Abstract
Hypoxia-inducible transcription factors (HIF) protect cells against oxygen deprivation, and HIF stabilization before ischemia mitigates tissue injury. Because ischemic acute kidney injury (AKI) often involves the thick ascending limb (TAL), modulation of HIF in this segment may be protective. Here, we generated mice with targeted TAL deletion of the von Hippel-Lindau protein (Vhl), which mediates HIF degradation under normoxia, using Tamm-Horsfall protein (Thp)-driven Cre expression. These mice showed strong expression of HIF-1α in TALs but no changes in kidney morphology or function under control conditions. Deficiency of Vhl in the TAL markedly attenuated proximal tubular injury and preserved TAL function following ischemia-reperfusion, which may be partially a result of enhanced expression of glycolytic enzymes and lactate metabolism. These results highlight the importance of the thick ascending limb in the pathogenesis of AKI and suggest that pharmacologically targeting the HIF system may have potential to prevent and mitigate AKI.
Acute kidney injury (AKI) is associated with high morbidity and mortality.1 Tubular ischemia is believed to be centrally involved in the initiation and establishment of AKI because intrarenal oxygen tensions are low and further reduced by hypoperfusion.2,3 Tubular injury in AKI has mainly been found in two segments located in the outer renal medulla: the late proximal tubule (S3 segment) and the thick ascending limb (TAL) of Henle's loop.4–6 Vulnerability of both segments has partially been explained by their low capacity for anaerobic glycolysis, although TAL cells have comparatively higher levels of glycolytic gene expression than proximal tubules.2,6,7 The relative importance of both segments in the pathogenesis of AKI remains controversial and may depend on the models used.4 Inhibition of sodium reabsorption and hence oxygen consumption in TALs using furosemide reduced TAL injury in some,8–10 but not all experimental settings11–15 and failed to reduce the incidence of AKI in humans.16
Hypoxia-inducible transcription factors (HIF) mediate cellular protection against reduced oxygen delivery by regulating cellular pathways and functions, including glycolysis, angiogenesis, erythropoiesis, and cell survival decisions.17–19 The oxygen-regulated HIFα subunit is continuously synthesized in cells but rapidly degraded in the presence of oxygen after hydroxylation by specific prolyl-hydroxylases (PHDs). Hydroxylated HIF is targeted for proteasomal degradation after binding to the von Hippel-Lindau (VHL) E3 ubiquitin ligase.20–23 In the kidney, baseline HIF expression is low, but both isoforms, HIF-1α and HIF-2α, increase rapidly in response to hypoxia, with HIF-1α occurring in tubular and HIF-2α in peritubular and glomerular cells.24 Tubular HIF-1α accumulation corresponds well to expression of the three PHDs and the factor inhibiting HIF (FIH-1) in distal tubular cells and in the medulla.25,26
Previous studies have shown that systemic hypoxia or pharmacologic approaches to stabilize HIF can protect the kidney against subsequent ischemic or toxic injury.27–31 In addition, Vhl knockout strategies have been successfully applied to stabilize HIF genetically32 and acute ubiquitous inactivation of Vhl has recently been shown to ameliorate ischemic AKI.33 All of these approaches have so far not allowed defining the role of specific parts of the nephron in AKI protection. To test for effects of selective distal tubular Vhl knockout in the course of experimental AKI, we generated transgenic mice with conditional knockout of Vhl in TALs using a Cre-recombinase driven by the Tamm-Horsfall protein (Thp) promoter, which is selectively expressed in TALs.
RESULTS
Generation of Mice with TAL-Specific Inactivation of Vhl
To investigate the functional consequences of Vhl deletion in TAL segments, we first generated mice, which express the Cre-recombinase gene under the control of the murine Thp-promoter (Figure 1A). Founder mice were generated by pronuclear injections of the Thp-Cre transgene and screened for transgene integration by Southern blot. Seven transgenic mice were intercrossed with ROSA26-LacZ reporter mice and kidneys were examined for β-galactosidase activity indicating Cre-mediated recombination. Reverse transcription-polymerase chain reaction (RT-PCR) showed expression of Cre-recombinase mRNA only in the kidneys of Thp-Cre+ mice and in no other organs, demonstrating kidney specificity of the Thp-promoter construct (Figure 1B). β-Galactosidase signals were only seen in TAL segments but in no other regions of the kidney, other visceral organs investigated, or ROSA26-LacZ mice lacking the Thp-Cre transgene (not shown). Line 27, which showed the highest efficiency for recombination and full specificity to the TAL, was used for all subsequent experiments.
Figure 1.
Generation of mice deficient for Vhl in thick ascending limbs of Henle's loop. (A) Scheme of mouse Tamm-Horsfall protein (Thp) promoter Cre-recombinase DNA construct. (B) Detection of Cre mRNA by RT-PCR in different organs of mice heterozygous for the Thp-Cre construct. The gel is representative of results obtained from offsprings derived from line 27 Thp-Cre mice. (C) RT-PCR analysis of genomic DNA isolated from different organs of Thp-Cre+;Vhl+f/+f mice with primers amplifying the nonrecombined (2-lox) and the recombined (1-lox) Vhl conditional allele. Lanes were run on the same gel but were noncontiguous. (D) Deletion efficiency of the loxP-flanked Vhl allele (Cre+; n = 3) in microdissected TAL cells was determined by real-time PCR in comparison to the WT allele (Cre−; n = 3). Representative consecutive kidney sections (magnification from the corticomedullary junction) displaying immunohistochemistries for HIF-1α (E) and THP (F) of 3-month-old Vhl knockout mice. Original magnification, ×400.
Thp-Cre mice were then crossed with mice, homozygous for a conditional loxP-flanked Vhl allele,34 to generate mice which are deficient for Vhl only in TAL cells (Thp-Cre+;Vhl+f/+f). RT-PCR of genomic DNA showed recombination of the Vhl conditional allele only in kidneys but in no other organs of Thp-Cre+;Vhl+f/+f mice (Figure 1C). Quantitative PCR demonstrated Vhl deletion in 93.3 ± 1.5% of microdissected TAL segments (Figure 1D).
HIFα Is Induced by Targeted Deletion of Vhl in the TAL
Inactivation of VHL is known to result in constitutive HIF stabilization and increased expression of HIF target genes.20 Thp-Cre+;Vhl+f/+f (knockout) mice showed marked nuclear accumulation of HIF-1α in tubular epithelial cells of the renal cortex and outer medulla (Figure 1E), colocalizing with THP (Figure 1F) in consecutive kidney sections. This indicated selective genetic inactivation of Vhl and consecutive HIF-1α stabilization in TALs.
In comparison, Thp-Cre−;Vhl+f/+f (wild type, WT) mice showed only weak HIF-1α signals in collecting duct cells (Supplemental Figure 1), which corresponded to HIF expression patterns seen in normal C57BL/6 mice (not shown). No significant HIF-2α signals were obtained in TAL cells of Vhl knockout mice at the age of 3 months (not shown).
Vhl Inactivation in the TAL Does Not Alter Renal Morphology
Microscopic analysis of kidneys from mice with TAL-specific Vhl deficiency showed normal morphology without any obvious differences to C57BL/6 or WT littermates (not shown). Previous studies demonstrated that inactivation of Vhl in proximal tubules can result in renal cyst development in aged mice35 or after combined deletion of the tumor suppressor PTEN.36 TAL-restricted Vhl inactivation in our study did not lead to renal cysts or renal cell carcinomas in mice up to an age of 18 months.
Because Vhl deletion can promote renal fibrosis in aged mice and enhance tubulointerstitial fibrosis in chronic kidney injury models,37,38 we analyzed collagen and matrix deposition and profibrotic gene expression. Semiquantitative assessment of Sirius Red stained kidney sections revealed significant age-related accumulation of interstitial collagen in 18 months compared with 3-month-old animals, but WT and Vhl knockout animals did not differ at either time point (Supplemental Figure 2A). Consistently, mRNA expression of the α2-chain of type-1 collagen (Col1a2), fibroblast-specific protein-1 (Fsp1), and TGF-β1 (Tgfb1) in Vhl deficient kidneys were comparable to WT mice at 3 and 18 months (Supplemental Figure 2B).
As Vhl inactivation and HIF stabilization is also known to induce angiogenesis in different organs and to be associated with highly vascularized tumors,39,40 we stained peritubular capillaries with an antibody against CD34 and quantified signals in the renal cortex and outer medulla. There were no differences in CD34 staining observed in 3-month-old Vhl knockout and WT mice (Supplemental Figure 3A), despite increased mRNA expression of vascular endothelial growth factor A (Vegfa) in microdissected TALs of Vhl deficient mice (Supplemental Figure 3B). We also did not find any vascular ectasias or hemangiomas in both groups of mice.
Deletion of Vhl in the TAL Does Not Alter Renal Function
Biologic parameters of Vhl knockout mice were similar to age- and gender-matched WT animals (Table 1). In particular, kidney/body weight ratios, kidney function, and urine-concentrating ability were not significantly different in WT and knockout animals. To test for differences in tubular function, the diuretic response to furosemide was evaluated as an index of TAL-specific activity of the sodium-potassium-chloride cotransporter 2 (Nkcc2).41 Single bolus injection of furosemide reduced urine osmolality from 1158 ± 76 to 357 ± 3 mosmol/L in WT and from 1469 ± 134 to 346 ± 6 mosmol/L in knockout mice, whereas urinary flow increased from 3.8 ± 0.7 μl/min to a maximum of 26.7 ± 0.9 μl/min in WT and from 3.5 ± 0.5 to 27.8 ± 1.5 μl/min in Vhl deficient mice (Figure 2) and were not different at any time point between WT and knockout animals. Thus, TAL-specific sodium reabsorption appeared not to be affected by Vhl deficiency.
Table 1.
Biologic parameters in WT (Cre−; n = 10 to 27) and Vhl knockout mice (Cre+; n = 10 to 34)
| Unit | Cre− |
Cre+ |
P | |||
|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | |||
| Parameter | ||||||
| 2-kidney/body weight | % | 1.10 | 0.03 | 1.04 | 0.03 | NS |
| water intake | ml/24 h | 5.0 | 0.4 | 4.8 | 0.3 | NS |
| food intake | g/24 h | 2.8 | 0.2 | 2.7 | 0.2 | NS |
| Blood count | ||||||
| hemoglobin | g/dl | 15.8 | 0.3 | 15.5 | 0.3 | NS |
| hematocrit | % | 48.3 | 0.8 | 47.5 | 0.8 | NS |
| Plasma | ||||||
| Na+ | mmol/L | 145.8 | 0.3 | 145.8 | 0.4 | NS |
| K+ | mmol/L | 3.8 | 0.1 | 4.0 | 0.2 | NS |
| creatinine | mg/dl | 0.10 | 0.00 | 0.10 | 0.01 | NS |
| urea | mg/dl | 46.77 | 1.79 | 51.44 | 2.43 | NS |
| glucose | mg/dl | 151.9 | 3.9 | 148.9 | 7.9 | NS |
| osmolality | mosmol/kg | 307.6 | 1.1 | 308.2 | 1.5 | NS |
| aldosterone | pg/ml | 245.0 | 38.6 | 233.8 | 38.3 | NS |
| Urine | ||||||
| volume | ml/24 h | 1.0 | 0.2 | 1.0 | 0.1 | NS |
| creatinine | mg/dl | 22.6 | 3.4 | 24.2 | 2.2 | NS |
| Na+ | mmol/L | 119.44 | 11.76 | 127.88 | 24.07 | NS |
| K+ | mmol/L | 222.58 | 32.05 | 226.89 | 27.87 | NS |
| Cl− | mmol/L | 152.55 | 20.27 | 153.47 | 17.41 | NS |
| Ca2+ | mmol/L | 2.05 | 0.29 | 2.09 | 0.30 | NS |
| osmolality | mosmol/kg | 1380 | 426 | 1441 | 226 | NS |
| protein | mg/mg creatinine | 47.8 | 3.6 | 47.0 | 4.4 | NS |
NS, not significant.
Figure 2.
Diuretic response to furosemide was not affected by Vhl deficiency. Urine osmolality (A) and urine flow rates (B) after intraperitoneal administration of 40 mg/kg furosemide in WT (Cre−; n = 19) and Vhl knockout mice (Cre+; n = 16).
Deletion of Vhl in TALs Does Not Induce Erythropoiesis
Previous investigations in humans and animal models have shown that loss of VHL or PHD2 and HIF stabilizing mutations can induce erythropoetin (EPO) synthesis, leading to polycythemia.39 High levels of EPO have been associated with protective effects in diverse organs including the kidney and brain.42 However, in 6-month-old Vhl knockout mice hemoglobin and hematocrit levels were not different from WT (Table 1). Consistently, Epo mRNA expression in total kidney tissue determined by real-time PCR was not significantly different in Vhl deficient and WT mice (not shown).
Vhl Deletion in TALs Results in Enhanced Expression of HIF Target Genes
To test for HIF-target gene induction, we microdissected mouse cortical nephron segments. Identity and homogeneity of tubular preparations were verified by segment specific markers (Supplemental Figure 4). Next, we analyzed the segment-specific RNA expression of glycolytic genes, which are known to be HIF targets. In TAL segments of Vhl deficient mice mRNA transcripts of glucose transporter 1 (Glut1), phosphoglycerate kinase 1 (Pgk1), pyruvate dehydrogenase kinase 1 (Pdk1), and lactate dehydrogenase A (Ldha) were significantly upregulated compared with TAL isolates from WT mice. Induction of target genes was only seen in TAL, but not in proximal tubule isolates of knockout mice, again confirming regional specificity of Vhl deletion (Figure 3A).
Figure 3.
Loss of Vhl in TAL cells induced HIF target genes and increased anaerobic glucose utilization. (A) mRNA expression of HIF target genes Glut1, Pgk1, Pdk1, and Ldha in microdissected proximal convoluted tubules (PCT) and TALs of WT (Cre−; n = 4) and Vhl deficient mice (Cre+; n = 7) analyzed by real-time PCR. Lactate (B) and ATP (C) concentrations in total kidney tissue of WT (Cre−; n = 9) and Vhl knockout animals (Cre+; n = 9) after abdominal laparotomy (left panel) and 5 minutes after warm ischemia (right panel), simulated by incubation in 37°C water bath. *, P < 0.05, Cre− versus Cre+.
Metabolic Shift toward Anaerobic Glycolysis in Kidneys of Vhl Deficient Thp-Cre Mice
Pdk1 regulates the entry of glycolytic intermediates into the TCA cycle and reduces glucose oxidation and mitochondrial oxygen consumption by enhancing anaerobic glycolysis.43 To determine functional consequences of Pdk1 upregulation in Vhl knockout mice, we measured lactate and ATP concentrations in total kidney tissue. Lactate concentrations in Vhl deficient kidneys were significantly higher than in matched WT kidneys (P = 0.0017) under control conditions (Figure 3B), indicating an increase in anaerobic glycolytic rate in knockout mice. After 5 minutes of warm ischemia lactate concentrations increased in both groups, but in Vhl deficient kidneys lactate concentration was still higher than in WT (P = 0.006). Tissue levels of ATP were similar in both groups under control conditions. In postischemic kidneys of knockout mice ATP content tended to be higher than that in WT, but the difference did not gain statistical significance in total kidney extracts (Figure 3C).
Vhl Deletion in TALs Ameliorates Ischemic Kidney Injury
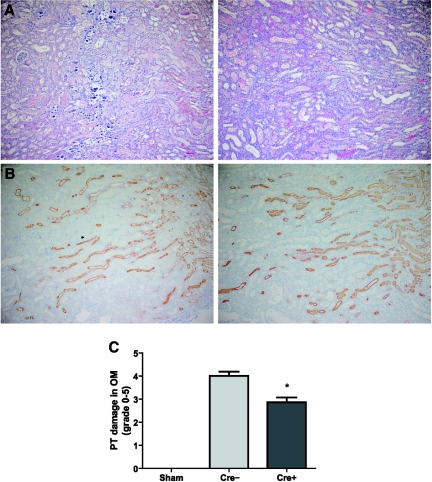
To determine effects of Vhl deletion in TALs in experimental AKI, we performed bilateral renal ischemia for 25 minutes followed by 72 hours of reperfusion. Generally, morphologic evaluation of H&E stained postischemic kidney specimens predominantly detects proximal tubular injury in the outer medulla; other tubules, including the TAL, show more limited injury, which is difficult to quantify in morphologic analysis.44 In Vhl knockout mice morphologic damage of proximal tubules in the outer medulla was significantly reduced in comparison to WT animals (score 2.84 ± 0.21 versus 4.00 ± 0.19; P = 0.0002) (Figure 4, A and C). This included the degree of tubular necrosis as well as the type of injury; that is, tubular calcification was only seen in WT animals. Evaluation of THP stained kidney sections 3 days after ischemia led to the conclusion that in both groups—in spite of the extensive necrosis in the adjacent proximal tubules—the TAL was relatively preserved, at least from the standpoint of THP maintenance (Figure 4B). Kidney parenchymas of sham-operated WT and knockout mice were morphologically indistinguishable from not operated animals (not shown).
Figure 4.
Kidney histomorphology was significantly ameliorated in knockout mice after renal ischemia-reperfusion. Representative microphotographs of H&E staining (A) and immunostaining for THP (B) in kidney sections (magnification from outer medulla) of WT (left) and Vhl deficient mice (right) 3 days after ischemia-reperfusion. Note that the calcification was present only in the WT group. Original magnification, ×100. (C) Semiquantitative analysis of proximal tubular (PT) damage in outer medulla (OM) of WT (Cre−; n = 18) and Vhl knockout mice (Cre+; n = 22) in comparison to sham-operated Cre− (n = 4) and Cre+ mice (n = 4). *, P < 0.05, Cre− versus Cre+.
Three days after renal ischemia plasma creatinine increased to 0.77 ± 0.11 mg/dl and plasma urea to 457.87 ± 59.88 mg/dl in WT animals, whereas these levels were approximately 60% lower in Vhl knockout mice (0.30 ± 0.03 and 186.86 ± 18.98 mg/dl, respectively; P = 0.0006 for creatinine and P = 0.0004 for urea) (Figure 5A). Kidney function in sham-operated WT and knockout animals was not different from unoperated mice.
Figure 5.
Kidney function was significantly improved in knockout mice after renal ischemia-reperfusion. (A) Plasma creatinine (left panel) and urea (right panel) 3 days after kidney ischemia in WT (Cre−; n = 19) and Vhl knockout mice (Cre+; n = 19). Both sham-operated WT (n = 3) and Vhl deficient mice (n = 3) served as controls. (B) mRNA expression of the renal biomarkers Ngal (left) und Kim1 (right) measured by real-time PCR in postischemic total kidney extracts of WT (n = 5) and Vhl deficient mice (n = 5) in comparison to sham-operated Cre− (n = 3) and Cre+ mice (n = 2). (C) Time course of urine osmolality after intraperitoneal administration of 40 mg/kg furosemide in WT (Cre−; n = 5) and Vhl deficient (Cre+; n = 5) mice 3 days after renal ischemia-reperfusion. (D) Proliferating cells per TAL cross section in postischemic WT (Cre−; n = 8) and Vhl knockout kidneys (Cre+; n = 8) as well as sham-operated Cre− (n = 2) and Cre+ kidneys (n = 2). Immunhistochemistry for PCNA and THP was performed on consecutive sections. (E) Tubular apoptosis in the outer medulla of postischemic WT (Cre−; n = 5) and knockout mice (Cre+; n = 5) assessed by TUNEL staining. Sham-operated Cre− (n = 2) and Cre+ kidneys (n = 1) served as controls. *, P < 0.05, Cre− versus Cre+.
To discriminate tubular segments mostly affected by renal ischemia, we analyzed mRNA expression of the biomarkers Ngal (expressed in TALs and collecting ducts),45 and Kim1 (expressed in proximal tubules).46,47 In kidneys of Vhl deficient mice the postischemic increase of Ngal mRNA was significantly reduced by 87% compared with WT (P = 0.03), potentially indicating less damage of distal tubular segments. In contrast, Kim1 was equivalently upregulated by ischemia-reperfusion in both groups (Figure 5B) and thus did not reflect improved morphologic scoring of Vhl knockout animals.
To test for functional differences of postischemic kidneys, we measured urine osmolality in response to furosemide after ischemia-reperfusion injury. Both groups showed a decreased osmolality compared with unoperated animals (Figure 2), but furosemide further reduced osmolality only in knockout mice (Figure 5C), indicating better preservation of TAL concentrating function in Vhl deficient postischemic kidneys.
In postischemic kidneys cellular proliferation was evaluated by immunostaining for proliferating cell nuclear antigen (PCNA). Vhl deficient mice presented a significant increase in PCNA-positive cells per TAL cross section compared with WT (P = 0.03) (Figure 5D).
To examine whether Vhl deletion had an effect on postischemic apoptosis, we used TUNEL staining to identify apoptotic tubular cells. Apoptotic cells were most numerous in the outer medulla of both WT and knockout mice without significant difference (Figure 5E).
Postischemic Inflammation Is Unchanged in Mice Deficient for Vhl in TAL
Because infiltration of inflammatory cells is an important event in AKI,48 we assessed inflammatory responses in postischemic kidneys and quantified renal cell infiltrates and cytokine levels. H&E staining showed comparable levels of inflammatory infiltrates in the kidneys of WT and Vhl knockout mice (Figure 6A). F4/80 positive macrophages were mainly detected in the interstitial area of the outer medulla in both WT and knockout mice, without significant difference between both groups (Figure 6B). RT-PCR analysis revealed similar upregulation of Tnfa, II1b, II6, and Mcp1 in renal tissues of WT and knockout mice (Figure 6C). Thus, the inflammatory reaction in postischemic kidneys seemed not to be influenced by Vhl deletion in TALs.
Figure 6.
The inflammatory reaction in postischemic kidneys was not affected by Vhl deletion. (A) Semiquantitative analysis of inflammatory cell infiltration in H&E stained kidney sections of WT (Cre−) and Vhl knockout (Cre+) mice after renal ischemic (Cre− n = 18, and Cre+ n = 22) or sham (Cre− n = 4, and Cre+ n = 4) surgeries. (B) Infiltration with F4/80-positive macrophages in postischemic kidneys of WT (Cre−; n = 6) and Vhl deficient mice (Cre+; n = 6) as well as sham-operated Cre− (n = 2) and Cre+ mice (n = 2). (C) mRNA levels of the inflammatory cytokines Tnfα, Il1ß, Il6, and Mcp1 in total kidney extracts of WT (Cre−; n = 5) and Vhl knockout mice (Cre+; n = 5) as measured by real-time PCR. Sham-operated Cre− (n = 2) and Cre+ kidneys (n = 2) served as controls.
Glycolytic Gene Expression Is Preserved in Postischemic Kidneys Mice with Vhl Deletion in TAL
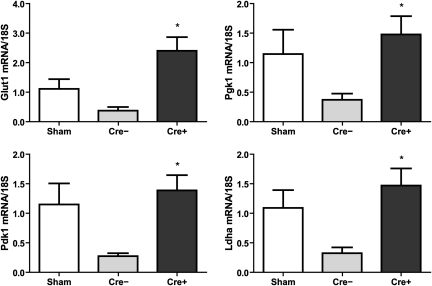
Because HIF tightly regulates glycolytic flux and energy metabolism, we next analyzed glycolytic enzyme mRNA levels in postischemic kidneys. Glut1, Pgk1, Pdk1, and Ldha mRNA were downregulated 3 days after renal ischemia-reperfusion in WT kidneys compared with sham-operated mice. In contrast, the expression of these enzymes was preserved in Vhl deficient kidneys compared with sham-operated controls (Figure 7). Postischemic preservation of glycolytic gene expression may therefore contribute to preserved ATP generation as a prerequisite for cell survival.
Figure 7.
Expression of glycolytic enzymes was preserved in postischemic kidneys of knockout mice. Relative mRNA expression of the glycolytic enzymes Glut1, Pgk1, Pdk1, and Ldha in total kidney extracts of WT (Cre−; n = 6) and Vhl knockout (Cre+; n = 6) mice 3 days after ischemia-reperfusion as well as sham-operated Cre− (n = 2) and Cre+ (n = 2) mice. *, P < 0.05, Cre− versus Cre+.
DISCUSSION
Tubular damage is a central component of AKI, but how it translates into a reduction in kidney function is far from clear. This study demonstrates that (1) targeted deletion of Vhl in the TAL associated with increased HIF-mediated gene transcription conveys substantial protection against ischemic injury and that (2) protection of this comparatively small nephron segment translates into a marked preservation of whole kidney function. Although these findings complement previous data showing that systemic and nonselective cellular stabilization of HIF protects against ischemic29,31 and toxic renal injury,28,30 this study extends these results by demonstrating that Vhl deletion in a specific nephron segment is sufficient to achieve renal protection. Moreover, in contrast to previously published mice with cell type specific inactivation of Vhl,32 the phenotype of animals with Vhl deletion in TALs was surprisingly normal, indicating that Vhl pathology is context-dependent.
Although the TAL has long been considered to play a role in the pathogenesis of AKI, its sensitivity to damage and its role in causing impairment of renal function may vary depending on the experimental or clinical circumstances.6,49 In ischemia-reperfusion models some authors have postulated that proximal tubular injury plays a more significant role, whereas others have concluded on the basis of morphologic changes and functional parameters that TAL damage is critical.4 Notably, also in humans the distal tubular medullary segments seem to be more susceptible in AKI than proximal tubular segments.4 This study shows that selective Vhl deletion in TAL has major effects in ischemic AKI pathophysiology, providing direct experimental support for a key role of this segment. This complements data demonstrating that, conversely, selective induction of apoptosis in TAL cells is sufficient to induce severe AKI.50
In ischemia-reperfusion experiments we found a significant functional improvement in renal waste excretion and sensitivity to furosemide as well as increased postischemic proliferation capacities in mice with TAL-restricted Vhl deletion. Interestingly, these functional improvements were not associated with improved morphology of the TAL. THP staining suggested preserved structural integrity of the TAL in both groups and revealed no morphologic differences between animals with Vhl deletion and WT. This indicates that TAL injury of both groups and protective effects of the Vhl knockouts seem to rely more on molecular regulatory processes, which did not translate into microscopically visible morphologic alterations, which corresponds to previous findings.51 Recently, Paragas et al.52 observed that distal nephron generated Ngal predicted the onset and resolution of ischemic and nephrotoxic injuries, although in these models morphologic damage is also mainly confined to the proximal tubule. Functional preservation of the TAL in our studies was in fact associated with lower levels of Ngal. Intriguingly, in animals with Vhl deletion in the TAL morphologic scores of proximal tubular injury were improved. In contrast, the levels of Kim1, presumably originating from the proximal tubule, were comparable in both groups and thus did not reflect improved epithelial morphology. In general, it is not clear so far, whether and to what extent the increase of epithelium derived biomarkers in kidney tissues parallels differences in morphologic scoring and extent of injury. It is possible for example that dystrophic calcification, present only in WT mice, affected Kim1 generation. The pathways by which postischemic TAL activation supported proximal tubular cell integrity in Vhl knockout mice53 need further investigation.
Vhl deletion in TAL did not alter the inflammatory response to ischemia-reperfusion, possibly because the degree of inflammatory infiltrate is governed more by effects on the endothelium rather than tubular damage. Inhibiting the infiltration of T cells and macrophages has been shown to ameliorate the decline in renal function after ischemia-reperfusion,54 but our study implies that a reduction in the inflammatory response is not a prerequisite for protecting renal function.
The observation that an endothelial HIF-2α knockout mouse was more susceptible to renal injury55 has raised the possibility that enhancing HIF-2α activity in the endothelium could underlie the reported protective effects of HIF activators. Our experiments imply, however, that endothelial HIF induction is not required for protection via HIF activation.
Because HIF is a master switch of transcriptional regulation, many target genes could contribute to protection from hypoxic injury. EPO, as the prototype HIF target gene, has been reported to be protective in models of AKI56 and is a strong candidate for mediating protective effects of HIF stabilization. But in TAL-restricted Vhl deficient mice we found no evidence for increased production of EPO, consistent with the fact that EPO is controlled by HIF-2α in peritubular fibroblasts.57,58
Although we did not detect a reduction in sodium transport, which could have affected oxygen consumption, we did find evidence for increased energy generation through anaerobic glycolysis. Noteworthy, the TAL has a higher capacity for glycolysis than the proximal tubule, with higher expression of glycolytic enzymes.7 This expression was further augmented in Vhl knockout animals and was preserved after ischemia-reperfusion. We also found increased renal lactate concentrations in knockout animals, suggesting that a higher glycolytic capacity supported TAL function and permitted ATP-consuming transport processes and maintenance of the osmotic gradient in the renal medulla.59–61 We could not detect significant differences in ATP levels, but interpretation of ATP measurements in total kidney extracts is limited. Biju et al. previously underscored the importance of HIF for glycolysis to counteract hypoxia-induced cell death.62 The association of protective effects of the HIF/PHD system against ischemia and enhanced glycolytic rates has also been observed in skeletal muscles.63
The findings of this study have potential clinical implications. Prolyl-hydroxylase inhibitors, which can stabilize HIF in the presence of oxygen, are under clinical development64–66 and these drugs offer a novel opportunity for tissue protection. This study underscores the potential of this approach and suggests direct protective effects of these compounds on tubular cells beyond EPO induction and protection from endothelial damage. The study also emphasizes the importance of the TAL segment in the pathogenesis of AKI and suggests that this part of the nephron deserves further exploration as a cellular target for strategies to prevent and mitigate AKI.
CONCISE METHODS
All chemicals were purchased from Sigma (Taufkirchen, Germany), unless indicated otherwise.
Animals
Animal experiments were approved by the local institutional review board for the care of animal subjects and were performed in accordance with National Institutes of Health guidelines. Animals were hosted on a 12:12 hour light-dark cycle under constant temperature (24 ± 1°C) in standard cages, fed a standard diet, and had free access to tap water. To measure water intake and collect urine samples, mice were kept in metabolic cages for 24 hours.
Generation and Genotyping of Thp-Cre Mutant Mice
3034 bp from the 5′ region of the mouse Tamm-Horsfall protein (Thp) gene was amplified by PCR using the primers 5′-TCCCCGCGGCCAGAGATCCAAGTCTCCTTC-3′ and 5′-GGAAGATCTGGTCCAGTCACAAGTAAGTG-3′. The product was digested with SacII and BglII and used to replace the CMV promoter fragment in pOG231 (a gift from Stephen O'Gorman, Salk Institute, San Diego) so that the translation start site of mouse Thp was fused to Cre-recombinase. The mouse Thp promoter in the final construct was sequenced completely. The plasmid backbone was removed and DNA injected into fertilized zygotes.
Genotyping of mice carrying the loxP-flanked conditional alleles of Vhl was described previously.35 The primer sequence to detect Thp-Cre is specified in Supplemental Table 1.
DNA Analysis
DNA from tissue was extracted with peqGOLD Trifast (Peqlab, Erlangen, Germany) according to the manufacturer's protocol. Twenty-five nanograms of genomic DNA was amplified by PCR with primers specific for WT and floxed Vhl alleles.35 PCR products were visualized on 2.5% agarose gels containing 0.005% ethidium bromide. To determine relative gene deletion by Cre-mediated excision in TALs, copies of the floxed allele quantified by real-time PCR were compared with a WT allele as described previously.67
RNA Analysis
Total RNA from homogenized mouse tissues was isolated with peqGOLD Trifast and analyzed by reverse transcription (RT)- or real-time RT-PCR as indicated. One microgram of total RNA was reverse-transcribed with M-MuLV-reverse transcriptase (Fermentas, St. Leon-Rot, Germany) and amplified in SYBR Green/Rox Master Mix (Fermentas) with an ABI Prism 7000 Sequence Detection System (Applied Biosystems, Foster City, CA). mRNA expression levels were normalized to 18S rRNA using the ΔΔCt method. All primer sequences are listed in Supplemental Table 1.
Immunohistochemistry
Kidneys were fixed by perfusion or immersion with 4% paraformaldehyde in PBS (pH 7.4) and processed for paraffin embedding. Paraffin sections (2 to 4 μm) were incubated with the following primary antibodies: monoclonal mouse anti-human HIF-1α (α67, Novus Biologicals, 1:10,000), polyclonal rabbit anti-mouse Hif-2α (PM9, 1:10,000,68 goat anti-human THP (ICN Pharmaceuticals, 1:500), monoclonal rat anti-mouse F4/80 (clone CI:A3-1, AbD Serotec, 1:500), monoclonal rat anti-mouse CD34 (Novus Biologicals, 1:100), monoclonal mouse anti-human PCNA (clone PC10, Dako, 1:50), and polyclonal rabbit anti-human GLUT-1 (Alpha Diagnostics, 1:10,000). Biotinylated polyclonal rabbit anti-rat IgG and rabbit anti-goat IgG antibodies (Dako, Hamburg, Germany) were detected using the Vectastain Elite ABC Kit (Vector Laboratories). For signal amplification, a catalyzed signal amplification system (Dako) was used. Anti-PCNA immunostaining was performed using MOM staining kit (Vector Laboratories).
Renal Ischemia-Reperfusion Injury
Mice were anesthetized by isoflurane inhalation. After a midline abdominal incision, both renal pedicles were clamped for 25 minutes with atraumatic microvascular clips (FE 723 K, Aesculap, Tuttlingen, Germany) to induce ischemia, which was verified by the change of renal color. In the sham group, clamps were applied but immediately removed. During the period of ischemia the abdomen was sutured, and core body temperature was maintained constant at 36°C by placing the mice on water recirculating heating pads and covering with a thermal blanket. After removal of the clamps, the kidneys were inspected for restoration of blood flow, returning to their original color. Before closing the abdomen in two layers, buprenorphine (0.05 to 0.1 mg/kg) was administered intraperitoneally. Mice were sacrificed 3 days after reperfusion under deep isoflurane anesthesia. Blood was obtained by inferior vena cava puncture. Kidney tissues were divided to be either snap frozen for subsequent mRNA extraction or to be fixed in 3% neutral-buffered paraformaldehyde for paraffin embedding.
Morphologic Analysis and Histologic Scoring
Hematoxylin and eosin (H&E) stained kidney sections were evaluated by two nephropathologists independently in a blinded manner. The magnitude of proximal tubular epithelial cell loss, necrosis, intratubular debris, and tubular cast formation was scored based on the percentage of affected tubules at 400-fold magnification: 0, no damage; 1, 0 to 25%; 2, 25 to 50%; 3, 50 to 75%; 4, 75 to 100%; 5, 75 to 100% plus calcification.
Fibrosis was assessed in Sirius Red stained sections and scored as follows: 0, none; 1, weak; 2, moderate; and 3, severe staining per section.
Digital images of F4/80 stained sections at 400-fold magnification were superimposed on a grid, and the number of grid points overlapping dark brown F4/80 positive macrophages in cortex and outer medulla was counted for each field.
To assess vascularization CD34 stained endothelial areas of peritubular capillaries were quantified using ImageJ software (The National Institutes of Health, Bethesda, Maryland) at 400-fold magnification. Glomerular capillaries were not included.
To investigate proliferation after the ischemic renal injury, consecutive kidney sections were immunostained for PCNA and THP, respectively. The number of PCNA-positive nuclei in THP-stained tubules was counted per section, and counts were expressed as ratio to number of TAL tubules.
TUNEL Assay
Apoptotic cells were detected using an in situ cell death detection kit (Roche, Mannheim, Germany). The number of apoptotic cells was quantified using ImageJ software at 200-fold magnification. To confirm the occurrence of apoptosis, characteristic morphologic changes, including cell shrinkage and nuclear condensation, were detected by light microscopy.
Urine and Plasma Analyses
Sodium, potassium, chloride, calcium, creatinine, urea, and glucose were measured in plasma and/or urine on a Cobas Integra 800 autoanalyzer (Roche). Plasma aldosterone was analyzed using a commercially available RIA kit (Aldosterone MAIA, Radim). Measurements of hemoglobin and hematocrit were performed on a blood gas analyzer (ABL 700 series, Radiometer Trading Co., Copenhagen, Denmark). Urinary osmolality was determined by freezing point depression using the osmometer OM 801 (Vogel, Giessen, Germany). Urinary protein was assessed in 24-hour collected urine samples with Bradford's method.
Microdissection of Nephron Segments
Nephron segments were microdissected from mouse renal cortex as described by Vitzthum et al.69 Tubular segments were identified by their morphologic appearance. Collected tubules were dissolved in 400 μl of guanidine thiocyanate solution (4 mol/L) and total RNA was extracted. Quantitative real-time PCR amplification was done with primers sets as indicated in Supplemental Table 1. Identity and homogeneity of proximal tubular and TAL cell preparations were verified by sodium-phosphate cotransporter 2a (Npt2a) and sodium-glucose cotransporter 2 (Sglt2) for proximal tubules as well as Nkcc2 and Thp mRNA detection for TAL segments.
Furosemide-Response Test
To test for TAL-specific urine concentrating abilities, furosemide (40 mg/kg body wt) together with 1 ml of glucose 5% for volume replacement was injected intraperitoneally. Then spot urine samples were continuously gathered over a time period of 3 hours and each sample was analyzed for osmolality.
Metabolite Assay
Under deep isoflurane anesthesia, kidneys were removed 40 seconds after median laparotomy as standardization (right) or subjected to consecutive 5 minutes of warm ischemia in a warm water bath at 37°C (left). Then kidneys were instantaneously snap-frozen in liquid nitrogen and stored at −70°C until further processing. Frozen kidney tissue was immersed in ice cold 8% perchloric acid in 40% (vol/vol) ethanol and homogenized with an Ultra-Turrax homogenizer followed by centrifugation for 10 minutes at 10,000 rpm. The supernatant was neutralized with 3.75 M K2CO3 containing 0.5 M triethanolamine hydrochloride. Lactate levels were measured in a Cobas Integra 800 autoanalyzer. ATP content was determined using an ATP bioluminescence assay kit (Roche).
Statistical Analysis
All data are expressed as means ± SEM. Two groups were compared using two-tailed, unpaired t test (Prism software; GraphPad, San Diego). Significance level was defined as P < 0.05. As sham-operated WT and Vhl knockout mice did not differ in morphologic and functional parameters, data were pooled for statistical analysis.
DISCLOSURES
None.
Supplementary Material
Acknowledgments
This work was supported by grants from the Deutsche-Forschungsgemeinschaft (SFB 423, TP A14) and Cancer Research UK. We thank Andrea Dengler, Hans Fees, Marcela Loza, Miriam Reutelshöfer, Brigitte Rogge, Barbara Teschemacher, and Reiko Ueki for excellent technical assistance.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
REFERENCES
- 1. Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML: Incidence and outcomes of acute kidney injury in intensive care units: A Veterans Administration study. Crit Care Med 37: 2552–2558, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Brezis M, Rosen S: Hypoxia of the renal medulla–its implications for disease. N Engl J Med 332: 647–655, 1995 [DOI] [PubMed] [Google Scholar]
- 3. Eckardt KU, Bernhardt WM, Weidemann A, Warnecke C, Rosenberger C, Wiesener MS, Willam C: Role of hypoxia in the pathogenesis of renal disease. Kidney Int Suppl: S46–S51, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Heyman SN, Rosenberger C, Rosen S: Experimental ischemia-reperfusion: Biases and myths-the proximal vs. distal hypoxic tubular injury debate revisited. Kidney Int 77: 9–16, 2010 [DOI] [PubMed] [Google Scholar]
- 5. Heyman SN, Shina A, Brezis M, Rosen S: Proximal tubular injury attenuates outer medullary hypoxic damage: studies in perfused rat kidneys. Exp Nephrol 10: 259–266, 2002 [DOI] [PubMed] [Google Scholar]
- 6. Lieberthal W, Nigam SK: Acute renal failure. I. Relative importance of proximal vs. distal tubular injury. Am J Physiol 275: F623–F631, 1998 [DOI] [PubMed] [Google Scholar]
- 7. Ross BD, Espinal J, Silva P: Glucose metabolism in renal tubular function. Kidney Int 29: 54–67, 1986 [DOI] [PubMed] [Google Scholar]
- 8. Aravindan N, Shaw A: Effect of furosemide infusion on renal hemodynamics and angiogenesis gene expression in acute renal ischemia/reperfusion. Ren Fail 28: 25–35, 2006 [DOI] [PubMed] [Google Scholar]
- 9. Brezis M, Rosen S, Silva P, Epstein FH: Transport activity modifies thick ascending limb damage in the isolated perfused kidney. Kidney Int 25: 65–72, 1984 [DOI] [PubMed] [Google Scholar]
- 10. Patak RV, Fadem SZ, Lifschitz MD, Stein JH: Study of factors which modify the development of norepinephrine-induced acute renal failure in the dog. Kidney Int 15: 227–237, 1979 [DOI] [PubMed] [Google Scholar]
- 11. Aydin G, Okiye SE, Zincke H: A comparative study of several agents alone and combined in protection of the rodent kidney from warm ischaemia: Methylprednisolone, propranolol, furosemide, mannitol, and adenosine triphosphate-magnesium chloride. Urol Res 11: 105–109, 1983 [DOI] [PubMed] [Google Scholar]
- 12. Epstein FH: Oxygen and renal metabolism. Kidney Int 51: 381–385, 1997 [DOI] [PubMed] [Google Scholar]
- 13. Lewis RM, Rice JH, Patton MK, Barnes JL, Nickel AE, Osgood RW, Fried T, Stein JH: Renal ischemic injury in the dog: Characterization and effect of various pharmacologic agents. J Lab Clin Med 104: 470–479, 1984 [PubMed] [Google Scholar]
- 14. Mason J, Kain H, Welsch J, Schnermann J: The early phase of experimental acute renal failure. VI. The influence of furosemide. Pflugers Arch 392: 125–133, 1981 [DOI] [PubMed] [Google Scholar]
- 15. Ufferman RC, Jaenike JR, Freeman RB, Pabico RC: Effects of furosemide on low-dose mercuric chloride acute renal failure in the rat. Kidney Int 8: 362–367, 1975 [DOI] [PubMed] [Google Scholar]
- 16. Solomon R, Werner C, Mann D, D'Elia J, Silva P: Effects of saline, mannitol, and furosemide to prevent acute decreases in renal function induced by radiocontrast agents. N Engl J Med 331: 1416–1420, 1994 [DOI] [PubMed] [Google Scholar]
- 17. Kaelin WG, Jr., Ratcliffe PJ: Oxygen sensing by metazoans: The central role of the HIF hydroxylase pathway. Mol Cell 30: 393–402, 2008 [DOI] [PubMed] [Google Scholar]
- 18. Semenza GL: HIF-1 inhibitors for cancer therapy: From gene expression to drug discovery. Curr Pharm Des 15: 3839–3843, 2009 [DOI] [PubMed] [Google Scholar]
- 19. Haase VH: Hypoxic regulation of erythropoiesis and iron metabolism. Am J Physiol Renal Physiol 299: F1–F13, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Maxwell PH, Wiesener MS, Chang GW, Clifford SC, Vaux EC, Cockman ME, Wykoff CC, Pugh CW, Maher ER, Ratcliffe PJ: The tumour suppressor protein VHL targets hypoxia-inducible factors for oxygen-dependent proteolysis. Nature 399: 271–275, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield CJ, Maxwell PH, Pugh CW, Ratcliffe PJ: Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science 292: 468–472, 2001 [DOI] [PubMed] [Google Scholar]
- 22. Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG, Jr.: HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science 292: 464–468, 2001 [DOI] [PubMed] [Google Scholar]
- 23. Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, Tian YM, Masson N, Hamilton DL, Jaakkola P, Barstead R, Hodgkin J, Maxwell PH, Pugh CW, Schofield CJ, Ratcliffe PJ: C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell 107: 43–54, 2001 [DOI] [PubMed] [Google Scholar]
- 24. Rosenberger C, Mandriota S, Jurgensen JS, Wiesener MS, Horstrup JH, Frei U, Ratcliffe PJ, Maxwell PH, Bachmann S, Eckardt KU: Expression of hypoxia-inducible factor-1alpha and −2alpha in hypoxic and ischemic rat kidneys. J Am Soc Nephrol 13: 1721–1732, 2002 [DOI] [PubMed] [Google Scholar]
- 25. Schödel J, Bohr D, Klanke B, Schley G, Schlotzer-Schrehardt U, Warnecke C, Kurtz A, Amann K, Eckardt KU, Willam C: Factor inhibiting HIF limits the expression of hypoxia-inducible genes in podocytes and distal tubular cells. Kidney Int 78: 857–867, 2010 [DOI] [PubMed] [Google Scholar]
- 26. Schödel J, Klanke B, Weidemann A, Buchholz B, Bernhardt W, Bertog M, Amann K, Korbmacher C, Wiesener M, Warnecke C, Kurtz A, Eckardt KU, Willam C: HIF-prolyl hydroxylases in the rat kidney: Physiologic expression patterns and regulation in acute kidney injury. Am J Pathol 174: 1663–1674, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Matsumoto M, Makino Y, Tanaka T, Tanaka H, Ishizaka N, Noiri E, Fujita T, Nangaku M: Induction of renoprotective gene expression by cobalt ameliorates ischemic injury of the kidney in rats. J Am Soc Nephrol 14: 1825–1832, 2003 [DOI] [PubMed] [Google Scholar]
- 28. Tanaka T, Kojima I, Ohse T, Inagi R, Miyata T, Ingelfinger JR, Fujita T, Nangaku M: Hypoxia-inducible factor modulates tubular cell survival in cisplatin nephrotoxicity. Am J Physiol Renal Physiol 289: F1123–F1133, 2005 [DOI] [PubMed] [Google Scholar]
- 29. Bernhardt WM, Campean V, Kany S, Jurgensen JS, Weidemann A, Warnecke C, Arend M, Klaus S, Gunzler V, Amann K, Willam C, Wiesener MS, Eckardt KU: Preconditional activation of hypoxia-inducible factors ameliorates ischemic acute renal failure. J Am Soc Nephrol 17: 1970–1978, 2006 [DOI] [PubMed] [Google Scholar]
- 30. Weidemann A, Bernhardt WM, Klanke B, Daniel C, Buchholz B, Campean V, Amann K, Warnecke C, Wiesener MS, Eckardt KU, Willam C: HIF activation protects from acute kidney injury. J Am Soc Nephrol 19: 486–494, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hill P, Shukla D, Tran MG, Aragones J, Cook HT, Carmeliet P, Maxwell PH: Inhibition of hypoxia inducible factor hydroxylases protects against renal ischemia-reperfusion injury. J Am Soc Nephrol 19: 39–46, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kapitsinou PP, Haase VH: The VHL tumor suppressor and HIF: Insights from genetic studies in mice. Cell Death Differ 15: 650–659, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Iguchi M, Kakinuma Y, Kurabayashi A, Sato T, Shuin T, Hong SB, Schmidt LS, Furihata M: Acute inactivation of the VHL gene contributes to protective effects of ischemic preconditioning in the mouse kidney. Nephron Exp Nephrol 110: e82–e90, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Haase VH, Glickman JN, Socolovsky M, Jaenisch R: Vascular tumors in livers with targeted inactivation of the von Hippel-Lindau tumor suppressor. Proc Natl Acad Sci U S A 98: 1583–1588, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rankin EB, Tomaszewski JE, Haase VH: Renal cyst development in mice with conditional inactivation of the von Hippel-Lindau tumor suppressor. Cancer Res 66: 2576–2583, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Frew IJ, Thoma CR, Georgiev S, Minola A, Hitz M, Montani M, Moch H, Krek W: pVHL and PTEN tumour suppressor proteins cooperatively suppress kidney cyst formation. Embo J 27: 1747–1757, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Higgins DF, Kimura K, Bernhardt WM, Shrimanker N, Akai Y, Hohenstein B, Saito Y, Johnson RS, Kretzler M, Cohen CD, Eckardt KU, Iwano M, Haase VH: Hypoxia promotes fibrogenesis in vivo via HIF-1 stimulation of epithelial-to-mesenchymal transition. J Clin Invest 117: 3810–3820, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kimura K, Iwano M, Higgins DF, Yamaguchi Y, Nakatani K, Harada K, Kubo A, Akai Y, Rankin EB, Neilson EG, Haase VH, Saito Y: Stable expression of HIF-1alpha in tubular epithelial cells promotes interstitial fibrosis. Am J Physiol Renal Physiol 295: F1023–F1029, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kaelin WG, Jr.: Molecular basis of the VHL hereditary cancer syndrome. Nat Rev Cancer 2: 673–682, 2002 [DOI] [PubMed] [Google Scholar]
- 40. Willam C, Masson N, Tian YM, Mahmood SA, Wilson MI, Bicknell R, Eckardt KU, Maxwell PH, Ratcliffe PJ, Pugh CW: Peptide blockade of HIFalpha degradation modulates cellular metabolism and angiogenesis. Proc Natl Acad Sci U S A 99: 10423–10428, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Riazi S, Tiwari S, Sharma N, Rash A, Ecelbarger CM: Abundance of the Na-K-2Cl cotransporter NKCC2 is increased by high-fat feeding in Fischer 344 X Brown Norway (F1) rats. Am J Physiol Renal Physiol 296: F762–F770, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brines M, Cerami A: Discovering erythropoietin's extra-hematopoietic functions: Biology and clinical promise. Kidney Int 70: 246–250, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Sugden MC, Holness MJ: Recent advances in mechanisms regulating glucose oxidation at the level of the pyruvate dehydrogenase complex by PDKs. Am J Physiol Endocrinol Metab 284: E855–E862, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Shanley PF, Rosen MD, Brezis M, Silva P, Epstein FH, Rosen S: Topography of focal proximal tubular necrosis after ischemia with reflow in the rat kidney. Am J Pathol 122: 462–468, 1986 [PMC free article] [PubMed] [Google Scholar]
- 45. Mori K, Lee HT, Rapoport D, Drexler IR, Foster K, Yang J, Schmidt-Ott KM, Chen X, Li JY, Weiss S, Mishra J, Cheema FH, Markowitz G, Suganami T, Sawai K, Mukoyama M, Kunis C, D'Agati V, Devarajan P, Barasch J: Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest 115: 610–621, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ichimura T, Bonventre JV, Bailly V, Wei H, Hession CA, Cate RL, Sanicola M: Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem 273: 4135–4142, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV: Urinary kidney injury molecule-1: A sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol 290: F517–F529, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Bonventre JV, Zuk A: Ischemic acute renal failure: An inflammatory disease? Kidney Int 66: 480–485, 2004 [DOI] [PubMed] [Google Scholar]
- 49. Lieberthal W, Nigam SK: Acute renal failure. II. Experimental models of acute renal failure: Imperfect but indispensable. Am J Physiol Renal Physiol 278: F1–F12, 2000 [DOI] [PubMed] [Google Scholar]
- 50. Srichai MB, Hao C, Davis L, Golovin A, Zhao M, Moeckel G, Dunn S, Bulus N, Harris RC, Zent R, Breyer MD: Apoptosis of the thick ascending limb results in acute kidney injury. J Am Soc Nephrol 19: 1538–1546, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Safirstein R: Gene expression in nephrotoxic and ischemic acute renal failure. J Am Soc Nephrol 4: 1387–1395, 1994 [DOI] [PubMed] [Google Scholar]
- 52. Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D'Agati V, Lin CS, Barasch J: The Ngal reporter mouse detects the response of the kidney to injury in real time. Nat Med 17: 216–222, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gobe GC, Johnson DW: Distal tubular epithelial cells of the kidney: Potential support for proximal tubular cell survival after renal injury. Int J Biochem Cell Biol 39: 1551–1561, 2007 [DOI] [PubMed] [Google Scholar]
- 54. Jang HR, Rabb H: The innate immune response in ischemic acute kidney injury. Clin Immunol 130: 41–50, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kojima I, Tanaka T, Inagi R, Kato H, Yamashita T, Sakiyama A, Ohneda O, Takeda N, Sata M, Miyata T, Fujita T, Nangaku M: Protective role of hypoxia-inducible factor-2alpha against ischemic damage and oxidative stress in the kidney. J Am Soc Nephrol 18: 1218–1226, 2007 [DOI] [PubMed] [Google Scholar]
- 56. Bernhardt WM, Eckardt KU: Physiological basis for the use of erythropoietin in critically ill patients at risk for acute kidney injury. Curr Opin Crit Care 14: 621–626, 2008 [DOI] [PubMed] [Google Scholar]
- 57. Rankin EB, Biju MP, Liu Q, Unger TL, Rha J, Johnson RS, Simon MC, Keith B, Haase VH: Hypoxia-inducible factor-2 (HIF-2) regulates hepatic erythropoietin in vivo. J Clin Invest 117: 1068–1077, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Warnecke C, Weidemann A, Volke M, Schietke R, Wu X, Knaup KX, Hackenbeck T, Bernhardt W, Willam C, Eckardt KU, Wiesener MS: The specific contribution of hypoxia-inducible factor-2alpha to hypoxic gene expression in vitro is limited and modulated by cell type-specific and exogenous factors. Exp Cell Res 314: 2016–2027, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Brezis M, Agmon Y, Epstein FH: Determinants of intrarenal oxygenation. I. Effects of diuretics. Am J Physiol 267: F1059–F1062, 1994 [DOI] [PubMed] [Google Scholar]
- 60. Brezis M, Heyman SN, Epstein FH: Determinants of intrarenal oxygenation. II. Hemodynamic effects. Am J Physiol 267: F1063–F1068, 1994 [DOI] [PubMed] [Google Scholar]
- 61. Katz AI, Doucet A, Morel F: Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Physiol 237: F114–F120, 1979 [DOI] [PubMed] [Google Scholar]
- 62. Biju MP, Akai Y, Shrimanker N, Haase VH: Protection of HIF-1-deficient primary renal tubular epithelial cells from hypoxia-induced cell death is glucose dependent. Am J Physiol Renal Physiol 289: F1217–F1226, 2005 [DOI] [PubMed] [Google Scholar]
- 63. Aragones J, Schneider M, Van Geyte K, Fraisl P, Dresselaers T, Mazzone M, Dirkx R, Zacchigna S, Lemieux H, Jeoung NH, Lambrechts D, Bishop T, Lafuste P, Diez-Juan A, Harten SK, Van Noten P, De Bock K, Willam C, Tjwa M, Grosfeld A, Navet R, Moons L, Vandendriessche T, Deroose C, Wijeyekoon B, Nuyts J, Jordan B, Silasi-Mansat R, Lupu F, Dewerchin M, Pugh C, Salmon P, Mortelmans L, Gallez B, Gorus F, Buyse J, Sluse F, Harris RA, Gnaiger E, Hespel P, Van Hecke P, Schuit F, Van Veldhoven P, Ratcliffe P, Baes M, Maxwell P, Carmeliet P: Deficiency or inhibition of oxygen sensor Phd1 induces hypoxia tolerance by reprogramming basal metabolism. Nat Genet 40: 170–180, 2008 [DOI] [PubMed] [Google Scholar]
- 64. Nangaku M, Kojima I, Tanaka T, Ohse T, Kato H, Fujita T: Novel drugs and the response to hypoxia: HIF stabilizers and prolyl hydroxylase. Recent Patents Cardiovasc Drug Discov 1: 129–139, 2006 [DOI] [PubMed] [Google Scholar]
- 65. Yan L, Colandrea VJ, Hale JJ: Prolyl hydroxylase domain-containing protein inhibitors as stabilizers of hypoxia-inducible factor: Small molecule-based therapeutics for anemia. Expert Opin Ther Pat 20: 1219–1245, 2010 [DOI] [PubMed] [Google Scholar]
- 66. Bernhardt WM, Wiesener MS, Scigalla P, Chou J, Schmieder RE, Gunzler V, Eckardt KU: Inhibition of prolyl hydroxylases increases erythropoietin production in ESRD. J Am Soc Nephrol 21: 2151–2156, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Weidemann A, Krohne TU, Aguilar E, Kurihara T, Takeda N, Dorrell MI, Simon MC, Haase VH, Friedlander M, Johnson RS: Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 58: 1177–1185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wiesener MS, Turley H, Allen WE, Willam C, Eckardt KU, Talks KL, Wood SM, Gatter KC, Harris AL, Pugh CW, Ratcliffe PJ, Maxwell PH: Induction of endothelial PAS domain protein-1 by hypoxia: Characterization and comparison with hypoxia-inducible factor-1alpha. Blood 92: 2260–2268, 1998 [PubMed] [Google Scholar]
- 69. Vitzthum H, Castrop H, Meier-Meitinger M, Riegger GA, Kurtz A, Kramer BK, Wolf K: Nephron specific regulation of chloride channel CLC-K2 mRNA in the rat. Kidney Int 61: 547–554, 2002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.