ff genotype (homozygote of infequent allele) generates two fragments of 198 and 74 bp. The heterozygote displays three fragments of 272, 198 and 74 bp, designated as Ff.
Statistical analysis
Patient survival was expressed by analysis according to the Kaplan–Meier method, using time zero as the date of thoracotomy and death as the endpoint. Perioperative deaths were included in survival analysis. Prognostic factors were evaluated in completely resected (R0) patients. Differences in survival were determined using the log-rank test in the univariate analysis, and multivariate analysis was done using the Cox proportional hazards regression model. For polymorphism analysis, the odds ratios and the confidence intervals were calculated as an estimate of the relative risk. Chi-square test was used for different alleles. Results were considered significant at P less than 0.05.
RESULTS
The mean age of patients was 56.7 ± 10.1 years (19–84 years). One-hundred-ninety-one patients (20.6%) had at least one comorbidity in addition to lung cancer. Pneumonectomy was performed in 240 patients (34.4%), sleeve lobectomy/bilobectomy was performed in 34 patients (4.9%), bilobectomy was performed in 71 patients (10.2%) and lobectomy was performed in 353 patients (50.1%). Postoperative mortality rates were 3.3% (n = 23). Complete resection (R0) was defined as the removal of all detectable disease by the surgeon and histologic confirmation of tumour-free resection margins. Complete resection was achieved in 665 cases (95.2%).
Twenty-three patients (3.3%) died postoperatively (30-day mortality). The completely resected patients had a 5-year survival rate of 48.7% with a median survival time of 60 months (95% confidence interval: 44–81 months). The 5-year survival rates of patients with T1, T2 and T3 disease were 65%, 51% and 39%, respectively. The surgical–pathological stages of patients were depicted in Table 1. T and N factors were found to be important prognosticators in univariate analysis (P = 0.01 and 0.0003, respectively). The number of patients who were operated during winter were greater than those who had undergone resection during spring. However, it has been found that, distribution of stages were not associated with surgery season (Table 1; P = 0.42).
Table 1:
Stage distribution of patients according to surgery season (P = 0.42)
| Seasons | Stage IA (%) | Stage IB (%) | Stage IIA (%) | Stage IIB (%) | Stage IIIA (%) | Stage IIIB (%) | Stage IV (%) | Total (%) |
|---|---|---|---|---|---|---|---|---|
| Spring | ||||||||
| Mar–May | 20 (2.9) | 40 (5.7) | 15 (2.1) | 49 (7.0) | 29 (4.2) | 7 (1.0) | 3 (0.4) | 163 (23.4) |
| Summer | ||||||||
| June–Aug | 21 (3.0) | 36 (5.2) | 14 (2.0) | 33 (4.7) | 31 (4.4) | 4 (0.6) | 3 (0.4) | 142 (20.3) |
| Autumn | ||||||||
| Sep–Nov | 18 (2.6) | 20 (2.9) | 13 (1.9) | 44 (6.3) | 40 (5.7) | 10 (1.4) | 3 (0.3) | 148 (21.2) |
| Winter | ||||||||
| Dec–Feb | 28 (4.0) | 54 (7.7) | 19 (2.7) | 55 (7.9) | 64 (9.1) | 20 (2.9) | 5 (0.7) | 245 (35.1) |
| Total | 87 (12.5) | 150 (21.5) | 61 (8.7) | 181 (25.8) | 164 (23.5) | 41 (5.7) | 14 (2.0) | 698 (100) |
Effect of season
Fig. 1 shows the survival curves of patients regarding the season of surgery. The survival rate of patients who had undergone resectional surgery during summer was higher than that of those who underwent resection in winter (Fig. 1; P = 0.03). The difference was also found statistically significant when N0 patients were evaluated (P = 0.0004). Spring or autumn seasons were not associated with statistically significant survival difference. The median survival of N0 patients who underwent resection in summer was 131 months (95% CI: 100–160 months), whereas N2 patients who had undergone anatomic resection in winter was calculated to have 14 months (95% CI: 11–46 months) median survival. The difference was found statistically significant (P = 0.0004).
Figure 1:
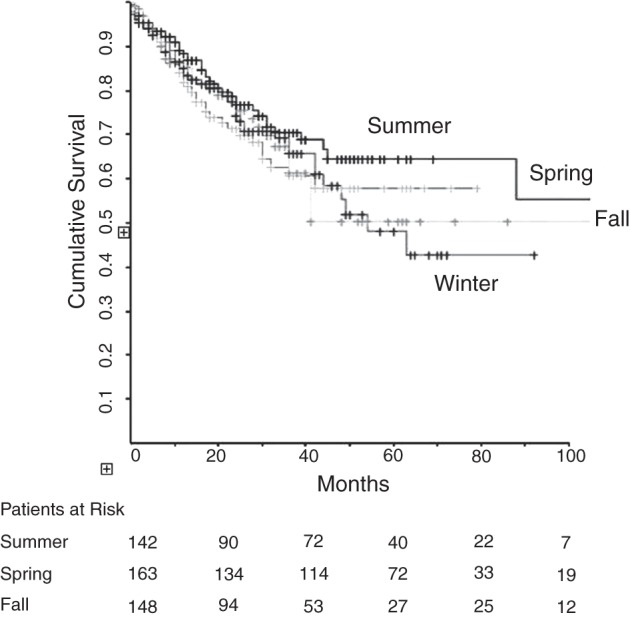
Survival curves of patients according to surgery seasons.
Effect of vitamin D receptor polymorphism
The distribution of stages of patients in whom the VDR gene polymorphisms is shown in Table 2. The stage distribution, FEV1, FVC and FEV1/FVC and total number of smoked cigarettes were comparable between total resected group (n = 698) and patients in whom VDR polymorphism was analysed (n = 62) (P = 0.2, 0.8, 1, 0.8 and 0.9, respectively). Table 3 shows the distribution of all combined genotypes. The genotype distribution for TaqI polymorphism was different between NSCLC patients and controls (Table 4; P = 0.015). VDR TaqI TT genotype was significantly associated with a increased risk of NSCLC in our study groups (odds ratio, OR = 1.714; 95% CI: 1.149–2.556; P = 0.007).There was no statistically significant association between F allele and NSCLC (Table 4; P = 0.57). On combined genotype analysis, the TTFF/TTFf combined genotype was associated with NSCLC risk compared with other genotypes (Table 5; OR = 1.788; 95% CI: 1.189–2.690; P = 0.004). Survival analysis disclosed that TTFF/TTfF combined genotype was associated with dismal survival (OR: 2.46 [95% confidence interval: 1.71–5.68]; P = 0.04). Genotype, N factor, T factor and season of surgery parameters were entered into the Cox proportional (multivariate) analysis. Multivariate analysis revealed that, N factor, season of surgery and VDR polymorphism (TTFF combined genotype) were found as independent prognostic factors (Table 6).
Table 2:
TaqI alleles are found in lung cancer and healthy subjects
| Group/polymorphism | Presence of T n (%) | Presence of t n (%) | Total | P-value |
|---|---|---|---|---|
| Lung cancer (n = 62) | 91 (73.4) | 33 (26.6) | 0,002 | |
| IA | 32 | |||
| IB | 10 | |||
| IIA | 3 | |||
| IIB | 4 | |||
| IIIA | 8 | |||
| IIIB | 6 | |||
| Control (healthy subjects) (n = 75) | 84 (56.0) | 66 (44.0) |
The occurrence of alleles is two times the number of patients since it shows the alleles. T allele was found to be associated with lung cancer risk (P = 0.002).
Table 3:
Distribution of all combined genotypes in NSCLC patients (n = 62) and control group (n = 75)
| Genotypes | NSCLC patients n (%) | Control n (%) |
|---|---|---|
| TTff | 0 (0) | 1 (1.3) |
| Ttff | 3 (4.8) | 1 (1.3) |
| TTFf | 20 (32.3) | 16 (21.3) |
| TtFf | 13 (21) | 20 (26.7) |
| ttFf | 1 (1.6) | 4 (5.3) |
| TTFF | 14 (22.6) | 7 (9.3) |
| TtFF | 7 (11.3) | 15 (20) |
| ttFF | 3 (4.8) | 10 (13.3) |
| ttff | 1 (1.6) | 1 (1.3) |
N, number of subjects.
Table 4:
Number and percentage of NSCLC cancer patients and controls with each TaqI and FokI VDR genotype and alleles
| Group | Controls n (%) | NSCLC cancer patients n (%) | ORa (95% CI) | P-values |
|---|---|---|---|---|
| VDR TaqI genotypes | ||||
| TT | 24 (32) | 34 (54.8) | 1.714 (1.149–2.556) | P = 0.007 |
| Tt | 36 (48) | 23 (37.1) | 0.773 (0.518–1.154) | P = 0.200 |
| tt | 15 (20) | 5 (8.1) | 0.403 (0.155–1.047) | P = 0.049 |
| P = 0.015 | ||||
| TaqI alleles | ||||
| T | 84 (56) | 91 (73.4) | ||
| t | 66 (44) | 33 (26.6) | ||
| P = 0.002 | ||||
| VDR FokI genotypes | ||||
| FF | 32 (42.7) | 24 (38.7) | 0.907 (0.603–1.365) | P = 0.639 |
| Ff | 40 (53.3) | 34 (54.8) | 1.512 (0.860–2.66) | P = 0.148 |
| ff | 3 (4) | 4 (6.5) | 1.613 (0.375–6.936) | P = 0.701 |
| P = 0.762 | ||||
| FokI alleles | ||||
| F | 104 (69.3) | 82 (66.1) | ||
| f | 46 (30.7) | 42 (33.9) | ||
| P = 0.57 | ||||
N, number of individuals.
aOdds ratio computed between selected genotype versus all other genotypes in corresponding group with the Tt or Ff genotype set at 1.0.
Table 5:
Comparison of patients with TTFf/TtFf genotypes with all other combined genotypes in NSCLC patients and control group
| Group | Subjects with all other TaqI/FokI combinations N (%) | Subjects with TTFF/TTFf genotypes N (%) |
|---|---|---|
| Control | 52 (69.3) | 23 (30.7) |
| NSCLC patients | 28 (45.2) | 34 (54.8)a |
aχ2 = 8.163; P = 0.004; (OR = 1.788;95% CI: 1.189–2.690).
Table 6:
Multivariate associations between clinicopathologic factors and survival
| HR | 95% Confidence interval | P-value | |
|---|---|---|---|
| Pathologic N stage | |||
| N0 (n = 632) | 1 | 1.637–4.458 | <0.0001 |
| N2 (n = 66) | 2.701 | ||
| Surgery season | |||
| Summer | 1 | 1.71–5.68 | 0,04 |
| Winter | 2.46 | ||
| VDR polymorphism all other | 1 | 1.23–3.48 | 0,04 |
| TaqI/FokI combinations | 1.81 | ||
| TTFf/TtFf genotypes | |||
DISCUSSION
Despite all efforts to define the survival rates of resected NSCLC, variations have been observed in patients with same stage [1]. The reasons for variations could be speculated to be due to different staging techniques, different lymph node dissections, staging modalities, adjuvant therapy protocols, patient populations and environmental factors. Zhou et al. [11] showed that, patients who had surgery during summer with highest vitamin D intake had better survival and recurrence-free survival than patients who had surgery during winter. We similarly demonstrated that resections occurring during the winter season had a negative association with survival compared to those occurring in the Summer with a hazard ratio of 2.46. Multivariate analysis concluded that surgery season and N stage were other independent prognostic factors. Roychoudhuri et al. [5] concluded that the finding of higher survival in cancer patients diagnosed in the summer and autumn is unlikely attributable to the seasonal variation in serum vitamin D concentration. It would be proposed that, daylight may effect cancer survival via unknown mechanisms. On the other hand, Zhou et al. [11] suggested that the joint effects of surgery season and recent vitamin D intake seemed to be associated with the survival of early-stage NSCLC. As Roychoudhuri suggested, summer season might be associated with earlier stage of the disease and it may in turn cause a better survival [8]. However, our analysis excluded that possibility since there was no statistically significant association between stage and season of surgery and season has been found to be an independent prognostic factor.
Many studies have investigated individual VDR polymorphisms in isolation when assessing the association with different disease states, but these results are often nonsignificant or conflicting [12]. However, a small number of studies, such as in this study, have carried out cross-genotyping analysis on the VDR polymorphisms and have found that this can reveal a positive association with disease status [10, 12].
We tested a different hypothesis and we investigated whether VDR polymorphism could have an independent prognostic value or not. We found that TTFF combined genotype of VDR was associated with worse survival in resected NSCLC patients. We also realized that TTFF combined genotype is an independent risk factor in resected NSCLC patients. To our knowledge, this relationship has not been reported before. The mechanism through which these polymorphisms affect the VDR is still unclear, as the polymorphisms do not alter the amino acid sequence of VDR protein [12]. It is plausible to speculate that, this polymorphism may reduce the intracellular signal of vitamin D. Although we did not test the vitamin D levels in NSCLC patients, we could speculate in the lights of data reported by Zhou et al. [11], the intracellular signalling of VDR may play a role in the pathway which is effective in tumour survival, whereas vitamin D levels is of no significant importance.
LaPar et al. [13] elegantly reported that, the morbidity and mortality of patients was independently influenced by operative season, being the lowest in patients operated on in spring. They found that, this effect was likely unrelated to underlying patients disease. They hypothesize that, the effect could be attributable to concomitant influence of several environmental stressors, seasonal infections or biophysiologic influences regarding seasonal change. However, they did not assess any of these hypothetical parameters. On the other hand, we did not analyse morbidity or mortality in our patients.
VDR might be in effect through immunologic anti-tumoural mechanisms. Selvaraj et al. [14] reported that VDR which in turn regulates the action of vitamin D3 and modulate the immune functions to Mycobacterium tuberculosis via macrophage phagocytosis and lymphoproliferative response. The theory that vitamin D can help prevent cancer is biologically plausible. Such findings suggest, but do not prove, that vitamin D has a role in preventing the development of cancer, promotes cell differentiation, inhibits cancer-cell proliferation exhibits anti-inflammatory, proapoptotic and antiangiogenic properties [15]. However, no study has demonstrated the possible anti-tumoural efficacy of VDR.
There are some limitations in our study which must be addressed. First of all, we did not include all possible parameters which may affect survival in resected NSCLC patients. However, it has been widely accepted that, stage of tumour, T and N factors have been universally accepted as prognostic parameters. Despite all efforts , International Association for Study of Lung Cancer was not able to add additional variable for new staging system which may be extracted by the analysis of more than 100 000 patients [1]. For this reason, we included these parameters into our multi-variable analysis. We also did not measure the vitamin D levels in patient. We also studied VDR polymorphism in a fraction of resected patients. However, the patients in whom VDR polymorphism was analysed was not different from the all resected population in terms of stage, FEV1, FVC, FEV1/FVC and smoking habit.
Nevertheless, we concluded that, surgery season, N factor and VDR polymorphism seemed to be independent prognostic factors in NSCLC patients who underwent resectional surgery. Further studies in order to unveil the mechanisms, the possible relationship between VDR polymorphism and serum vitamin D levels and lung cancer risk, are warranted.
Conflict of interest: none declared.
APPENDIX A: CONFERENCE DISCUSSION
Dr B. Kubisa (Szczecin, Poland): Eight years ago I worked on vitamin D, and I have proven that vitamin D is a very strong immunosuppressant and that it acts against acute rejection in lung transplantation in rats. And I find some kind of coincidence with what you have found, because, as we know, vitamin D is a cholecalciferol and it has been hydroxylized in the kidney and in the skin, and after the hydroxylation in the skin it becomes a hormone, calcitriol, and this has a very strong immunologic effect. What effect? That should be investigated.
Dr T. Grodzki (Sczcecin, Poland): I would be interested in the differences between surgeons, because it is obvious that the surgeon is a factor in survival. Can you comment on this particular factor? Because I agree with you that there is a coincidence, but I am afraid that vitamin D is not a key factor in this role.
Dr A. Turna (Istanbul, Turkey): I didn't look at the surgeon as a parameter and didn't add that parameter to the multivariate analysis. You may be right. But a couple of years ago I analyzed the effect of surgeon in our department in an approximately 500-case series, and I didn't find any significant effect of surgeon. And we performed an analysis of complications in resected non-small cell lung cancer. We examined whether a resident performing lung cancer resection is a factor for complication or survival, and we didn't find any important or significant effect in this respect.
Dr L. Lampl (Augsburg, Germany): I am wondering why you didn't consider respiratory infections, which are in this season much more frequent, and why you didn't consider the long-term consequences of repeated respiratory infections in such a postoperative period?
Dr A. Turna: It is a good idea but we didn't look at this parameter. But I suspect that respiratory complications can be a prognostic factor for lung cancer patients, too.
Dr B. Al-Alao (Dublin, Ireland): You say your difference between summer and winter is on univariate analysis, and even when you adjusted on multivariate, you didn't adjust for very clear and significant prognostic factors such as age and gender and histology type. Your odds ratio there is just based on adjusting not two important factors like age and gender and histology type. You haven't adjusted for those to come up with a significant odds ratio. So what would you say about that?
Dr A. Turna: We actually performed the univariate analysis first and we included those parameters, and we only selected the parameters with a P value lower than 0.20. So we didn't include all the parameters in the multivariate analysis.
Dr Al-Alao: What I am trying to say is regarding the difference between the summer group and winter group, they might be two different high-risk groups. The winter group might be very high-risk based on age or gender or surgery; they might have pneumonectomy instead of lobectomy. So you didn't show us the characteristics of each group. You haven't matched them at least in between.
Dr E. Stoelben (Cologne, Germany): Following your presentation, I got the impression that you are only operating on patients in winter and summer, but we hear nothing about the results in spring and autumn. Perhaps they would be disturbing your results.
Dr A. Turna: I selected some graphs and tables for the sake of time here, and we found a difference between the patients who underwent surgery in winter and spring also. We didn't find any difference between summer and autumn, but we found a difference between winter and spring. But in order to clarify the effect and in order to clearly show the seasonal effect, I chose to present those tables and the graphs.
REFERENCES
- 1.Goldstraw P, Crowley J, Chansky K, Giroux DJ, Groome PA, Rami-Porta R, et al. The IASLC Lung cancer staging project: proposals for the revision of the TNM stage groupings in the Forthcoming (Seventh) edition of the TNM classification of malignant tumours. J Thorac Oncol. 2007;2:706–14. doi: 10.1097/JTO.0b013e31812f3c1a. [DOI] [PubMed] [Google Scholar]
- 2.Robsahm TE, Tretli S, Dahlback A, Moan J. Vitamin D3 from sunlight may improve the prognosis of breast, colon and prostate cancer (Norway) Cancer Causes Control. 2004;15:149–58. doi: 10.1023/B:CACO.0000019494.34403.09. [DOI] [PubMed] [Google Scholar]
- 3.Lim HS, Roychoudhuri R, Peto J, Schwartz G, Baade P, Møller H. Cancer survival is dependent on season of diagnosis and sunlight exposure. Int J Cancer. 2006;119:1530–6. doi: 10.1002/ijc.22052. [DOI] [PubMed] [Google Scholar]
- 4.Porojnicu AC, Dahlback A, Moan J. Sun exposure and cancer survival in Norway: changes in the risk of death with season of diagnosis and latitude. Adv Exp Med Biol. 2008;624:43–54. doi: 10.1007/978-0-387-77574-6_4. [DOI] [PubMed] [Google Scholar]
- 5.Roychoudhuri R, Robinson D, Coupland V, Holmberg L, Moller H. Season of cancer diagnosis exerts distinct effects upon short and long-term survival. Int J Cancer. 2009;124:2436–41. doi: 10.1002/ijc.24213. [DOI] [PubMed] [Google Scholar]
- 6.Grant WB. An estimate of premature cancer mortality in the U.S. due to inadequate doses of solar ultraviolet radiation. Cancer. 2002;94:1867–75. doi: 10.1002/cncr.10427. [DOI] [PubMed] [Google Scholar]
- 7.Hanchette CL, Schwartz GG. Geographic patterns of prostate cancer mortality. Evidence for a protective effect of ultraviolet radiation. Cancer. 1992;70:2861–9. doi: 10.1002/1097-0142(19921215)70:12<2861::aid-cncr2820701224>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 8.Mountain CF. Revisions in the international system for staging lung cancer. Chest. 1997;111:1710. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 9.Miller SA, Dykes DD, Polesky HS. Simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acid Res. 1988;16:1215–9. doi: 10.1093/nar/16.3.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curran JE, Vaughan T, Lea RA, Weinstein SR, Morrrison NA, Griffiths LR. Association of a vitamin D receptor polymorphism with sporadic breast cancer development. Int J Cancer. 1999;83:723–26. doi: 10.1002/(sici)1097-0215(19991210)83:6<723::aid-ijc4>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Zhou W, Suk R, Liu G, Park S, Neuberg DS, Wain JC, et al. Vitamin D is associated with improved survival in early stage non-small cell lung cancer patients. Cancer Epidemiol Biomarkers Prev. 2005;14(10):2303–9. doi: 10.1158/1055-9965.EPI-05-0335. [DOI] [PubMed] [Google Scholar]
- 12.Zmuda JM, Cauley JA, Ferrel RE. Molecular epidemiology of vitamin D receptor gene variants. Epidemiol Rev. 2000;22(2):203–17. doi: 10.1093/oxfordjournals.epirev.a018033. [DOI] [PubMed] [Google Scholar]
- 13.LaPar DJ, Nagji AS, Bhamidipati CM, Kozower BD, Lau CL, Ailawadi G, et al. Seasonal variation influences outcomes following lung cancer resections. Eur J Cardiothorac Surg. 2011;40:83–90. doi: 10.1016/j.ejcts.2010.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Selvaraj P, Chandra G, Jawahar MS, Rani MV, Rajeshwari DN, Narayanan PR. Regulatory role of vitamin D receptor gene variants of Bsm I, Apa I, Taq I, and Fok I polymorphisms on macrophage phagocytosis and lymphoproliferative response to Mycobacterium tuberculosis antigen in pulmonary tuberculosis. J Clin Immunol. 2004;24:523–32. doi: 10.1023/B:JOCI.0000040923.07879.31. [DOI] [PubMed] [Google Scholar]
- 15.Manson JE, Mayne ST, Clinton SK. Vitamin D and prevention of cancer-ready for prime time? N Engl J Med. 2011;14:1385–7. doi: 10.1056/NEJMp1102022. [DOI] [PubMed] [Google Scholar]


