Summary
Background and objectives
Renal phosphate wasting occurs early postkidney transplantation as a result of an accumulation of parathyroid hormone and fibroblast growth factor 23 from the CKD period. Serum phosphate, parathyroid hormone, and fibroblast growth factor 23 return to baseline 1 year postkidney transplantation. What happens beyond this period is unknown.
Design, setting, participants, & measurements
Mineral parameters were obtained from 229 kidney transplant recipients at least 1 year posttransplantation; 46 normal subjects and 202 CKD patients with similar GFR served as controls. Factors associated with phosphate metabolism were analyzed.
Results
Despite the reduced graft function, most kidney transplant recipients had lower serum phosphate than normal subjects accompanied by renal phosphate loss. Fibroblast growth factor 23 was mostly lower or comparable with normal subjects, whereas parathyroid hormone was elevated in most patients. Hyperparathyroidism is also more common among kidney transplant recipients compared with CKD patients. Both parathyroid hormone and fibroblast growth factor 23 showed relationships with renal phosphate excretion, but only parathyroid hormone displayed an independent association. Parathyroid hormone showed the highest area under the curve in predicting renal phosphate leak. When patients were categorized according to parathyroid hormone and fibroblast growth factor 23 levels, only subset of patients with high parathyroid hormone had an increased renal phosphate excretion.
Conclusions
Relatively low serum phosphate from renal phosphate leak continued to present in long-term kidney transplantation. Both parathyroid hormone and fibroblast growth factor 23 participated in renal tubular phosphate handling, but persistent hyperparathyroidism seemed to have a greater influence in this setting.
Introduction
Hypophosphatemia commonly occurs early after kidney transplantation (KT) as a result of renal phosphate (Pi) wasting (1). In CKD, as GFR declines, phosphaturic hormones such as parathyroid hormone (PTH) and fibroblast growth factor 23 (FGF-23) accumulate in an attempt at inhibiting renal tubular Pi reabsorption counteracting Pi retention (2). Thus, serum Pi is kept within the normal range until later in the course of CKD. With a profound reduction of the kidney mass, despite a marked increase in FGF-23 and PTH, hyperphosphatemia ensues (3). As kidney function recovers after KT, the accumulated FGF-23 and PTH exaggerate renal tubular Pi leak, leading to Pi depletion (4,5). Serum Pi gradually increases, reaching the normal limits within 12 months in association with the decline in PTH and FGF-23 toward baseline (1,4).
What happens to Pi metabolism beyond this first year is not known. Serum Pi seems to be within the normal range, and PTH and FGF-23 may have reached the baseline. The data on renal tubular Pi handling in this later period is not well characterized. The present study explored Pi metabolism in long-term KT recipients who were at least 1 year postsurgery. Factors associated with renal Pi handling were analyzed. Normal subjects and CKD patients with equivalent GFR served as controls.
Materials and Methods
This study was approved by the ethical committee for research involving human subjects of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University, and it was conducted according to the Declaration of Helsinki. Informed consents were obtained from all participants.
Study Population
All patients were from the outpatient clinic of Ramathibodi Hospital and recruited consecutively according to the following inclusion criteria: KT recipients at least 1 year post-transplantation and predialysis CKD patients with serum creatinine ≥1.1 mg/dl in female or ≥1.2 mg/dl in male patients. Exclusion criteria were acute illness, acute kidney injury, or change of serum creatinine >10% within the past 3 months. None of the patients received noncalcium-containing phosphate binder, vitamin D analog, or calcimimetic, because the availability of these drugs in Thailand was limited. Active vitamin D available was calcitriol or alfacalcidol. Normal subjects were healthcare personnel of Ramathibodi Hospital who had a normal urinalysis and did not have a history of diabetes and/or kidney disease.
Biochemical Assessments
Fasting blood samples were collected between 7:00 and 9:00 am in all subjects. Serum calcium (Ca) was corrected based on the following equation: corrected Ca (mg/dl) = serum Ca + [(40 − serum albumin)/(10 × 0.8)]. Estimated GFR was calculated using an Modification of Diet in Renal Disease formula: eGFR (mL/min per 1.73 m2) = (186.3 × serum Cr−1.154 × age−0.203)(×0.742 if female). FGF-23 was analyzed by a two-site ELISA, which detected both intact molecules and C-terminal fragments (Immutopics, San Clemente, CA). The assay has been used in KT and CKD populations (2,5–8). 25-Hydroxyvitamin D (25-OH-D) was determined by a chemiluminescent immunoassay. 1,25-Dihydroxyvitamin D (1,25-OH-D) was measured in KT recipients who were not receiving active vitamin D and had sufficient blood samples for analysis (n=126) by a radioimmunoassay (Diasorin, Vercelli, Italy). Fractional excretion of Pi (FePi) was calculated from a 24-hour urine collection (between 7:00 am on the previous day and 7:00 am the next day) using the following equation: FePi (%) = [UPi × PCr × 100]/[PPi × UCr], as described previously (9,10). Tubular maximum Pi reabsorption/GFR was calculated as follows: tubular maximum phosphate reabsorption (TmP)/GFR (mg/dl) = serum Pi − (urine Pi/urine Cr) × serum Cr (11).
Statistical Analyses
Data are presented as mean ± SD or median (interquartile range) depending on if they pass a normality test. A t test was used to compare differences between two continuous variables. For non-normal distribution data, a Mann–Whitney U test was applied. The significance of trend of multiple groups was derived from linear by linear association in a chi-squared test. Univariate and multivariate regression analyses were used to assess associations between baseline demographics and all laboratory values. Log transformation was applied to non-normal distribution data. Receiver operating characteristic (ROC) curves were used to determine the area under the curve (AUC) of factors predicting renal Pi excretion. P<0.05 is considered statistically significant. All computations were performed using the PASW Statistics 18 (SPSS, Chicago, IL).
Results
Baseline Parameters
Baseline characteristics and laboratory values of all subjects are shown in Table 1. Serum Pi was within normal limits in 213 (93%) patients, below in 13 (6%) patients, and above in 3 (1%) patients. Serum Ca was within the normal range in 193 (84%) patients, low in 20 (9%) patients, and high in 16 (7%) patients. PTH was within normal limits (15–65 pg/ml) in 89 (39%) patients and high in 140 (61%) patients. FGF-23 was above 50 RU/ml in 22 (9.6%) patients and 100 RU/ml in 7 (3.1%) patients; 30% had 25-OH-D levels >30 ng/dl, 62% had levels between 15 and 30 ng/dl, and 8% had levels <15 ng/dl. According to the Kidney Disease Outcomes Quality Initiative guideline, 5 (2.2%) KT patients were in CKD stage 1, 95 (41.5%) patients were in stage 2, 106 (46.3%) patients were in stage 3, 20 (8.7%) patients were in stage 4, and 3 (1.3%) patients were in stage 5 (12). The average GFR of KT was higher than the GFR of CKD, because more KT recipients were in CKD stages 1 and 2 and fewer were in stages 3–5. All patients received corticosteroid with calcineurin inhibitors and/or mammalian target of rapamycin (mTOR) inhibitors. The third drug was mycophenolate mofetil or azathioprine.
Table 1.
Baseline characteristics and laboratory values of all subjects
| Parameters | KT (n=229) | Normal (n=46) | CKD (n=202) |
|---|---|---|---|
| Age (year) | 48.9±11.7 | 45.5±9.2 | 54.5±11.5 |
| Male sex (n/%) | 149 (65) | 22 (47.8) | 128 (63.4) |
| Body weight (kg) | 63±12.1 | 62.8±19.5 | 68.5±14.7 |
| DM (n/%) | 58 (25.3) | — | 104 (51.5) |
| Cadaveric KT (n/%) | 109 (48) | — | — |
| Duration of KT (yr)a | 5.7 (2.4–10) | — | — |
| Active vitamin D (n/%) | 21 (9.2) | — | 6 (3) |
| Nutritional vitamin D (n/%) | 0 (0) | — | 19 (9.4) |
| Calcium supplement (n/%) | 56 (12) | — | 47 (23.3) |
| Immunosuppressive drugs | |||
| corticosteroid (n/%) | 229 (100) | — | — |
| calcineurin inhibitor (n/%) | 201 (88) | — | — |
| mTOR inhibitor (n/%) | 31 (13) | — | — |
| Laboratory values | |||
| calcium (mg/dl)b | 9.6±0.7 | 9±0.4 | 9.3±0.5 |
| phosphate (mg/dl) | 3.3±0.6 | 3.8±0.5 | 3.7±0.6 |
| albumin (g/l) | 40.5±4.5 | 42.6±2.9 | 37.9±5.6 |
| PTH (pg/ml)a | 75 (54–110) | 36 (29–42) | 60 (39–84) |
| FGF-23 (RU/ml)a | 19 (11–29) | 18 (14–24) | — |
| 25-OH-D (ng/dl) | 26.1±8.6 | — | 22.1±8.7 |
| 1-25-OH-D (pg/ml)c | 48.5±25.5 | — | — |
| 24-hour urine Pi (g/day) | 0.53±0.2 | 0.45±0.2 | — |
| FePi (%) | 22.3±10 | 8.2±3.2 | — |
| TmP/GFR (mg/dl) | 2.6±0.6 | 3.5±0.5 | — |
| eGFR (ml/min per 1.73 m2)d | 55.7±19.3 | 99.4±22.3 | 43.5±16.3 |
| CKD stages (n/%) | |||
| stages 1 and 2 | 100 (43.7) | — | 30 (14.9) |
| stage 3 | 106 (46.3) | — | 126 (62.3) |
| stages 4 and 5 | 23 (10) | — | 46 (22.8) |
KT, kidney transplantation; DM, diabetes mellitus; mTOR, target of rapamycin; PTH, parathyroid hormone; FGF-23, fibroblast growth factor 23; 25-OH-D, 25-hydroxyvitamin D; 1,25-OH-D, 1,25-dihydroxyvitamin D; FePi, fractional excretion of phosphate; TmP/GFR; tubular maximum phosphate reabsorption/GFR.
Median (interquartile range).
Corrected calcium.
n=126 (Materials and Methods).
Calculated by the Modification of Diet in Renal Disease formula.
Mineral Parameters
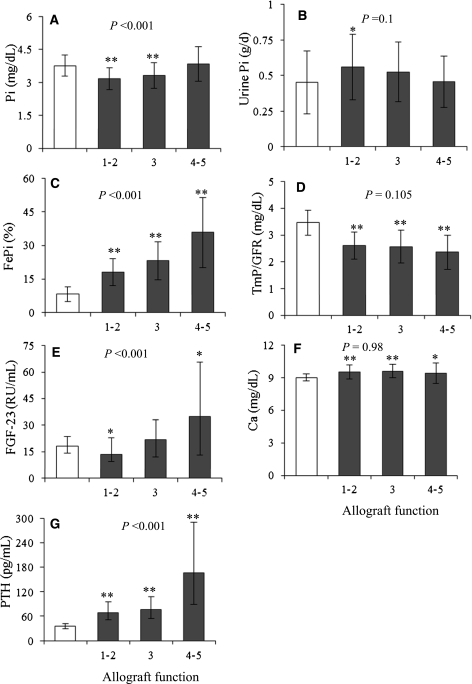
To characterize Pi metabolism in KT, mineral parameters were first compared with normal subjects according to the stages of allograft function (Figure 1). Serum Pi was mostly lower than the serum Pi of normal subjects (stages 1 and 2: 3.2±0.5 mg/dl, P<0.001; stage 3: 3.3±0.6 mg/dl, P<0.001) until stage 4 (3.9±0.8 mg/dl, P=0.6) when the difference became insignificant. Despite the lower serum Pi, 24-hour urine Pi (normal versus all KT, P=0.05) and FePi (normal versus all KT, P<0.001) were higher, and TmP/GFR (normal versus all KT, P<0.001) was lower, suggesting renal Pi leak. FGF-23 level was lower than the level of normal subjects in stages 1 and 2 (13 [9–23] RU/ml, P=0.009), comparable in stage 3 (22 [12–33] RU/ml, P=0.93), and only became elevated in stages 4 and 5 (35 [13–66] RU/ml, P=0.004) corresponding to serum Pi. However, PTH (normal versus all KT, P<0.001) as well as serum Ca (normal versus all KT, P<0.001) was higher than PTH and serum calcium of normal subjects at all levels of allograft function, indicating hyperparathyroidism (HPT). Despite the dysregulation of mineral metabolism, the relationship between the decline in allograft function and the increase in serum Pi, FePi, FGF-23 and PTH were preserved, denoted by the significance of P values for trends. Because of the presence of HPT and the absence of increased FGF-23 in most KT recipients, the magnitude of HPT was compared with predialysis CKD with equivalent GFR. CKD patients were slightly older and more diabetic compared with KT (P<0.01). Because there was no CKD patient in stage 1, five KT patients in stage 1 were excluded from the comparison. Three KT patients in stage 5 were analyzed together with those patients in stage 4. Serum PTH in KT was higher than in CKD at all levels of kidney function (Table 2). HPT is also more common among KT recipients. Higher PTH was associated with lower serum Pi and higher serum Ca in most KT patients, indicating a more severe degree of HPT. 25-OH-D in KT was mostly higher than in CKD, and vitamin D deficiency was less common among KT. FGF-23 in CKD was not obtained because of the absence of elevated FGF-23 in most KT recipients. Studies in predialysis CKD have documented a rise in FGF-23 starting from CKD stage 2; therefore, the lack of an increase in FGF-23 in KT argued against its accumulation in this later period (6,13,14).
Figure 1.
Comparisons of mineral parameters between normal subjects (white bars, n=46) and kidney transplantation (KT) recipients (black bars) categorized by the stages of kidney function (stages 1 and 2: n=99; stage 3: n=106; stages 4 and 5: n=23). (A) Serum phosphate, (B) 24-hour urine phosphate, (C) fractional excretion of phosphate, (D) tubular maximum phosphate reabsorption/GFR, (E) fibroblast growth factor 23 (FGF-23), (F) serum calcium, and (G) parathyroid hormone (PTH). P values in the graphs represent the significance of trends for allograft function stages 1–5. *P<0.05 versus normal subjects; **P<0.001 versus normal subjects.
Table 2.
Comparisons of mineral parameters between KT recipients and CKD patients according to stages of kidney function
| Parameters | Stage 2a | Stage 3b | Stages 4 and 5c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| CKD (n=30) | KT (n=95) | P Value | CKD (n=126) | KT (n=106) | P Value | CKD (n=43) | KT (n=23) | P Value | |
| Calcium (mg/dl) | 9.23±0.67 | 9.48±0.63 | 0.07 | 9.28±0.42 | 9.62±0.6 | <0.001 | 9.28±0.43 | 9.4±0.94 | 0.48 |
| N (% hypercalcemia) | 2 (6.7) | 3 (3.2) | 0.39 | 1 (0.8) | 10 (9.4) | 0.002 | 0 (0) | 1 (4.3) | 0.16 |
| Phosphate (mg/dl) | 3.52±0.56 | 3.19±0.49 | 0.002 | 3.66±0.53 | 3.31±0.59 | <0.001 | 4.08±0.58 | 3.85±0.79 | 0.22 |
| N (% hypophosphatemia) | 0 (0) | 2 (2.1) | 0.42 | 0 (0) | 9 (8.5) | 0.001 | 0 (0) | 1 (4.3) | 0.16 |
| PTH (pg/ml)d | 32 (27–70) | 69 (51–92) | <0.001 | 59 (39–79) | 76 (54–107) | <0.001 | 108 (59–168) | 167 (89–291) | 0.02 |
| N (% HPT) | 8 (27.6) | 51 (54.3) | 0.01 | 51 (41.8) | 69 (64.8) | 0.001 | 33 (73.3) | 20 (87) | 0.20 |
| 25-OH-D (ng/dl) | 23.5±11 | 25.6±8.6 | 0.35 | 21.9±8.9 | 26.6±8.3 | <0.001 | 21.9±6 | 27.1±10.5 | 0.03 |
| N (% insufficiency)e | 24 (80) | 67 (70.5) | 0.31 | 99 (79.8) | 74 (70.5) | 0.10 | 40 (87) | 14 (60.9) | 0.01 |
| eGFR (ml/min per 1.73 m2) | 68.8±8.3 | 71.2±8.1 | 0.16 | 45.4±8.4 | 47.2±8.8 | 0.11 | 21.9±6.3 | 22±6.2 | 0.98 |
HPT, hyperparathyroidism; eGFR, estimated GFR.
eGFR=60–89 ml/min per 1.73 m2.
eGFR=30–59 ml/min per 1.73 m2.
eGFR=0–29 ml/min per 1.73 m2.
Median (interquartile range).
25-OH-D<30 ng/dl.
Factors Associated with Pi Metabolism in KT
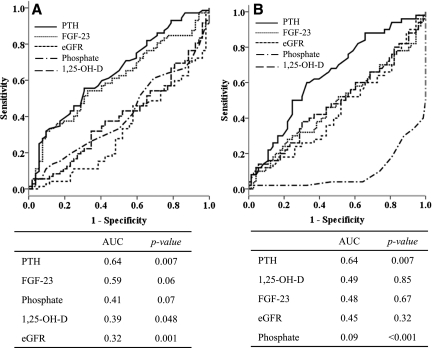
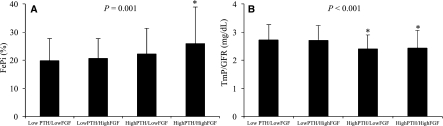
Regression analyses were performed to determine important factors associated with Pi metabolism in KT (Table 3). In univariate analysis, serum Pi showed an inverse relationship with GFR and serum Ca but correlated positively with the duration of KT and FGF-23. After adjustment, the decline in GFR and prolonged duration of KT were independent predictors of serum Pi. FePi increased as GFR declined in association with an increase in PTH and FGF-23. In multivariate models, only PTH and GFR were independently associated with FePi. The loss of significance of FGF-23 after adjustment suggested that PTH had a greater influence on renal Pi loss in this setting. As for TmP/GFR, significant association was observed with only serum Pi (r=0.692, P<0.001) and PTH (r=−0.249, P<0.001). There was no relationship between TmP/GFR and FGF-23 or other mineral parameters. Factors associated with PTH were next analyzed. Apart from FePi and TmP/GFR, high PTH also exhibited a positive relationship with FGF-23 and correlated negatively with the duration of KT, GFR, and 25-OH-D. Multivariate analysis revealed FGF-23, duration of KT, and 25-OH-D as independent predictors. These data indicated that PTH continued to decline with the prolonged duration of KT and the importance of 25-OH-D deficiency on a severity of HPT. Moreover, the close relationship between PTH and FGF-23 was shown. As for factors associated with FGF-23, in addition to positive relationships with serum Pi, PTH, and FePi mentioned above, FGF-23 also increased in association with a decline in GFR and a reduction in 1,25-OH-D. After adjustment, only PTH and 1,25-OH-D were significantly associated with FGF-23. FGF-23 suppresses 1,25-OH-D synthesis, and the inverse relationship is expected. The independent association between FGF-23 and PTH is again illustrated. There were no associations between mineral parameters and other demographic data. ROC curves analysis was used to determine important factors predicting renal Pi leak. Normal subjects in the present study had FePi ranging from 1.93% to 19.6% (other studies=0–20%) (9) and TmP/GFR between 2.5 and 4.4 mg/dl (other studies=2.3–4.3 mg/dl) (15); therefore, high FePi and low TmP/GFR were defined as FePi above the upper limit (>19.6) and TmP/GFR below the lower limit of normal subjects (<2.5). Variables that showed significant correlations with the parameters of renal Pi excretion were selected for ROC analyses. PTH displayed the highest AUC followed by FGF-23 in both models, indicating a greater influence of PTH on renal tubular Pi reabsorption (Figure 2). To further explore the roles of PTH and FGF-23 in this setting, KT patients were categorized according PTH and FGF-23 levels into low (below median) or high (above median) (Figure 3). Only subjects with increased PTH displayed an increase in FePi and a decrease TmP/GFR compared with subjects with low PTH and FGF-23. Nevertheless, trends to an increase in renal Pi excretion with increasing FGF and PTH were discernable. Excluding patients who were taking vitamin D from analyses did not change the study results.
Table 3.
Regression analyses of factors associated with phosphate metabolism in KT recipients (n=229)
| Independent Variables | Serum Phosphate | FePi | Log PTH | Log FGF-23 | ||||
|---|---|---|---|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | Univariate | Multivariate | |
| Log durationa | 0.2 (0.002) | 0.24 (0.005) | 0.02 (0.78) | — | −0.13 (0.048) | −0.15 (0.009) | 0.006 (0.93) | — |
| Calcium | −0.18 (0.008) | −0.012 (0.88) | 0.06 (0.38) | — | 0.01 (0.86) | — | 0.02 (0.8) | — |
| Phosphate | — | — | 0.02 (0.78) | — | 0.05 (0.5) | — | 0.13 (0.045) | 0.15 (0.1) |
| Log PTH | 0.05 (0.5) | — | 0.43 (<0.001) | 0.32 (<0.001) | — | — | 0.25 (<0.001) | 0.32 (0.001) |
| Log FGF-23 | 0.13 (0.045) | 0.15 (0.1) | 0.18 (0.006) | −0.05 (0.55) | 0.26 (<0.001) | 0.17 (0.006) | — | — |
| 1,25-OH-D | −0.12 (0.2) | 0.13 (0.16) | −0.26 (0.004) | −0.15 (0.09) | 0.03 (0.77) | — | −0.33 (<0.001) | −0.32 (0.001) |
| 25-OH-D | −0.01 (0.89) | — | −0.05 (0.47) | — | −0.22 (0.001) | −0.2 (0.001) | −0.05 (0.47) | — |
| eGFR | −0.36 (<0.001) | −0.36 (<0.001) | −0.55 (<0.001) | −0.34 (<0.001) | −0.35 (<0.001) | −0.13 (0.053) | −0.29 (<0.001) | −0.04 (0.71) |
| FePi | 0.02 (0.78) | — | — | — | 0.43 (<0.001) | 0.32 (<0.001) | 0.18 (0.006) | −0.03 (0.73) |
Results are presented as correlation coefficient (P value). Only variables that have significant association with P<0.2 were included in multivariate analysis.
Duration of KT.
Figure 2.
Receiver operating characteristic curves analyses of factors associated with renal tubular phosphate leak in KT recipients defined by fractional excretion of phosphate (FePi) and tubular maximum phosphate reabsorption/GFR (TmP/GFR) above and below the normal limits of normal subjects, respectively. (A) FePi and (B) TmP/GFR. AUC, area under the curve.
Figure 3.
Parameters of renal tubular phosphate excretion according to PTH and FGF-23 levels (low, below median; high, above median). (A) FePi and (B) TmP/GFR. *P<0.05 versus low PTH/low FGF-23. P values in the graphs represent the significance of trends of all groups.
Role of mTOR Inhibitor in Renal Tubular Pi Handling in KT
A recent study suggested that the mTOR inhibitor may promote renal Pi leak (16); therefore, factors associated with Pi metabolism were compared between subgroups of patients who were taking and not taking the drug (Table 4). In the present study, there was no difference in serum phosphate, PTH, FGF-23, and parameters of renal Pi excretion in KT recipients who were on and not on mTOR inhibitor.
Table 4.
Comparisons of factors associated with phosphate metabolism in KT recipients with regard to the use of mTOR inhibitors
| Parameters | Stages 1 and 2a | Stage 3b | Stages 4 and 5c | ||||||
|---|---|---|---|---|---|---|---|---|---|
| (+) mTOR (n=5) | (−) mTOR (n=94) | P Value | (+) mTOR (n=20) | (−) mTOR (n=87) | P Value | (+) mTOR (n=6) | (−) mTOR (n=17) | P Value | |
| Calcium (mg/dl) | 9.6±0.58 | 9.51±0.65 | 0.76 | 9.46±0.57 | 9.66±0.61 | 0.17 | 9±0.83 | 9.5±0.96 | 0.26 |
| Phosphate (mg/dl) | 2.94±0.66 | 3.19±0.49 | 0.45 | 3.36±0.56 | 3.31±0.6 | 0.73 | 4±1 | 3.8±0.73 | 0.67 |
| PTH (pg/ml)d | 53 (32-82) | 69 (51–96) | 0.29 | 69 (55–122) | 78 (53–106) | 0.83 | 162 (89–491) | 167 (90–327) | 0.81 |
| FGF-23 (RU/ml)d | 13 (2–25) | 14 (9–23) | 0.44 | 18 (10–31) | 23 (13–34) | 0.29 | 13 (9–91) | 41 (20–65) | 0.36 |
| 24-Hour urine Pi (g/d) | 0.38±0.16 | 0.56±0.23 | 0.07 | 0.56±0.22 | 0.53±0.21 | 0.59 | 0.48±0.19 | 0.44±0.19 | 0.68 |
| FePi (%) | 16.9±7.7 | 18.2±6.1 | 0.72 | 26.5±11.4 | 22.5±7.6 | 0.15 | 44±20.7 | 32.9±12.9 | 0.26 |
| TmP/GFR (mg/dl) | 2.5±0.73 | 2.62±0.5 | 0.74 | 2.49±0.66 | 2.59±0.6 | 0.54 | 2.12±0.8 | 2.45±0.57 | 0.38 |
| eGFR (ml/min per 1.73 m2) | 73±9.3 | 72.8±11.8 | 0.97 | 44.4±10 | 47.9±8.4 | 0.16 | 20.4±9.5 | 22.5±4.8 | 0.63 |
eGFR, estimated GFR.
eGFR≥60 ml/min per 1.73 m2.
eGFR=30–59 ml/min per 1.73 m2.
eGFR=0–29 ml/min per 1.73 m2.
Median (interquartile range).
Discussion
The present study revealed the evidence of continuing renal Pi loss in long-term KT. Most KT patients had lower serum Pi than normal subjects in association with a decrease in renal tubular Pi reabsorption. FGF-23 was lower or comparable with normal subjects, whereas PTH was elevated in most patients. KT patients also had a greater degree of HPT compared with CKD. Both PTH and FGF-23 showed relationships with renal Pi excretion, but only PTH showed an independent association. PTH also displayed the highest AUC in predicting renal Pi leak. Only a subset of patients with increased PTH displayed an increase in renal Pi excretion.
Hypophosphatemia in the early period post-KT is well characterized and seems to relate to renal Pi wasting. Renal tubular Pi reabsorption recovers rapidly during the first 3 months and continues to increase thereafter, reaching the level of CKD with a comparable degree of renal impairment approximately 12 months after KT (1,17,18). The state of mineral metabolism after this first year has not been extensively studied. The present study revealed the evidence suggesting a continuing impairment in renal Pi reabsorption after the first year. Overall, serum Pi of KT patients was mostly within a normal range, with few patients having low values. Compared with normal subjects, despite the reduced graft function, the decrease in serum Pi became evident. This was associated with a reduction in renal tubular Pi reabsorption. Serum Pi in KT recipients was even lower than in CKD patients with equivalent GFR. A previous study that followed KT recipients for 1 year documented lower serum Pi relative to CKD (1). Another study reported a return of serum Pi to normal limits after 1 year, with some displaying low levels along with a generalized decrease in TmP/GFR, suggesting renal Pi leak (19). Similarly, in the present study, the decrease in Pi reabsorption was documented based on high FePi and low TmP/GFR in the presence of relatively low serum Pi.
Additional analyses of factors associated with renal Pi excretion revealed relatively low FGF-23 but elevated PTH compared with normal subjects. Both PTH and FGF-23 displayed relationships with renal Pi excretion in univariate analysis; however, after adjustment, only PTH retained a significant association, suggesting a greater influence of PTH over FGF-23. With patients categorized according to PTH and FGF-23 levels, only those patients with increased PTH displayed the evidence of increased renal Pi loss. Nevertheless, the role of FGF-23 in renal tubular Pi handling was still discernible, which was suggested by the trend to an increase in renal Pi excretion with increasing FGF-23. This observation is in contrast to the earlier period post-KT, when FGF-23 was reportedly an independent predictor of FePi and hypophosphatemia was attributed to an excessive FGF-23 level (1,5,18). A recent study that followed patients after KT for 1 year also observed a decline in FGF-23 toward baseline, whereas PTH remained elevated in most patients (19). Despite the authors’ conclusion that persistent HPT was responsible for low serum Pi, pretransplant FGF-23 still had the power in predicting low serum Pi at 1 year. Another study described 10 hypophosphatemic patients 3–14 years post-KT, and a high prevalence of persistent HPT (80%) was observed (20). Similarly, in the present study, up to 61% of patients had PTH above the upper normal limit. Compared with CKD, higher PTH in association with lower serum Pi and higher serum Ca was observed in KT, which was likely the result of persistent HPT from the dialysis period. The loss of a significant relationship between PTH and GFR in the multivariate model confirmed the importance of pre-existing HPT, which never completely resolved despite the recovery of kidney function. Nevertheless, data also suggested continuing decline in PTH with the prolonged duration of KT, and the resolution of HPT may take several years. Two other studies in long-term KT reported a high prevalence of HPT ranging from 45% to 81% (21,22). The relationship between low 25-OH-D and HPT has been well documented (23–26). Despite the presence of pre-existing HPT, the inverse relationship between 25-OH-D and PTH was maintained, suggesting that an inadequate vitamin D store posed a significant influence on the severity of HPT. Because of the higher prevalence of diabetes in CKD in the present study, most KT recipients seemed to have higher 25-OH-D levels compared with CKD. However, despite the superior vitamin D store, HPT is more common among KT. FGF-23 suppresses 1,25-OH-D synthesis, and their opposing relationship has often been documented (27,28). The association between FGF-23 and PTH has been described in CKD (14). FGF-23 may directly inhibit PTH secretion, and a direct feedback loop between the two hormones may exist (29,30).
Ca, Pi, PTH, and FGF-23 have been shown to predict several important outcomes, including all-cause and cardiovascular mortality (7,31). Increasing serum Pi even within the normal range was reportedly related to an increased mortality in CKD, KT, and general population, whereas low normal serum Pi was associated with an improved survival (32–34). Recent data in KT also suggested that low FGF-23 may be associated with better graft and patient survival (35). However, chronically low serum Pi is associated with glucose intolerance and obesity (36). Phosphaturia in the presence of hypercalciuria may also lead to negative calcium and phosphate balance and osteoporosis. Whether the alteration in Pi metabolism in long-term KT affects outcomes requires additional study.
Few differences in baseline characteristics were present; for example, CKD patients were older than KT recipients. In the general population, serum Pi declines slightly with age (37). However, despite younger age, serum Pi in KT was lower than in CKD. The use of C-terminal FGF-23 assay may raise concern regarding the accumulation of C-terminal fragments in subjects with reduced kidney function. To date, there has been no concrete evidence supporting this notion, and C-terminal assay has been used extensively in studies that included subjects with reduced kidney function (2,5–8). Moreover, FGF-23 level in KT was low, which argued against its accumulation.
In conclusion, relatively low serum Pi and the evidence of renal Pi leak continued to present in long-term KT. Both PTH and FGF-23 participated in renal tubular Pi handling; however, persistent HPT seemed to have a greater influence on renal Pi loss in this later period.
Disclosures
None.
Acknowledgments
This work was supported by Thai Transplantation Society, Faculty of Medicine, Ramathibodi Hospital, Mahidol University Grant 52056/2009 and the Kidney Foundation of Thailand.
Part of the abstract was presented at the American Society of Nephrology Renal Week 2010 and the 12th Congress of the Asian Society of Transplantation.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Evenepoel P, Meijers BK, de Jonge H, Naesens M, Bammens B, Claes K, Kuypers D, Vanrenterghem Y: Recovery of hyperphosphatoninism and renal phosphorus wasting one year after successful renal transplantation. Clin J Am Soc Nephrol 3: 1829–1836, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, Juppner H, Wolf M: Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol 16: 2205–2215, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Imanishi Y, Inaba M, Nakatsuka K, Nagasue K, Okuno S, Yoshihara A, Miura M, Miyauchi A, Kobayashi K, Miki T, Shoji T, Ishimura E, Nishizawa Y: FGF-23 in patients with end-stage renal disease on hemodialysis. Kidney Int 65: 1943–1946, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Claesson K, Hellman P, Frodin L, Rastad J: Prospective study of calcium homeostasis after renal transplantation. World J Surg 22: 635–641, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Bhan I, Shah A, Holmes J, Isakova T, Gutierrez O, Burnett SM, Juppner H, Wolf M: Post-transplant hypophosphatemia: Tertiary 'hyper-phosphatoninism'? Kidney Int 70: 1486–1494, 2006 [DOI] [PubMed] [Google Scholar]
- 6.Larsson T, Nisbeth U, Ljunggren O, Juppner H, Jonsson KB: Circulating concentration of FGF-23 increases as renal function declines in patients with chronic kidney disease, but does not change in response to variation in phosphate intake in healthy volunteers. Kidney Int 64: 2272–2279, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Gutiérrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, Smith K, Lee H, Thadhani R, Juppner H, Wolf M: Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med 359: 584–592, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu CY, Lash J, Leonard M, Kusek JW, Feldman HI, Wolf M: Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA 305: 2432–2439, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS: Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 21: 1187–1196, 2006 [DOI] [PubMed] [Google Scholar]
- 10.Evenepoel P, Meijers B, Viaene L, Bammens B, Claes K, Kuypers D, Vanderschueren D, Vanrenterghem Y: Fibroblast growth factor-23 in early chronic kidney disease: additional support in favor of a phosphate-centric paradigm for the pathogenesis of secondary hyperparathyroidism. Clin J Am Soc Nephrol 5: 1268–1276, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parfitt AM: Misconceptions IV—the hypophosphatemia of primary hyperparathyroidism is the result of renal phosphate wasting. Bone 35: 345–347, 2004 [DOI] [PubMed] [Google Scholar]
- 12.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am J Kidney Dis 39[2 Suppl 1]: S81–S112, 2002 [PubMed] [Google Scholar]
- 13.Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, Appleby D, Nessel L, Bellovich K, Chen J, Hamm L, Gadegbeku C, Horwitz E, Townsend RR, Anderson CA, Lash JP, Hsu CY, Leonard MB, Wolf M: Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int 79: 1370–1378, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fliser D, Kollerits B, Neyer U, Ankerst DP, Lhotta K, Lingenhel A, Ritz E, Kronenberg F, Kuen E, Konig P, Kraatz G, Mann JF, Muller GA, Kohler H, Riegler P: Fibroblast growth factor 23 (FGF23) predicts progression of chronic kidney disease: The Mild to Moderate Kidney Disease (MMKD) Study. J Am Soc Nephrol 18: 2600–2608, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Karim Z, Gerard B, Bakouh N, Alili R, Leroy C, Beck L, Silve C, Planelles G, Urena-Torres P, Grandchamp B, Friedlander G, Prie D: NHERF1 mutations and responsiveness of renal parathyroid hormone. N Engl J Med 359: 1128–1135, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Tataranni T, Biondi G, Cariello M, Mangino M, Colucci G, Rutigliano M, Ditonno P, Schena FP, Gesualdo L, Grandaliano G: Rapamycin-induced hypophosphatemia and insulin resistance are associated with mTORC2 activation and Klotho expression. Am J Transplant 11: 1656–1664, 2011 [DOI] [PubMed] [Google Scholar]
- 17.Kawarazaki H, Shibagaki Y, Shimizu H, Kawarazaki W, Ito N, Ishikawa A, Fukumoto S, Fujita T: Persistent high level of fibroblast growth factor 23 as a cause of post-renal transplant hypophosphatemia. Clin Exp Nephrol 11: 255–257, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Evenepoel P, Naesens M, Claes K, Kuypers D, Vanrenterghem Y: Tertiary 'hyperphosphatoninism' accentuates hypophosphatemia and suppresses calcitriol levels in renal transplant recipients. Am J Transplant 7: 1193–1200, 2007 [DOI] [PubMed] [Google Scholar]
- 19.Kawarazaki H, Shibagaki Y, Fukumoto S, Kido R, Nakajima I, Fuchinoue S, Fujita T, Fukagawa M, Teraoka S: The relative role of fibroblast growth factor 23 and parathyroid hormone in predicting future hypophosphatemia and hypercalcemia after living donor kidney transplantation: A 1-year prospective observational study. Nephrol Dial Transplant 26: 2691–2695, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Felsenfeld AJ, Gutman RA, Drezner M, Llach F: Hypophosphatemia in long-term renal transplant recipients: Effects on bone histology and 1,25-dihydroxycholecalciferol. Miner Electrolyte Metab 12: 333–341, 1986 [PubMed] [Google Scholar]
- 21.Cayco AV, Wysolmerski J, Simpson C, Mitnick MA, Gundberg C, Kliger A, Lorber M, Silver D, Basadonna G, Friedman A, Insogna K, Cruz D, Bia M: Posttransplant bone disease: Evidence for a high bone resorption state. Transplantation 70: 1722–1728, 2000 [DOI] [PubMed] [Google Scholar]
- 22.Giannini S, D'Angelo A, Carraro G, Antonello A, Di Landro D, Marchini F, Plebani M, Zaninotto M, Rigotti P, Sartori L, Crepaldi G: Persistently increased bone turnover and low bone density in long-term survivors to kidney transplantation. Clin Nephrol 56: 353–363, 2001 [PubMed] [Google Scholar]
- 23.Ewers B, Gasbjerg A, Moelgaard C, Frederiksen AM, Marckmann P: Vitamin D status in kidney transplant patients: Need for intensified routine supplementation. Am J Clin Nutr 87: 431–437, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Holick MF, Chen TC: Vitamin D deficiency: A worldwide problem with health consequences. Am J Clin Nutr 87: 1080S–1086S, 2008 [DOI] [PubMed] [Google Scholar]
- 25.LaClair RE, Hellman RN, Karp SL, Kraus M, Ofner S, Li Q, Graves KL, Moe SM: Prevalence of calcidiol deficiency in CKD: A cross-sectional study across latitudes in the United States. Am J Kidney Dis 45: 1026–1033, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Stavroulopoulos A, Cassidy MJ, Porter CJ, Hosking DJ, Roe SD: Vitamin D status in renal transplant recipients. Am J Transplant 7: 2546–2552, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Shimada T, Hasegawa H, Yamazaki Y, Muto T, Hino R, Takeuchi Y, Fujita T, Nakahara K, Fukumoto S, Yamashita T: FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res 19: 429–435, 2004 [DOI] [PubMed] [Google Scholar]
- 28.Hasegawa H, Nagano N, Urakawa I, Yamazaki Y, Iijima K, Fujita T, Yamashita T, Fukumoto S, Shimada T: Direct evidence for a causative role of FGF23 in the abnormal renal phosphate handling and vitamin D metabolism in rats with early-stage chronic kidney disease. Kidney Int 78: 975–980, 2010 [DOI] [PubMed] [Google Scholar]
- 29.Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J: The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117: 4003–4008, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krajisnik T, Bjorklund P, Marsell R, Ljunggren O, Akerstrom G, Jonsson KB, Westin G, Larsson TE: Fibroblast growth factor-23 regulates parathyroid hormone and 1alpha-hydroxylase expression in cultured bovine parathyroid cells. J Endocrinol 195: 125–131, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Kestenbaum B, Sampson JN, Rudser KD, Patterson DJ, Seliger SL, Young B, Sherrard DJ, Andress DL: Serum phosphate levels and mortality risk among people with chronic kidney disease. J Am Soc Nephrol 16: 520–528, 2005 [DOI] [PubMed] [Google Scholar]
- 33.Connolly GM, Cunningham R, McNamee PT, Young IS, Maxwell AP: Elevated serum phosphate predicts mortality in renal transplant recipients. Transplantation 87: 1040–1044, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Larsson TE, Olauson H, Hagstrom E, Ingelsson E, Arnlov J, Lind L, Sundstrom J: Conjoint effects of serum calcium and phosphate on risk of total, cardiovascular, and noncardiovascular mortality in the community. Arterioscler Thromb Vasc Biol 30: 333–339, 2010 [DOI] [PubMed] [Google Scholar]
- 35.Wolf M, Molnar MZ, Amaral AP, Czira ME, Rudas A, Ujszaszi A, Kiss I, Rosivall L, Kosa J, Lakatos P, Kovesdy CP, Mucsi I: Elevated fibroblast growth factor 23 is a risk factor for kidney transplant loss and mortality. J Am Soc Nephrol 22: 956–966, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunelli SM, Goldfarb S: Hypophosphatemia: Clinical consequences and management. J Am Soc Nephrol 18: 1999–2003, 2007 [DOI] [PubMed] [Google Scholar]
- 37.Cirillo M, Ciacci C, De Santo NG: Age, renal tubular phosphate reabsorption, and serum phosphate levels in adults. N Engl J Med 359: 864–866, 2008 [DOI] [PubMed] [Google Scholar]