Abstract
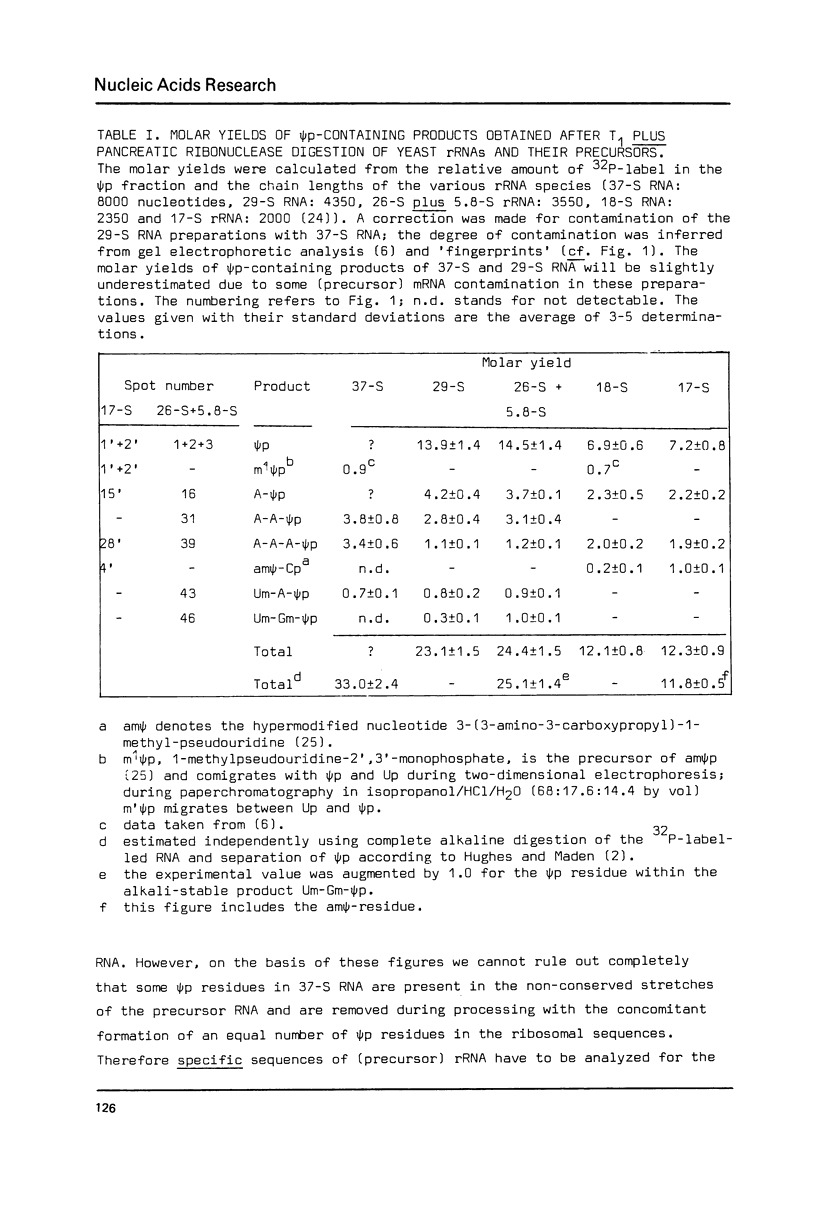
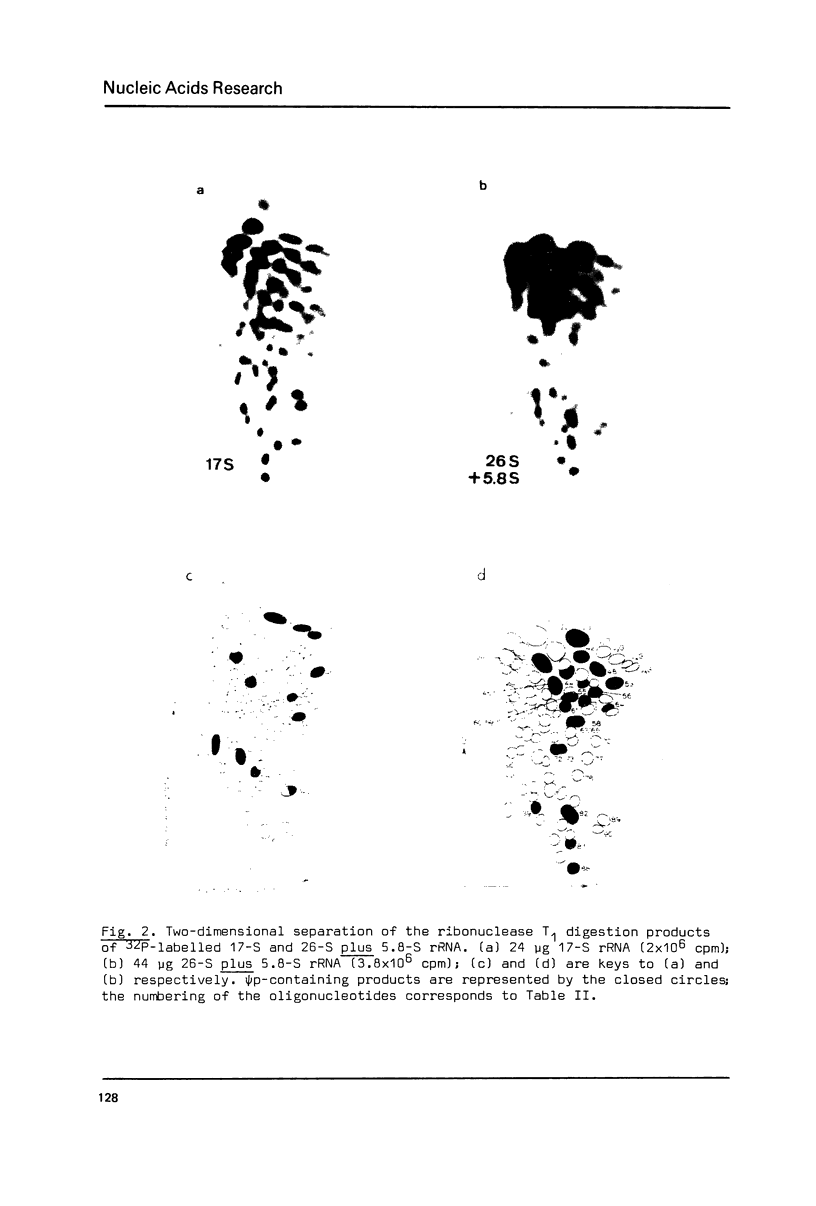
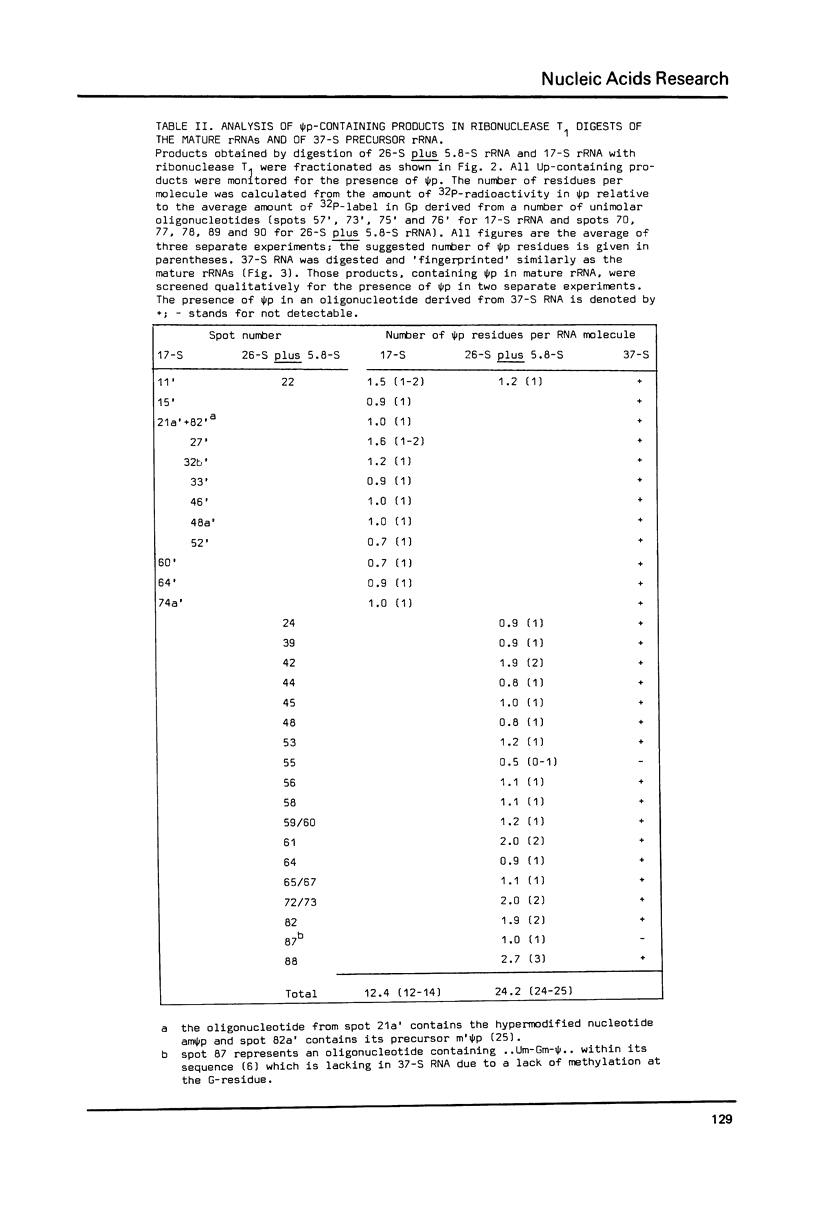
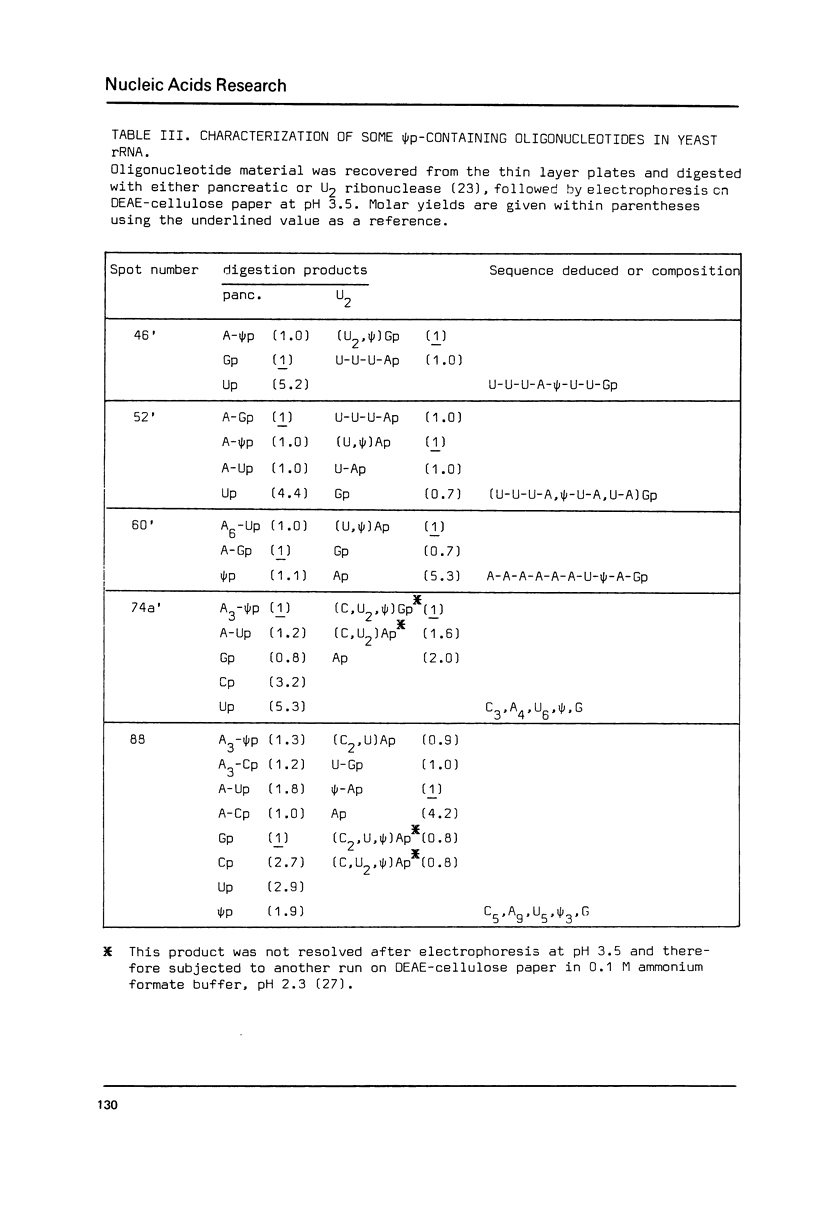
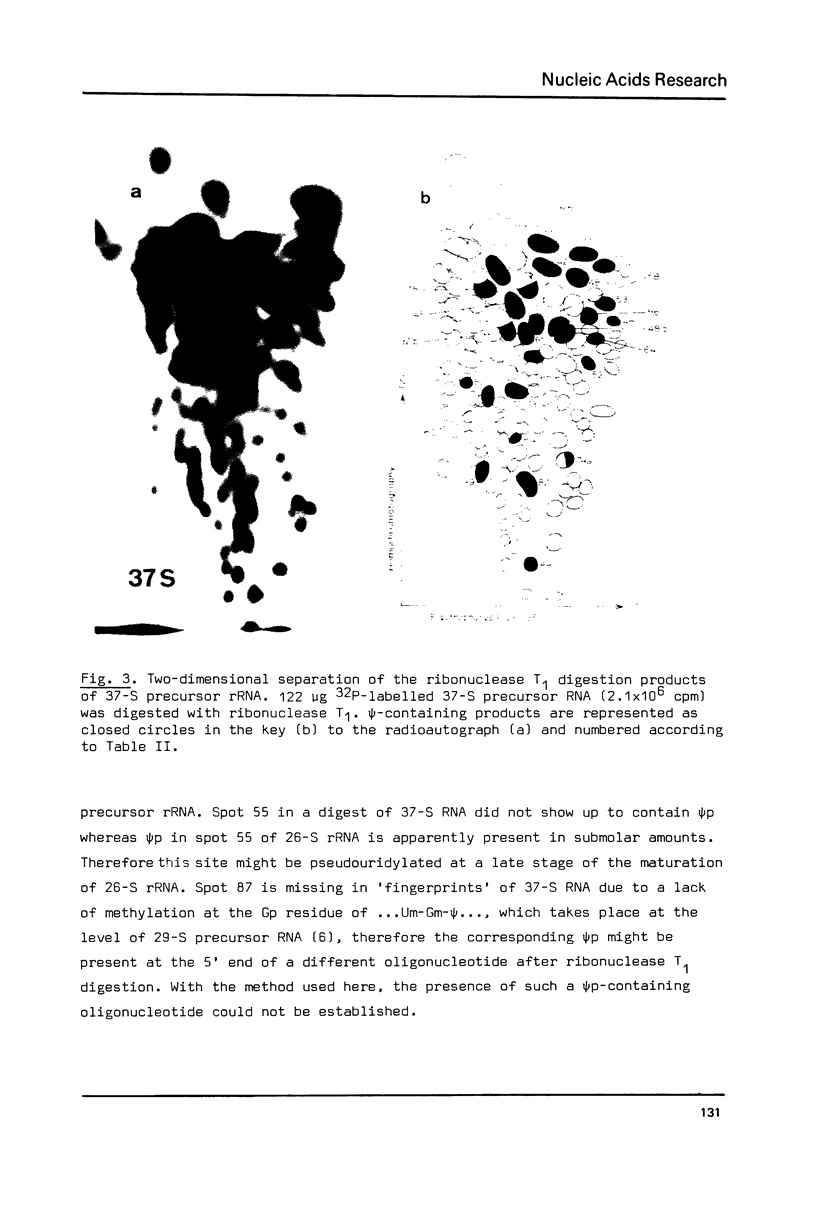
The pseudouridylation of ribosomal RNA of Saccharomyces carlsbergensis was investigated with respect to its timing during the maturation of rRNA and its sequence specificity. Analysis of 37-S RNA, the common precursor to 17-S, 5.8-S and 26-S rRNA and most probably the primary ribosomal transcript, shows that this RNA molecule contains already most if not all of the 36-37 pseudouridine residues found in the mature rRNAs. Thus pseudouridylation is, like 2'-0-ribosemethylation, an early event in the maturation of rRNA, taking place immediately after, or even during, transcription. The data presented show that the non-conserved sequences of 37-S precursor rRNA contain very few pseudouridine residues if any. The pseudouridine residues within the rRNA sequences are apparently clustered to a certain degree as can inferred from the occurrence of a single oligonucleotide containing 3 pseudouridines, which was obtained by digestion of 26-S rRNA with ribonuclease T1.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brand R. C., Klootwijk J., Planta R. J., Maden B. E. Biosynthesis of a hypermodified nucleotide in Saccharomyces carlsbergensis 17S and HeLa-cell 18S ribosomal ribonucleic acid. Biochem J. 1978 Jan 1;169(1):71–77. doi: 10.1042/bj1690071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand R. C., Klootwijk J., Van Steenbergen T. J., De Kok A. J., Planta R. J. Secondary methylation of yeast ribosomal precursor RNA. Eur J Biochem. 1977 May 2;75(1):311–318. doi: 10.1111/j.1432-1033.1977.tb11531.x. [DOI] [PubMed] [Google Scholar]
- Brand R. C., Planta R. J. The molecular weights of yeast ribosomal precursor RNAs. Mol Biol Rep. 1975 Dec;2(4):321–325. doi: 10.1007/BF00357019. [DOI] [PubMed] [Google Scholar]
- Brownlee G. G., Sanger F. Chromatography of 32P-labelled oligonucleotides on thin layers of DEAE-cellulose. Eur J Biochem. 1969 Dec;11(2):395–399. doi: 10.1111/j.1432-1033.1969.tb00786.x. [DOI] [PubMed] [Google Scholar]
- Caboche M., Bachellerie J. P. RNA methylation and control of eukaryotic RNA biosynthesis. Effects of cycloleucine, a specific inhibitor of methylation, on ribosomal RNA maturation. Eur J Biochem. 1977 Mar 15;74(1):19–29. doi: 10.1111/j.1432-1033.1977.tb11362.x. [DOI] [PubMed] [Google Scholar]
- Choi Y. C., Busch H. Modified nucleotides in T1 RNase oligonucleotides of 18S ribosomal RNA of the Novikoff hepatoma. Biochemistry. 1978 Jun 27;17(13):2551–2560. doi: 10.1021/bi00606a015. [DOI] [PubMed] [Google Scholar]
- De Jonge P., Klootwijk J., Planta R. J. Terminal nucleotide sequences of 17-S ribosomal RNA and its immediate precursor 18-S RNA in yeast. Eur J Biochem. 1977 Jan;72(2):361–369. doi: 10.1111/j.1432-1033.1977.tb11260.x. [DOI] [PubMed] [Google Scholar]
- Grummt I. The effects of histidine starvation on the methylation of ribosomal RNA. Eur J Biochem. 1977 Sep 15;79(1):133–141. doi: 10.1111/j.1432-1033.1977.tb11791.x. [DOI] [PubMed] [Google Scholar]
- Hughes D. G., Hughes S., Maden B. E. The pseudouridine content of hela cell ribosomal RNA. FEBS Lett. 1976 Dec 31;72(2):304–308. doi: 10.1016/0014-5793(76)80992-1. [DOI] [PubMed] [Google Scholar]
- Hughes D. G., Maden B. E. The pseudouridine contents of the ribosomal ribonucleic acids of three vertebrate species. Numerical correspondence between pseudouridine residues and 2'-O-methyl groups is not always conserved. Biochem J. 1978 Jun 1;171(3):781–786. doi: 10.1042/bj1710781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanteur P., Amaldi F., Attardi G. Partial sequence analysis of ribosomal RNA from HeLa cells. II. Evidence for sequences of non-ribosmal type in 45 and 32 s ribosomal RNA precursors. J Mol Biol. 1968 May 14;33(3):757–775. doi: 10.1016/0022-2836(68)90318-5. [DOI] [PubMed] [Google Scholar]
- Khan M. S., Salim M., Maden B. E. Extensive homologies between the methylated nucleotide sequences in several vertebrate ribosomal ribonucleic acids. Biochem J. 1978 Mar 1;169(3):531–542. doi: 10.1042/bj1690531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klootwijk J., Klein I., Grivell L. A. Minimal post-transcriptional modification of yeast mitochondrial ribosomal RNA. J Mol Biol. 1975 Sep 25;97(3):337–350. doi: 10.1016/s0022-2836(75)80044-1. [DOI] [PubMed] [Google Scholar]
- Klootwijk J., Planta R. J. Analysis of the methylation sites in yeast ribosomal RNA. Eur J Biochem. 1973 Nov 15;39(2):325–333. doi: 10.1111/j.1432-1033.1973.tb03130.x. [DOI] [PubMed] [Google Scholar]
- Maden B. E.H., Forbes J. Standard and non standard products in combined T(1) plus pancreatic RNAase fingerprints of HeLa cell rRNA and its precursors. FEBS Lett. 1972 Dec 15;28(3):289–292. doi: 10.1016/0014-5793(72)80733-6. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Salim M. The methylated nucleotide sequences in HELA cell ribosomal RNA and its precursors. J Mol Biol. 1974 Sep 5;88(1):133–152. doi: 10.1016/0022-2836(74)90299-x. [DOI] [PubMed] [Google Scholar]
- Perry R. P. Processing of RNA. Annu Rev Biochem. 1976;45:605–629. doi: 10.1146/annurev.bi.45.070176.003133. [DOI] [PubMed] [Google Scholar]
- Retèl J., Planta R. J. The investigation of the ribosomal RNA sites in yeast DNA by the hybridization technique. Biochim Biophys Acta. 1968 Dec 17;169(2):416–429. doi: 10.1016/0005-2787(68)90050-6. [DOI] [PubMed] [Google Scholar]
- Rubin G. M. The nucleotide sequence of Saccharomyces cerevisiae 5.8 S ribosomal ribonucleic acid. J Biol Chem. 1973 Jun 10;248(11):3860–3875. [PubMed] [Google Scholar]
- Sanger F., Brownlee G. G., Barrell B. G. A two-dimensional fractionation procedure for radioactive nucleotides. J Mol Biol. 1965 Sep;13(2):373–398. doi: 10.1016/s0022-2836(65)80104-8. [DOI] [PubMed] [Google Scholar]
- Vaughan M. H., Jr, Soeiro R., Warner J. R., Darnell J. E., Jr The effects of methionine deprivation on ribosome synthesis in HeLa cells. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1527–1534. doi: 10.1073/pnas.58.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volckaert G., Jou W. M., Fiers W. Analysis of 32P-labeled bacteriophage MS2 RNA by a mini-fingerprinting procedure. Anal Biochem. 1976 May 7;72:433–446. doi: 10.1016/0003-2697(76)90551-0. [DOI] [PubMed] [Google Scholar]
- van den Bos R. C., Klootwijk J., Planta R. J. Structural comparison of 17 S ribosomal RNA of yeast and its immediate precursor, 18 S RNA. FEBS Lett. 1972 Jul 15;24(1):93–97. doi: 10.1016/0014-5793(72)80834-2. [DOI] [PubMed] [Google Scholar]