Abstract
Autosomal dominant polycystic kidney disease (ADPKD) is typically a late-onset disease caused by mutations in PKD1 or PKD2, but about 2% of patients with ADPKD show an early and severe phenotype that can be clinically indistinguishable from autosomal recessive polycystic kidney disease (ARPKD). The high recurrence risk in pedigrees with early and severe PKD strongly suggests a common familial modifying background, but the mechanisms underlying the extensive phenotypic variability observed among affected family members remain unknown. Here, we describe severely affected patients with PKD who carry, in addition to their expected familial germ-line defect, additional mutations in PKD genes, including HNF-1β, which likely aggravate the phenotype. Our findings are consistent with a common pathogenesis and dosage theory for PKD and may propose a general concept for the modification of disease expression in other so-called monogenic disorders.
Autosomal dominant polycystic kidney disease (ADPKD) is the most frequently inherited renal disease and one of the most common Mendelian human disorders with a prevalence of 1/400 to 1/1000.1,2 This approximates to about 12.5 million affected individuals worldwide. About 5 to 10% of all patients requiring renal replacement therapy are affected by ADPKD. As the name implies, ADPKD is transmitted in an autosomal dominant, fully penetrant fashion, i.e. virtually all individuals who inherit a mutated PKD allele in their germ-line will develop sonographically detectable renal cysts by age 30 or slightly later. Clinical symptoms usually do not arise until middle age. The majority of ADPKD patients carry a germ-line mutation in the PKD1 gene on chromosome 16p13, and 15 to 20% of patients harbor a PKD2 mutation on 4q21. ADPKD1 causes more severe disease with an average age of 54 years (versus 74 years for ADPKD2) at ESRD.3
In a minor subset of patients, ADPKD manifests very early in life and as a much more severe disorder.4,5 We recently demonstrated that early and severe ADPKD is not strictly confined to persons with a PKD1 mutation as previously thought but can also occur in patients with a PKD2 mutation.6,7 Homozygosity for PKD1 and the coinheritance in trans of an incompletely penetrant PKD1 allele with an inactivating PKD1 mutation may explain some of the cases of early-onset PKD; however, other mechanisms for early-onset ADPKD are still unknown.8,9 Among those cases with early-onset PKD are fetuses with Potter sequence and significant perinatal/neonatal morbidity and mortality sometimes clinically indistinguishable from those with a typical presentation of autosomal recessive polycystic kidney disease (ARPKD) and mutations in the PKHD1 gene on chromosome 6p12.9 Several lines of evidence corroborate these clinical observations and suggest a common pathogenesis for these diseases.
First, most cystoproteins, among them the ADPKD proteins polycystin-1 and polycystin-2 and the ARPKD protein fibrocystin/polyductin, colocalize in primary cilia and related organelles. Polycystin-1 and polycystin-2 directly interact, and polycystin-2 and polyductin form a common protein complex in which polyductin is crucial for polycystin-2 channel function.10,11
On a cellular level, a recessive two-hit disease model has been proposed for ADPKD with a PKD1 or PKD2 germ-line mutation on one allele and a transheterozygous somatic PKD1 or PKD2 mutation. In keeping with a recessive disease model, Pkd1 mice harboring a hypomorphic missense change on both alleles develop severe PKD that interestingly is confined to distal nephron segments and thus mimic the pattern typically seen in ARPKD.12 Likewise, ARPKD patients and mice with cysts not confined to distal tubules and collecting ducts but present in all nephron segments are also known (our unpublished data).13 Hepatic ductal plate malformation is an invariable feature of ARPKD but can also be found in some patients with ADPKD.
Williams et al.13 described an ARPKD mouse model with a homozygous Pkhd1 mutation in which Pkd1 and Pkd2 expression was considerably reduced. Direct evidence for genetic interactions between ADPKD and ARPKD loci came from two other mouse studies in which Pkhd1/Pkd1 and Pkhd1/Pkd2 transmutants showed a much more severe renal cystic phenotype than mice bearing a mutation in only one of these genes.11,14
The high recurrence risk for early and severe polycystic kidney disease in affected pedigrees suggests a common familial modifying background.15 Here we present the data of eight pedigrees in which the severely affected patients are the only family members that carry other PKD alleles in trans and/or de novo in addition to the familial germ-line mutation (see Figures 1 through 8).
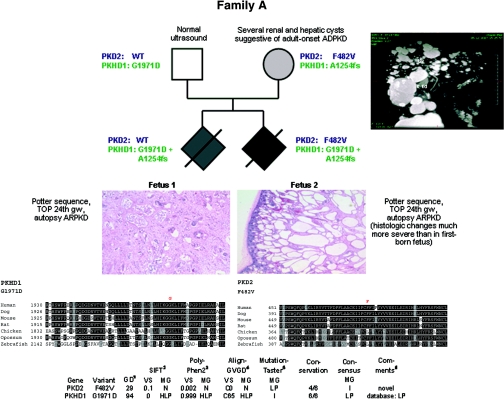
Figure 1.
Pedigree of family A with clinical information, genotypes, multisequence alignments, and bioinformatic data for both missense changes detected. Renal histology shows much more severe changes in the second-born fetus than in the first-born as regards size and number of renal cysts. (Top right panel) MR cholangiogram (T2-weighted gadolinium-enhanced coronal projection) of the mother of both fetuses in family A at 35 years of age showing multiple cysts of various sizes. The multiple hepatic cysts do not communicate with the biliary tree. Liv, Liver; R Kd, right kidney; S, superior; I, inferior; LA, left anterior; RP, right posterior.
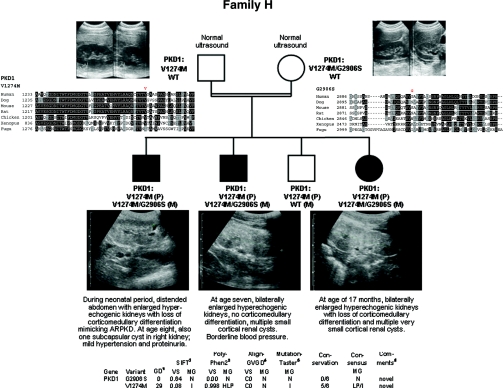
Figure 8.
Pedigree, ultrasonographic data, and genotypes of consanguineous family H. (Top left and right panels) Normal renal ultrasound data of the parents at age 25 (mother) and 29 (father). (Bottom panel) Renal ultrasonographic data of all three affected children of this family with enlarged polycystic kidneys with loss of corticomedullary differentiation and bioinformatic information on both missense changes identified in this family.
Family A
The mother showed several renal and hepatic cysts of various sizes in accordance with a clinical diagnosis of ADPKD (Figure 1). Both of her pregnancies were complicated by Potter sequence with enlarged hyperechogenic kidneys and oligo/anhydramnios and terminated in the 24th gestational week, respectively. Post mortem performed in both fetuses was in line with ARPKD. However, notably, pathologic changes were much more severe in the second-born fetus (Figure 1). By PKHD1 mutation analysis, two convincing mutations were found that both have been described in the literature: paternally, the nonconservative, an evolutionarily highly conserved amino acid affecting missense mutation c.5912G>A (p.G1971D), and maternally, the frameshifting mutation c.3761_3762delCC insG (p.A1254fs). However, heterozygosity for PKHD1 could not explain the clinical features suggestive of ADPKD in the mother. Thus, we proceeded with sequencing of PKD1 and PKD2 and detected the novel PKD2 missense mutation c.1444T>G (p.F482V) that was not present among 200 tested controls and alters a conserved polycystin-2 residue. In line with a dosage theory for PKD, only the more severely affected second fetus inherited this PKD2 allele.
As regards the next families discussed, it is of importance that HNF1β/TCF2 mutations can mimic polycystic kidney disease and that phenotypic variability, even within a family, is often extensive (our unpublished data).16–18 HNF1β directly regulates the transcription of PKD2 and PKHD1.19 In line, mice with renal-specific inactivation of Hnf1β develop polycystic kidney disease and renal cyst formation is accompanied by a drastic defect in Pkd2 and Pkhd1 expression.20 Thus, it can be postulated that early and severe PKD phenotypes in patients with HNF1β mutation may, at least in part, be due to in trans coinheritance of changes in the genes for ADPKD and ARPKD.
Family B
The couple's first pregnancy was affected with Potter sequence. The ultrasonographic “pepper-salt pattern” of the fetal kidneys was suggestive of ARPKD (Figure 2). The couple decided to terminate the pregnancy in the 26th gestational week. Post mortem showed cystic kidneys resembling ADPKD, but no evidence of ductal plate malformation in the liver. The mother displayed normal renal ultrasound, whereas the father had one small cyst in his left kidney and four cysts in the right kidney at the age of 33 years. Clinically, he showed slightly increased levels of creatinine, uric acid, liver transaminases, and glucose. His mother developed gestational diabetes and later on type 2 diabetes. At the age of 65 years, she showed morphologically normal kidneys slightly decreased in size by ultrasound and a creatinine of 1.5 mg/dl.
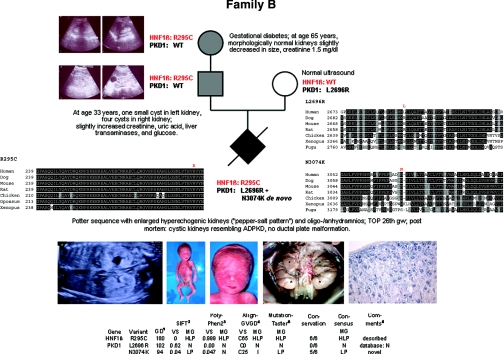
Figure 2.
Pedigree of family B with genotypes, clinical and ultrasonographic data, multisequence alignments, and bioinformatic data for all three missense changes identified in this family. (Top panel) At age 65 years, the grandmother's kidneys were slightly decreased in size but morphologically normal without any cyst. (Middle panel) The father's kidneys showed one small cyst on the left and four cysts on the right side at the age of 33 years. (Bottom left panel) Prenatal ultrasound revealed oligo/anhydramnios and enlarged hyperechogenic fetal kidneys with a so-called “pepper-salt pattern.” (Bottom middle panel) Post mortem findings in the severely affected male fetus of this family with facial features typical of Potter sequence (termination of pregnancy in the 26th week of gestation [TOP 26th gw]). Abdominal situs with bilaterally enlarged kidneys (weight, 12.5 g; reference range for respective gestational age, 5.5 to 9.3 g). *Kidneys; +adrenal glands; arrows, ureters. (Bottom right panel) Renal histology (2.5× hematoxylin and eosin staining) with small tubular and glomerular cysts. The collecting ducts were not considerably dilated.
Given the clinical data and family history, we started with mutation analysis of HNF1β and detected the missense mutation c.883C>T (p.R295C) in the fetus, father, and paternal grandmother. This mutation has been described in the literature, affects an evolutionarily conserved amino acid, and is bioinformatically predicted to be “highly likely pathogenic.”21 To explain the severe fetal phenotype suggestive of ADPKD, we additionally performed sequencing of the known PKD genes and identified the PKD1 missense change c.8087T>G (p.L2696R) on the maternal allele, classified as neutral in the ADPKD database, in line with the normal renal phenotype of the mother. In addition, only the fetus was shown to carry the novel PKD1 mutation c.9222C>G (p.N3074K) that arose de novo and affects an evolutionarily highly conserved residue. In accordance, in silico data predicts this mutation to be “likely pathogenic.”
Family C
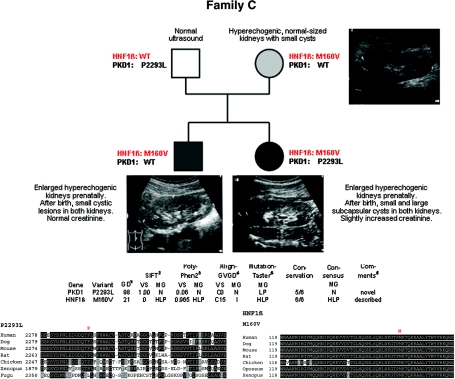
Both pregnancies of this couple were complicated by enlarged hyperechogenic kidneys. Postnatally, small cystic lesions were present in the son, whereas large subcapsular cystic lesions were additionally noted in the daughter (Figure 3). We initiated HNF1β mutation analysis because kidney biopsy revealed glomerular cysts. This resulted in detection of the novel missense mutation c.478A>G (p.M160V) that was absent from 200 controls, affects an evolutionarily highly conserved residue, and was categorized in silico as “(highly) likely pathogenic.” Segregation analysis revealed this mutation to be of maternal origin. The mother was clinically healthy but showed hyperechogenic, normal-sized kidneys with small cysts. No other HNF1β-related feature was present in her. Sequencing of the mother's parents proved the mutation to be de novo in her. To explain the clinical variability in this family, we proceeded with sequencing of PKD1, PKD2, and PKHD1. By this, we identified the novel and putatively hypomorphic PKD1 missense mutation c.6878C>T (p.P2293L) on the daughter's paternal allele, for which her brother and mother showed the wild-type sequence. In line with recent data on incomplete PKD1 penetrance and normal ultrasound data of the father in this family, heterozygous PKD1 alleles like P2293L are assumed to be not sufficient to cause ADPKD but can exert an aggravating effect in concert with other changes, a hypothesis that is further corroborated by the findings obtained in the pedigrees described below.8,9
Figure 3.
Pedigree of family C with genotypes, in silico information on both missense changes detected in this family, and clinical and ultrasonographic data. Mother and son presented with hyperechogenic normal-sized kidneys with small cysts, whereas the daughter additionally displayed some large subcapsular cystic lesions.
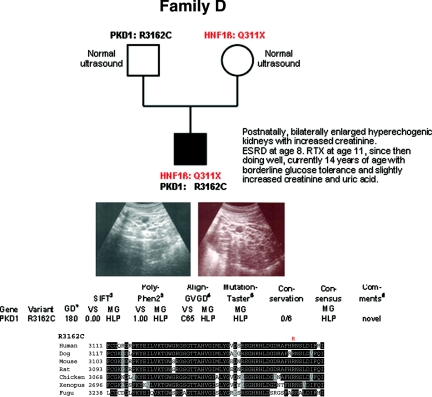
Family D
The family's propositus already displayed increased serum creatinine values and ultrasonographic features of PKD with bilaterally enlarged hyperechogenic kidneys postnatally (Figure 4). At the age of 8 years, he proceeded to ESRD and received peritoneal dialysis for almost 3 years before he was successfully transplanted. Since then he has been doing well with borderline glucose tolerance and slightly increased values for serum creatinine and uric acid at the age of 14 years. He carries the HNF1β nonsense mutation c.931C>T (p.Q311X) on his maternal allele, and the novel nonconservative PKD1 missense mutation c.9484C>T (p.R3162C) on his paternal allele. This change affects an evolutionarily highly conserved residue and is predicted by all bioinformatic tools used to be “highly likely pathogenic.” Surprisingly, neither the mother nor the father showed any clinical or ultrasonographic features in their mid-thirties.
Figure 4.
Pedigree and genotypes of family D. Renal ultrasound of the propositus at the age of 10 years with polycystic, considerably enlarged hyperechogenic kidneys. (Bottom panel) Bioinformatic data obtained for the PKD1 mutation R3162C.
In addition to the above pedigrees with mutations in more than one PKD gene, we also identified ADPKD families in which the early and severely affected index patient harbored an in trans or de novo PKD1 change in addition to the familiar PKD1 germ-line mutation (see Figures 5 through 8).
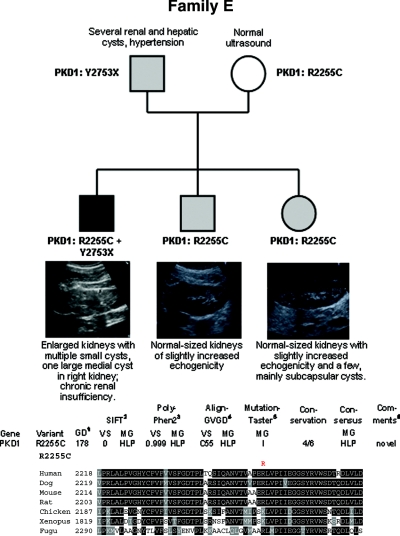
Figure 5.
Pedigree, genotypes, and renal ultrasound data of all children of family E. Enlarged kidneys with multiple small cysts and one large medial cyst in the right kidney were present in the eldest son, whereas his younger siblings demonstrated normal-sized kidneys with slightly increased echogenicity. A few mainly subcapsular cysts were also seen in the girl. (Bottom panel) In silico data obtained for the PKD1 mutation R2255C identified in this pedigree.
Family E
The most severely affected eldest son of this family (Figure 5) carries PKD1 mutations on both parental alleles: the nonsense mutation c.8259C>G (p.Y2753X) on his paternal allele and the nonconservative missense mutation c.6763C>T (p.R2255C) on his maternal allele, whereas his two siblings have only inherited the hypomorphic missense allele from her mother which obviously leads to mild disease with incomplete penetrance.
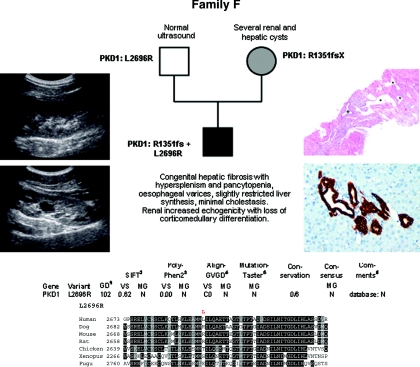
Family F
In this family (Figure 6), the novel 2-bp deletion in PKD1 c.4051_4052del (p.R1351fs) segregates with ADPKD, whereas the son's early and severe phenotype with histologically proven ductal plate malformation is thought to have been further aggravated by occurrence of the paternally inherited nonconservative PKD1 missense change c.8087T>G (p.L2696R) (same mutation as in Family B).
Figure 6.
Pedigree and genotypes of family F. (Left panel) Renal ultrasound of the propositus at the age of 15 years with increased echogenicity, one large cyst in the left kidney (bottom panel) and loss of corticomedullary differentiation. (Right top panel) Liver histology at the age of 9 years showing ductal plate malformation with irregularly distributed, dilated portal vein branches (asterisk), and dilated bile ducts (cross) in a fibrotic, expanded portal field (hematoxylin and eosin; original magnification, ×40). (Right bottom panel) Liver histology at the age of 15 years in line with ductal plate malformation and congenital hepatic fibrosis and demonstrating a portal tract with irregular, circular arrangement of widened bile ducts (brown) that extend into the hepatic lobules. Note absence of inflammation and fibrosis in this portal tract (Cytokeratin 7 immunoperoxidase staining; original magnification, ×200). (Bottom panel) Bioinformatic prediction scores and multiple sequence alignments obtained for the PKD1 change L2696R.
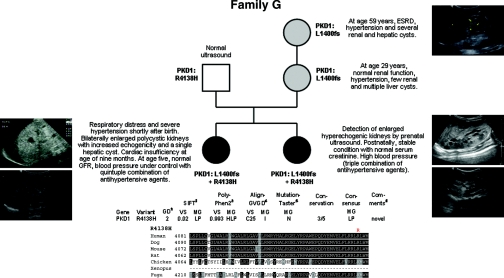
Family G
Patient 1 of this family (Figure 7) experienced respiratory distress and became cyanotic 1 h after birth. X-ray showed a pneumothorax on the right side and a white lung on the left side. Drainage of the pneumothorax and intubation with application of surfactant stabilized the patient. The girl's abdomen was distended and ultrasound revealed two enlarged polycystic kidneys with increased echogenicity and a singular cyst in the liver. Serum creatinine normalized within 4 weeks. Despite multiple antihypertensive drug therapy, the girl developed cardiac insufficiency at the age of 9 months. Addition of carvedilol and occlusion of a persistent ductus arteriosus improved cardiac function. At the age of 5 years, GFR is normal and BP is under control with a quintuple combination of antihypertensive agents. While the girl's 37-year-old father is healthy and showed normal abdominal ultrasound, the 25-year-old, clinically healthy mother displayed multiple cysts in the liver, but only a few in the kidneys. Abdominal ultrasound of the proposita's maternal grandmother was in line with ADPKD, at the birth of her granddaughter her serum creatinine was around 2.0 mg/dl, recently she reached ESRD. During the mother's second pregnancy, prenatal ultrasound revealed enlarged hyperechogenic kidneys. Postnatally, the patient was respiratory stable, and serum creatinine was in normal range, whereas the BP was elevated and is currently treated by amlodipine, atenolol, and captopril. Genetically, the novel PKD1 1-bp deletion c.4199del (p.L1400fs) segregates with ADPKD in this family. In contrast to their mother and maternal grandmother, both girls additionally carry a second PKD1 change (c.12413G>A, p.R4138H) on their other parental allele, which may have aggravated their clinical course.
Figure 7.
Pedigree and genotypes of family G. (Left panel) Renal ultrasound of proposita postnatally (left top panel) and at the age of 4 years (left bottom panel). (Right top panel) Renal ultrasound of maternal grandmother at the age of 59 years. (Middle and bottom right panels) Renal ultrasound of the second-born daughter postnatally (middle right panel) and at the age of 15 months (bottom right panel). (Bottom panel) Bioinformatic predictions and multiple sequence alignments for the PKD1 variant R4138H identified on the paternal allele of both severely affected girls.
Family H
Parental consanguinity and the clinical course of all three affected children were suggestive of ARPKD. However, haplotype analysis was incompatible with linkage to PKHD1. After HNF1β mutation analysis had been negative, we started with ADPKD diagnostics and detected the homozygous PKD1 missense change c.3820G>A (p.V1274M) in all affected children inherited in cis with c.8716G>A (p.G2906S) on the maternal disease allele (Figure 8). The parents, although still young (mother, 25 years old; father, 29 years old), showed no clinical or ultrasonographic abnormalities. Also, the family history was unremarkable for kidney diseases and related disorders.
The data obtained from the latter families further strengthens the message on incomplete penetrance and a dosage effect in ADPKD.8,9 Conclusively, heterozygous PKD1 alleles like V1274M in pedigree H, R4138H in family G, or L2696R in pedigrees B and F can be expected to be insufficient to cause ADPKD on their own but are most likely able to worsen the clinical course in affected individuals with other mutations. In family H, it is also tempting to speculate whether G2906S further impairs the functionality of the maternal disease allele.
Reconstruction of our data by animal models and/or functional assays may corroborate the message; however, these experiments are cumbersome and considerably hampered by the size and structure of PKD1 and PKHD1. According to the hypothesized recessive disease model on a cellular level in ADPKD, Piontek et al.22 recently demonstrated that the developmental age in which the second allele in Pkd1 is inactivated is crucial for disease severity. Inactivation of Pkd1 in mice before day 13 resulted in severely cystic kidneys, whereas later Pkd1 inactivation resulted in significantly milder and later onset of disease. These findings are in accordance with germ-line mutations identified in our early and severely affected patients and in contrast to ADPKD patients with a “typical” adult disease onset in which stochastically acquired somatic changes during later life are to be hypothesized.
Given these results and existing literature data, we find that there is sufficient evidence that our findings describe a general principle instead of being just a sequence of single cases. We postulate that additional PKD alleles in trans and/or de novo exert an aggravating effect and contribute to early and severe disease expression in polycystic kidney disease. Oligogenic inheritance with changes in different genes and converging pathomechanistic pathways is well known for syndromic ciliopathies like Bardet-Biedl syndrome but has not been described in a series of patients with polycystic kidney disease so far.23,24 We hypothesize that a reduced dosage of PKD proteins severely disturbs homeostasis and network integrity, and by this correlates with disease severity in PKD. It is likely that stochastic and environmental factors and changes in other (especially ciliary) genes/proteins that converge in common pathways may further influence the phenotype. Improved sequencing facilities (next-generation sequencing) will provide further rapidly growing insight in this issue and the mutational-load theory.
Elucidation of molecular mechanisms that may explain some of the phenotypic variability in these families is of importance for the understanding of polycystic kidney disease. Moreover, it is to be expected that similar findings may also apply for variable disease expression in other so-called monogenic conditions, most probably describing a general concept. These results and the aspect of early and severe disease manifestation in ADPKD deserve increased attention, e.g. in genetic counseling. Only few ADPKD patients and presumably also attending physicians know about early and severe disease expression and the considerably increased recurrence risk in affected families. Our findings may thus have important implications for the clinical and molecular understanding of the disease and the management of affected families.
CONCISE METHODS
The study was approved by the relevant review board and ethics committee, and the participants gave informed consent. For haplotype analysis of PKHD1, we used previously described markers and techniques.25 Mutation analysis involved direct sequencing of exonic and flanking intronic regions of PKD1, PKD2, HNF1β/TCF2, and PKHD1. All of the novel splice and missense mutations detected in one of the aforementioned genes were not present in 400 control chromosomes tested by DHPLC or restriction digestion analysis. The ADPKD and ARPKD mutation databases were used as a source of information about known PKD1, PKD2, and PKHD1 variants.26,27 Scoring of likely mutations was performed as described previously28 using a multisequence alignment of orthologs and different bioinformatic algorithms (Supplemental Table 1). The clinical and genetic data of all of the families discussed in this study are given in Supplemental Table 2.
DISCLOSURES
None.
Acknowledgments
The authors would like to thank the patients and their families for their cooperation and interest in the study. We thank Dr. Cecília Bagulho (Department of Radiology, Garcia de Orta Hospital, Almada, Portugal), Dr. Maximilian Kellner (Department of Radiology, Children's Hospital Cologne, Cologne, Germany), and Prof. Dr. Karl Schneider (Department of Pediatric Radiology, Dr. von Haunersches Children's Hospital Munich, Munich, Germany) for imaging data. The technical assistance of Elvira Golz-Staggemeyer, Edith von Heel, and Edith Bünger is gratefully acknowledged. This work was supported by the German Kidney Foundation, PKD Foundation, and the German Research Fund (DFG, SFB/TRR57).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental information for this article is available online at http://www.jasn.org/
REFERENCES
- 1. Wilson PD: Polycystic kidney disease. N Engl J Med 350: 151–164, 2004 [DOI] [PubMed] [Google Scholar]
- 2. Harris PC, Torres VE: Polycystic kidney disease. Annu Rev Med 60: 321–337, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 4. Bergmann C, Zerres K: Early manifestations of polycystic kidney disease. Lancet 369: 2157, 2007 [DOI] [PubMed] [Google Scholar]
- 5. Ogborn MR: Polycystic kidney disease: A truly pediatric problem. Pediatr Nephrol 8: 762–767, 1994 [DOI] [PubMed] [Google Scholar]
- 6. Bergmann C, Ortiz Brüchle N, Frank V, Rehder H, Zerres K: Perinatal deaths in a family with autosomal dominant polycystic kidney disease and a PKD2 mutation. N Engl J Med 359: 318–319, 2008 [DOI] [PubMed] [Google Scholar]
- 7. Bergmann C, Ortiz Brüchle N, Frank V, von Bothmer J, Zerres K: Early and severe disease manifestation in autosomal dominant polycystic kidney disease (ADPKD) [Abstract]. Med Gen 21: 62, 2009 [Google Scholar]
- 8. Rossetti S, Kubly VJ, Consugar MB, Hopp K, Roy S, Horsley SW, Chauveau D, Rees L, Barratt TM, van't Hoff WG, Niaudet P, Torres VE, Harris PC: Incompletely penetrant PKD1 alleles suggest a role for gene dosage in cyst initiation in polycystic kidney disease. Kidney Int 75: 848–855, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vujic M, Heyer CM, Ars E, Hopp K, Markoff A, Orndal C, Rudenhed B, Nasr SH, Torres VE, Torra R, Bogdanova N, Harris PC: Incompletely penetrant PKD1 alleles mimic the renal manifestations of ARPKD. J Am Soc Nephrol 21: 1097–1102, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J: Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol 27: 3241–3252, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim I, Fu Y, Hui K, Moeckel G, Mai W, Li C, Liang D, Zhao P, Ma J, Chen XZ, George AL, Jr., Coffey RJ, Feng ZP, Wu G: Fibrocystin/polyductin modulates renal tubular formation by regulating polycystin-2 expression and function. J Am Soc Nephrol 19: 455–468, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yu S, Hackmann K, Gao J, He X, Piontek K, Garcia-González MA, Menezes LF, Xu H, Germino GG, Zuo J, Qian F: Essential role of cleavage of Polycystin-1 at G protein-coupled receptor proteolytic site for kidney tubular structure. Proc Natl Acad Sci USA 104: 18688–18693, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Williams SS, Cobo-Stark P, James LR, Somlo S, Igarashi P: Kidney cysts, pancreatic cysts, and biliary disease in a mouse model of autosomal recessive polycystic kidney disease. Pediatr Nephrol 23: 733–741, 2008 [DOI] [PubMed] [Google Scholar]
- 14. Garcia-Gonzalez MA, Menezes LF, Piontek KB, Kaimori J, Huso DL, Watnick T, Onuchic LF, Guay-Woodford LM, Germino GG: Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet 16: 1940–1950, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zerres K, Rudnik-Schoneborn S, Deget F: Childhood onset autosomal dominant polycystic kidney disease in sibs: Clinical picture and recurrence risk. German Working Group on Paediatric Nephrology. J Med Genet 30: 583–588, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ulinski T, Lescure S, Beaufils S, Guigonis V, Decramer S, Morin D, Clauin S, Deschênes G, Bouissou F, Bensman A, Bellanné-Chantelot C: Renal phenotypes related to hepatocyte nuclear factor-1beta (TCF2) mutations in a pediatric cohort. J Am Soc Nephrol 17: 497–503, 2006 [DOI] [PubMed] [Google Scholar]
- 17. Faguer S, Bouissou F, Dumazer P, Guitard J, Bellannè-Chantelot C, Chauveau D: Massively enlarged polycystic kidneys in monozygotic twins with TCF2/HNF-1beta (hepatocyte nuclear factor-1beta) heterozygous whole-gene deletion. Am J Kidney Dis 50: 1023, 2007 [DOI] [PubMed] [Google Scholar]
- 18. Decramer S, Parant O, Beaufils S, Clauin S, Guillou C, Kessler S, Aziza J, Bandin F, Schanstra JP, Bellannè-Chantelot C: Anomalies of the TCF2 gene are the main cause of fetal bilateral hyperechogenic kidneys. J Am Soc Nephrol 18: 923–933, 2007 [DOI] [PubMed] [Google Scholar]
- 19. Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M: A transcriptional network in polycystic kidney disease. EMBO J 23: 1657–1668, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hiesberger T, Bai Y, Shao X, McNally BT, Sinclair AM, Tian X, Somlo S, Igarashi P: Mutation of hepatocyte nuclear factor-1β inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest 113: 814–825, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bellanné-Chantelot C, Clauin S, Chauveau D, Collin P, Daumont M, Douillard C, Dubois-Laforgue D, Dusselier L, Gautier JF, Jadoul M, Laloi-Michelin M, Jacquesson L, Larger E, Louis J, Nicolino M, Subra JF, Wilhem JM, Young J, Velho G, Timsit J: Large genomic rearrangements in the hepatocyte nuclear factor-1beta (TCF2) gene are the most frequent cause of maturity-onset diabetes of the young type 5. Diabetes 54: 3126–3132, 2005 [DOI] [PubMed] [Google Scholar]
- 22. Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG: A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med 13: 1490–1495, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fliegauf M, Benzing T, Omran H: When cilia go bad: Cilia defects and ciliopathies. Nat Rev Mol Cell Biol 8: 880–893, 2007 [DOI] [PubMed] [Google Scholar]
- 24. Zaghloul NA, Katsanis N: Functional modules, mutational load and human genetic disease. Trends Genet 26: 168–176, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bergmann C, Senderek J, Sedlacek B, Pegiazoglou I, Puglia P, Eggermann T, Rudnik-Schöneborn S, Furu L, Onuchic LF, De Baca M, Germino GG, Guay-Woodford L, Somlo S, Moser M, Büttner R, Zerres K: Spectrum of mutations in the gene for autosomal recessive polycystic kidney disease (ARPKD/PKHD1). J Am Soc Nephrol 14: 76–89, 2003 [DOI] [PubMed] [Google Scholar]
- 26. Harris PC, Rossetti S: Molecular diagnostics for autosomal dominant polycystic kidney disease. Nat Rev Nephrol 6: 197–206, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Bergmann C, Küpper F, Dornia C, Schneider F, Senderek J, Zerres K: Algorithm for efficient PKHD1 mutation screening in autosomal recessive polycystic kidney disease (ARPKD). Hum Mutat 25: 225–231, 2005 [DOI] [PubMed] [Google Scholar]
- 28. Dowdle WE, Robinson JF, Kneist A, Zerres K, Frints SGM, van Lijnschoten G, Mulders L, Verver DE, Salomé Sirerol-Piquer M, García-Verdugo JM, Corbit KC, Katsanis N, Bergmann C, Reiter JF: Disruption of a ciliary B9 protein complex causes Meckel Syndrome. Am J Hum Genet 89: 94–110, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]