Abstract
Surgical treatment of an abdominal aortic aneurysm in patients with a heart disease is risky. Aortic cross-clamping is featured by important consequences on cardiac, renal and gastrointestinal functions. Endovascular aortic repair is considered to be the gold standard in patients with severe comorbidities. However, in the case of unsuccessful endovascular treatment, surgery can be reconsidered with the use of extracorporeal membrane oxygenation, which seems to be a new tool for the management of cardiac and gastrointestinal events ensuring better post-operative outcomes.
Keywords: Aneurysm, Extracorporeal circulation, Great vessels
BACKGROUND
Cardiovascular complications after the surgical treatment of abdominal aortic aneurysms (AAAs) remain an issue. In high-risk patients with anatomical limitations, not suitable for endovascular aortic repair (EVAR), surgical treatment could be reconsidered by using extracorporeal membrane oxygenation (ECMO) allowing limited post-operative cardiac morbidity. We report the first two cases of the surgical treatment of AAAs with ECMO support.
REPORT
We report two cases of AAA in which the endovascular attempt failed. The first patient was an 82-year old man who underwent a coronary artery bypass grafting 7 years ago. The patient kept residual angina and NYHA class III dyspnoea associated with left ventricle dysfunction [left ventricular ejection fraction (LVEF) 40%]. An 8-cm AAA was diagnosed and the endovascular attempt failed because of huge calcifications of femoral and iliac arteries rendering the access impossible (Fig. 1). The second patient, a 70-year old man, presented with a history of myocardial infarction with an untreatable tritroncular disease associated with left ventricle dysfunction (LVEF 35%) and grade III aortic regurgitation. The endovascular exclusion of a 10-cm AAA failed because of tortuous iliac arteries with a 180° angulation between the common and external iliac arteries (Fig. 2). Surgical correction was decided in both cases using ECMO support. Low-dose heparinization (1 mg/kg) was used, and the cannulation of the inferior vena cava was performed through the right femoral vein. The optimal positioning of the vena cava cannula was assessed by transesophageal echocardiography (TEO). The right subclavian artery was surgically controlled and a latero-terminal anastomosis of a 10-mm Dacron tube was achieved. A median laparotomy was then performed and the arterio-venous ECMO support was activated just before infrarenal aortic cross-clamping. The flow rate was fixed at 2 l/min. Monitoring of cardiac function and output with TOE allowed adjustments of the ECMO flow. During cross-clamping, the flow was increased in order to ensure heart drainage and optimal output. After declamping, a fast refilling was obtained through the arterial cannula. At the end of the surgical procedure, ECMO was removed and despite of the laparotomy, a normal body temperature was maintained thanks to the heat exchanger. There was neither major post-operative bleeding nor renal failure, and troponin levels remained normal. Both patients were discharges on day 12 with a normal LVEF assessed by transthoracic echocardiography.
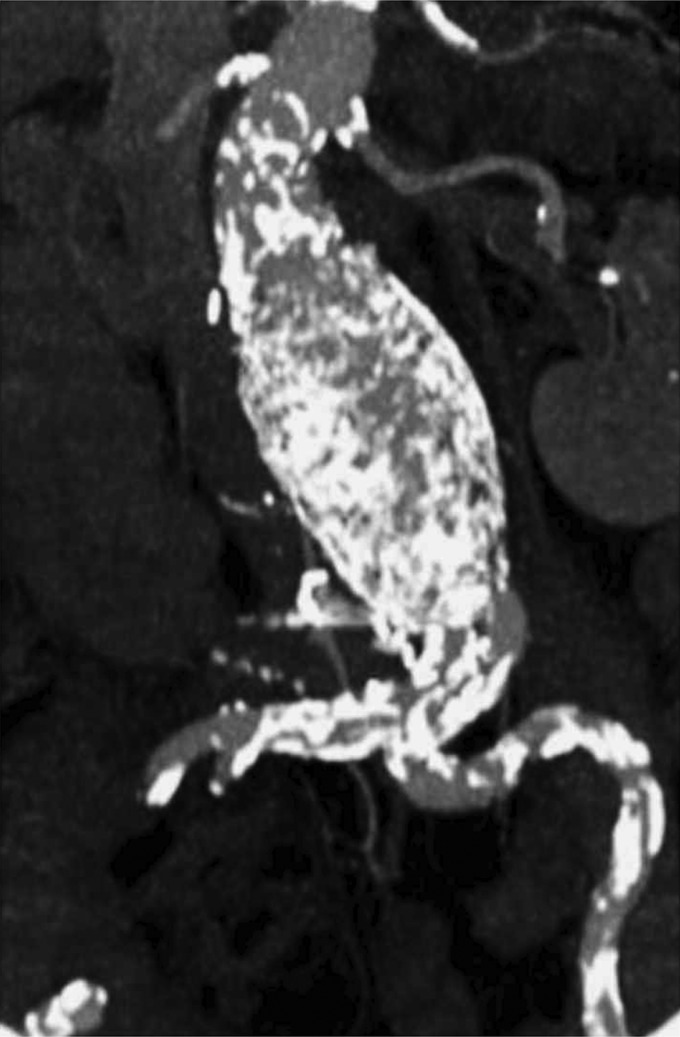
Figure 1:
MIP image showing a huge calcification of femoral arteries and a tortuous iliac arteries.
Figure 2:
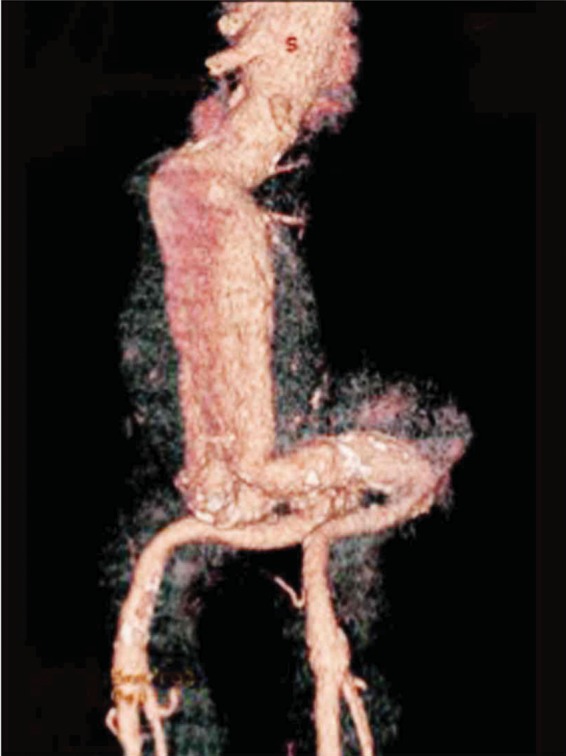
Volume rendering image showed a tortuous iliac artery with a 180° angulation between common and external iliac arteries.
DISCUSSION
Despite recent advances in the perioperative management of patients operated on for AAA, morbidity and mortality still remain high because of cardiovascular complications especially in the case of large diameter aneurysms [1]. The asymptomatic coronary artery disease can reach a prevalence of 20% in patients of more than 75 years old [2]. Aortic cross-clamping can increase the left ventricle inotropism and might lead to tachycardia, increasing the myocardial oxygen needs. In undiagnosed coronaropathic patients, this phenomenon may induce myocardial infarction. Pocar et al. [3] reported the first application of a totally axillo-femoral bypass to support cardiac function on the beating heart during resection of a giant AAA and ischaemic cardiomyopathy. It appeared to us that the use of ECMO was more advantageous than the total cardiopulmonary bypass, because of reduced heparinization and the absence of air/blood contact inducing inflammatory reactions. In our two cases, the goal was to reduce the cardiac overloading during aortic cross-clamping time, by deloading the right heart. Consequently, the left ventricular inotropism was decreased as well. TEO was very useful for monitoring the effective left ventricle output by the mean of the velocity time integral that permitted ECMO flow adaptation. The collaboration between anaesthesiologists and perfusionists is the key point of real-time ECMO flow's adaptation to the cardiac output and feeling. In addition, TEO can detect wall motion alterations which are the first sign of myocardial ischaemia. Concerning our second patient with significant aortic regurgitation, TEO showed that the aortic insufficiency remained stable and the flow rate provided by the ECMO system through the left subclavian artery was distributed mainly to the thoracic aorta, without left ventricular dilatation [4]. Renal function damage after aortic cross-clamping is another issue. Renal ischaemia following supra renal aorta cross-clamping has been widely incriminated in post-operative renal dysfunction leading to dialysis. Concerning infrarenal cross-clamping, perioperative low-flow and inflammatory factors' release due to ischaemia reperfusion injury could be the main mechanisms of the nephronic pool deterioration [5]. In the case of huge aneurismal sacs with non-thrombosed lumbar arteries, blood loss occurring while opening the aneurismal sac could lead to severe hypovolemia and low cardiac output. ECMO support can be a useful tool because it permits a rapid filling through the circuit which immediately corrects the hypovolemia.
Moreover, laparotomy-induced heat loss can be hazardous for metabolic and hemodynamic stabilization. Temperature stability can be maintained thanks to a heat exchanger which allows warming of transfused blood, avoiding metabolic disorders due to standard transfusion [6].
CONCLUSION
AAA surgery with ECMO support might be an alternative for high cardiac risk patients not amenable to conventional open repair or EVAR. This strategy requires close collaboration between vascular surgeons and anaesthesiologists and should be further evaluated by other studies.
Conflict of interest: none declared.
REFERENCES
- 1.Schouten O, Kok NF, Hoedt MT, van Laanen JH, Poldermans D. The influence of aneurysm size on perioperative cardiac outcome in elective open infrarenal aortic aneurysm repair. J Vasc Surg. 2006;44:435–41. doi: 10.1016/j.jvs.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 2.Welten GM, Schouten O, van Domburg RT, Feringa HH, Hoeks SE, Dunkelgrün M, et al. The influence of aging on the prognostic value of the revised cardiac risk index for postoperative cardiac complications in vascular surgery patients. Eur J Vasc Endovasc Surg. 2007;34:632–8. doi: 10.1016/j.ejvs.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Pocar M, Moneta A, Passolunghi D, Donatelli F. Femoro-axillary cardiopulmonary bypass for giant abdominal aortic aneurysm repair prior to staged cardiac operation for ischaemic cardiomyopathy. Eur J Cardiothorac Surg. 2010;37:972–4. doi: 10.1016/j.ejcts.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Bashore TM. Afterload reduction in chronic aortic regurgitation: it sure seems like a good idea. J Am Coll Cardiol. 2005;45:1031–3. doi: 10.1016/j.jacc.2004.12.052. [DOI] [PubMed] [Google Scholar]
- 5.Johnston K. Multicenter prospective study of non-ruptured abdominal aortic aneurysm. Part II. Variables predicting morbidity and mortality. J Vasc Surg. 1989;9:437–47. doi: 10.1067/mva.1989.vs0090437. [DOI] [PubMed] [Google Scholar]
- 6.Rogiers P, Sun Q, Dimopoulos G, Tu Z, Pauwels D, Manhaeghe C, et al. Blood warming during hemofiltration can improve hemodynamics and outcome in ovine septic shock. Anesthesiology. 2006;104:1216–22. doi: 10.1097/00000542-200606000-00017. [DOI] [PubMed] [Google Scholar]