Abstract
Surgical resection is currently a standard approach for isolated lung metastases from different primary tumours. The aim of the present analysis is to evaluate the outcome of patients submitted to complete resection of pulmonary metastases and to determine prognostic factors for long-term survival. A group of 440 consecutive patients previously diagnosed with primary malignant solid tumours and submitted to complete surgical resection of lung nodules with suspected or diagnosed metastatic lesion were retrospectively reviewed. The average follow-up time was 43.2 months (range: 0–192) and the 60-month O.S. was 43.7%. Univariate analysis: patients with adenocarcinoma presented the highest 5-year survival rates (53.4%, P = 0.0001); DFI >36 months (P < 0.0001), number of nodules on CT scan (P = 0.0052), number of malignant nodules resected (P = 0.0252) and the size of the largest resected nodule (P < 0.0001) were also significant. Multivariate analysis: number of malignant nodules resected (P = 0.01), size of the largest nodule resected (P = 0.001), DFI >36 months (P < 0.001) and histology of the primary tumour (P = 0.017) had significant impact on survival. The benefit of such an aggressive surgical approach is only limited to selected subgroups of patients. The decision to consider a patient for resection of metastastic disease should include factors beyond the feasibility of complete removal.
Keywords: Lung, Metastases, Metastasectomy, Prognostic factors
INTRODUCTION
Surgical resection is currently a standard approach for isolated lung metastases from different primary tumours, being associated with longer survival rates than available systemic therapies [1–3]. Considering the most frequent primary neoplasms, the mean 5 survival rates for patients with completely resected metastatic lesions reaches ∼40% [3–5]. Excision of metastatic pulmonary nodules is also considered for recurrent lesions, taking into account the low rates of surgical morbidity and mortality and the higher survival benefit for patients undergoing successive resections compared with patients undergoing only one operation or chemotherapy [1–3, 6]. Many authors reviewed their experience with lung metastasectomy searching for variables associated with better survival. Complete surgical resection, long disease-free interval (DFI) and number of resected metastases are considered the most important variables associated with better survival rates. The capability of complete surgical excision of the metastatic nodules has been shown to be the most significant prognostic factor, in most published studies [1, 3, 7–9].
The aim of the present analysis is to evaluate the outcome of patients submitted to complete resection of pulmonary metastases, treated in a single institution, and to determine prognostic factors for long-term survival in this selected favourable group of patients.
MATERIALS AND METHODS
We retrospectively reviewed 440 consecutive patients previously diagnosed with primary malignant solid tumours, and submitted to complete surgical resection of lung nodules with suspected or diagnosed metastatic lesions. All patients were admitted and treated in the Department of Thoracic Surgery of AC Camargo Hospital, from 1990 to 2008. The study was approved by the Ethics Committee of the institution. Data were collected from chart reviews of individual patients, by registering several characteristics of the primary tumour, such as date of surgical resection and histological type, and neoadjuvant or adjuvant treatment associated to resection; variables related to the pulmonary metastases, such as number of pulmonary nodules at CT scan, number of malignant pulmonary nodules resected, size of the largest nodule resected and laterality of pulmonary nodules were also collected. DFI was considered the period of time between the treatment of primary tumour and the diagnosis of pulmonary metastases. Overall survival was calculated from the date of the first metastasectomy.
Statistical analysis
Continuous data are presented as median, and categorical data are presented as percentages. Overall and disease-free survivals were estimated by the method of Kaplan and Meier, and log-rank and Breslow analyses were used to compare differences between groups. Overall survival was estimated from the date of operation to the date of last follow-up information or until death from any cause. The influence of each variable on overall and disease-free survival was calculated. Multivariate analysis to determine independent prognostic factors was determined by the Cox's proportional hazard model. Logistic regression was used to incorporate all the significant variables in the same model. The forward stepwise method was used to select the variables with independent prognostic value. Statistical analyses were performed using SPSS 11.5 for Windows. Significant differences were considered for P < 0.05.
Oncological assessment
The patients were evaluated for the diagnosis and treatment of lung nodules identified during pretreatment evaluation and staging of a neoplasm, or during routine follow-up after treatment of different primary tumours. All patients were submitted to a chest CT scan to evaluate resectability of pulmonary nodules. Reports of the radiologist were reviewed in each patient chart for information about the number of pulmonary nodules, size of the greater nodule and laterality. In the case of bilateral nodules on admission, we routinely performed a staged thoracotomy, starting by the side of the lungs with lesser chances of complete resection, either due to the number of nodules identified by chest CT scan or due to the anatomical position of one or more nodules relative to the pulmonary hilum, with the potential likelihood of a more complex and extensive lung resection.
Operative approach
Patients were selected for pulmonary metastasectomy if they presented the following characteristics: (i) primary tumour controlled or controllable, (ii) nodules confined to the lung parenchyma, (iii) nodules likely to get a complete surgical resection in the radiological preoperative evaluation, (iv) compatible pulmonary function and clinical condition with the planned operation, (v) predictable adequate lung function after resection, and (vi) absence of a more efficient systemic treatment, other than resection. Patients were submitted to general anaesthesia with selective single lung ventilation. Surgical access to thorax was performed by lateral thoracotomy to resect lung metastases even for patients with bilateral metastases. The surgeon routinely attempted to completely remove all lung nodules, preserving lung parenchyma by resecting the tumour with a small margin (5–10 mm). We did not routinely perform mediastinal lymph node dissection or sampling. On the other hand, in the presence of a single pulmonary nodule, frozen section analysis was mandatory. In the event of histological diagnosis of a possible primary lung tumour, the patient was preferably submitted to pulmonary lobectomy with radical mediastinal lymph-node dissection. Lung nodules were identified individually according to the site of resection and sent to histopathological analysis. Date of surgery, type of resection (complete or incomplete), number of malignant and benign resected nodules, size of the greatest nodule and type of lung resection (wedge, segmentectomy, lobectomy or pneumonectomy) were registered according to the surgical report in individual patient record. Some patients received chemotherapy, at the medical oncologist's discretion, before or after surgical resection of lung nodules. In that case, type of treatment and radiological response (complete, partial, stable or progression of disease, as defined by RECIST) were collected. After hospital discharge, patients were followed-up by clinical examination, radiological evaluation (chest X-rays and CT scans) every 3 months during the first 2 years post-resection, then every 6 months until the fifth year. Annual radiological follow-up was performed thereafter. Other ancillary tests were performed according to symptoms or clinical suspicion of recurrence in organs other than the lungs. We considered recurrence when new lesions were identified in the lungs or in other organs. When necessary, histological confirmation of malignancy was performed. When recurrence was limited to lung parenchyma, without evidence of other distant metastasis and the disease considered resectable by the attending thoracic surgeon, the patient was submitted to further thoracotomies for metastasectomy. After complete surgical resection, all patients were evaluated in the Department of Clinical Oncology, and systemic treatment was administered according to the discretion of the medical oncologist.
RESULTS
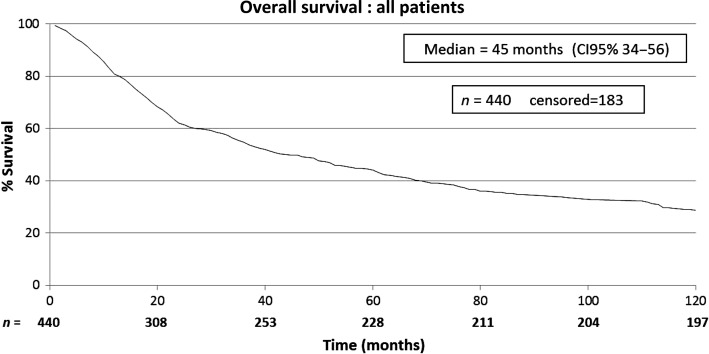
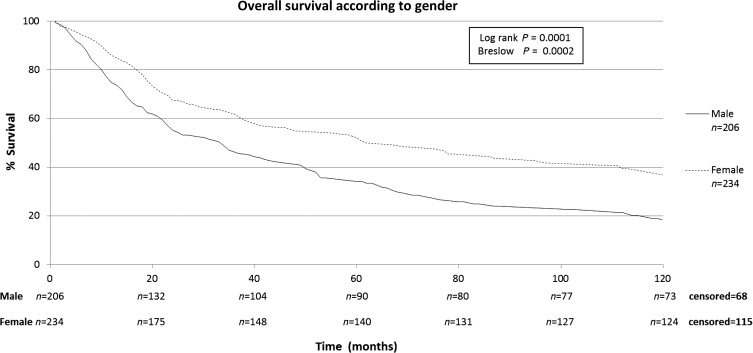
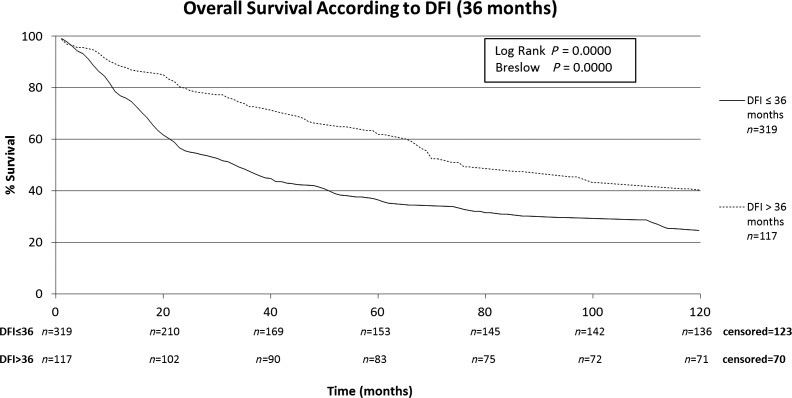
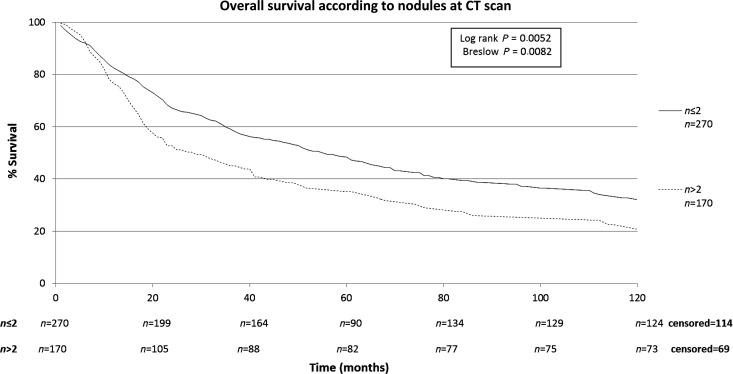
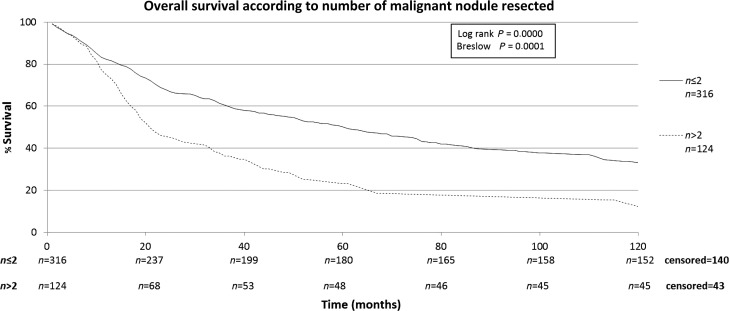
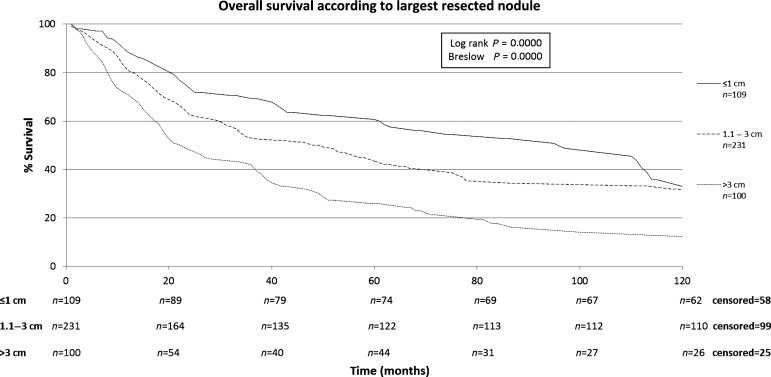
The characteristics of the patients included in this study are shown in Table 1. The patients included in this retrospective study (n = 440) were submitted to a total of 668 thoracotomies. The average follow-up time of all patients was 43.2 months, median of 24.3 months (range: 0–192 months). A total of four patients were lost from follow-up. The 5-year overall survival rate for all patients was 43.7% (Fig. 1). Male patients had lower survival rates than female (5-year survival rates of 34.2 vs. 51.4%), as shown on Fig. 2. Patients with adenocarcinoma (Table 2) presented the highest 5-year survival rates (53.4%). DFI significantly influenced outcome, with 5-year survival of patients with synchronous lung metastases (≤36 months of the primary tumour) of 34.8%, while in the same period the survival for patients who had DFI > 36 months was 69.74% (Fig. 3). The number of nodules identified on chest CT scan before thoracotomy was also significant (Fig. 4). Patients with the number of lesions ≤ 2 presented a 5-year survival rate of 47.8%; in patients with> 2 lesions, survival was 34.1%. The number of malignant nodules resected at the time of the operation had significant impact on long-term survival, as shown in Fig. 5. Patients with number of nodules ≤ 2 presented a 5-year survival rate of 49.67% and patients with >2 had survival rate of 23.2%. In addition, size of the largest resected nodule had significant impact on survival by the univariate analysis (Fig. 6). All other factors evaluated by univariate analysis, including age, response to adjuvant or neoadjuvant chemotherapy, number of resected nodules, laterality of the nodules at CT scan, type of resection, number of thoracotomies and completeness of last resection, did not reach statistical significance (P > 0.05). In our study, the number of nodules identified on CT and the number of malignant nodules resected are significant factors on survival, but the number of resected nodules is not a significant factor.
Table 1:
Patient characteristics (total, n = 440)
| Variables | n | % |
|---|---|---|
| Age (years) | ||
| 0–12 | 34 | 7.8 |
| 13–40 | 129 | 29 |
| 40–65 | 185 | 42.8 |
| >65 | 92 | 20.4 |
| Gender | ||
| Male | 206 | 46.3 |
| Female | 234 | 53.7 |
| Primary tumour site | ||
| Bone | 91 | 20.7 |
| Head-neck | 79 | 18 |
| Soft tissue | 53 | 12 |
| Breast | 58 | 13.2 |
| Colorectal | 51 | 11.6 |
| Skin | 37 | 8.4 |
| Uterus | 17 | 4 |
| Kidney | 14 | 3.2 |
| Stomach | 5 | 1.1 |
| Bladder | 2 | 0.4 |
| Other | 33 | 7.5 |
| Histology of the primary tumour | ||
| Adenocarcinoma | 133 | 53 |
| Osteosarcoma | 74 | 17 |
| Squamous-cell carcinoma | 63 | 14.4 |
| Soft tissue sarcoma | 60 | 13.6 |
| Malignant melanoma | 44 | 10 |
| Other | 66 | 15 |
| Disease-free interval (months) | ||
| ≤ 12 | 178 | 40.5 |
| >12 | 258 | 58.6 |
| Unknown | 4 | 0.9 |
| Disease-free interval (months) | ||
| ≤ 36 | 319 | 72.5 |
| >36 | 117 | 26.6 |
| Unknown | 4 | 0.9 |
| Number of nodules on CT scan | ||
| ≤2 | 270 | 61.4 |
| >2 | 170 | 39.6 |
| Laterality of the nodules | ||
| Right lung | 163 | 37 |
| Left lung | 112 | 11.6 |
| Bilateral | 165 | 51.4 |
| Type of resection | ||
| Pneumonectomy | 1 | 0.2 |
| Lobectomy | 4 | 1 |
| Segmentectomy | 58 | 13.2 |
| Wedge | 377 | 85.6 |
| Number of resected nodules | ||
| ≤2 | 233 | 53 |
| >2 | 207 | 47 |
| Number of malignant nodules resected | ||
| ≤2 | 316 | 71.8 |
| >2 | 124 | 28.2 |
| Diameter of greater nodule | ||
| ≤1 cm | 109 | 24.8 |
| 1.1–3 cm | 231 | 52.5 |
| >3 cm | 100 | 22.7 |
| Number of thoracotomies | ||
| 1 | 262 | 59.5 |
| 2 | 128 | 29.1 |
| 3 | 38 | 8.6 |
| 4 | 9 | 2.1 |
| 5 | 3 | 0.7 |
| Complete resection at last thoracotomy | ||
| Yes | 402 | 91.4 |
| No | 38 | 38.6 |
| Neoadjuvant chemotherapy | ||
| Yes | 126 | 28.6 |
| No | 314 | 71.4 |
| Adjuvant chemotherapy after lung resection | ||
| No | 192 | 43.6 |
| Yes | 245 | 55.7 |
| Unknown | 3 | 0.7 |
Figure 1:
Overall survival: all patients.
Figure 2:
Overall survival according to gender.
Table 2:
Overall survival according to histology (log rank P = 0.0001 Breslow P < 0.0001)
| Histology | Number of cases (n) | Median survival (months) | 95% CI (months) | 5-year survival rate (%) |
|---|---|---|---|---|
| Adenocarcinoma | 133 | 75 | (51; 98) | 53.45 |
| Osteosarcoma | 74 | 22 | (18; 25) | 27 |
| SCC | 63 | 19 | (8; 30) | 24.15 |
| STS | 60 | 48 | (29; 67) | 41.79 |
| Melanoma | 44 | 36 | (7; 65) | 40.1 |
| Other | 66 | 78 | (60; 97) | 60.64 |
CI, confidence interval; SCC, squamous cell carcinoma; STS, soft tissue sarcoma.
Figure 3:
Overall survival according to DFI (36 months).
Figure 4:
Overall survival according to number of nodules at CT scan.
Figure 5:
Overall survival according to number of malignant nodules resected.
Figure 6:
Overall survival according to largest resected nodule.
Multivariate analysis identified the number of malignant nodules resected (P = 0.01), the size of the largest nodule resected (P = 0.001), DFI> 36 months (P < 0.001) and histology of the primary tumour (P = 0.017) as independent factors with significant impact on overall survival. Consequently, patients with metastatic adenocarcinoma presenting with less than three nodules, the largest with a diameter <3 cm, and admitted with a DFI greater than 36 months, reached a 5-year survival rate of 61.4%.
DISCUSSION
Complete resection of metastatic lesions has long been established as an important prognostic factor in most studies, and is included nowadays among the major criteria to consider a patient for surgical management [1–3]. Microscopically negative resection margins minimize the probability of recurrence. Nevertheless, we could not find in the medical literature extensive studies evaluating prognostic factors in the subgroup of patients with the most favourable outcome perspectives, i.e. those submitted to complete resection. According to the present study, following complete resection of all pulmonary metastatic nodules, the following factors evaluated by univariate analyses significantly impacted patient outcome and long-term survival: gender, histology of the primary tumour, DFI, number of nodules identified on preoperative chest CT scan, number of malignant nodules and size of the largest resected nodule. Despite being considered important prognostic factors by several authors, response to adjuvant or neoadjuvant chemotherapy and overall number of resected nodules (that included benign lesions) were not statistically significant (P > 0.05). There was a difference between the number of nodules resected and those detected on CT scan. On the other hand, most undetected and resected nodules were benign. Multivariate analyses selected the number of malignant nodules resected, DFI and histology, as significant independent prognostic factors to predict better outcome. Recently, oncologists are questioning the indication of pulmonary metastasectomy, as a widespread procedure, due to the benefit limited to a small group of patients. In the present study, as in most published series [7–10], <50% of resected cases are expected to survive over 5 years. With the increasing effectiveness of systemic therapy, surgeons as well as oncologists are trying to better select the patients that would likely benefit from either approach. Up to the present date, despite the clear progress in histology, immunohistochemistry and molecular biology, this expanding knowledge has not been routinely applied to the selection of patients for surgical resection of metastatic disease. At the same time, few studies on metastasectomy included only patients submitted to the modern staging methods, such as 18F-FDG PET-CT scans [11]. At our institution, PET-CT scans were not routinely required for the present group of patients. It is obvious, nonetheless, that we have to make the utmost efforts in order to avoid unnecessary procedures, either surgical or systemic therapies. The present study emphasizes the impact of the intrinsic biology and behaviour of the tumour, rather than aspects of surgical techniques. As long as, by whichever resection technique, the patient was rendered free of gross detectable disease, tumour characteristics would be paramount to select the best subgroup of patients. Indication for postoperative adjuvant treatment, for example, should be discussed, and offered to patients with greater likelihood of early recurrence. Thus, avoiding by that strategy the treatment of patients with excellent prognosis. On the other hand, surgical operation should be thoroughly reassessed in patients that, despite complete resection, are predicted to have high risk of recurrence and low survival rates. Should this subgroup of limited prognosis be operated on at all? Should they receive intensive preoperative neoadjuvant chemotherapy, before considering surgical resection? The present study clearly shows that even following complete resection of pulmonary metastases, the benefit of such aggressive surgical approach is only limited to selected subgroups of patients. These results confirm that the decision of thoracic surgeons to consider a patient for resection of metastastic disease should include factors beyond the feasibility of complete removal of all nodules identified on preoperative imaging scans. The medical community should take advantage of the current knowledge of tumour biology to further select patients for multimodality strategies, including, if necessary, surgical resection.
Conflict of interest: none declared.
REFERENCES
- 1.Pastorino U, Buyse M, Frielder G. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg. 1997;113:37–49. doi: 10.1016/s0022-5223(97)70397-0. [DOI] [PubMed] [Google Scholar]
- 2.Kaifi JT, Gusani NJ, Deshaies I, Kimchi ET, Reed MF, Mahraj RP, et al. Indications and approach to surgical resection of lung metastases. J Surg Oncol. 2010;102:187–95. doi: 10.1002/jso.21596. [DOI] [PubMed] [Google Scholar]
- 3.Casiraghi M, De Pas T, Maisonneuve P, Brambilla D, Ciprandi B, Galetta D, et al. A 10-year single-center experience on 708 lung metastasectomies: the evidence of the International Registry of Lung Metastases. J Thorac Oncol. 2011 doi: 10.1097/JTO.0b013e3182208e58. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 4.Chen F, Sakai H, Miyahara R, Bando T, Okubo K, Date H. Repeat resection of pulmonary metastasis is beneficial for patients with colorectal carcinoma. World J Surg. 2010;34:2373–8. doi: 10.1007/s00268-010-0695-x. [DOI] [PubMed] [Google Scholar]
- 5.Younes RN, Haddad F, Ferreira F, Gross JL. Surgical removal of pulmonary metastases: a prospective study in 182 patients. Rev Assoc Med Bras. 1998;44:218–25. doi: 10.1590/s0104-42301998000300010. [DOI] [PubMed] [Google Scholar]
- 6.Pastorino U. Lung metastasectomy: why, when, how. Crit Rev Oncol Hematol. 1997;26:137–45. doi: 10.1016/s1040-8428(97)00017-6. [DOI] [PubMed] [Google Scholar]
- 7.Shiono S, Kawamura M, Sato T, Okumura S, Nakajima J, Yoshino I, et al. Metastatic Lung Tumor Study Group of Japan. Pulmonary metastasectomy for pulmonary metastases of head and neck squamous cell carcinomas. Ann Thorac Surg. 2009;88:856–60. doi: 10.1016/j.athoracsur.2009.04.040. [DOI] [PubMed] [Google Scholar]
- 8.Kanzaki R, Higashiyama M, Fujiwara A, Tokunaga T, Maeda J, Okami J, et al. Long-term results of surgical resection for pulmonary metastasis from renal cell carcinoma: a 25-year single-institution experience. Eur J Cardiothorac Surg. 2011;39:167–72. doi: 10.1016/j.ejcts.2010.05.021. [DOI] [PubMed] [Google Scholar]
- 9.García Franco CE, Torre W, Tamura A, Guillén-Grima F, San-Julian M, Martin-Algarra S, et al. Long-term results after resection for bone sarcoma pulmonary metastases. Eur J Cardiothorac Surg. 2010;37:1205–8. doi: 10.1016/j.ejcts.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 10.Kemp CD, Kitano M, Kerkar S, Ripley RT, Marquardt JU, Schrump DS, et al. Pulmonary resection for metastatic gastric cancer. J Thorac Oncol. 2010;5:1796–805. doi: 10.1097/JTO.0b013e3181ed3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaira K, Okumura T, Ohde Y, Takahashi T, Murakami H, Oriuchi N, et al. Correlation between 18F-FDG uptake on PET and molecular biology in metastatic pulmonary tumors. J Nucl Med. 2011;52:705–11. doi: 10.2967/jnumed.111.087676. [DOI] [PubMed] [Google Scholar]
- 12.Younes RN, Gross JL, Taira AM, Martins AAC, Neves GS. Surgical resection of lung metastases: results from 529 patients. Clinics. 2009;64:535–41. doi: 10.1590/S1807-59322009000600008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younes RN, Gross JL, Silva JF, Fernandez JA, Kowalski LP. Surgical treatment of lung metastases of head and neck tumors. Am J Surg. 1997;174:499–502. doi: 10.1016/s0002-9610(97)00164-5. [DOI] [PubMed] [Google Scholar]
- 14.Chen F, Fujinaga T, Sato K, Sonobe M, Shoji T, Sakai H, et al. Clinical features of surgical resection for pulmonary metastasis from breast cancer. Eur J Surg Oncol. 2009;35:393–7. doi: 10.1016/j.ejso.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 15.Younes RN, Gross JL, Deheinzelin D. Surgical resection of unilateral lung metastases: is bilateral thoracotomy necessary? World J Surg. 2002;26:1112–6. doi: 10.1007/s00268-002-6209-8. [DOI] [PubMed] [Google Scholar]