Abstract
Acute exacerbation (AE) of idiopathic pulmonary fibrosis (IPF) in lung cancer patients is a critical factor in post-operative mortality. The cause of AE development is unknown and AE may occur in patients without the diagnosis of IPF. We have conducted a retrospective study of consecutive patients who underwent lung cancer surgery since January 2004. Sixty-two patients with fibrous findings in preoperative high-resolution computed tomography were enrolled in the present study and clinicopathological factors were analysed. AE was observed in 6 of 62 patients. The frequency of AE according to the type of fibrous changes classification was 1/7 in the usual interstitial pneumonia (UIP) pattern, 1/16 in the cellular non-specific interstitial pneumonia (NSIP) pattern, 4/25 in the fibrotic NSIP pattern and 0/14 in the unclassified or focal fibrous changes pattern. Preoperative Krebs von den Lungen-6 (KL-6) was higher in patients with AE than in those without AE. In patients who underwent partial resection, AE did not develop even with high KL-6 levels. In conclusion, in patients with both the UIP and the NSIP patterns, AE development is possible. In patients with a high risk of AE, such as those with high KL-6 values, limited surgery may be an option to prevent AE development.
Keywords: Idiopathic pulmonary fibrosis, Acute exacerbation, Lung cancer, KL-6
INTRODUCTION
In lung cancer patients who undergo lung resection, it has been reported that post-operative pulmonary morbidity and mortality of the patients with idiopathic pulmonary fibrosis (IPF)/usual interstitial pneumonia (UIP) were higher than in those without IPF/UIP [1]. The Japanese Association for Thoracic Surgery has conducted a survey of lung cancer surgery, which was published as an annual report [2]. In 1036 of 27 881 lung cancer patients, interstitial pneumonia (IP) was diagnosed as an associated disease. Sixty-eight patients died of IP within 30 days post-operatively and this IP, probably with acute exacerbation (AE), was the most frequent cause of the post-operative deaths. In the perioperative morbidity, AE of IP is one of the most critical factors in IP patients. Some candidates have been considered as triggers of AE: for example, a high concentration of inspired oxygen causes lung injury [3]. Enomoto et al. [4] reported on high peripheral white blood cell counts (WBC) and high Krebs von den Lungen-6 (KL-6). Recently, KL-6 has been considered as one of the most reliable predictive factors of AE of IPF [5]. Preoperative percentage vital capacity (%VC) and serum lactate dehydrogenase (LDH) have been reported as predictive factors for AE of UIP [6]. However, the causes or detailed mechanisms of AE are still unclear. If it were possible to predict AE of IP using a preoperative examination, the post-operative mortality of lung cancer operations could be decreased.
We have retrospectively reviewed lung cancer patients who underwent lung resection and analysed the relation between the preoperative CT findings, other clinicopathological factors and post-operative AE development.
PATIENTS AND METHODS
Patients and methods
A total of 480 patients with non-small cell lung cancer were treated surgically at Nagoya City University Hospital between January 2004 and December 2009. In the present study, the patients who underwent segmentectomy or partial resection (wedge resection) have been included. These surgical procedures are not appropriate oncologically [7]. However, in the patients with IP shadow, radiotherapy or chemotherapy is difficult to be selected. Limited resection has been performed palliatively to consider the pulmonary function or elder age.
Sixty-two patients were preoperatively diagnosed with fibrous findings at various degrees by high-resolution computed tomography (HRCT). The HRCT radiological findings were examined by two radiologists who specialized in diagnosing pulmonary lesions and diagnosed as an UIP or non-specific interstitial pneumonia (NSIP) pattern [8–11]. The NSIP pattern was classified into cellular NSIP (c-NSIP) pattern, fibrotic NSIP (f-NSIP) pattern [10, 11] or the unclassified or focal fibrous changes (UFC) pattern. In these 62 patients, the clinicopathological factors, radiological findings and surgical factors were analysed to predict post-operative AE.
Statistical analyses
Survival analysis was performed by the Kaplan–Meier method and a univariate log-rank test. An outcome measure was utilized with overall survival. The significance of differences between categorized groups was evaluated using a χ2 test. Pathological stages and operative procedures were used to grade and were assessed using the Mann–Whitney U-test. Parametric values were presented as mean ± standard deviation and analysed by the non-paired t-test using Scheffe's method. Statistical significance was defined as P < 0.05.
RESULTS
Patients and clinicopathological characteristics
The patient characteristics were as follows. The median age was 69 years (range, 58–85 years), and 55 patients were males and 7 were females. Fifty-eight patients were current or former smokers and four patients were non-smokers. The most common histopathological types of lung cancer were adenocarcinoma (n = 29) and squamous cell carcinoma (n = 22). Haematological examinations related to pulmonary fibrosis, WBC, C-reactive protein (CRP), LDH and KL-6 were assessed preoperatively. The means and standard deviations of WBC, CRP, LDH and KL-6 were 6.91 ± 2.03 × 103/µl, 1.08 ± 2.64 mg/dl, 2.00 ± 0.40 × 102 and 6.73 ± 4.38 × 102 U/ml, respectively. KL-6 was measured only in 51 of 62 patients. Preoperative %VC and forced expiratory volume (FEV1)/forced vital capacity were 99.1 ± 17.5 and 77.8 ± 14.2%, respectively. The preoperative HRCT patterns of fibrosis were UIP in 7, c-NSIP in 16, f-NSIP in 25 and UFC in 14 patients. In addition to fibrosis, emphysema was seen in 38 patients, bullae in 11 patients and old inflammation in 9 patients. The operation time was 196 ± 69 min and the blood loss was 381 ± 522 ml. The operative procedures consisted of lobectomy in 44 patients, segmentectomy in 6 patients and partial resection in 12 patients. The pathological stages were diagnosed as IA (n = 24), IB (n = 13), IIA (n = 7), IIB (n = 9), IIIA (n = 8) and IV (n = 1).
Post-operative exacerbation and survival
AE was observed in six patients and became evident on post-operative days 2, 5, 5, 6, 35 and 120, respectively. We used a broad definition of post-operative AE and included all six patients in the present study.
The mean observation period was 26.1 months after the operation. In the prognosis, 21 of 62 patients died of lung cancer (n = 14), AE (n = 3), respiratory failure (n = 2), post-operative multiple organ failure (n = 1) and liver cirrhosis (n = 1), respectively. Only one case with liver cirrhosis died within 30 days after the operation. Within 90 days, two cases died of AE.
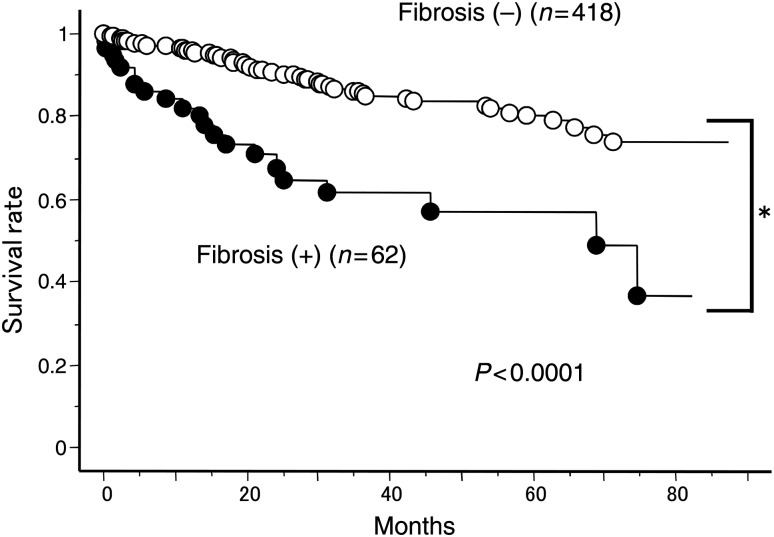
The overall survival in 62 patients was 83.8, 60.3 and 55.7% at 1, 3 and 5 years after the primary operation. The prognosis of the 62 patients was significantly worse than the 418 patients without fibrosis who underwent the operation during the same period (96.4, 85.8 and 81.3% at 1, 3 and 5 years after the primary operation, P < 0.0001) (Fig. 1).
Figure 1:
Overall survival of lung cancer patients with or without fibrotic findings.
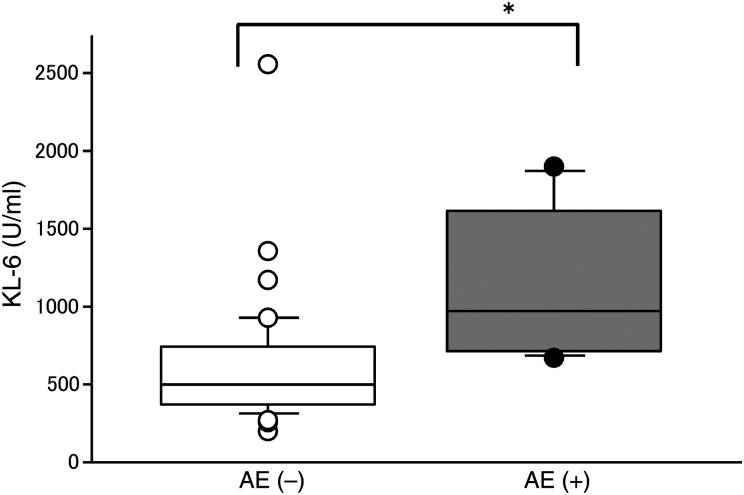
The relationships between AE and clinicopathological factors were analysed (Table 1). Only the preoperative KL-6 in patients with post-operative AE (1143 ± 518 U/ml) was significantly higher than in patients without post-operative AE (610 ± 392 U/ml; Fig. 2). Also, preoperative %VC in patients with post-operative AE was lower than in those without post-operative AE. Other clinicopathological factors did not significantly affect post-operative AE (Table 1). In patients with preoperative fibrosis findings, AE developed in patients with UIP (1/7), f-NSIP (4/21) and c-NSIP (1/15) patterns. In patients with the UFC pattern, AE did not occur.
Table 1:
Clinicopathological factors and AE
| Factors | AE (−) | AE (+) | P-values | |
|---|---|---|---|---|
| Total (n) | 62 | 56 | 6 | — |
| Age (58–85) | 70 ± 6 | 70 ± 6 | 72 ± 8 | 0.509 |
| Gender (n = 62) | 0.264 | |||
| Male | 55 | 51 | 4 | |
| Female | 7 | 5 | 2 | |
| CRP (n = 62) | 1.08 ± 2.64 | 0.905 ± 2.15 | 2.67 ± 5.54 | 0.121 |
| WBC (×103) (n = 62) | 6.91 ± 2.03 | 6.82 ± 1.99 | 7.82 ± 2.92 | 0.254 |
| LDH (n = 62) | 200 ± 40 | 198 ± 37 | 222 ± 61 | 0.164 |
| KL-6 (n = 51) | 673 ± 438 | 610 ± 392 | 1143 ± 518 | 0.004 |
| Operation time (n = 62) (min) | 196 ± 69 | 191 ± 68 | 238 ± 69 | 0.112 |
| Blood loss (n = 62) (ml) | 381 ± 522 | 364 ± 535 | 545 ± 387 | 0.423 |
| %VC (n = 62) (%) | 99.1 ± 17.5 | 100.5 ± 17.7 | 86.2 ± 9.4 | 0.056 |
| FEV1.0% (n = 62) (%) | 77.8 ± 14.2 | 77.8 ± 14.8 | 77.8 ± 5.4 | 0.995 |
| Alcohol (n = 62) | 0.999 | |||
| − | 32 | 29 | 3 | |
| + | 30 | 27 | 3 | |
| Emphysema (n = 62) | 0.999 | |||
| − | 24 | 22 | 2 | |
| + | 38 | 34 | 4 | |
| Bulla (n = 62) | 0.624 | |||
| − | 51 | 47 | 4 | |
| + | 11 | 9 | 2 | |
| Old inflammation (n = 62) | 0.999 | |||
| − | 53 | 48 | 5 | |
| + | 9 | 8 | 1 | |
| Fibrosis (n = 62) | 0.112 | |||
| UIP | 7 | 6 | 1 | |
| f-NSIP | 25 | 21 | 4 | |
| c-NSIP | 16 | 15 | 1 | |
| UFC | 14 | 14 | 0 | |
| Operative procedure (n = 62) | 0.414 | |||
| Lobectomy | 44 | 39 | 5 | |
| Segmentectomy | 6 | 5 | 1 | |
| Partial resection | 12 | 12 | 0 | |
| Pathology (n = 62) | 0.518 | |||
| Adenoca | 29 | 25 | 4 | |
| Squamous | 22 | 21 | 1 | |
| Others | 11 | 10 | 1 | |
| Pathological stage (n = 62) | 0.606 | |||
| IA | 24 | 21 | 3 | |
| IB | 13 | 12 | 1 | |
| IIA | 7 | 7 | 0 | |
| IIB | 9 | 7 | 2 | |
| IIIA | 8 | 8 | 0 | |
| IIIB | 0 | 0 | 0 | |
| IV | 1 | 1 | 0 |
Figure 2:
Preoperative KL-6 and post-operative AE (*P < 0.05).
KL-6 and other factors
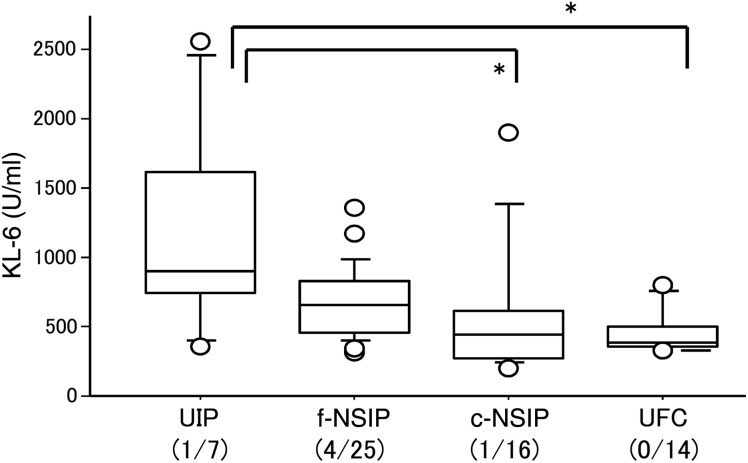
Figure 3 shows the relation between preoperative KL-6 and preoperative fibrosis findings. KL-6 values were 1180 ± 790 in patients with an UIP pattern, 676 ± 264 with an f-NSIP pattern, 592 ± 489 with a c-NSIP pattern and 460 ± 165 with an UFC pattern. The KL-6 value in patients with an UIP pattern was higher than those with a c-NSIP (P = 0.046) or an UFC (P = 0.012) pattern.
Figure 3:
Preoperative KL-6 and a fibrotic pattern on HRCT (*P < 0.05).
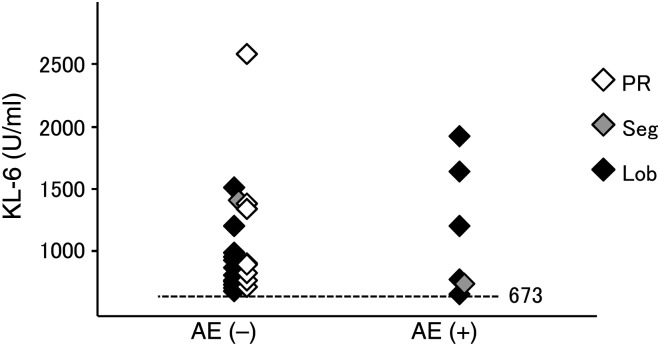
In regard to operative procedures, AE developed in patients who underwent lobectomy (5/44) and segmentectomy (1/5) but did not occur in patients who underwent partial resection. In patients with post-operative AE, the preoperative KL-6 values were 680, 722, 756, 1180, 1620 and 1900 U/ml, respectively, so all values were higher than the mean value of KL-6 (673 U/ml) in 51 patients. Twenty-one of the 51 patients with preoperative KL-6 examinations showed results higher than 673 U/ml. Of these 21 patients, KL-6 values, operative procedures and AE development are plotted in Fig. 4. Even in patients with high KL-6 values, AE did not develop in those who underwent partial resection.
Figure 4:
Preoperative KL-6, post-operative AE and operative procedures.
DISCUSSION
In the present study, AE of IP in patients with lung cancer who underwent lung resection seems to be closely related to high values of preoperative KL-6. None of the patients who underwent partial resection of the lung developed AE, suggesting that a lesser operative insult may be related to a reduced risk of AE. Collard et al. [5] recently reported that KL-6 was one of the most important predictive factors of AE development in IP patients without lung cancer. Enomoto et al. [4] reported high KL-6, high peripheral WBC and low pulmonary function as predictive factors of AE development in UIP after lung biopsy using video-assisted thoracic surgery (VATS). Also in patients with lung cancer, KL-6 seems to be one of the most predictive factors of AE. Shintani et al. reported predictive factors of post-operative AE in UIP, including KL-6. However, in their report, %VC and LDH were more influential factors [6]. Though the P-value of %VC was 0.056 in the present study, it may be significant and %VC may be one of the prognostic factors of AE as Shintani's report because the number of cases was so small in the present study.
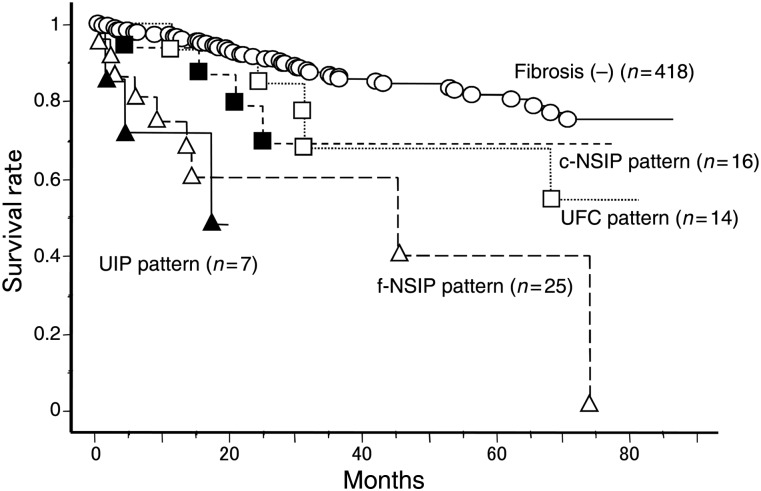
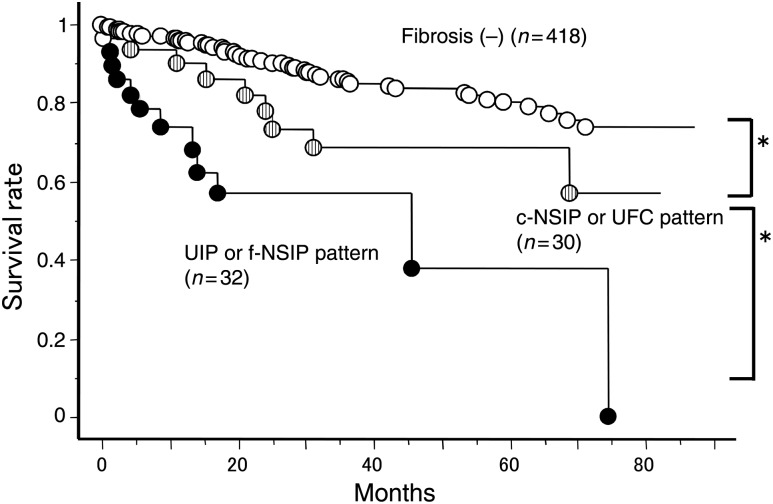
In the present study, overall survival in the 62 patients was significantly worse than the 418 lung cancer patients without a fibrotic shadow who underwent the operation during the same period. There was no difference of pathological stage between groups. We have suggested that the fibrotic shadow on HRCT was a worse prognostic factor. In addition, the prognosis of the patients with UIP and f-NSIP patterns was similar (Fig. 5). The prognosis of the patients with c-NSIP and UFC was similar (Fig. 5). When we divide the groups into patients without a fibrotic shadow, with an UIP or an f-NSIP pattern and with a c-NSIP or an UFC pattern (Fig. 6), there were significant differences of overall survival between groups. These data suggest the importance of preoperative careful diagnosis of a fibrotic shadow.
Figure 5:
Overall survival of lung cancer patients with or without fibrotic findings. Fibriotic findings were classified into four patterns: UIP, f-NSIP, c-NSIP and UFC patterns.
Figure 6:
Overall survival of lung cancer patients with or without fibrotic findings. Fibriotic findings were classified into two patterns: UIP or f-NSIP and c-NSIP or UFC patterns (*P < 0.05).
In our study, we have shown that AE can develop in patients with an NSIP pattern. Though a relationship between AE and IPF/UIP has been reported [8, 12], an association between post-operative AE in lung cancer patients and NSIP or other types of IP has been reported to be rare [13]. Park IN reported that AE occurred in patients with NSIP at a frequency of 4.2% in 1 year [13]. In the present study, in patients with an f-NSIP pattern, AE may develop more frequently than in patients with a c-NSIP pattern.
In the present study, we speculate that a part or most of IP with an f-NSIP pattern may diagnose as UIP pathologically. Because both groups with an UIP or an f-NSIP pattern were similar in AE development and overall survival. To consider the KL-6 values in fibrotic patterns, the group with an f-NSIP pattern may include the early stage of UIP without characteristic UIP findings; honeycomb formation.
Unfortunately, we could not demonstrate an agreement between radiological and pathological findings of IP. Indeed, we could not perform resection or biopsy of the fibrotic part in all cases. We have no data of pathological diagnosis of IP. In the present study, the most important subject is to determine the prognostic factors of post-operative AE before the operation. It usually takes much time to diagnose IP pathologically. If AE of IP develops post-operatively, the patients may become critical before diagnosis of IP. Post-operative pathological analysis was definitely important. However, we would like to determine the prognostic factors of AE using preoperative radiological findings and preoperative examination data.
We have also found that the volume of the resected lung or the degree of the operative insult may be related to post-operative AE. Koizumi et al. compared three surgical approaches in lung cancer in patients with IP, and VATS did not prevent AE of IP. Yano et al. [3] reviewed five reports on surgery for lung cancer with IP in the Japanese literature and reported a high rate of AE in pneumonectomy and no cases of AE in wedge resection. The volume of resection seemed to have more influence on the occurrence of post-operative AE than did a surgical approach. The advantages of partial resection may depend on many factors, such as a smaller volume of the lung resection, shorter anaesthesia time, shorter operation time, less bleeding and less surgical stress. However, even after the surgical biopsy of IP, AE has been reported in 2.1–22.2% of cases with IP [4, 14]. Limited resection, especially wedge resection was not adequate procedure oncologically [7]. However, in the patients with IP shadow, radiotherapy or chemotherapy is difficult to be selected. Wedge resection has been performed palliatively to consider the pulmonary function or elder age. It is a dilemma. We select partial resection to decrease AE and this limited resection may show a worse prognosis oncologically. Fujimoto et al. [15] showed a worse prognosis of lung cancer patients with IPF/UIP who underwent surgical resection. They performed lobectomy (n = 17), segmentectomy (n = 2) and wedge resection (n = 2). They suggested the importance of pathological diagnosis of IPF/UIP before the lung cancer operation and validity of wedge resection for the tumour. There are still many problems left in the surgical resection of the lung cancer with IP: surgical procedure, operative indication of the stage or operative indication itself. In the present study, we have included the patients with advanced disease IIIA (n = 8) and IV (n = 1). This stage IV disease is a case with contralateral pulmonary metastasis. We usually operate such a case to diagnose multiple lung cancer or contralateral pulmonary metastasis. We also included pN2 lung cancer (IIIA disease) but IP patients often show lymph node swelling without metastasis. Fujimoto et al. [15] reported the prognosis with or without lymph node metastasis, and they showed no differences with or without lymph node metastasis.
In conclusion, we have suggested that AE may develop not only in patients with an UIP pattern but also in those with an NSIP pattern, especially if they show an f-NSIP pattern in preoperative computed tomography. KL-6 is a candidate prognostic factor for the occurrence of post-operative AE, and partial resection may be an option to prevent AE in patients with high values of KL-6.
Conflict of interest: none declared.
REFERENCES
- 1.Kawasaki H, Nagai K, Yoshida J, Nishimura M, Nishiwaki Y. Postoperative morbidity, mortality, and survival in lung cancer associated with idiopathic pulmonary fibrosis. J Surg Oncol. 2002;81:33–7. doi: 10.1002/jso.10145. doi:10.1002/jso.10145. [DOI] [PubMed] [Google Scholar]
- 2.Sakata R, Fujii Y, Kuwano H. Thoracic and cardiovascular surgery in Japan during 2008: annual report by The Japanese Association for Thoracic Surgery. Gen Thorac Cardiovasc Surg. 2010;58:356–83. doi: 10.1007/s11748-010-0604-0. doi:10.1007/s11748-010-0604-0. [DOI] [PubMed] [Google Scholar]
- 3.Yano T, Koga T, Ninomiya S, Taketomi A, Yoshida T, Matsumata T. A review of Japanese literatures concerning surgery for lung cancer with idiopathic interstitial pneumonia. Kyobu Geka. 2002;55:131–3. Abstract in English. [PubMed] [Google Scholar]
- 4.Enomoto T, Kawamoto M, Kunugi S, Hiramatsu K, Sakakibara K, Usuki J, et al. Clinicopathological analysis of patients with idiopathic pulmonary fibrosis which became acutely exacerbated after video-assisted thoracoscopic surgical lung biopsy. Nihon Kokyuki Gakkai Zasshi. 2002;40:806–11. Abstract in English. [PubMed] [Google Scholar]
- 5.Collard HR, Calfee CS, Wolters PJ, Song JW, Hong SB, Brady S, et al. Plasma biomarker profiles in acute exacerbation of idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2010;299:L3–7. doi: 10.1152/ajplung.90637.2008. doi:10.1152/ajplung.90637.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shintani Y, Ohta M, Iwasaki T, Ikeda N, Tomita E, Kawahara K, et al. Predictive factors for postoperative acute exacerbation of interstitial pneumonia combined with lung cancer. Gen Thorac Cardiovasc Surg. 2010;58:182–5. doi: 10.1007/s11748-009-0569-z. doi:10.1007/s11748-009-0569-z. [DOI] [PubMed] [Google Scholar]
- 7.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. Ann Thorac Surg. 1995;60:615–22. doi: 10.1016/0003-4975(95)00537-u. doi:10.1016/0003-4975(95)00537-U. [DOI] [PubMed] [Google Scholar]
- 8.Travis WD, Matsui K, Moss J, Ferrans VJ. Idiopathic nonspecific interstitial pneumonia: prognostic significance of cellular and fibrosing patterns: survival comparison with usual interstitial pneumonia and desquamative interstitial pneumonia. Am J Surg Pathol. 2000;24:19–33. doi: 10.1097/00000478-200001000-00003. doi:10.1097/00000478-200001000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Sumikawa H, Johkoh T, Colby TV, Ichikado K, Suga M, Taniguchi H, et al. Computed tomography findings in pathological usual interstitial pneumonia: relationship to survival. Am J Respir Crit Care Med. 2008;177:433–9. doi: 10.1164/rccm.200611-1696OC. doi:10.1164/rccm.200611-1696OC. [DOI] [PubMed] [Google Scholar]
- 10.Nagai S, Kitaichi M, Itoh H, Nishimura K, Izumi T, Colby TV. Idiopathic nonspecific interstitial pneumonia/fibrosis: comparison with idiopathic pulmonary fibrosis and BOOP. Eur Respir J. 1998;12:1010–9. doi: 10.1183/09031936.98.12051010. doi:10.1183/09031936.98.12051010. [DOI] [PubMed] [Google Scholar]
- 11.Johkoh T, Müller NL, Colby TV, Ichikado K, Taniguchi H, Kondoh Y, et al. Nonspecific interstitial pneumonia: correlation between thin-section CT findings and pathologic subgroups in 55 patients. Radiology. 2002;225:199–204. doi: 10.1148/radiol.2251011555. doi:10.1148/radiol.2251011555. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto S, Homma S, Mun M, Fujii T, Kurosaki A, Yoshimura K. Acute exacerbation of idiopathic interstitial pneumonia following lung surgery in 3 of 68 consecutive patients: a retrospective study. Intern Med. 2011;50:77–85. doi: 10.2169/internalmedicine.50.3390. doi:10.2169/internalmedicine.50.3390. [DOI] [PubMed] [Google Scholar]
- 13.Park IN, Kim DS, Shim TS, Lim CM, Lee SD, Koh Y, et al. Acute exacerbation of interstitial pneumonia other than idiopathic pulmonary fibrosis. Chest. 2007;132:214–20. doi: 10.1378/chest.07-0323. doi:10.1378/chest.07-0323. [DOI] [PubMed] [Google Scholar]
- 14.Kondoh Y, Taniguchi H, Kitaichi M, Yokoi T, Johkoh T, Oishi T, et al. Acute exacerbation of interstitial pneumonia following surgical lung biopsy. Respir Med. 2006;100:1753–9. doi: 10.1016/j.rmed.2006.02.002. doi:10.1016/j.rmed.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 15.Fujimoto T, Okazaki T, Matsukura T, Hanawa T, Yamashita N, Nishimura K, et al. Operation for lung cancer in patients with idiopathic pulmonary fibrosis: surgical contraindication? Ann Thorac Surg. 2003;76:1674–8. doi: 10.1016/s0003-4975(03)00966-4. doi:10.1016/S0003-4975(03)00966-4. [DOI] [PubMed] [Google Scholar]