Summary
Background and objectives
Renal hemodynamic effects of inhibitors of the renin-angiotensin system can increase the risk of acute kidney injury under certain conditions. The BP-lowering effects of the renin inhibitor aliskiren are sustained 3–4 weeks after withdrawal. In this study, the reversibility of the renal hemodynamic effects of aliskiren was tested.
Design, setting, participants, & measurements
In this open-label study, renal perfusion was measured by 1.5-T magnetic resonance imaging–arterial spin labeling in 34 subjects with arterial hypertension before aliskiren (pre-aliskiren), after 4 weeks of aliskiren treatment (300 mg), and 4–5 days (∼2.5–3.0× plasma half-life) after withdrawal (post-aliskiren).
Results
Aliskiren reduced systolic BP from 152 ± 14 to 139 ± 16 mmHg (P<0.0001), which was sustained post-aliskiren (136 ± 13 mmHg, P=1.00 versus aliskiren). Aliskiren significantly altered renal perfusion (P=0.005), increasing from 272 ± 25 pre-aliskiren to 287 ± 29 ml/min per 100 g during aliskiren (P=0.03). This increase in renal perfusion was completely reversed post-aliskiren (272 ± 26 ml/min per 100 g, P=0.03 versus aliskiren, P=0.63 versus pre-aliskiren). No changes were noted in urinary angiotensinogen levels. Plasma renin activity was reduced by aliskiren, which was sustained post-aliskiren. Angiotensin II and aldosterone were reduced by aliskiren but recovered post-aliskiren to pre-aliskiren levels.
Conclusions
After withdrawal of aliskiren, the effects on BP were sustained, whereas increase in renal perfusion was reversed, which was associated with recovery of angiotensin II and aldosterone to pretreatment levels. Renal hemodynamic effects are more readily reversible than systemic effects of aliskiren.
Introduction
Renin enzyme inhibitors such as aliskiren (ALI) lower plasma renin activity and reduce the synthesis of angiotensin I and II (1). ALI has potent antihypertensive effects (2,3), and there is expanding evidence for the organoprotective potential of this treatment, which was recently shown for patients with diabetic nephropathy (4) and patients with congestive heart failure (5). ALI is excreted through the hepatobiliary route, with a plasma half-life of ∼40 hours (6). However, the antihypertensive effects of ALI last up to 3–4 weeks after treatment withdrawal (7,8). This time is substantially longer than expected based on plasma half-life, and it has been proposed to be attributable to drug accumulation in tissues (9). The slower rebound of hypertension after withdrawal of ALI compared with other drugs may be an advantage in situations where patients miss a dose of their antihypertensive medication (7).
Inhibitors of the renin-angiotensin system (RAS) cause preferential vasodilation of the efferent arteriole with subsequent lowering of glomerular filtration pressure. Although this action is generally beneficial in patients with arterial hypertension or renal disease, the reduction in filtration pressure is thought to increase the risk of acute kidney injury under circumstances such as sepsis or in the context of the administration of iodine-based contrast agents for imaging purposes. Under these circumstances, treatment with inhibitors of the RAS is generally stopped, aiming for reversal of the renal actions.
As a marker for their renal actions, the effects on renal perfusion of these drugs can be studied. In the kidney, a previous study has shown that administration of ALI causes acute renal vasodilation, exceeding the vasodilatory responses of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers (10). In that study, persistence of a degree of renal vasodilation was observed at a subsequent visit 48 hours after a single dose of ALI, perhaps suggesting that the prolonged actions seen in the systemic circulation also extend to the kidney. However, 48 hours is still close to plasma half-life, and some residual action of the drug would be expected at that time point.
We hypothesized that, if no tissue accumulation existed in humans or if it was irrelevant for the actions of ALI on the kidney, one should see a significant reversal of renal vasodilation as early as 4–5 days (∼2.5–3.0× plasma half-life) after ALI withdrawal. Magnetic resonance imaging (MRI)-based arterial spin labeling (ASL) techniques have been developed to measure renal perfusion (11). A key advantage of these techniques is that they do not require contrast media such as iodine or gadolinium, thereby avoiding potential and serious complications such as acute kidney injury and nephrogenic systemic fibrosis. To this end, we measured renal perfusion by MRI-ASL in patients with arterial hypertension before ALI (pre-ALI), after 4 weeks of treatment with ALI (300 mg), and 4–5 days after withdrawal (post-ALI).
Materials and Methods
Study Design and Participants
In this open-label study, renal perfusion was measured by 1.5T MRI-ASL technique in subjects with arterial hypertension before ALI (pre-ALI), after 4 weeks of treatment with ALI (300 mg), and 4–5 days after discontinuation (post-ALI). According to our previous studies, a sample size of 34 subjects was required to exclude a difference in renal perfusion of ≥10 ml/min per 100 g kidney tissue by paired comparison based on an SD of 20 ml/min per 100 g, a P value of <0.05, and a power of ≥0.8 (12). Inclusion criteria were a mean BP >140 mmHg systolic or >90 mmHg diastolic measured on two different occasions or treated arterial hypertension. Exclusion criteria were clinically significant liver or kidney disease, atrial fibrillation, atrioventricular blockade grade II or higher, and current treatment with ALI. The study was approved by the ethics committee of the University of Erlangen-Nuremberg and performed according to good clinical practice guidelines. Written informed consent was obtained from all patients before study entry.
Renal Perfusion by MRI
MRI-ASL was performed on a 1.5-T scanner (Magnetom Avanto; Siemens, Erlangen, Germany) using a flow-sensitive alternating inversion recovery (FAIR) True FISP (fast imaging with steady state processing) sequence, as described previously (12). The FAIR and True-FISP approach combines a FAIR perfusion preparation and a True-FISP data acquisition strategy. The perfusion measurement is based on two data acquisitions, one with a global inversion prepulse followed by one with a slice-selective prepulse (FAIR). The prepulses lead to a labeling of blood water spins. In contrast to the global inversion prepulse, the slice-selective inversion prepulse only labels the blood water spins inside the kidney but not the inflowing blood water spins. Subtraction of both images, therefore, reflects the local perfusion. A third image without the FAIR preparation pulse was measured to normalize the signal intensities on each patient. The technical and theoretical background of the sequence has been previously described in detail (11). All patients were examined in supine position with a body-phased array coil (Siemens) combined with the spine coil (Siemens). The FAIR True-FISP parameters were repetition time=4.9 ms; echo time=2.5 ms, effective inversion time=1200 ms, flip angle=70°, bandwidth=650 Hz/pixel, field of view=360 mm, and matrix=128×256 resulting in an in-plane resolution of 2.8×1.4 mm.
All images were measured during expiration in breath hold. Breath hold time was 18 s. The FAIR True-FISP sequence was measured four times. An M0 True-FISP image with the same scan parameters as the FAIR True-FISP sequence but without the inversion pulse was obtained after the second FAIR True-FISP acquisition. Whole scan time was about 5 minutes with five breath holds. Slices were positioned in an oblique coronal orientation to match the longitudinal axis of both kidneys. Slice thickness was 8 mm. Care was given to similarly position the slices in all subjects, and crucial attention was made to match the same slice position at all three study visits within each subject. The perfusion of each kidney was assessed pixel by pixel, and the average perfusion of the whole area of the kidney within the slice was calculated. Pixels with nonphysiologic high perfusion (>600 ml/min per 100 g; e.g., blood vessels) and pixels without perfusion (<3 ml/min per 100 g; e.g., pyelon) were excluded from the evaluation. The average of the perfusion values of both kidneys was used for analyses. In all subjects, MRI-ASL data were analyzed and postprocessed by the same radiologist (J.R. has 10 years of experience in MRI and 3 years of experience in MRI perfusion techniques), who was blinded to the date of the examinations and the clinical data of the study participants. MRI-ASL data were analyzed on an external Windows-based computer using dedicated software (J. Hornegger, Informatik 5; University of Erlangen-Nuremberg, Erlangen, Germany).
Biochemistry
Routine methods were used for the determination of serum concentrations of total cholesterol, HDL, LDL, triglycerides, and glucose as well as plasma and urinary creatinine and sodium concentrations. Fractional urinary excretion of sodium (FENa) was then calculated as (urinary sodium concentration×plasma creatinine concentration)/(plasma sodium concentration×urinary creatinine concentration) ×100. High-sensitivity C-reactive protein was determined using a polystyrene-enhanced nephelometric assay (Dade-Behring, Marburg, Germany). Urinary angiotensinogen (UAGT) was measured in diluted urine (1:4) by solid phase sandwich ELISA (IBL-International, Hamburg, Germany). Plasma aldosterone (Aldo) was measured in serum by RIA (Adaltis Italia S.p.A., Casalecchio di Reno, Italy). Plasma renin activity (PRA) was determined by measuring the generation of angiotensin I (Ang I) by RIA using a rabbit antibody (Bachem, Weil am Rhein, Germany). After extraction from plasma, Ang II was measured by RIA with a rabbit antibody (Celine C3; provided by D. Ganten, Berlin, Germany). All measurements were performed in duplicate, and mean values are presented.
Statistical Analyses
Analyses were performed using SPSS Software (release 18.0; SPSS Inc., Chicago, IL). Normal distribution was tested by Kolmogorov–Smirnov tests, and nonparametric data were logarithmically transformed where indicated (lnUAGT). Parametric data are presented as mean ± SD, and nonparametric data are presented as median and interquartile range. Differences in the measured parameters between study visits were analyzed by one-way ANOVA. If a significant difference was found, Bonferroni corrections were used for paired comparisons between pre-ALI and ALI, ALI and post-ALI, and pre-ALI and post-ALI. The P values given for Bonferroni corrections have been adjusted for these multiple comparisons. A two-sided P<0.05 was considered statistically significant. Data are presented as mean ± SD in the text and mean ± SEM in the figures.
Results
Clinical Characteristics
The clinical characteristics of the study participants, as determined on the day of the screening visit, are shown in Table 1. The majority of subjects were male and overweight. Data on number and type of antihypertensive medications are summarized in Table 2. Almost one-half of all included subjects were treated with a single antihypertensive drug. The remaining subjects were either not on any antihypertensive treatment or on two drugs. Only one patient was on three drugs, and one patient was on four antihypertensive drugs. The most commonly used drugs were calcium channel blockers and diuretics followed by angiotensin-converting enzyme inhibitors and angiotensin receptor blockers. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers were stopped in those patients treated with these medications to allow us to study the impact of ALI on the RAS. These drugs were stopped 36 days (IQR=19–57 days) before the beginning of the study. During the study, antihypertensive medication was kept constant.
Table 1.
Clinical characteristics of the study population
| Parameter | Mean ± SD (Total Range) |
|---|---|
| Age (yr) | 54 ± 9 (28–71) |
| Sex (male/female) | 27/7 |
| Height (cm) | 175 ± 10 (156–198) |
| Weight (kg) | 89 ± 18 (62–126) |
| BMI (kg/m2) | 29 ± 5 (20–38) |
| Waist circumference (cm) | 102 ± 13 (73–120) |
| Systolic BP (mmHg) | 145 ± 13 (120–194) |
| Diastolic BP (mmHg) | 90 ± 9 (66–118) |
| Heart rate (bpm) | 70 ± 9 (47–91) |
| Total cholesterol (mg/dl) | 221 ± 44 (135–343) |
| HDL cholesterol (mg/dl) | 58 ± 18 (31–104) |
| LDL cholesterol (mg/dl) | 140 ± 36 (73–240) |
| Triglycerides (mg/dl) | 123 ± 59 (39–313) |
| Glucose (mg/dl) | 95 ± 13 (74–124) |
| HbA1c (%) | 5.9 ± 0.3 (5.1–6.8) |
| Creatinine (mg/dl) | 0.9 ± 0.2 (0.5–1.2) |
BMI, body mass index.
Table 2.
Number and types of antihypertensive medications
| Number of Patients | |
|---|---|
| Number of antihypertensives | |
| zero antihypertensives | 8 |
| one antihypertensives | 15 |
| two antihypertensives | 9 |
| three antihypertensives | 1 |
| four antihypertensives | 1 |
| Drug | |
| calcium channel blocker | 10 |
| diuretic | 10 |
| angiotensin-converting enzyme inhibitor | 7 |
| angiotensin receptor blocker | 7 |
| β-blocker | 5 |
| sympatholytic | 1 |
Note that angiotensin-converting enzyme inhibitors and angiotensin receptor blockers were subsequently stopped.
BP and Renal Parameters
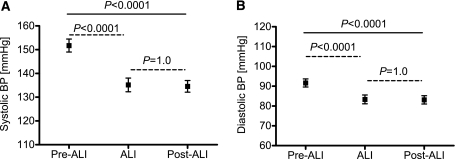
The results on BP and renal perfusion before, during, and 4–5 days after ALI are presented in Figures 1 and 2, respectively. ALI reduced systolic BP significantly from pre-ALI levels (from 152 ± 14 to 139 ± 16 mmHg, P<0.0001), and this effect was sustained after withdrawal of ALI (136 ± 13 mmHg, P=1.0 versus ALI) (Figure 1A). Similarly, ALI reduced diastolic BP significantly from pre-ALI levels (from 91 ± 11 to 85 ± 11 mmHg, P<0.0001) and this effect was also sustained after withdrawal of ALI (84 ± 10 mmHg, P=1.0 versus ALI) (Figure 1B).
Figure 1.
Sustained reduction of BP after aliskiren (ALI) withdrawal. Systolic (A) and diastolic (B) BP before treatment with ALI (pre-ALI), during treatment with ALI, and after treatment with ALI (post-ALI; result of one-way ANOVA, straight line; Bonferroni corrections, dashed lines).
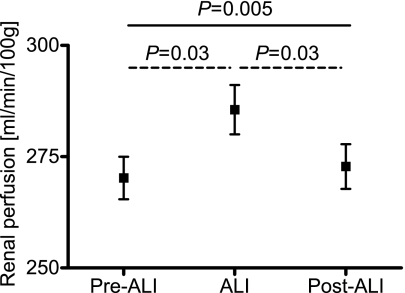
Figure 2.
Reversal of renal perfusion after aliskiren withdrawal. Renal perfusion measured by 1.5-T magnetic resonance imaging before (pre-ALI), during (ALI), and after (post-ALI) treatment with ALI (result of one-way ANOVA, straight line; Bonferroni corrections, dashed lines).
Renal perfusion was measured by MRI-ASL. Based on 80 measurements, the intra-observer coefficient of variation of this method was found to be 2.7%. Furthermore, as a measure of reproducibility/accuracy, we found a high correlation between two separate measurements on the same day in 10 healthy subjects (r=0.84, P<0.01; unpublished data). In the current study, renal perfusion increased during treatment with ALI compared with pre-ALI levels (from 272 ± 25 to 287 ± 29 ml/min per 100 g, P=0.03) (Figure 2). As the main result and in contrast to the BP response, renal vasodilation was reversed post-ALI (272 ± 26 ml/min per 100 g, P=0.03 versus ALI, P=0.63 versus pre-ALI) (Figure 2). Figure 3 shows examples of original MRI-ASL images with no prepulse (Figure 3A) (control), nonselective prepulse (Figure 3B), and slice-selective prepulse (Figure 3C). Figure 4 shows typical calculated perfusion maps with color coding obtained during pre-ALI (Figure 4A), ALI (Figure 4B), and post-ALI (Figure 4C), showing increased perfusion during ALI. Finally, FENa, as a second parameter of renal function, was also significantly affected by the withdrawal of ALI (from 0.60 ± 0.34% pre-ALI to 0.54 ± 0.32% during ALI, P=0.18, and to 0.70 ± 0.44% post-ALI, P=0.05 versus ALI, P=0.31 versus pre-ALI).
Figure 3.
Renal perfusion imaging with magnetic resonance imaging with arterial spin labeling (MRI-ASL). MRI-ASL: image without flow-sensitive alternating inversion recovery preparation (A), image with nonselective prepulse (B; no perfusion), and image with slice-selective prepulse (C; perfusion image).
Figure 4.
Color-encoded perfusion maps before, during, and after aliskiren (ALI). Calculated perfusion map with color-encoding before (A; pre-ALI), during (B; ALI), and after (C; post-ALI) treatment with ALI. White, perfusion <3 or >600 ml/min per 100 g; blue, perfusion <100 ml/min per 100 g; yellow, perfusion >100 and <250 ml/min per 100 g; red, perfusion >250 and <600 ml/min per 100 g.
RAS Measurements
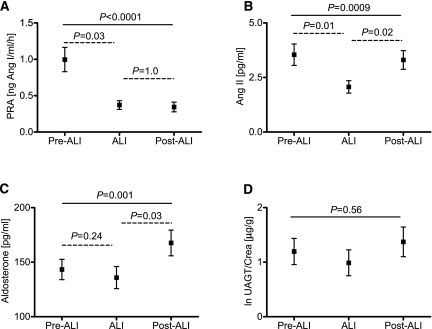
The results of the measurement of the parameters of the RAS are shown in Figure 4. Of note, the coefficient of variation of all biochemical measurements was <10%. PRA was significantly reduced by ALI (from 0.96 ± 0.80 to 0.34 ± 0.28 ng Ang I/ml per h, P=0.03), which was sustained after withdrawal (0.37 ± 0.32 ng Ang I/ml per h, P=1.00 versus ALI) (Figure 5A). Plasma Ang II levels were also reduced by ALI, but these levels recovered post-ALI (from 3.7 ± 2.4 pre-ALI to 2.1 ± 1.4 pg/ml during ALI, P=0.01, and to 3.2 ± 2.0 pg/ml post-ALI, P=0.02 versus ALI) (Figure 5B). There was no difference in Ang II levels between pre-ALI and post-ALI (P=0.72 by Bonferroni test). Plasma Aldo levels increased after withdrawal from ALI (from 140 ± 47 pre-ALI to 135 ± 50 pg/ml during ALI, P=0.24, and to 166 ± 58 pg/ml post-ALI, P=0.03 versus ALI) (Figure 5C), and there were no differences between pre-ALI and post-ALI levels (P=0.10 by Bonferroni). UAGT was not different across treatment phases (from 1.4 ± 1.3 pre-ALI to 1.1 ± 1.3 µg/g creatinine during ALI and to 1.4 ± 1.3 µg/g creatinine post-ALI, P=0.56 by one-way ANOVA) (Figure 5D). No direct correlation was found between the changes in these parameters and changes in renal perfusion.
Figure 5.
Components of the renin-angiotensin system before, during, and after aliskiren (ALI). (A) Plasma renin activity (PRA), (B) plasma angiotensin II (Ang II), (C) plasma aldosterone, and (D) urinary angiotensinogen (lnUAGT/Crea) before (pre-ALI), during (ALI), and after (post-ALI) treatment with ALI (result of one-way ANOVA, straight line; Bonferroni corrections, dashed lines).
Discussion
The key finding of this study is that renal vasodilation after administration of ALI is reversed 4–5 days after withdrawal of ALI, whereas BP-lowering actions of ALI are still sustained. This finding was associated with a recovery of plasma Ang II and Aldo to pretreatment levels. Thus, several components of the RAS are no longer suppressed shortly after withdrawal of ALI, and this process may contribute to the reversal of the renal response.
Previous clinical trials have shown that antihypertensive effects of ALI can be shown up to 3–4 weeks after treatment withdrawal (7,8). This time is considerably longer than expected based on plasma half-life, which is ∼40 hours (6). In contrast to other antihypertensives, missing one dose of ALI has little effect on BP control. Animal studies are in keeping with extensive tissue distribution of ALI, including deposition in the kidney (9). In vitro studies also suggest that ALI might inhibit renin even before it is secreted from the renin secretory granules of the juxtaglomerular apparatus (13). It has been argued, however, that the plasma concentrations achieved with recommended treatment doses of ALI are far too low to inhibit intracellular renin and that these in vitro data are not relevant clinically (14).
A previous study in 20 healthy volunteers has compared the acute renal effects of oral administration of ALI of 75, 150, 300, and 600 mg with the angiotensin-converting enzyme inhibitor captopril (25 mg) (10). The study participants were kept on a very low sodium diet before the investigations to activate the RAS and maximize the renal vascular response to the RAS inhibitors. The increase of renal perfusion, measured by the para-aminohippurate clearance technique, was greatest with the highest dose of 600 mg ALI, with an increase in renal plasma flow of ∼30%. The renal clearance studies were separated by 48 hours, and it was noted that there was some degree of persistent renal vasodilation from the previous administration of ALI, which seemed dose-dependent. There seemed to be little persistent renal vasodilation with doses of 75 or 150 mg, but with ALI at 300 mg, a residual vasodilation of ∼80 ml/min per 1.73 m2 was seen 48 hours later (∼14% above the baseline of the previous investigation). However, 48 hours is very close to the plasma half-life of the drug (∼40 hours) (6), and the presence of circulating drug could, therefore, explain the residual renal vasodilation after 48 hours. In fact, a previous study has shown that, after a single dose, ALI can still be detected in plasma after 48 hours and in urine even after 6 days, particularly when a higher dose of ALI had been administered (1).
Because these data in healthy volunteers on a very low sodium diet and with one time only administration of ALI do not reflect the typical clinical scenario (no steady state), we studied renal perfusion before ALI, after 4 weeks of treatment with ALI, and 4–5 days (∼2.5–3.0× plasma half-life) after withdrawal of ALI in patients with arterial hypertension. We measured renal perfusion by an MRI-ASL technique, which has the advantage that no contrast agents are required. We have previously shown that this technique correlates well with the renal clearance of para-aminohippurate, showing that this technique measures renal perfusion (12). Furthermore, Artz et al. (15) have recently shown that the MRI-ASL technique has excellent repeatability/accuracy. Although the respiratory-gated technique used by Artz et al. (15), which may be advantageous in patients with limited breath hold capacity, is slightly different from our method, we have observed a similarly good repeatability using a breath hold technique. Using this MRI-ASL technique for measuring renal perfusion in the current study, we showed that ALI significantly increased renal perfusion to an extent that was similar to what we have previously observed with an angiotensin receptor blocker (12). Our main result is that renal vasodilation is clearly reversed 4–5 days after withdrawal of ALI, whereas BP-lowering actions of ALI are sustained. Furthermore, FENa, as a second parameter of renal function, was affected similar to renal perfusion in that we observed an increase in FENa after ALI withdrawal. Even if there was long-lasting deposition of ALI in the kidney, our data show that this deposition does not affect the reversal of the renal response.
Of note, concurrent antihypertensive therapy, which consisted of diuretics, calcium channel blockers, β-blockers, or sympatholytics, had no apparent influence on the results of our renal perfusion measurements. As an example, when restricting the analysis to subjects treated with a diuretic (n=10), renal perfusion increased from 280 ± 23 ml/min per 100 g from pre-ALI to 292 ± 24 ml/min per 100 g during ALI (P=0.05), and subsequently, it recovered to 275 ± 16 ml/min per 100 g post-ALI (P=0.02 versus ALI, P=0.63 versus pre-ALI). Similar results were found for patients treated with calcium channel blockers (n=10): from 275 ± 25 ml/min per 100 g pre-ALI to 292 ± 36 ml/min per 100 g during ALI (P=0.04) to recovery to 273 ± 26 ml/min per 100 g post-ALI (P=0.04 versus ALI, P=0.43 versus pre-ALI). However, because of the low sample sizes, these subanalyses must be treated with caution.
Finally, we studied changes in the various components of the RAS. After 4 weeks of treatment with ALI, we found a marked suppression of PRA (∼65% reduction from pre-ALI). This suppression was sustained 4–5 days after stopping ALI. The degree of suppression and the persistence of suppression after withdrawal of PRA are in line with previous reports on the effects of treatment and withdrawal of ALI in patients with arterial hypertension (16). Furthermore, we found that Ang II and Aldo levels recovered to pretreatment levels 4–5 days after withdrawal, which is also consistent with previous studies (1,17). Of note, these previous studies have also noted a slower recovery of PRA than Ang II. The mechanism behind this recovery requires additional study. One would argue that active renin must be available somewhere to drive the recovery of Ang II and Aldo. In line with the reversal of the renal hemodynamic response observed in the current study, it seems possible that renin becomes available locally in the kidney sooner than circulating PRA would suggest. Furthermore, both of the changes in Ang II and Aldo could have contributed to the reversal of the renal vasodilation. However, we did not find any direct correlations between changes of Ang II or Aldo with the reversal of the renal response. This finding may be because of complex interactions between these mediators or local levels in the kidney differing from the measured plasma levels. The involvement of factors that we have not measured, such as endothelin, and limitations of our relatively small sample size for correlative analyses of this kind are other possibilities.
We also studied the response of UAGT. UAGT has been proposed as a novel biomarker of the intrarenal activation of the RAS (18,19). AGT is not filtered by glomeruli and therefore, derives solely from the kidney and not from the systemic circulation. By a positive feedback mechanism, Ang II increased AGT expression within the kidney, which subsequently led to additional increase of intratubular Ang II formation (18). In agreement with the concept that UAGT reflects intrarenal activation of the RAS, UAGT was found to correlate with high BP (20). Furthermore, inhibitors of the RAS are associated with reduced UAGT excretion (19). In the current study, we found no significant effect of ALI on UAGT. Previous studies, however, showed a large variation of this measurement (19,20), and our study may have simply been underpowered to detect a significant effect of ALI on this parameter.
In summary, our data show that renal vasodilation is clearly reversed 4–5 days after withdrawal of ALI, whereas BP-lowering actions are sustained. The reversal of the renal response was associated with recovery of plasma Ang II and Aldo to pretreatment levels. This reversal might be advantageous in circumstances where renal actions need to be reversed, such as in patients with a potential risk of acute kidney injury in sepsis or the context of administration of iodine-based contrast agents for imaging purposes. Renal hemodynamic effects are, therefore, more readily reversible than the systemic effects of ALI.
Disclosures
M.P.S. and R.E.S. have received lecture fees from Novartis Pharma GmbH, Nuremberg, Germany.
Acknowledgments
This work was supported by Deutsche Forschungsgemeinschaft Grant SFB TP B5 (to M.P.S. and R.E.S.) and Novartis Pharma GmbH, Nuremberg, Germany.
Footnotes
M.P.S. and R.J. contributed equally to this work.
Published online ahead of print. Publication date available at www.cjasn.org.
Access to UpToDate on-line is available for additional clinical information at www.cjasn.org.
References
- 1.Nussberger J, Wuerzner G, Jensen C, Brunner HR: Angiotensin II suppression in humans by the orally active renin inhibitor Aliskiren (SPP100): Comparison with enalapril. Hypertension 39: E1–E8, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Schmieder RE, Philipp T, Guerediaga J, Gorostidi M, Smith B, Weissbach N, Maboudian M, Botha J, van Ingen H: Long-term antihypertensive efficacy and safety of the oral direct renin inhibitor aliskiren: A 12-month randomized, double-blind comparator trial with hydrochlorothiazide. Circulation 119: 417–425, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Gradman AH, Schmieder RE, Lins RL, Nussberger J, Chiang Y, Bedigian MP: Aliskiren, a novel orally effective renin inhibitor, provides dose-dependent antihypertensive efficacy and placebo-like tolerability in hypertensive patients. Circulation 111: 1012–1018, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Parving HH, Persson F, Lewis JB, Lewis EJ, Hollenberg NK. AVOID Study Investigators: Aliskiren combined with losartan in type 2 diabetes and nephropathy. N Engl J Med 358: 2433–2446, 2008 [DOI] [PubMed] [Google Scholar]
- 5.McMurray JJ, Pitt B, Latini R, Maggioni AP, Solomon SD, Keefe DL, Ford J, Verma A, Lewsey J. Aliskiren Observation of Heart Failure Treatment (ALOFT) Investigators: Effects of the oral direct renin inhibitor aliskiren in patients with symptomatic heart failure. Circ Heart Fail 1: 17–24, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Vaidyanathan S, Jarugula V, Dieterich HA, Howard D, Dole WP: Clinical pharmacokinetics and pharmacodynamics of aliskiren. Clin Pharmacokinet 47: 515–531, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Andersen K, Weinberger MH, Egan B, Constance CM, Ali MA, Jin J, Keefe DL: Comparative efficacy and safety of aliskiren, an oral direct renin inhibitor, and ramipril in hypertension: A 6-month, randomized, double-blind trial. J Hypertens 26: 589–599, 2008 [DOI] [PubMed] [Google Scholar]
- 8.Oh BH, Mitchell J, Herron JR, Chung J, Khan M, Keefe DL: Aliskiren, an oral renin inhibitor, provides dose-dependent efficacy and sustained 24-hour blood pressure control in patients with hypertension. J Am Coll Cardiol 49: 1157–1163, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Feldman DL, Jin L, Xuan H, Contrepas A, Zhou Y, Webb RL, Mueller DN, Feldt S, Cumin F, Maniara W, Persohn E, Schuetz H, Jan Danser AH, Nguyen G: Effects of aliskiren on blood pressure, albuminuria, and (pro)renin receptor expression in diabetic TG(mRen-2)27 rats. Hypertension 52: 130–136, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Fisher ND, Jan Danser AH, Nussberger J, Dole WP, Hollenberg NK: Renal and hormonal responses to direct renin inhibition with aliskiren in healthy humans. Circulation 117: 3199–3205, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Martirosian P, Klose U, Mader I, Schick F: FAIR true-FISP perfusion imaging of the kidneys. Magn Reson Med 51: 353–361, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Ritt M, Janka R, Schneider MP, Martirosian P, Hornegger J, Bautz W, Uder M, Schmieder RE: Measurement of kidney perfusion by magnetic resonance imaging: comparison of MRI with arterial spin labeling to para-aminohippuric acid plasma clearance in male subjects with metabolic syndrome. Nephrol Dial Transplant 25: 1126–1133, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Krop M, Garrelds IM, de Bruin RJ, van Gool JM, Fisher ND, Hollenberg NK, Jan Danser AH: Aliskiren accumulates in Renin secretory granules and binds plasma prorenin. Hypertension 52: 1076–1083, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Campbell DJ: Aliskiren therapy will have minimal effect on intracellular renin of renin-producing cells. Hypertension 53: e17, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Artz NS, Sadowski EA, Wentland AL, Grist TM, Seo S, Djamali A, Fain SB: Arterial spin labeling MRI for assessment of perfusion in native and transplanted kidneys. Magn Reson Imaging 29: 74–82, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andersen K, Weinberger MH, Constance CM, Ali MA, Jin J, Prescott MF, Keefe DL: Comparative effects of aliskiren-based and ramipril-based therapy on the renin system during long-term (6 months) treatment and withdrawal in patients with hypertension. J Renin Angiotensin Aldosterone Syst 10: 157–167, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Azizi M, Ménard J, Bissery A, Guyenne TT, Bura-Rivière A, Vaidyanathan S, Camisasca RP: Pharmacologic demonstration of the synergistic effects of a combination of the renin inhibitor aliskiren and the AT1 receptor antagonist valsartan on the angiotensin II-renin feedback interruption. J Am Soc Nephrol 15: 3126–3133, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA: Intratubular renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kobori H, Alper AB, Jr, Shenava R, Katsurada A, Saito T, Ohashi N, Urushihara M, Miyata K, Satou R, Hamm LL, Navar LG: Urinary angiotensinogen as a novel biomarker of the intrarenal renin-angiotensin system status in hypertensive patients. Hypertension 53: 344–350, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kobori H, Urushihara M, Xu JH, Berenson GS, Navar LG: Urinary angiotensinogen is correlated with blood pressure in men (Bogalusa Heart Study). J Hypertens 28: 1422–1428, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]