Abstract
Objective:
Cerebral vasospasm is the commonest cause for mortality and morbidity in patients following clipping of a ruptured aneurysm. Selective phosphodiesterase (PDE) inhibitor like sildenafil acts as a vasodilator. The objective of this study was to evaluate the safety and feasibility of oral sildenafil citrate in patients with symptomatic refractory vasospasm.
Methods:
A total of 832 patients with aneurysmal subarachnoid bleed were operated in 4 years. Two hundred and seventy-three patients had vasospasm. Of these, 72 patients had refractory cerebral vasospasm. Vasospasm was defined as refractory when institution of “HHH” failed to reverse the transcranial Doppler (TCD) values even after 24 hours. Computed tomography (CT) scan showed no infarct, hematoma, or hydrocephalus, and the serum electrolytes were within normal limits. They received 100–150 mg of sildenafil every 4 hours. Response was evaluated by 2-hourly TCD.
Results:
Eight patients had sustained (TCD values normal for >48 hours) and four had temporary relief in vasospasm, as suggested. Four patients developed complications significant enough to terminate the therapy.
Conclusions:
Sildenafil citrate may be effective in patients with refractory symptomatic vasospasm. It calls upon the pharmacologists and scientists to discover newer supraselective PDE inhibitors, specific to PDE receptors in brain vessels.
Keywords: Sildenafil citrate, subarachnoid hemorrhage, vasospasm
INTRODUCTION
Vasospasm is a common complication after subarachnoid hemorrhage (SAH) and influences both mortality and morbidity. Conventionally, the management of symptomatic vasospasm is based on (hypertension, hypervolemia, hemodilution) HHH therapy and nimodipine.[5,11] Papaverine, nimodipine, and magnesium sulfate are some of the available chemotherapeutic agents used in clinical practice. However, the results of these therapies are not always satisfactory.[12] Sildenafil is a selective oral phosphodiesterase (PDE) inhibitor that has a vasodilator effect and has never been tried in humans with cerebral vasospasm.
The objective of this study was to evaluate the safety and efficacy of sildenafil citrate in humans with symptomatic refractory vasospasm.
MATERIALS AND METHODS
The study was started in October 2006 after obtaining clearance from the institute ethics committee. All patients underwent cerebral digital subtraction angiogram before surgery. They underwent pterional craniotomy, trans-sylvian approach, and clipping of aneurysm. The inclusion criteria were the following: 1) Patients older than 18 and less than 60 years of age 2) Case of intracranial aneurysm operated within 72 hours of SAH. 3) Clinically symptomatic vasospasm was defined as a patient who after surgery worsened neurologically as compared to the preoperative grade. The worsening was either in sensorium or a new focal deficit. Clinically symptomatic refractory vasospasm was defined as no improvement after 24 hours following HHH therapy. The HHH therapy constituted of hypertension, hemodilution, and hypervolemia. The hypervolemia and hemodilution were achieved by giving crystalloids to maintain a central venous pressure of 12–14 cm of saline . The systolic blood pressure was maintained around 180 mm Hg by adding dopamine or dobutamine if necessary Other causes of deterioration as mentioned in point 5 (vide infra) were ruled out. All the patients were deemed to have vasospasm based on transcranial Doppler (TCD) and none were subjected to a repeat Digital Subtraction Angiogram (DSA). Vasospasm was defined as “refractory” when institution of HHH failed to reverse the TCD values (increased flow velocities) even after 24 hours. 4) Vasospasm was corroborated on TCD velocity middle cerebral artery (v-MCA) >120 cm/s. The TCD was done using 2 MHz probe through the temporal bone window. 5) Computed tomography (CT) scan showed no infarct, hematoma, or hydrocephalus, and the serum electrolytes were within normal limits. Nimodipine (60 mg 4 hourly per orally) was given to all patients with aneurysmal SAH. Dexamethasone, nimodipine, low–molecular-weight dextran and HHH were continued in all, as this has been the protocol of our center. This protocol was not changed as per the ethics committee directive. Consequently, these confounding factors were not eliminated from this study. None of the patients underwent intracranial pressure monitoring.
A total of 832 patients with aneurysmal subarachnoid bleed were operated from October 2006 to September 2010. Two hundred and seventy-three patients had vasospasm in the postoperative period. Seventy-two patients of these 273 patients had clinically refractory symptomatic vasospasm and were included in the study group. The clinical, radiological, and operative details are detailed in Table 1. They were given sildanefil. The dose was 100 mg every 4 hours for patients weighing up to 70 kg and 150 mg every 4 hours for those weighing more. The route of administration for all was enteral. It was given through the nasogastric tube in patients who were not conscious. TCD was done every 30 minutes for 8 hours and then every 2 hours till the end point. Insonation of both ipsilateral and contralateral internal carotid artery (ICA), MCA, and anterior cerebral artery (ACA) was tried, but only ICA and MCA were utilized for measurement.
Table 1.
Preoperative and operative details of patients with refractory vasospasm in whom the therapy was instituted
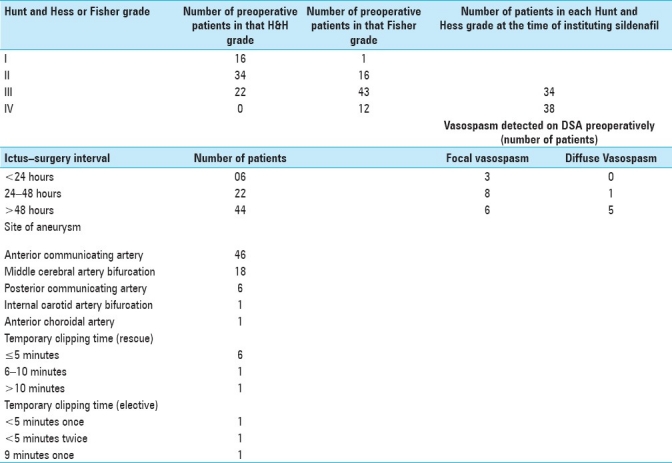
The end points of this therapy were: 1) no response for 48 hours, i.e. if the fall of TCD values was less than 40 cm/s; 2) normalization of TCD values for 2 days or 1 week after clipping, whichever occurs later; and 3) adverse reaction (defined as any new symptom, including increase in severity of headache or deficits after starting sildenafil in whom no obvious organic cause like infarct, hematoma, hydrocephalus, metabolic disturbance was found).
Sustained reversal of vasospasm was defined as decrease of TCD values by more than 40 cm/s for more than 48 hours and it was transient if there were intermittent readings of vMCA of fall less than 40 cm/s interspersed with readings of fall more than 40 cm/s.
RESULTS
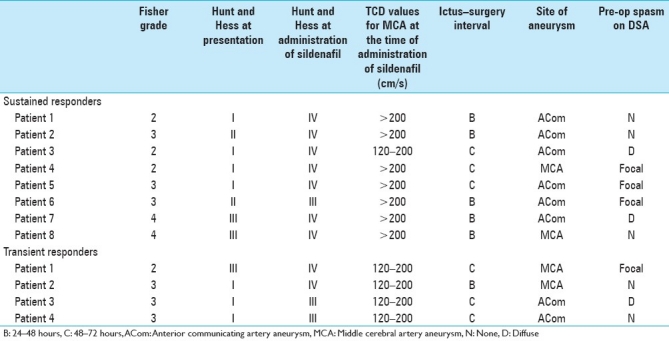
Sustained reversal was seen in eight patients (TCD values normal for >48 hours) and transient reversal in four patients [Table 2]. Of the eight patients who developed sustained relief, seven had vMCA >200 cm/s and one patient had vMCA between 120 and 200 cm/s. All the eight patients had a Lindegaard ratio of >3. On the other hand, all four patients with transient reversal had velocities between 120 and 200 cm/s and Lindegaard ratio of <3.
Table 2.
Details of patients who responded to sildenafil therapy
Four patients developed side effects severe enough to terminate the therapy. One patient had severe hypotension (possibly due to dehydration and concomitant dehydration), two patients had severe headache, and one patient had visual obscuration. The patient who developed hypotension was found to have a central venous pressure of 0–2 cm suggestive of significant volume depletion. This was despite giving HHH therapy. The patient with severe visual obscuration would complain of visual blurring lasting for several minutes with no other features of raised intracranial pressure.
The milder side effects were headache in 12, flushing in 7, and abnormal vision in 1 patient. Any complaint of headache was attributed to sildenafil. The mild abnormality of vision was manifested as seeing colored halo around light source.
DISCUSSION
With early recognition and clipping of aneurysms, cerebral vasospasm has become the most feared acute complication following the rupture of an aneurysm. Vasospasm pathophysiology is complex and not fully understood.[8] Delayed ischemic neurological deficit secondary to vasospasm represents a frequent cause of disability and death. Without a proper therapy, cerebral vasospasm is associated with a 34% permanent disability and 30% mortality.[5,6] Papaverine is a nonspecific phosphodiesterase (PDE) inhibitor that has been used in vasospasm when applied topically during surgery. When given intra-arterially, it effectively dilates the spastic arteries. However, the effect is short-lived and vasospasm usually recurs.
Nitric oxide (NO) is a primary endogenous vasodilator that affects smooth muscle relaxation. NO regulates the relaxation of vascular smooth muscle cells through activation of soluble guanylate cyclase. The cyclase converts guanosine triphosphate to cyclic guanosine monophosphate (cGMP) in the cells of cerebral arteries. Accumulation of cGMP relaxes the smooth muscles and its reduction leads to vasoconstriction. On the contrary, PDE hydrolyzes cGMP and cyclic adenosine monophosphate (cAMP). Type 5 PDE isoenzyme causes vasoconstriction. Thus, the amount of cGMP in the vascular smooth muscle cells is influenced by both NO and PDE.[3]
Currently, 11 families of PDEs have been identified in humans. PDE1 and PDE5 are present in vascular smooth muscle cells. Brain tissue has PDE1, PDE2, PDE4, and PDE10.[2] Sildenafil has good selectivity for PDE5, but the selectivity is not exclusive as it has some action on PDE1, PDE2, and PDE4. The selectivity ratio for PDE5 to PDE1, PDE2, and PDE4 is 1:80:19,000:2057, respectively.[4]
Sildanefil was originally used for angina and later for erectile dysfunction. It has been used in pulmonary hypertension, Raynaud's phenomenon and vertebro-basilar insufficiency.[3,17]
The bioavailability is approximately 40% and the peak level is usually reached in 30–120 minutes. The terminal half-life is 4 hours. Considering the bioavailability and the half-life, 100 mg every 4 hours was the dose given to patients weighing less than 70 kg and 150 mg was given every 4 hours for persons weighing more.
The mechanism of action of sildenafil may be more complex. Rats administered sildenafil in the lateral ventricles had remarkable tachycardia without significant change in basal arterial pressure.[7] Sildenafil elicited an increase in sympathetic nerve activity that is not baroreflex mediated, suggesting that this drug is able to elicit an autonomic imbalance of central origin.
Certain clinical studies and reports, however, have revealed contrary results. Sildenafil administration in stroke patients showed significantly more areas with diminished perfusion compared to baseline.[13] Our results are contrary to these. Though a perfusion study was not carried out in our patients, we presume that sildenafil administration may have improved the cerebral blood flow in responders. SAH has also been reported after usage of sildenafil.[9]
A critical review of the clinical data supports the conclusion that nimodipine decreases the severity of neurologic deficits and improves outcome after SAH. All our patients received nimodipine. One may argue that nimodipine per se would have helped in overcoming vasospasm. The mechanisms by which mortality and morbidity are reduced are still controversial. First, the frequency of vasospasm is not altered. Second, the consistent reversal of vasospasm once present has not been demonstrated either angiographically or by noninvasive cerebral blood flow studies. These observations suggest that there is either modification of microcirculatory flow (i.e., dilation of pial conducting vessels or decreased platelet aggregation) or a direct neuronal protective effect. Thus, nimodipine is unlikely to cause the improvement in the flow velocity on TCD.[14]
Natural resolution of vasospasm over a period of time or delayed response to HHH (>24 hours) cannot be ruled out, as there was no control group.
It has been shown that inhibitors of PDE 2, PDE4, PDE5, PDE9, and PDE10 improve a wide range of cognitive processes, including information processing, attention, learning, memory, executive functioning, and response inhibition, in various behavioral models within different species. It is argued that it is unlikely that blood flow is the mechanism underlying these procognitive effects. It is felt that long-term potentiation appears to be a better substrate for the cognition-enhancing properties of PDE inhibitors.[16]
Nitrates, which increase cGMP by increasing NO, have been used in cerebral vasospasm by intrathecal and intraventricular routes.[10,15] The approach is invasive with accompanying complications and infection. Administering oral PDE inhibitor in the form of sildenafil is a novel approach. It is easier to administer with less side effects. Sildenafil citrate is documented to be useful to treat cerebral vasospasm in animal studies.[1] This is the first human study, though there are a few limitations. It lacks outcome measures and there is no control group. Larger studies may be carried out to find out the efficacy of sildenafil in refractory vasospasm.
CONCLUSIONS
In this report, we present our initial results on the use of oral sildenafil in cerebral vasospasm. It may be useful in severe refractory vasospasm. Till date, no human trials have been published. This is a feasibility study and opens up the possibility of further trials in humans. Also, it calls upon the pharmacologists and scientists to discover newer supraselective PDE inhibitors specific to PDE receptors in brain vessels.
ACKNOWLEDGMENTS
The authors would like to acknowledge Late Prof. Y. Suzuki of Nagoya, Japan, and Prof. A. D. Mendelow of New Castle for encouraging this study.
Contributor Information
Kanchan K. Mukherjee, Email: kk_mukherjee@hotmail.com.
Shrawan K. Singh, Email: shrawanksingh2002@yahoo.com.
Virender K. Khosla, Email: khosla_vk@yahoo.com.
Sandeep Mohindra, Email: sandeepmohindra@gmail.com.
Pravin Salunke, Email: drpravin_salunke@yahoo.co.uk.
REFERENCES
- 1.Atalay B, Caner H, Cekinmez M, Ozen O, Celasun B, Altinors N. Systemic administration of phosphodiesterase V inhibitor, sildenafil citrate, for attenuation of cerebral vasospasm after experimental subarachnoid hemorrhage. Neurosurgery. 2006;59:1102–7. doi: 10.1227/01.NEU.0000245605.22817.44. [DOI] [PubMed] [Google Scholar]
- 2.Bischoff E. Pharmacology of phosphodiesterase inhibitor. In: Broderick G, editor. Oral Pharmacotherapy for Male Sexual Dysfunction: A Guide to Clinical Management. 1st ed. New Jersey: Humana Press Inc.; 2005. p. 47. [Google Scholar]
- 3.Bozgeyik Z, Berilgen S, Ozdemir H, Tekatas A, Ogur E. Evaluation of the effects of sildenafil citrate (viagra) on vertebral artery blood flow in patients with vertebro-basilar insufficiency. Korean J Radiol. 2008;9:477–80. doi: 10.3348/kjr.2008.9.6.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carson C., 3rd . Sildenafil: First in the therapeutic class of phosphodiesterase type 5 inhibitors. In: Carson C 3rd, Kirby R, Goldstein I, Wyllie M, editors. Text Book of Erectile Dysfunction. New York: Informa Healthcare; 2009. pp. 231–5. [Google Scholar]
- 5.Dorsch N. Therapeutic approaches to vasospasm in subarachnoid hemorrhage. Curr Opin Crit Care. 2002;8:128–33. doi: 10.1097/00075198-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Dorsch NW. A review of cerebral vasospasm in aneurismal subarachnoid hemorrhage. Part II: Management. J Clin Neurosci. 1994;1:78–92. doi: 10.1016/0967-5868(94)90080-9. [DOI] [PubMed] [Google Scholar]
- 7.Fazan R, Jr, Huber DA, Silva CA, Dias da Silva VJ, Salgado MC, Salgado HC. Sildenafil acts on the central nervous system increasing sympathetic activity. J Appl Physiol. 2008;104:1683–9. doi: 10.1152/japplphysiol.01142.2007. [DOI] [PubMed] [Google Scholar]
- 8.Hansen-Schwartz J. Cerebral vasospasm. A consideration of the various cellular mechanism involved in the pathophysiology. Neurocrit Care. 2004;1:235–46. doi: 10.1385/NCC:1:2:235. [DOI] [PubMed] [Google Scholar]
- 9.Kaneria MV, Pagar S, Samant H, Yeole S, Patil S. Subarachnoid haemorrhage: Possibly caused by the illegitimate use of sildenafil citrate. Assoc Physicians India. 2008;56:809–11. [PubMed] [Google Scholar]
- 10.Kumar R, Pathak A, Mathuriya SN, Khandelwal N. Intraventricular sodium nitroprusside therapy: A future promise for refractory subarachnoid hemorrhage induced vasospasm. Neurol India. 2003;51:197–202. [PubMed] [Google Scholar]
- 11.Lee KH. “Triple-H” therapy for cerebral vasospasm following subarachnoid hemorrhage. Neurocrit Care. 2006;4:68–76. doi: 10.1385/NCC:4:1:068. [DOI] [PubMed] [Google Scholar]
- 12.Liu JK, Couldwell WT. Intra-arterial papaverine infusions for the treatment of cerebral vasospasm induced by aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2005;2:124–32. doi: 10.1385/NCC:2:2:124. [DOI] [PubMed] [Google Scholar]
- 13.Lorberboym M, Mena I, Wainstein J, Boaz M, Lampl Y. The effect of sildenafil citrate (Viagra) on cerebral blood flow in patients with cerebrovascular risk factors. Acta Neurol Scand. 2010;121:370–6. doi: 10.1111/j.1600-0404.2009.01307.x. [DOI] [PubMed] [Google Scholar]
- 14.Meyer FB. Calcium antagonists and vasospasm. Neurosurg Clin N Am. 1990;1:367–76. [PubMed] [Google Scholar]
- 15.Pathak A, Mathuriya SN, Khandelwal N, Verma K. Intermittent low dose intrathecal sodium nitroprusside therapy for treatment of symptomatic aneurismal SAH-induced vasospasm. Br J Neurosurg. 2003;17:306–10. doi: 10.1080/02688690310001601180. [DOI] [PubMed] [Google Scholar]
- 16.Reneerkens OA, Rutten K, Steinbusch HW, Blokland A, Prickaerts J. Selective phosphodiesterase inhibitors: A promising target for cognition enhancement. Psychopharmacology (Berl) 2009;202:419–43. doi: 10.1007/s00213-008-1273-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenkranz S, Caglayan E, Erdmann E. Novel indications for phosphodiesterase type 5 inhibitors. Med Klin (Munich) 2007;15:617–30. doi: 10.1007/s00063-007-1078-4. [DOI] [PubMed] [Google Scholar]


