Abstract
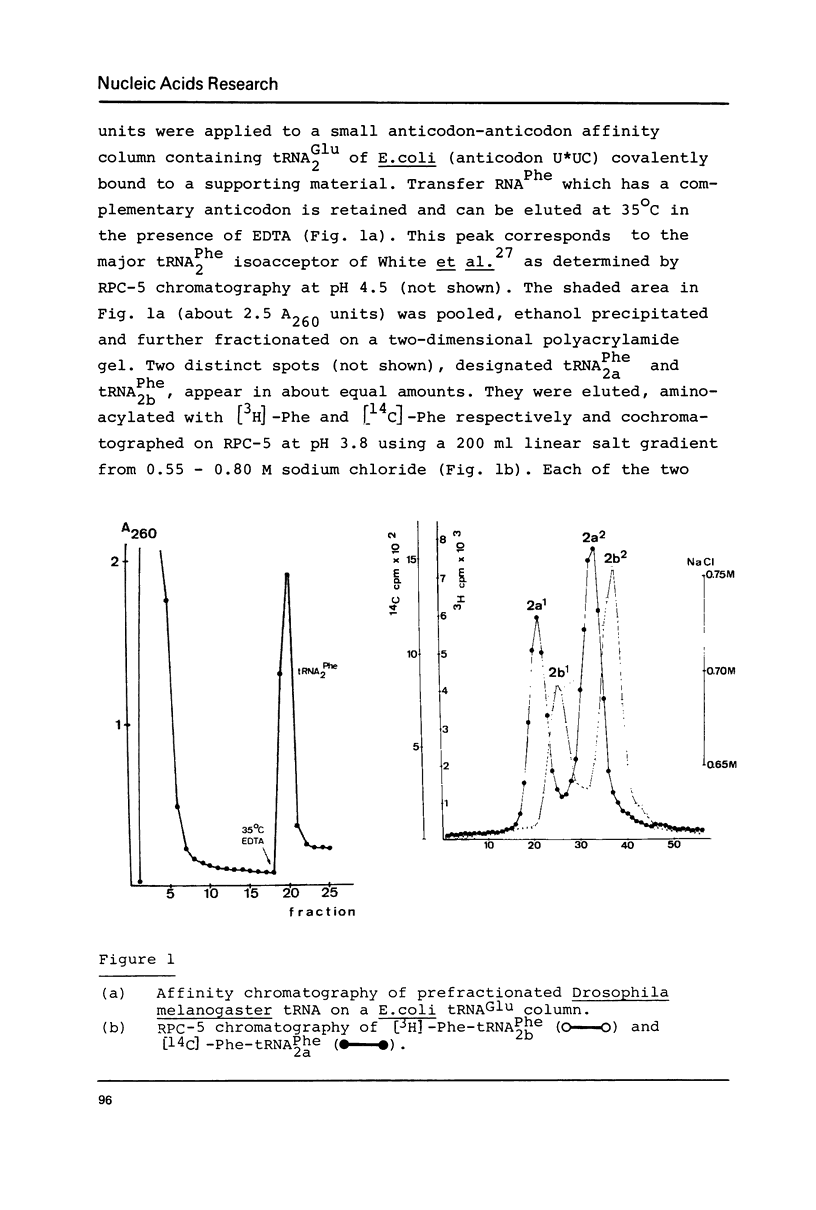
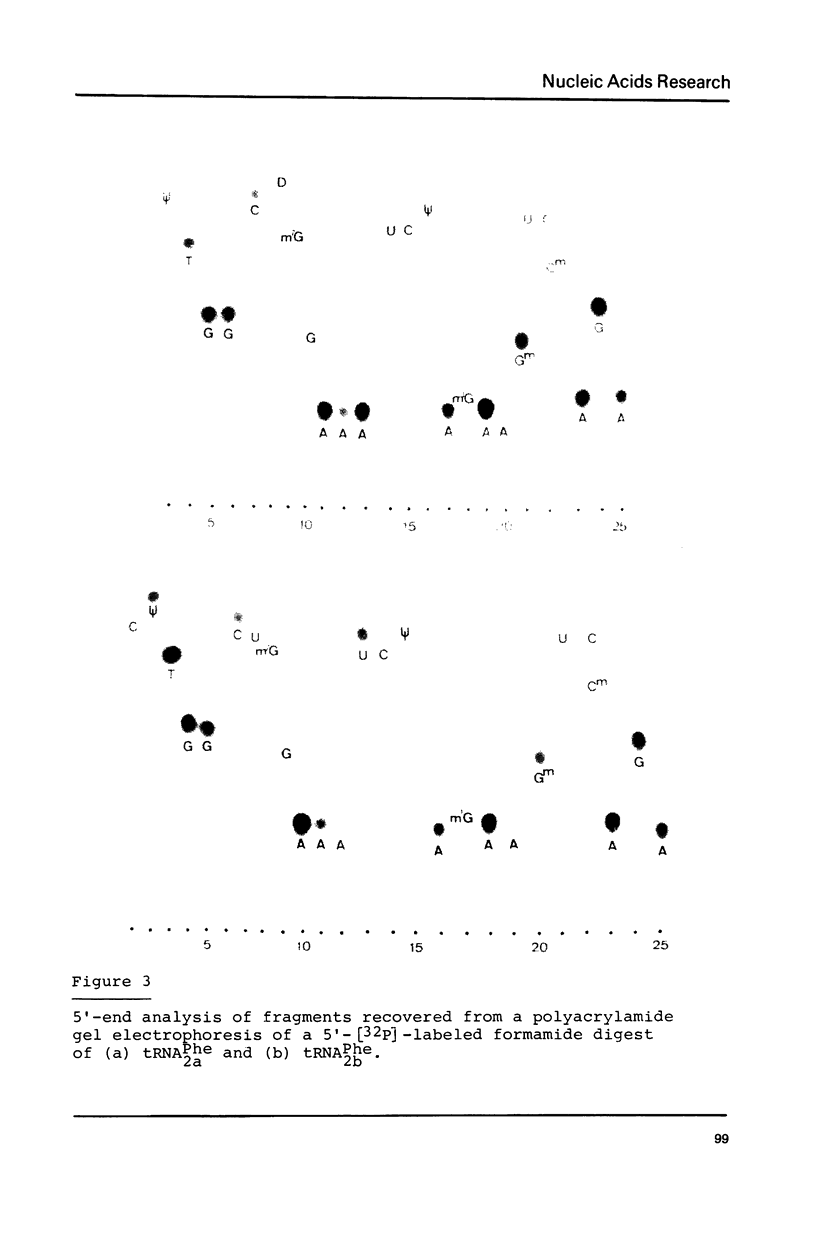
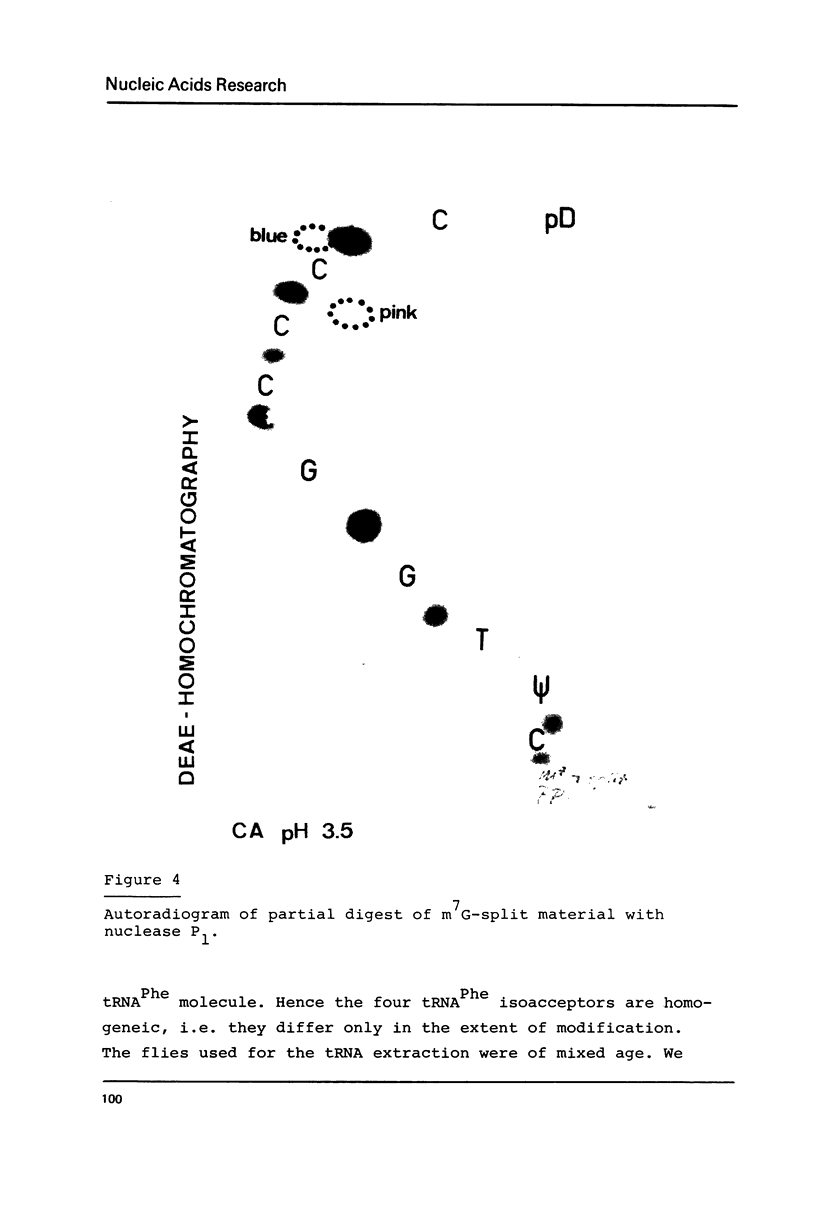
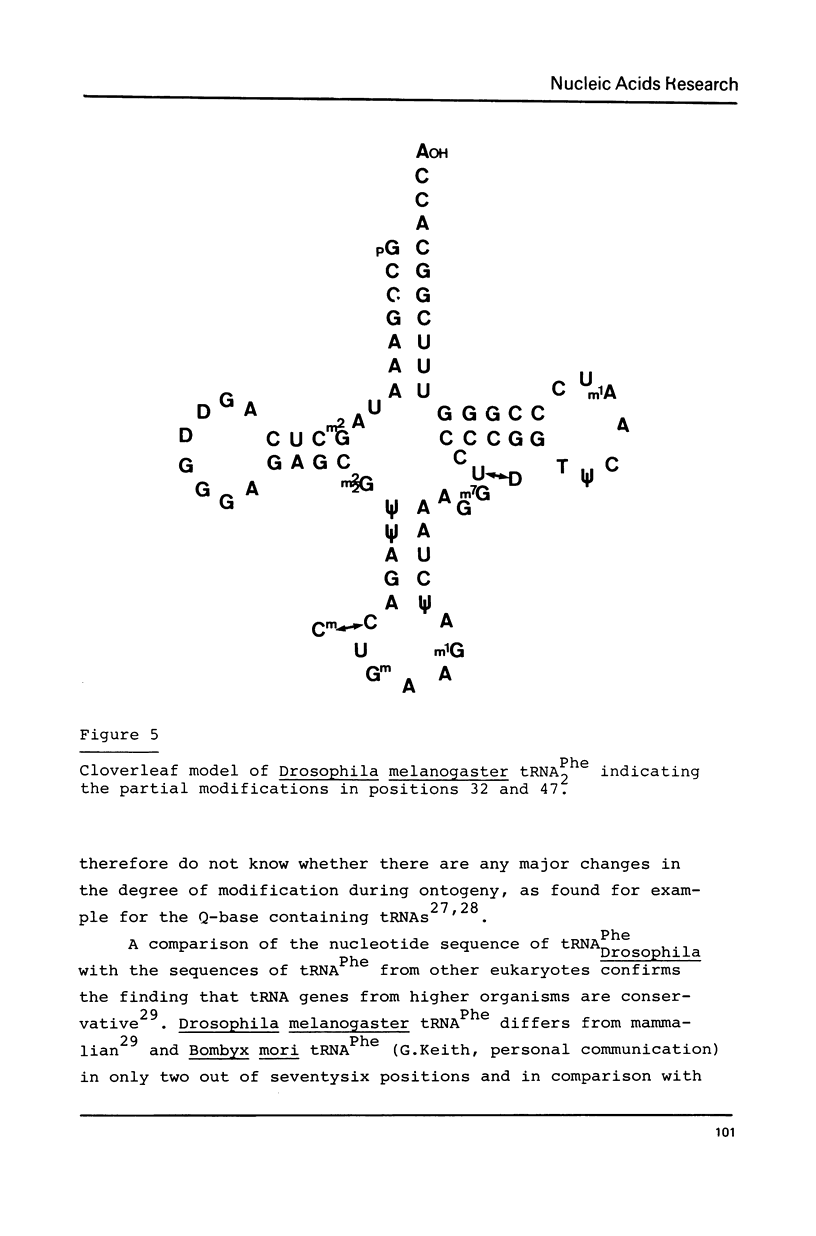
The nucleotide sequence of Drosophila melanogaster phenylalanine tRNA2 was determined to be: pG-C-C-G-A-A-A-U-A-M2G-C-U-C-A-G-D-D-G-G-G-A-G-A-G-C-m22G-psi-psi-A-G-A-C(m)-U-Gm-A-A-mlG-A-psi-C-U-A-A-A-G-m7G-U(D)-C-C-C-C-G-G-T-psi-C-A-mlA-U-C-C-G-G-G-U-U-U-C-G-G-C-A-C-C-AOH. Upon RPC-5 chromatography at pH 3.8 tRNA2Phe can be separated into four isoacceptors due to the partial modifications in positions 32 and 47. Thus the posttranscriptional modification of tRNA2Phe transcribed from one gene (or many genes with identical sequences results in four isoacceptors with the same basic sequence.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chovnick A., Gelbart W., McCarron M. Organization of the Rosy locus in Drosophila melanogaster. Cell. 1977 May;11(1):1–10. doi: 10.1016/0092-8674(77)90312-9. [DOI] [PubMed] [Google Scholar]
- Cortese R., Landsberg R., Haar R. A., Umbarger H. E., Ames B. N. Pleiotropy of hisT mutants blocked in pseudouridine synthesis in tRNA: leucine and isoleucine-valine operons. Proc Natl Acad Sci U S A. 1974 May;71(5):1857–1861. doi: 10.1073/pnas.71.5.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davanloo P., Sprinzl M., Watanabe K., Albani M., Kersten H. Role of ribothymidine in the thermal stability of transfer RNA as monitored by proton magnetic resonance. Nucleic Acids Res. 1979 Apr;6(4):1571–1581. doi: 10.1093/nar/6.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn R., Addison W. R., Gillam I. C., Tener G. M. The purification and properties of valine tRNAs of Drosophila melanogaster. Can J Biochem. 1978 Jun;56(6):618–623. doi: 10.1139/o78-093. [DOI] [PubMed] [Google Scholar]
- Fradin A., Gruhl H., Feldmann H. Mapping of yeast tRNAs by two-dimensional electrophoresis on polyacrylamide gels. FEBS Lett. 1975 Feb 1;50(2):185–189. doi: 10.1016/0014-5793(75)80485-6. [DOI] [PubMed] [Google Scholar]
- Gauss D. H., Grüter F., Sprinzl M. Compilation of tRNA sequences. Nucleic Acids Res. 1979 Jan;6(1):r1–r19. [PMC free article] [PubMed] [Google Scholar]
- Grigliatti T. A., White B. N., Tener G. M., Kaufman T. C., Suzuki D. T. The localization of transfer RNA5Lys genes in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3527–3531. doi: 10.1073/pnas.71.9.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H. J., de Henau S., Crothers D. M. On the physical basis for ambiguity in genetic coding interactions. Proc Natl Acad Sci U S A. 1978 Feb;75(2):610–614. doi: 10.1073/pnas.75.2.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosjean H., Takada C., Petre J. Complex formation between transfer RNAs with complementary anticodons: use of matrix bound tRNA. Biochem Biophys Res Commun. 1973 Aug 6;53(3):882–893. doi: 10.1016/0006-291x(73)90175-7. [DOI] [PubMed] [Google Scholar]
- Hosbach H. A., Kubli E. Transfer RNA in aging Drosophila: II. Isoacceptor patterns. Mech Ageing Dev. 1979 Apr;10(1-2):141–149. doi: 10.1016/0047-6374(79)90077-0. [DOI] [PubMed] [Google Scholar]
- Kimball M. E., Szeto K. S., Soll D. The nucleotide sequence of phenylalanine tRNA from Mycoplasma sp. (Kid). Nucleic Acids Res. 1974 Dec;1(12):1721–1732. [PMC free article] [PubMed] [Google Scholar]
- Kubli E., Schmidt T. The localization of tRNA4Glu genes from Drosophila melanogaster by "in situ" hybridization. Nucleic Acids Res. 1978 May;5(5):1465–1478. doi: 10.1093/nar/5.5.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley D. L., Sandler L., Baker B. S., Carpenter A. T., Denell R. E., Hall J. C., Jacobs P. A., Miklos G. L., Davis B. K., Gethmann R. C. Segmental aneuploidy and the genetic gross structure of the Drosophila genome. Genetics. 1972 May;71(1):157–184. doi: 10.1093/genetics/71.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcu K. B., Dudock B. S. Effect of ribothymidine in specific eukaryotic tRNAs on their efficiency in in vitro protein synthesis. Nature. 1976 May 13;261(5556):159–162. doi: 10.1038/261159a0. [DOI] [PubMed] [Google Scholar]
- Norby S. A specific nutritional requirement for pyrimidines in rudimentary mutants of Drosophila melanogaster. Hereditas. 1970;66(2):205–214. doi: 10.1111/j.1601-5223.1970.tb02346.x. [DOI] [PubMed] [Google Scholar]
- O'Donnell J., Gerace L., Leister F., Sofer W. Chemical selection of mutants that affect alcohol dehydrogenase in Drosophila. II. Use of 1-pentyne-3-ol. Genetics. 1975 Jan;79(1):73–83. doi: 10.1093/genetics/79.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe B. A., Tsen H. Y. Role of ribothymidine in mammalian tRNAPhe. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3696–3700. doi: 10.1073/pnas.74.9.3696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt O., Mao J. I., Silverman S., Hovemann B., Söll D. Specific transcription of eukaryotic tRNA genes in Xenopus germinal vesicle extracts. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4819–4823. doi: 10.1073/pnas.75.10.4819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T., Egg A. H., Kubli E. The localization of tRNAAsp2 genes from Drosophila melanogaster by "in situ" hybridization. Mol Gen Genet. 1978 Sep 8;164(3):249–254. doi: 10.1007/BF00333153. [DOI] [PubMed] [Google Scholar]
- Silberklang M., Gillum A. M., RajBhandary U. L. Use of in vitro 32P labeling in the sequence analysis of nonradioactive tRNAs. Methods Enzymol. 1979;59:58–109. doi: 10.1016/0076-6879(79)59072-7. [DOI] [PubMed] [Google Scholar]
- Sinsheimer R. L. Recombinant DNA. Annu Rev Biochem. 1977;46:415–438. doi: 10.1146/annurev.bi.46.070177.002215. [DOI] [PubMed] [Google Scholar]
- Stanley J., Vassilenko S. A different approach to RNA sequencing. Nature. 1978 Jul 6;274(5666):87–89. doi: 10.1038/274087a0. [DOI] [PubMed] [Google Scholar]
- Strehler B., Hirsch G., Gusseck D., Johnson R., Bick M. Codon-restriction theory by aging and development. J Theor Biol. 1971 Dec;33(3):429–474. doi: 10.1016/0022-5193(71)90091-9. [DOI] [PubMed] [Google Scholar]
- Sueoka N., Kano-Sueoka T. Transfer RNA and cell differentiation. Prog Nucleic Acid Res Mol Biol. 1970;10:23–55. doi: 10.1016/s0079-6603(08)60560-7. [DOI] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Chromatography of Drosophila tRNA on BD-cellulose. Can J Biochem. 1973 Jun;51(6):896–902. doi: 10.1139/o73-111. [DOI] [PubMed] [Google Scholar]
- White B. N., Tener G. M., Holden J., Suzuki D. T. Analysis of tRNAs during the development of Drosophila. Dev Biol. 1973 Jul;33(1):185–195. doi: 10.1016/0012-1606(73)90173-5. [DOI] [PubMed] [Google Scholar]
- White B. N., Tener G. M. Properties of tRNAPhe from Drosophila. Biochim Biophys Acta. 1973 Jun 23;312(2):267–275. doi: 10.1016/0005-2787(73)90372-9. [DOI] [PubMed] [Google Scholar]
- Yen P. H., Sodja A., Cohen M., Jr, Conrad S. E., Wu M., Davidson N. Sequence arrangement of tRNA genes on a fragment of Drosophila melanogaster DNA cloned in E. coli. Cell. 1977 Aug;11(4):763–777. doi: 10.1016/0092-8674(77)90290-2. [DOI] [PubMed] [Google Scholar]
- Yoshida M., Takeishi K., Ukita T. Anticodon structure of GAA-specific glutamic acid tRNA from yeast. Biochem Biophys Res Commun. 1970 Jun 5;39(5):852–857. doi: 10.1016/0006-291x(70)90401-8. [DOI] [PubMed] [Google Scholar]






