Summary
Background and objectives
Fibroblast growth factor 23 (FGF23) levels are elevated in patients with autosomal dominant polycystic kidney disease (ADPKD) and X-linked hypophosphatemia (XLH), but only the latter is characterized by a renal phosphate wasting phenotype. This study explored potential mechanisms underlying resistance to FGF23 in ADPKD.
Design, setting, participants, & measurements
FGF23 and Klotho levels were measured, and renal phosphate transport was evaluated by calculating the ratio of the maximum rate of tubular phosphate reabsorption to GFR (TmP/GFR) in 99 ADPKD patients, 32 CKD patients, 12 XLH patients, and 20 healthy volunteers. ADPKD and CKD patients were classified by estimated GFR (CKD stage 1, ≥90 ml/min per 1.73 m2; CKD stage 2, 60–89 ml/min per 1.73 m2).
Results
ADPKD patients had 50% higher FGF23 levels than did XLH patients; TmP/GFR was near normal in most ADPKD patients and very low in XLH patients. Serum Klotho levels were lowest in the ADPKD group, whereas the CKD and XLH groups and volunteers had similar levels. ADPKD patients with an apparent renal phosphate leak had two-fold higher Klotho levels than those without. Serum Klotho values correlated inversely with cyst volume and kidney growth.
Conclusions
Loss of Klotho might be a consequence of cyst growth and constrain the phosphaturic effect of FGF23 in most patients with ADPKD. Normal serum Klotho levels were associated with normal FGF23 biologic activity in all XLH patients and a minority of ADPKD patients. Loss of Klotho and FGF23 increase appear to exceed and precede the changes that can be explained by loss of GFR in patients with ADPKD.
Introduction
Autosomal dominant polycystic kidney disease (ADPKD) is a genetic disorder characterized by bilateral growth of numerous cysts (1,2). Between age 20 and 40 years, the cysts replace approximately 50% of the normal parenchyma (3). In most patients, GFR remains preserved up to age 40 because glomerular hyperfiltration of remaining nephrons compensates for the ongoing loss of functional renal tissue (4,5). ADPKD can easily be detected early in the course of the disease and is an ideal condition to study mineral metabolism at an early stage of CKD because GFR remains stable for a long time, and the disease course is generally not confounded by therapies.
Fibroblast growth factor 23 (FGF23) is a phosphaturic hormone secreted by osteocytes (6,7). Serum levels of this 30-kD peptide were elevated in tumor-induced osteomalacia (8,9), as well as in several genetic diseases, such as autosomal dominant hypophosphatemic rickets secondary to a mutation of the gene that encodes for FGF23 (10,11), autosomal recessive hypophosphatemic rickets secondary to an inactivating mutation of dentin matrix acidic phosphoprotein (DMP1) (12,13), and X-linked hypophosphatemia (XLH) secondary to inactivating mutations of PHEX (14). As a result of these mutations, FGF23 is less accessible to degradation, and as a consequence, the FGF23 accumulation. leads to severe renal phosphate wasting. In contrast, elevated FGF23 levels in patients with CKD may play an essential role in maintaining normal serum phosphate levels at CKD stages 1 and 2 (15–17). Recently we reported that serum levels of FGF23 are markedly elevated in patients with ADPKD (18). As has been seen with tumor-induced osteomalacia, autosomal dominant hypophosphatemic rickets, autosomal recessive hypophosphatemic rickets, and XLH, this occurred even in the presence of a normal GFR. However, the marked hypophosphatemia typical for tubular disorders is not present in ADPKD, suggesting a resistance to FGF23 in this disease.
The gene Klotho encodes for a 130-kD protein with a single transmembrane domain that is mainly expressed in the kidney, the parathyroid glands, and the choroid plexus (19). Once Klotho protein is translocated from the endosome to the cell membrane, the extracellular domain of Klotho (soluble α-Klotho) may be cleaved and released into the blood stream (20).
FGF23 needs to bind Klotho protein by its C-terminal part to activate the canonical receptor FGFR1c, which leads to reduced membrane expression of the NaPiIIa and NaPiIIc cotransporters as well as downregulation of 1-α hydroxylase (21). Thus, peripheral resistance to FGF23 could be triggered by lower local or circulating levels of Klotho. However, a reliable assay for soluble Klotho has not been available until recently, and data on the expression, function, and regulatory mechanism of soluble α-Klotho are scarce (22).
This study explored potential mechanisms underlying peripheral resistance to FGF23 in ADPKD. We studied the relationship between FGF23, serum Klotho, and renal phosphate handling in patients affected by ADPKD with CKD stage 1 and 2 (ADPKD1-2) and compared it with that in patients with CKD stages 1 and 2 who did not have polycystic kidney disease (CKD1-2), patients with XLH, and healthy volunteers.
Materials and Methods
Study Participants and Procedures
Patients with ADPKD belonged to the well characterized longitudinal observational SUISSE ADPKD cohort (23,24) and were eligible for this study if they had an estimated GFR ≥ than 60 ml/min per 1.73 m2 according to the CKD Epidemiology Collaboration formula. For comparison, we studied a group of healthy volunteers with age and gender distribution similar to that of the ADPKD cohort, patients with CKD who did not have polycystic kidney disease, and patients with XLH. The ADPKD and CKD patients were classified according to estimated GFR (CKD stage 1, ≥ 90 ml/min per 1.73 m2; CKD stage 2, 60–89 ml/min per 1.73 m2). XLH was diagnosed in all patients during childhood when they presented with rickets; diagnosis was based on the presence of clinical and radiographic findings, bowing deformities of the legs, and the laboratory finding of hypophosphatemia due to renal phosphate wasting. Eight of the 12 patients also had a positive family history, and 6 of the 12 underwent corrective orthopedic surgery for their leg deformities. Growth was often impaired despite treatment. Eight of 12 XLH patients were receiving treatment with calcitriol and phosphate (median daily doses of 0.5 μg and 1.25 g, respectively) when the samples were obtained for analysis.
Blood samples were drawn in the morning after an overnight fasting period. The second fasting spot urine samples were collected after the patient voided the first urine of the day before the clinic visit. Blood drawing and spot urine collections were scheduled at the outpatient clinic between 8:00 a.m. and 10:00 a.m. For α-Klotho measurements, aliquots of samples were stored at −80°C before analysis; the exceptions were serum samples from patients with XLH, which were stored at −20°C.
Patients with ADPKD1-2 underwent serial renal magnetic resonance imaging without contrast material every 6 months. Total kidney volumes were estimated from magnetic resonance imaging sequences, as reported elsewhere (3). The healthy volunteers underwent renal ultrasonography, and their kidney volumes were estimated by applying the ellipsoid formula (25).
The study was carried out in accordance with the ethical principles of the Declaration of Helsinki, the Good Clinical Practice guidelines of the International Conference on Harmonization, local regulatory requirements, and local medical ethics committee approval. Patients provided written informed consent.
Analytical Methods
The levels of FGF23 (Immutopics, San Clemente, CA), human soluble α-Klotho (Immuno-Biologic Laboratories Co., Ltd. Japan), and intact parathyroid hormone (Biomerica, Newport Beach, CA) were measured by ELISA according to the manufacturer’s protocol.
The novel ELISA method detecting human soluble α-Klotho was developed by first establishing a monoclonal antibody with strong affinity for human α-Klotho protein, recognizing with high selectivity the tertiary protein structure of its extracellular domain. The established protein detection method has been tested by comparing the serum α-Klotho levels of human healthy volunteers with a human case in which the α-Klotho gene carries a mutation that hinders the expression of α-Klotho in the test subject (26). The results of the analysis indicated that the ELISA system can specifically detect and measure the circulating serum α-Klotho levels in humans (22). The ELISA assay was based on the antibodies and substrates designed, used, and described by Yamazaki and colleagues (22). The mean ± SD intra-assay coefficient of variation was 3.6%±1.3%.
In addition to the validation conducted by Yamazaki and colleagues, we performed a sample measurement validation for accuracy at our facility. We performed multiple analyses, measuring human serum Klotho levels from human serum samples that were exposed to different conditions. Samples stored at −80°C with no freeze/thaw cycle for more than 1 year were compared with samples stored at −80°C with one to three freeze/thaw cycles for less than 1 year in order to evaluate thawing stability; freshly collected serum samples were compared with samples kept at room temperature for more than 4 hours in order to evaluate stability at room temperature. The analysis showed that samples with two freeze/thaw cycles remained stable, but further cycles should be avoided. Serum samples kept longer than 4 hours at room temperature showed a slight decrease in reactivity compared with freshly collected serum samples. Finally, we compared samples from various storage conditions (stored at 4°C vs. −20°C) with freshly collected samples. We found no significant differences. Further analyses regarding Klotho expression on the levels of renal tissue could not be performed because we had no access to tissue samples from either study group.
Phosphate and creatinine (isotope dilution mass spectrometry traceable modified Jaffé method) concentrations were measured in serum and urine. The ratio of the maximum rate of tubular phosphate reabsorption to the GFR (TmP/GFR) was calculated as follows:
where PP, UP, PCrea, and UCrea refer to the plasma and urinary concentration of phosphate and creatinine, respectively (27). TmP/GFR allows evaluation of renal phosphate transport and is called the theoretical renal phosphate threshold. This value corresponds to the theoretical lower limit of plasma phosphate below which all filtered phosphate would be absorbed (normal range, 0.80–1.35 mmol/L). TmP/GFR is independent of dietary phosphorus intake, tissue release of phosphorus, and GFR (28). Renal phosphate leak was defined as TmP/GFR < 0.6 mmol/L. The GFR was estimated by using the CKD Epidemiology Collaboration equation (29).
Statistical Analyses
A general linear model approach was applied to calculate least-square means and simultaneous 95% confidence intervals. P values were adjusted for multiple comparisons by applying the Dunnett post hoc test. Univariate Pearson correlation was used to test for association between continuous variables. Statistical analyses were performed using SAS statistical software, version 9.2 (SAS Institute Inc., Cary, NC).
Results
Study Participants
A total of 99 patients ADPKD1-2, 32 patients with CKD1-2, 12 patients with XLH, and 20 healthy volunteers were enrolled in the study at the University Hospital Zurich between January 2004 and May 2011. Most patients were in their third or fourth decade of life and had an estimated GFR of 80–120 mL/min per 1.73 m2 (Table 1). Causes of nephropathies in patients with CKD1-2 were IgA nephropathy (nine patients [eight biopsy proven]), Alport or thin basement disease (three patients [two biopsy proven]), minimal change nephropathy (three patients [all biopsy proven]), lupus nephritis (three patients [all biopsy proven]), focal segmental glomerulosclerosis (two patients [all biopsy proven]), other chronic glomerulonephritis (six patients [four biopsy proven]), interstitial nephritis (one patient [biopsy proven]), and hypertensive nephropathy (three patients [none biopsy proven]). As expected, patients with CKD1-2 had higher urinary albumin excretion, and patients with XLH were predominantly female and had lower height than the other groups. The kidneys of the healthy volunteers were normal per ultrasonography, with a mean total kidney volume of 360 cm3. None of the ADPKD1-2 patients, CKD1-2 patients, or volunteers received nutritional or activated vitamin D metabolites, calcium supplementation, phosphate binders, or bisphosphonates, but most of the patients with XLH were receiving treatment with calcitriol and phosphate supplements (for details, see the Methods section).
Table 1.
Characteristics of patients according to study group
| Characteristic | ADPKD1-2 (n=99) | CKD1-2 (n=32) | XLH (n=12) | Volunteer (n=20) |
|---|---|---|---|---|
| Age (yr) | 31±6 | 40±14 | 31±11 | 32±6 |
| Sex, n (%) | ||||
| female | 36 (36) | 18 (56) | 9 (75) | 8 (40) |
| male | 63 (64) | 14 (44) | 3 (25) | 12 (60) |
| Height (cm) | 177±9 | 171±10 | 158±9 | 176±9 |
| Body mass index (kg/m2) | 24±4 | 26±5 | 24±4 | 22±2 |
| eGFRa (ml/min per 1.73 m2) | 94±17 | 94±21 | 113±18 | 99±12 |
| BP (mm Hg) | ||||
| systolic | 131±16 | 135±12 | 109±14 | 123±7 |
| diastolic | 83±11 | 84±8 | 73±13 | 75±6 |
| Albumin-to-creatinine ratio (mg/mmol)b | 2 (1–3) | 3 (1–36) | 1 (1–1) | 0 (0–1) |
Values expressed with a plus/minus sign are the mean ± SD. ADPKD1-2, autosomal dominant polycystic kidney disease with CKD stage 1 and 2; CKD1-2, CKD stage 1 and 2 without polycystic kidney disease; XLH, X-linked hypophosphatemia; eGFR, estimated GFR.
GFR was estimated by using the CKD Epidemiology Collaboration equation (29).
Median (interquartile range).
Resistance to the Phosphaturic Effect of FGF23 in Polycystic Kidneys
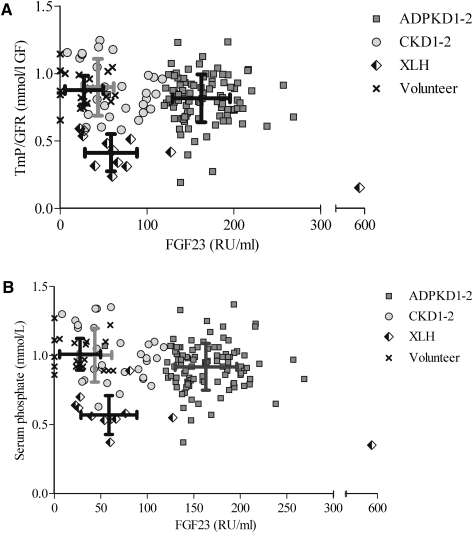
The FGF23 levels were highest among patients with ADPKD1-2, with values approximately 100% higher than those observed in patients with CKD1-2 and 50% higher than those observed in patients with XLH. FGF23 is thought to promote renal phosphate excretion by inhibiting tubular phosphate reabsorption. Thus, we would expect to observe lower TmP/GFR values and serum phosphate levels among patients with ADPKD1-2 than among those with CKD1-2 and those with XLH. However, TmP/GFR values were generally not decreased in patients with ADPKD1-2; they were similar to those found in patients with CKD1-2 and much higher than the values found in patients with XLH (Figure 1A). Notably, TmP/GFR values were inversely (negatively) correlated with FGF23 levels in XLH (r=−0.63; P=0.03), whereas no correlation could be detected for ADPKD1-2 or CKD1-2. Furthermore, Figure 1B demonstrates that patients with ADPKD1-2 had higher serum phosphate levels than those with XLH and only modestly reduced serum phosphate levels (mean difference, 0.07 mmol/L) compared with CKD1-2 patients and healthy volunteers. Thus, in patients with ADPKD1-2, although serum FGF23 levels were higher than in XLH patients, both the renal threshold of phosphate and serum phosphate levels were higher in polycystic patients, suggesting that the biologic activity of FGF23 was impaired in patients with ADPKD1-2.
Figure 1.
Fibroblast growth factor 23 (FGF23) versus tubular maximum phosphate reabsorption and serum phosphate. Scatter plots of FGF23 values versus (A) ratio of the maximum rate of tubular phosphate reabsorption to the GFR (TmP/GFR) and (B) serum phosphate of 99 patients with autosomal dominant polycystic kidney disease and CKD stage 1 and 2 (ADPKD1-2), 32 patients with CKD stage 1 and 2 not affected by polycystic kidney disease (CKD1-2), 12 patients with X-linked hypophosphatemia (XLH), and 20 healthy volunteers. Symbols represent individual patients, and lines indicate SDs.
Disturbance of the Hormonal Network Regulating Phosphate Metabolism in ADPKD
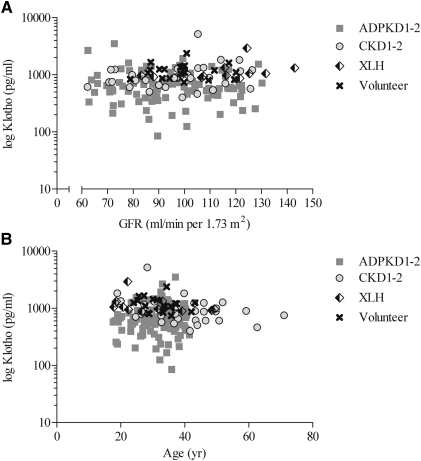
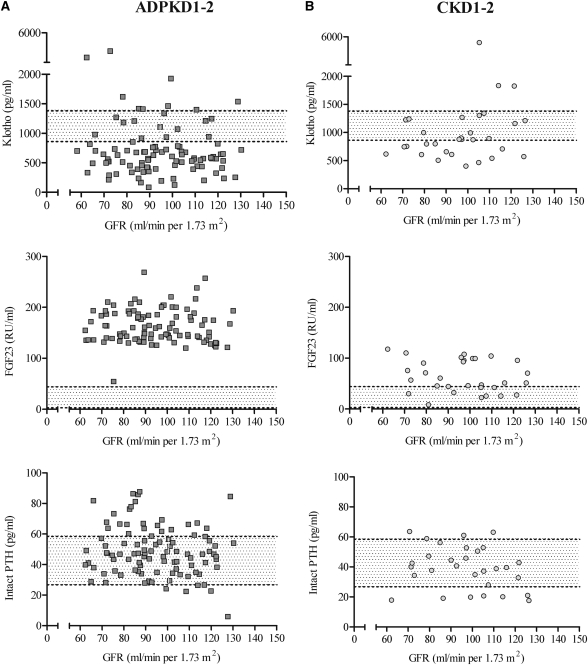
Patients with ADPKD1-2 had the lowest mean serum level of Klotho, the highest mean serum level of FGF23, and by far the highest serum FGF23-to-Klotho ratio, whereas patients with XLH had the lowest serum phosphate levels and the lowest value for TmP/GFR (Table 2). Parathyroid hormone levels were similar in all groups. Klotho levels were independent of GFR and age (Figure 2) in all groups. Figure 3 summarizes, for ADPKD1-2 (Figure 3A) and CKD1-2 patients (Figure 3B), the individual values of serum Klotho, FGF23 and parathyroid hormone values plotted as a function of GFR over the range of 60–130 mL/min per 1.73 m2; no correlation was observed. However, it is obvious from the graphs that patients with ADPKD1-2 had lower Klotho levels and higher FGF23 levels than CKD1-2 patients over the whole range of GFR. We also conducted a subset analysis measuring serum Klotho levels in six ADPKD patients with CKD stage 3. These results showed mean ± SD serum levels of 299.0±77.4 pg/ml. This finding concurs with the initial hypothesis that Klotho levels decrease as renal damage progresses.
Table 2.
Phosphate metabolism variables according to study group
| Variable | ADPKD1-2 (n=99) | CKD1-2 (n=32) | XLH (n=12) | Volunteer (n=20) |
|---|---|---|---|---|
| Klotho (pg/ml) | 719±503 | 1037±839 | 1187±569 | 1200±385 |
| mean difference from ADPKD (95% CI) | Reference | 318 (34 to 602)a | 468 (42 to 895)a | 481 (140 to 824)a |
| FGF23 (RU/ml) | 163±33 | 67±36 | 99±143 | 28±22 |
| mean difference from ADPKD (95% CI) | Reference | −96 (−120 to −72)a | −64 (−101 to −28)a | −136 (165 to −107)a |
| FGF23-to-Klotho ratio (RU/pg) | 0.33±0.25 | 0.09±0.07 | 0.08±0.11 | 0.02±0.02 |
| mean difference from ADPKD (95% CI) | Reference | −0.23 (−0.33 to -0.14)a | −0.24 (−0.39 to −0.10)a | −0.30 (−0.41 to −0.18)a |
| Parathyroid hormone (ng/ml) | 49±16 | 43±23 | 57±20 | 43±16 |
| mean difference from ADPKD (95% CI) | Reference | −6 (−15 to 2) | 8 (−5 to 20) | −6 (−16 to 5) |
| Serum phosphate (mmol/L) | 0.92±0.17 | 1.01±0.19 | 0.57±0.14 | 1.01±0.12 |
| mean difference from ADPKD (95% CI) | Reference | 0.08 (0.00 to 0.16)a | −0.35 (−0.47 to −0.23)a | 0.09 (−0.01 to 0.18) |
| TmP/GFR (mmol/L) | 0.81±0.18 | 0.89±0.20 | 0.41±0.13 | 0.89±0.12 |
| mean difference from ADPKD (95% CI) | Reference | 0.07 (−0.02 to 0.16) | −0.40 (−0.53 to −0.28)a | 0.07 (−0.03 to 0.17) |
Values expressed with a plus/minus sign are the least-square mean ± SD. ADPKD1-2, autosomal dominant polycystic kidney disease with CKD stage 1 and 2; CKD1-2, CKD stage 1 and 2 without polycystic kidney disease; XLH, X-linked hypophosphatemia; CI, confidence interval; FGF23, fibroblast growth factor 23; TmP/GFR, ratio of the maximum rate of tubular phosphate reabsorption to the GFR.
Significant at an α level of 0.05, post hoc Dunnett test with ADPKD values as reference.
Figure 2.
Klotho versus GFR and age. Scatter plots of values of serum α-Klotho versus (A) estimated GFR and (B) age of 99 patients with autosomal dominant polycystic kidney disease and CKD stage 1 and 2 (ADPKD1-2), 32 patients with CKD stage 1 and 2 not affected by polycystic kidney disease (CKD1-2), 12 patients with X-linked hypophosphatemia (XLH), and 20 healthy volunteers. Symbols represent individual patients.
Figure 3.
Klotho, fibroblast growth factor 23 (FGF23), and parathyroid hormone versus estimated GFR. Serum α-Klotho, FGF23, and intact parathyroid hormone (PTH) values versus estimated GFR in (A) 99 patients with autosomal dominant polycystic kidney disease and CKD stage 1 and 2 (ADPKD1-2) and (B) 32 patients with CKD stage 1 and 2 not affected by polycystic kidney disease (CKD1-2). Symbols represent individual patients, and dotted lines represent 25% and 75% quartiles of controls.
Low Soluble Klotho Levels Can Be Associated with Low FGF23 Activity in ADPKD
The data were stratified for a functional renal leak of phosphate (TmP/GFR ≥ 0.6 or < 0.6 mmol/L) (Table 3): Thirteen percent of ADPKD1-2 patients, 9% of CKD1-2 patients, 100% of XLH patients, and none of the volunteers had an apparent renal leak of phosphate. Strikingly, among the patients with an apparent renal leak of phosphate, serum levels of Klotho, FGF23, and parathyroid hormone were similar between the groups. On the other hand, among patients without renal leak of phosphate, ADPKD1-2 patients had significantly lower serum Klotho levels, higher serum FGF23 levels, and higher serum FGF23-to-Klotho ratios than CKD1-2 patients or healthy volunteers. In addition, serum Klotho levels were lower in ADPKD1-2 patients without renal phosphate leak than in those with renal leak (659 pg/ml in ADPKD1-2 patients without leak and 1105 pg/ml in ADPKD1-2 patients with leak; mean difference, 446 pg/ml [95% confidence interval, 161–731 pg/ml]). Age, GFR, gender distribution, and frequencies of angiotensin-converting enzyme inhibitor and angiotensin-receptor blocker use were similar in ADPKD1-2 patients with and in those without renal phosphate leak (data not shown).
Table 3.
Phosphate metabolism variables in the study groups, according to presence of renal phosphate leak
| Variable | No Apparent Renal Phosphate Leak (TmP/GFR ≥ 0.6 mmol/L) | Apparent Renal Phosphate Leak (TmP/GFR < 0.6 mmol/L) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ADPKD1-2 (n=86) | CKD1-2 (n=28) | Volunteer (n=20) | P Value | ADPKD1-2 (n=13) | CKD1-2 (n=4) | XLH (n=12) | P Value | |||
| TmP/GFR (mmol/L) | 0.84 | 0.90 | 0.88 | 0.45 | 0.58 | 0.41 | ||||
| Klotho (pg/ml) | 659 | 1016a | 1201a | <0.0001 | 1105 | 1185 | 1187 | 0.9 | ||
| mean difference from ADPKD (95% CI) | Reference | 357 (80 to 634) | 542 | (226 to 857) | Reference | 80 (−732 to 891) | 82 | (−486 to 651) | ||
| FGF23 (RU/ml) | 165 | 68a | 28a | <0.0001 | 152 | 59 | 99 | 0.2 | ||
| mean difference from ADPKD (95% CI) | Reference | −97 (−113 to −80) | −137 (−156 to −119) | Reference | −93 (−221 to 34) | −53 | (−143 to 36) | |||
| — | ||||||||||
| FGF23-to-Klotho ratio | 0.34 | 0.09a | 0.02a | <0.0001 | 0.20 | 0.06 | 0.08 | 0.05 | ||
| mean difference from ADPKD (95% CI) | Reference | −0.25 (−0.35 to −0.15) | −0.32 (−0.44 to −0.20) | Reference | −0.12 (−0.24 to 0.00) | −0.14 | (−0.32 to 0.03) | |||
| Parathyroid hormone (ng/ml) | 49 | 43 | 43 | 0.2 | 46 | 38 | 57 | 0.2 | ||
| mean difference from ADPKD (95% CI) | Reference | −6.0 (−14.9 to 3.0) | −6.0 (−16.0 to 4.0) | Reference | −8 (−32 to 16) | 10 (−6 to 27) | ||||
Values expressed with a plus/minus sign are the least-square mean. TMP/GFR, ratio of the maximum rate of tubular phosphate reabsorption to the GFR; ADPKD1-2, autosomal dominant polycystic kidney disease with CKD stage 1 and 2; CKD1-2, CKD stage 1 and 2 without polycystic kidney disease; XLH, X-linked hypophosphatemia; CI, confidence interval; FGF23, fibroblast growth factor 23.
Significant at an α level of 0.05, post hoc Dunnett test with ADPKD1-2 values as reference.
ADPKD Phenotype and Klotho
Patients with ADPKD1-2 had a mean ± SD total kidney volume of 970±533 cm3 and a mean total cyst volume of 561±426 cm3. The annual mean absolute and percentage kidney growth rates were 78±98 cm3 and 6.3%±6.9% per year, respectively. The annual mean absolute and percentage cyst growth rates were 98±91 cm3 and 23%±24% per year, respectively, for a median (interquartile range) follow-up of 36 (25–38) months.
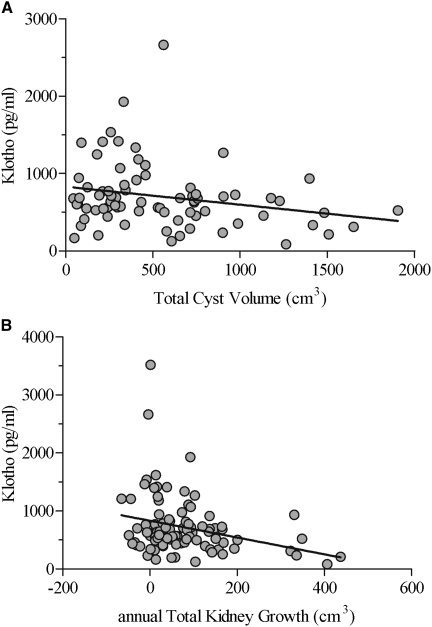
Figure 4 shows that serum Klotho values were inversely correlated with total cyst volume (r=−0.24; P<0.05) and with annual total kidney growth, both absolute (r=−0.29; P<0.01 [shown]) and percentage (r=−0.27; P<0.01 [not shown]). FGF23 values were not correlated with kidney volume or growth, whereas the FGF23-to-Klotho ratios were positively correlated with total cyst volume (r=0.22; P<0.05) as well as with absolute (r=0.39; P<0.0001) and percentage (r=0.28; P<0.01) kidney growth.
Figure 4.
Klotho versus total cyst volume and annual kidney growth. Serum α-Klotho values versus (A) total cyst volume and (B) annual kidney growth (median follow-up, 3 years) in 99 patients with autosomal dominant polycystic kidney disease and CKD 1 and 2 (ADPKD1-2). Symbols represent individual patients, and linear regression lines are drawn.
Discussion
Our study reveals a reduced biologic activity of FGF23 in patients with ADPKD1-2 that is characterized by low renal phosphate excretion in the face of excessive FGF23 levels. This finding was accompanied by relatively low serum Klotho levels, suggesting that the resistance to the phosphaturic effect of FGF23 could be mediated by a reduction of Klotho.
FGF23 is secreted by osteocytes and acts on the kidney to promote phosphate excretion into the urine. Higher serum levels of FGF23 have been described in several genetic diseases associated with high urinary phosphate excretion and hypophosphatemia, including patients with XLH. Our XLH patients displayed the characteristic features of renal phosphate wasting (“phosphate diabetes”; TmP/GFR was half that found in healthy volunteers), hypophosphatemia, high FGF23 serum levels (four-fold higher than the levels found in volunteers), short stature, and rickets in childhood; most of them still had impaired bone mineralization in adulthood despite treatment. In patients with XLH we also observed a positive relationship between FGF23 and phosphate wasting (inverse with TmP/GFR), whereas these associations were not present in patients with ADPKD1-2. Furthermore, patients with ADPKD1-2 had even higher FGF23 levels than patients with XLH, but the former did not exhibit renal phosphate wasting. These findings suggest a potential renal resistance to FGF23.
Studies in Klotho-deficient and FGF23-deficient mice have revealed that cofactor binding such as Klotho is essential to initiate FGFR1c activation and thus for FGF23 biologic activity (30). Even extremely high serum FGF23 levels in mice lacking Klotho do not cause a phosphate wasting phenotype, illustrating that the absence of Klotho confers resistance to FGF23 (31). Our data concur with the concept that Klotho is required in humans to permit FGF23 to accomplish its biologic activity. Excessive FGF23 secretion was seen in all patients with ADPKD1-2, but none of these patients had a phosphate wasting phenotype and only 13% had a low TmP/GFR. Remarkably, ADPKD1-2 patients with an elevated renal phosphate excretion had normal serum Klotho levels. However, the majority of patients with ADPKD1-2 had low serum Klotho levels, and we infer that this prevented phosphate wasting. Therefore, we conclude that in this particular inherited kidney disease associated with excessive FGF23 secretion, the loss of Klotho may have contributed to FGF23 resistance.
The mechanisms by which kidney disease decreases Klotho levels remain unknown. Klotho is expressed in tubular epithelial cell of the kidney, with predominant expression in the distal convoluted tubule (19,32). Likewise, cysts evolve from tubular epithelial cells of all parts of the nephrons and may start predominantly in the thick ascending loops of Henle, distal tubules, and collecting ducts in the corticomedullary region and outer medulla (32–34). Intriguingly, serum Klotho levels and cyst volume were inversely correlated with each other. Because we had no access to additional renal tissue samples to measure the level of molecular expression of Klotho as a transmembrane molecule in our patients, we could not clarify whether the level of transmembrane expression of Klotho is specifically reduced in patients with ADPKD. The extracellular domain of Klotho is shed on the cell surface after being cleaved by membrane-anchored proteases (ADAM 10 and 17) and is detected in the blood and cerebrospinal fluid in mice and humans (35,36). Serum (and urine) Klotho levels correlated with Klotho expression in the kidney of Klotho knock-in and knock-out mice, indicating that circulating Klotho may represent renal Klotho expression (37). This shedded soluble Klotho molecule acts as a hormone on the renal proximal tubule, where it has recently been postulated to pass through the basolateral membrane and to migrate through the cytosol; it ultimately reaches the brush-border membrane, where it causes endocytosis of the Na-Pi cotransporters (32). Thus, the low production of Klotho might be related to cyst-induced damage and be responsible, at least in part, for the absence of renal leak of phosphate despite the high levels of FGF23.
Our study results must be interpreted in the context of the study design: We had no access to kidney tissue. It remains speculative to declare at this current stage of knowledge that soluble Klotho levels mirror kidney Klotho expression in the kidney. Our cross-sectional study was also not designed to elucidate the sequential order of the observed disturbance of the FGF23 and Klotho secretion, and the mechanism causing excessive FGF23 secretion in ADPKD is still not well understood. We speculate that excessive FGF23 secretion and loss of Klotho are two independent processes in which the bone is the origin of the FGF23 production and the loss of Klotho in the polycystic kidney constrains the phosphaturic effect of FGF23.
In conclusion, the renal pattern of ADPKD, classically characterized by cyst development and growth, appears to include, in the early course of the disease, decreased Klotho availability constraining FGF23 activity. Loss of Klotho and the concomitant increase in FGF23 appear to exceed and precede changes mediated by the loss of GFR in patients with ADPKD and could be regarded as early markers of the renal disease in ADPKD.
Disclosures
None.
Acknowledgments
The authors thank Julia Hofmann, Ruth Russi, and Marian Struker for excellent technical assistance; Cornelia Zwimpfer for performing FGF23 assays in sera from XLH patients; and especially Marieluise Wipperman at TecoMedical Group for support with the Klotho test assays.
Funding was provided by the Swiss National Science Foundation (3100030_132597/1) to A.S. and the Center for Integrative Human Physiology at the University of Zurich to A.S. and C.W.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Fick GM, Gabow PA: Hereditary and acquired cystic disease of the kidney. Kidney Int 46: 951–964, 1994 [DOI] [PubMed] [Google Scholar]
- 2.Torres VE, Harris PC, Pirson Y: Autosomal dominant polycystic kidney disease. Lancet 369: 1287–1301, 2007 [DOI] [PubMed] [Google Scholar]
- 3.Kistler AD, Poster D, Krauer F, Weishaupt D, Raina S, Senn O, Binet I, Spanaus K, Wuthrich RP, Serra AL. Increases in kidney volume in autosomal dominant polycystic kidney disease can be detected within 6 months. Kidney Int 75:235–241, 2009. [DOI] [PubMed]
- 4.Poster D, Kistler AD, Krauer F, Blumenfeld JD, Rennert H, Weishaupt D, Wüthrich RP, Serra AL: Kidney function and volume progression in unilateral autosomal dominant polycystic kidney disease with contralateral renal agenesis or hypoplasia: A case series. Am J Kidney Dis 54: 450–458, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Wong H, Vivian L, Weiler G, Filler G: Patients with autosomal dominant polycystic kidney disease hyperfiltrate early in their disease. Am J Kidney Dis 43: 624–628, 2004 [DOI] [PubMed] [Google Scholar]
- 6.Ubaidus S, Li M, Sultana S, de Freitas PHL, Oda K, Maeda T, Takagi R, Amizuka N: FGF23 is mainly synthesized by osteocytes in the regularly distributed osteocytic lacunar canalicular system established after physiological bone remodeling. J Electron Microsc (Tokyo) 58: 381–392, 2009 [DOI] [PubMed] [Google Scholar]
- 7.Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD: Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab 291: E38–E49, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Shimada T, Mizutani S, Muto T, Yoneya T, Hino R, Takeda S, Takeuchi Y, Fujita T, Fukumoto S, Yamashita T: Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 98: 6500–6505, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jonsson KB, Zahradnik R, Larsson T, White KE, Sugimoto T, Imanishi Y, Yamamoto T, Hampson G, Koshiyama H, Ljunggren O, Oba K, Yang IM, Miyauchi A, Econs MJ, Lavigne J, Jüppner H: Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 348: 1656–1663, 2003 [DOI] [PubMed] [Google Scholar]
- 10.White KE, Evans WE, O'Riordan JLH, Speer MC, Econs MJ, Lorenz-Depiereux B, Grabowski M, Meitinger T, Strom TM. ADHR Consortium: Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 26: 345–348, 2000 [DOI] [PubMed] [Google Scholar]
- 11.White KE, Carn G, Lorenz-Depiereux B, Benet-Pages A, Strom TM, Econs MJ: Autosomal-dominant hypophosphatemic rickets (ADHR) mutations stabilize FGF-23. Kidney Int 60: 2079–2086, 2001 [DOI] [PubMed] [Google Scholar]
- 12.Lorenz-Depiereux B, Bastepe M, Benet-Pagès A, Amyere M, Wagenstaller J, Müller-Barth U, Badenhoop K, Kaiser SM, Rittmaster RS, Shlossberg AH, Olivares JL, Loris C, Ramos FJ, Glorieux F, Vikkula M, Jüppner H, Strom TM: DMP1 mutations in autosomal recessive hypophosphatemia implicate a bone matrix protein in the regulation of phosphate homeostasis. Nat Genet 38: 1248–1250, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE: Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38: 1310–1315, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Francis F, Hennig S, Korn B, Reinhardt R, de Jong P, Poustka A, Lehrach H, Rowe PSN, Goulding JN, Summerfield T, Mountford R, Read AP, Popowska E, Pronicka E, Davies KE, O'Riordan JLH, Econs MJ, Nesbitt T, Drezner MK, Oudet C, Pannetier S, Hanauer A, Strom TM, Meindl A, Lorenz B, Cagnoli B, Mohnike KL, Murken J, Meitinger T. The HYP Consortium: A gene (PEX) with homologies to endopeptidases is mutated in patients with X-linked hypophosphatemic rickets. Nat Genet 11: 130–136, 1995 [DOI] [PubMed] [Google Scholar]
- 15.Prié D, Ureña Torres P, Friedlander G: Latest findings in phosphate homeostasis. Kidney Int 75: 882–889, 2009 [DOI] [PubMed] [Google Scholar]
- 16.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T: Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010 [DOI] [PubMed] [Google Scholar]
- 17.Isakova T, Wahl P, Vargas GS, Gutiérrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CAM, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavik I, Jaeger P, Kistler AD, Poster D, Krauer F, Cavelti-Weder C, Rentsch KM, Wüthrich RP, Serra AL: Patients with autosomal dominant polycystic kidney disease have elevated fibroblast growth factor 23 levels and a renal leak of phosphate. Kidney Int 79: 234–240, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, Iwasaki H, Iida A, Shiraki-Iida T, Nishikawa S, Nagai R, Nabeshima YI: Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45–51, 1997 [DOI] [PubMed] [Google Scholar]
- 20.Matsumura Y, Aizawa H, Shiraki-Iida T, Nagai R, Kuro-o M, Nabeshima Y-i: Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 242: 626–630, 1998 [DOI] [PubMed] [Google Scholar]
- 21.Imura A, Tsuji Y, Murata M, Maeda R, Kubota K, Iwano A, Obuse C, Togashi K, Tominaga M, Kita N, Tomiyama K-i, Iijima J, Nabeshima Y, Fujioka M, Asato R, Tanaka S, Kojima K, Ito J, Nozaki K, Hashimoto N, Ito T, Nishio T, Uchiyama T, Fujimori T, Nabeshima Y-i: alpha-Klotho as a regulator of calcium homeostasis. Science 316: 1615–1618, 2007 [DOI] [PubMed] [Google Scholar]
- 22.Yamazaki Y, Imura A, Urakawa I, Shimada T, Murakami J, Aono Y, Hasegawa H, Yamashita T, Nakatani K, Saito Y, Okamoto N, Kurumatani N, Namba N, Kitaoka T, Ozono K, Sakai T, Hataya H, Ichikawa S, Imel EA, Econs MJ, Nabeshima Y-i: Establishment of sandwich ELISA for soluble alpha-Klotho measurement: Age-dependent change of soluble alpha-Klotho levels in healthy subjects. Biochem Biophys Res Commun 398: 513–518, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Serra AL, Kistler AD, Poster D, Struker M, Wüthrich RP, Weishaupt D, Tschirch F: Clinical proof-of-concept trial to assess the therapeutic effect of sirolimus in patients with autosomal dominant polycystic kidney disease: SUISSE ADPKD study. BMC Nephrol 8: 13, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P, Scheffel H, Weishaupt D, Wüthrich RP: Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363: 820–829, 2010 [DOI] [PubMed] [Google Scholar]
- 25.Bakker J, Olree M, Kaatee R, de Lange EE, Moons KGM, Beutler JJ, Beek FJA: Renal volume measurements: Accuracy and repeatability of US compared with that of MR imaging. Radiology 211: 623–628, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Ichikawa S, Imel EA, Kreiter ML, Yu X, Mackenzie DS, Sorenson AH, Goetz R, Mohammadi M, White KE, Econs MJ: A homozygous missense mutation in human KLOTHO causes severe tumoral calcinosis. J Clin Invest 117: 2684–2691, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodehl J, Krause A, Hoyer PF: Assessment of maximal tubular phosphate reabsorption: Comparison of direct measurement with the nomogram of Bijvoet. Pediatr Nephrol 2: 183–189, 1988 [DOI] [PubMed] [Google Scholar]
- 28.Payne RB: Renal tubular reabsorption of phosphate (TmP/GFR): Indications and interpretation. Ann Clin Biochem 35: 201–206, 1998 [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration): A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Urakawa I, Yamazaki Y, Shimada T, Iijima K, Hasegawa H, Okawa K, Fujita T, Fukumoto S, Yamashita T: Klotho converts canonical FGF receptor into a specific receptor for FGF23. Nature 444: 770–774, 2006 [DOI] [PubMed] [Google Scholar]
- 31.Nakatani T, Sarraj B, Ohnishi M, Densmore MJ, Taguchi T, Goetz R, Mohammadi M, Lanske B, Razzaque MS: In vivo genetic evidence for klotho-dependent, fibroblast growth factor 23 (Fgf23)-mediated regulation of systemic phosphate homeostasis. FASEB J 23: 433–441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu MC, Shi M, Zhang J, Pastor J, Nakatani T, Lanske B, Razzaque MS, Rosenblatt KP, Baum MG, Kuro-o M, Moe OW: Klotho: A novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 24: 3438–3450, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murcia NS, Sweeney WE, Jr, Avner ED: New insights into the molecular pathophysiology of polycystic kidney disease. Kidney Int 55: 1187–1197, 1999 [DOI] [PubMed] [Google Scholar]
- 34.Lager DJ, Qian Q, Bengal RJ, Ishibashi M, Torres VE: The pck rat: a new model that resembles human autosomal dominant polycystic kidney and liver disease. Kidney Int 59: 126–136, 2001 [DOI] [PubMed] [Google Scholar]
- 35.Kurosu H, Yamamoto M, Clark JD, Pastor JV, Nandi A, Gurnani P, McGuinness OP, Chikuda H, Yamaguchi M, Kawaguchi H, Shimomura I, Takayama Y, Herz J, Kahn CR, Rosenblatt KP, Kuro-o M: Suppression of aging in mice by the hormone Klotho. Science 309: 1829–1833, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen C-D, Podvin S, Gillespie E, Leeman SE, Abraham CR: Insulin stimulates the cleavage and release of the extracellular domain of Klotho by ADAM10 and ADAM17. Proc Natl Acad Sci USA 104: 19796–19801, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu MC, Shi M, Zhang J, Quiñones H, Griffith C, Kuro-o M, Moe OW: Klotho deficiency causes vascular calcification in chronic kidney disease. J Am Soc Nephrol 22: 124–136, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]