Summary
Background and objectives
Both prolactin clearance and production are altered in CKD. In nonrenal populations, emerging evidence suggests that prolactin participates in the atherosclerotic process. Given the elevated cardiovascular risk of CKD, this study examined links between prolactinemia, vascular derangements, and outcomes.
Design, setting, participants, & measurements
This observational study was conducted in two cohorts: one with 457 nondialyzed CKD patients (mean age 52±12 years; 229 men) with measurements of flow-mediated dilation (FMD) and carotid intima-media thickness and one with 173 hemodialysis patients (65±12 years; 111 men) with measurements of pulse wave velocity (PWV). Patients were followed for cardiovascular events (n=146, nondialyzed cohort) or death (n=79, hemodialysis cohort).
Results
Prolactin levels increased along with reduced kidney function. Prolactin significantly and independently contributed to explain the variance of both FMD (in nondialyzed patients) and PWV (in hemodialysis patients), but not intima-media thickness. In Cox analyses, the risk of cardiovascular events in nondialyzed patients increased by 27% (hazard ratio [HR], 1.27; 95% confidence interval [95% CI], 1.17–1.38) for each 10 ng/ml increment of prolactin. Similarly, the risk for all-cause and cardiovascular mortality in hemodialysis patients increased by 12% (HR, 1.12; 95% CI, 1.06–1.17) and 15% (HR, 1.15; 95% CI, 1.08–1.21), respectively. This was true after multivariate adjustment for confounders and after adjustment within the purported causal pathway (FMD or PWV).
Conclusions
Prolactin levels directly associated with endothelial dysfunction/stiffness and with increased risk of cardiovascular events and mortality in two independent cohorts of CKD patients.
Introduction
CKD and progression toward ESRD expose patients to premature vascular disease and excess cardiovascular morbidity and mortality (1,2). The reasons for the elevated risk of cardiovascular disease (CVD) in patients with CKD are not fully elucidated (3,4). Prolactin is a hormone secreted by various tissues in addition to the anterior pituitary gland and its biologic action in women is to control breast development and lactation. However, the role of prolactin in men remains unclear. In CKD, prolactin levels appear substantially elevated, with a prevalence of hyperprolactinemia ranging from 30% to 65% (5–7). Hyperprolactinemia in CKD is the consequence of both reduced renal clearance (7) and increased production (8,9).
Emerging, yet incipient, evidence indicates that prolactin may have several biologic actions that participate in the atherosclerotic process. For example, prolactin elevations occur in essential hypertension (10) and during the acute phase of coronary syndromes, ischemic strokes, transient ischemic attacks (11–13), and preeclampsia (14,15), playing a causative role in the heart failure that accompanies postpartum cardiomyopathy (16). Prolactin levels predicted major cardiovascular events in men with erectile dysfunction (17), and increased expression of prolactin receptors was found in advanced human atherosclerotic plaques (18). At present, little is known about the implications of hyperprolactinemia in CKD. This may be relevant because testosterone deficiency, partly a consequence of prolactin-induced inhibition of gonadotropins, has been associated with cardiovascular risk factors and mortality in CKD (19–24). We hypothesized that prolactin retention in CKD may be a contributing factor to vascular derangements and worse (cardiovascular) outcomes, and we tested this in two independent materials with prospective follow-up, including a cohort of nondialysis CKD patients with assessments of flow-mediated dilation (FMD) and carotid intima-media thickness (IMT), as well as a cohort of prevalent hemodialysis patients with assessments of pulse wave velocity (PWV).
Materials and Methods
Patients
This is an observational study performed in two existing patient cohorts with prospective follow-up and in which prolactin levels were assessed a posteriori from stored samples. The first cohort is composed of prevalent patients that were referred to the Renal Unit of the Gulhane School of Medicine Medical Center, Ankara, Turkey, for the first time between January 2005 and August 2010 because of suspected or manifest renal failure. All patients were diagnosed as having CKD according to their estimated GFR (eGFR) (25) and the presence of kidney injury as defined by National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines. The protocol for this patient material was described in more detail elsewhere (26), and was approved by the local ethical committee. This cohort was designed to study determinants of endothelial function on nondialyzed CKD patients; therefore, a priori exclusion criteria included use of angiotensin converting enzyme inhibitors, angiotensin receptor blockers, statins, erythropoietin, or supplemental vitamin pills. Additional exclusion criteria considered acute infections and unwillingness to participate. In total, 457 patients with a mean age of 52±12 years were included. The causes of CKD were diabetes (n=109), GN (n=67), hypertensive nephropathy (n=83), reflux nephropathy (n=16), autosomal polycystic kidney disease (n=39), and unknown (n=143). At the time of determination, 59 patients were receiving antihypertensive therapy and 109 patients were receiving antidiabetic therapy. Twenty-three (5%) patients were consuming medication reported to stimulate prolactin production (27), including risperidone (n=5), olanzapine (n=7), verapamil (n=6), and metoclopramide (n=5). Eighty-six patients (19%) had a history of CVD, estimated according to symptoms (angina pain, claudication), cardiovascular imaging (ischemic electrocardiogram findings, positive angiography results), or medical history of acute coronary syndrome, transient cerebral ischemia, or peripheral or coronary revascularization.
The second cohort included prevalent hemodialysis patients from the dialysis units of the University Hospital of Heraklion, General Hospital of Chania and General Hospital of Chios, Greece. Between October 2005 and December 2006, all patients receiving hemodialysis were invited to participate. Exclusion criteria were hemodialysis treatment of <6 months, concurrent inflammatory illness, malignancy, overt endocrine disease, and use of exogenous hormones and bromocriptine before study enrolment. We enrolled 173 patients (111 men). Dialysis treatments were hemodialysis thrice weekly (minimum 4 hours) using bicarbonate dialysate and either high-flux (28%) or low-flux membranes. This cohort was designed to investigate the effect of sex hormones on CVD risk markers and especially on PWV. The protocol for this cohort was described in more detail elsewhere (24) and was approved by the scientific committees of the participating centers. Data pertaining to history of CVD (n=84), diabetes (n=31), current smoking status (n=22), prescribed antihypertensive medications (n=70), and lipid lowering agents (statins; n=55) were retrieved from patients’ medical charts. CVD was defined as a medical history of myocardial infarction, angina pectoris, percutaneous coronary intervention, and coronary artery bypass surgery; the development of symptoms (intermittent claudication and transient ischemic attack); therapeutic interventions (revascularization and amputation); and artery stenosis >60% in imaging studies.
Follow-Up and Adjudication of Outcomes
Follow-up data were retrieved from clinical records and/or death certificates by personnel blind to vascular assessments and before prolactin measurements were performed. Nonhemodialyzed patients were followed until cardiovascular event or death, whichever came first. Hemodialysis patients were followed for fatal outcomes. Cardiovascular-related death included deaths as a result of coronary heart disease, sudden death, stroke, or complicated peripheral vascular disease (septic shock from critical limb ischemia and/or gangrene and fatal amputation). Cardiovascular-related events included stroke, transient ischemic event, acute coronary syndrome (including unstable angina and ST or non-ST-myocardial infarction) and peripheral vascular disease (including new cases of claudication, revascularization, and amputations).
Laboratory Measurements
In both cohorts, samples were obtained between 7:00 and 11:00 am, and after 12 hours of fasting. Samples were kept frozen at −70°C if not analyzed immediately. In hemodialysis patients, blood extraction occurred in a nondialysis day. For nondialyzed patients, routine biochemical measurements included serum albumin, hemoglobin, parathyroid hormone (PTH), calcium, phosphate, and total cholesterol. High-sensitivity C-reactive protein (CRP) was measured by a photometric method (26). Serum prolactin levels were quantified by the Modular Analytics E 170 Module (Roche Diagnostics, Indianapolis, IN). The lower limit of detection for prolactin was 0.05 ng/ml and the inter- and intra-assay coefficients of variation were 2.8%–4.3% and 1.8%–2.6%, respectively. For hemodialysis patients, serum calcium, phosphorus, total and LDL cholesterol, albumin, CRP, and hemoglobin were measured routinely, at least once monthly, and PTH at least quarterly, using standard laboratory techniques at the hospitals. The average of the last 6-month laboratory data from medical charts were used for analysis. Prolactin was measured with a PRL-IRMA immunoradiometric assay kit from Biosource Europe S.A. (Nivelles, Belgium). The lower limit of detection for prolactin was 0.35 ng/ml and the inter- and intra-assay coefficients of variation were 4.5% and 3.3%, respectively.
Vascular Assessments
In both cohorts, arterial BP was measured in the morning after a 15-minute resting period by a physician three consecutive times, and mean values were calculated for systolic and diastolic pressure in both cohorts. Mean arterial pressure (MAP) was calculated as diastolic pressure + 1/3 (diastolic − systolic pressure).
In the nondialyzed cohort, FMD and nitroglycerine-mediated dilation (NMD) of the brachial artery were assessed noninvasively, using high-resolution ultrasound (26). Measurements were made by a single observer using an ATL 5000 ultrasound system (Advanced Technology Laboratories Inc, Bothell, WA) with a 12-mHz probe. The maximum FMD and NMD dilation diameters were calculated as the average of the three consecutive diameter measurements. The FMD and NMD were then calculated as the percentage change in diameter compared with baseline resting diameters. IMT was also assessed in all participants (26) with a high-resolution B-mode ultrasound of the common carotid arteries with scanning of the longitudinal axis until the bifurcation of the transversal axis using an instrument generating a wide-band ultrasonic pulse. IMT and FMD measurements were performed ideally in the same day or within 7 days from blood extraction and biochemical assessment. The intraobserver coefficients of variation for FMD and IMT were 4.9% and 5.8%, respectively,
In the hemodialysis cohort, PWV measurements were performed in the supine position and before blood sampling for hormone determination by the Pulse Trace 6000 system (MicroMedical Ltd, Kent, UK), as described previously (24). Short-term reproducibility of the method was assessed in 20 HD patients by obtaining two PWV measurements on two consecutive mid-week HD sessions under identical conditions. Bland-Altman agreement analysis showed acceptable consistency between the measures, with a mean difference of 0.28 m/s (95% confidence interval [95% CI], −1.06, 1.62).
Statistical Analyses
Statistical analyses were performed with STATA software (version 11.1; Stata Corp, College Station, TX). Non-normally distributed variables were expressed as interquartile range and normally distributed variables were as mean ± SD. A P value <0.05 was considered to be statistically significant. Between-group comparisons were assessed with the chi-squared and Kruskal–Wallis tests. Univariate correlations were used to determine associations between variables, and partial correlations examined these associations after controlling for GFR in the nondialysis cohort. Multivariate regression analysis (showing both unstandardized and standardized [β] coefficients) was used to assess the predictors for FMD and PWV levels, including all variables associated with these in univariate analysis. In addition, age, sex, and MAP were forced into these models. Time-to-event analysis of cardiovascular outcomes or mortality was done with the Kaplan–Meier curves or the Cox proportional hazards model, including adjustment for potential confounders on the basis of traditional risk factors affecting the prognosis of these patients. In a final step, adjustment within the purported causal pathway (e.g., FMD, IMT, or PWV) was done in search of potential mechanisms of action. The proportionality assumptions were checked through inspection of the log of the incidence rates. Data are presented in the form of hazard ratios (HRs) and 95% CIs.
Results
General Characteristics
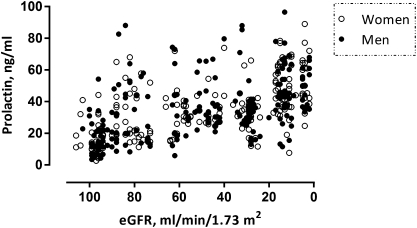
The demographic and clinical characteristics of the cohorts are given in Tables 1 and 2, categorized according to the sex-specific median prolactin values. In nondialyzed CKD, patients with prolactin concentration above the median value were more often diabetic and presented a lower eGFR, hemoglobin, and calcium as well as higher CRP, PTH, and phosphate. IMT was increased and FMD decreased. Figure 1 shows the inverse univariate correlation between prolactin levels and kidney function (eGFR) in these patients. In prevalent hemodialysis patients, those with prolactin concentration above the median value more often had cardiovascular comorbidity and smoked. These individuals presented higher BMI, phosphate, and calcium values as well as lower albumin and (borderline significant) hemoglobin. PWV was significantly increased in those hemodialysis patients with a prolactin concentration above the median value.
Table 1.
Demographic, hemodynamic, and biochemical characteristics in 457 nondialyzed CKD according to sex-specific median prolactin values
| Characteristic | ≤ Median (n=229) | > Median (n=228) | P Value |
|---|---|---|---|
| Age (yr) | 52 (43–61) | 52 (44–64) | 0.25 |
| Cardiovascular disease (%) | 48 | 52 | 0.82 |
| Diabetes (%) | 19 | 28 | 0.03 |
| Body mass index (kg/m2) | 26.1±2.5 | 25.9±2.9 | 0.59 |
| Mean arterial pressure (mmHg) | 100±4.5 | 102±5.5 | 0.04 |
| Smoking (%) | 46 | 44 | 0.20 |
| Estimated GFR (ml/min per 1.73 m2) | 78 (44–95) | 26 (12–51) | <0.001 |
| Proteinuria (g/d per 24 h) | 1.66±0.83 | 1.91±1.1 | 0.06 |
| Hemoglobin (g/dl) | 12.3±2.3 | 11.3±2.1 | 0.006 |
| Albumin (g/dl) | 4.0±0.3 | 3.9±0.3 | 0.14 |
| C-reactive protein (mg/L) | 11.5 (8.0–18.3) | 19.0 (12.0–24.0) | <0.001 |
| Calcium (mg/dl) | 8.6±0.6 | 8.2±0.5 | <0.001 |
| Phosphate (mg/dl) | 4.5±1.1 | 5.6±1.5 | <0.001 |
| Parathyroid hormone (pg/ml) | 101±68 | 181±75 | <0.001 |
| Intima-media thickness (mm) | 0.67 (0.56–0.8) | 0.81 (0.7–0.9) | <0.001 |
| Flow-mediated dilation (%) | 7.6 (6.8–8.2) | 6.4 (5.7–7.2) | <0.001 |
| Nitroglycerine-mediated dilation (%) | 13.0 (12.7–13.2) | 13.1 (12.3–13.2) | 0.11 |
| Prolactin (ng/ml) | 19.8 (13.4–27.3) | 44.1 (38.0–58.2) | — |
Median values of prolactin were 35 ng/ml (20–45) for women and 33 ng/ml (17–40) for men. Continuous values are mean ± SD or median (interquartile range).
Table 2.
Demographic, hemodynamic, and biochemical characteristics in 173 prevalent hemodialysis patients according to sex-specific median prolactin values
| Characteristic | ≤ Median (n=86) | > Median (n=87) | P Value |
|---|---|---|---|
| Age (yr) | 65±13 | 66±12 | 0.51 |
| Cardiovascular disease (%) | 41 | 56 | 0.04 |
| Diabetes (%) | 13 | 23 | 0.08 |
| Body mass index (kg/m2) | 24.5±3.6 | 25.6±3.8 | 0.04 |
| Smoking (%) | 7 | 18 | 0.02 |
| Hemodialysis vintage (mo) | 48 (24–92) | 50 (22–75) | 0.95 |
| Hemoglobin (g/dl) | 11.6±1.2 | 11.2±1.1 | 0.05 |
| Albumin (g/dl) | 4.1±0.4 | 4.0±0.4 | 0.01 |
| C-reactive protein (mg/L) | 4.4 (3.2–12.6) | 6.3 (3.2–12.6) | 0.11 |
| Calcium (mg/dl) | 9.2±0.8 | 9.5±0.9 | 0.03 |
| Phosphate (mg/dl) | 5.1±1.2 | 5.5±1.1 | 0.02 |
| Parathyroid hormone (pg/ml) | 221 (102–370) | 185 (102–327) | 0.48 |
| Pulse wave velocity (m/s) | 10.6±1.7 | 11.2±1.4 | 0.008 |
| Prolactin (ng/ml) | 9.9 (7.1–14.6) | 33.1 (26–53.7) | — |
Median values of prolactin were 25 ng/ml (14–35) for women and 16 ng/ml (8–33) for men. Continuous values are mean ± SD or median (interquartile range).
Figure 1.
The association between prolactin levels and estimated GFR (eGFR) in nondialyzed CKD patients.
Multivariate Regression Models for FMD, IMT, and PWV
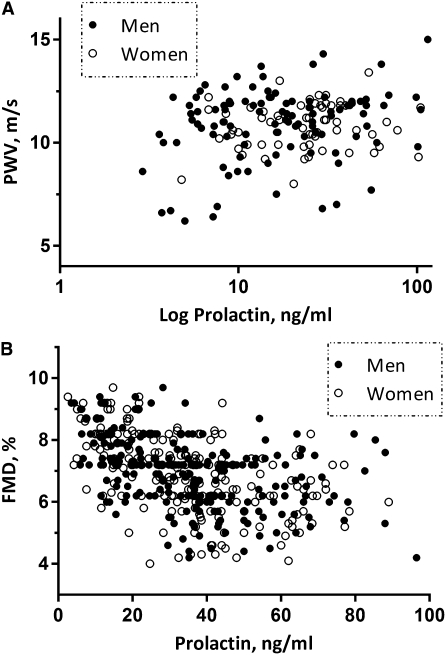
In univariate analysis, prolactin levels in the nondialyzed cohort correlated with FMD (Pearson’s correlation −0.47, P<0.0001; partial correlation adjusted by GFR −0.11, P=0.02) (Figure 2). A significant association was also observed between prolactin and IMT in univariate analysis (Pearson’s correlation 0.39, P<0.0001), but this association was dependent on GFR (partial correlation adjusted by GFR 0.01, P=0.81) and therefore no multivariate adjustment was performed. Prolactin levels also had a positive association with MAP values (Pearson’s correlation 0.13, P=0.01; partial correlation adjusted by GFR 0.11, P=0.02). In multiple regression analysis (Table 3), adjustment for covariates that were univariately associated with FMD did not alter the statistical significance of the FMD-prolactin association. Exclusion of 23 patients consuming prolactin-stimulating drugs did not change the results.
Figure 2.
The association between prolactin levels and endothelial dysfunction as assessed by (A) arterial stiffness by pulse wave velocity (PWV) in hemodialysis patients and (B) flow-mediated vasodilation (FMD) in nondialyzed CKD patients.
Table 3.
Multiple regression model for flow-mediated dilation (per % increase) in nondialyzed CKD patients
| Covariate | Standardized β | Coefficient (95% Confidence Interval) | P Value |
|---|---|---|---|
| Prolactin (ng/ml) | −0.078 | −0.005 (−0.009, −0.001) | 0.01 |
| Age (yr) | −0.013 | −0.001 (−0.007, 0.004) | 0.59 |
| Sex (men) | −0.004 | −0.010 (−0.107, 0.132) | 0.86 |
| Diabetes (presence) | −0.011 | −0.033 (−0.194, 0.125) | 0.66 |
| Cardiovascular disease (presence) | −0.018 | −0.057 (−0.085, 0.082) | 0.47 |
| Mean arterial pressure (mmHg) | −0.022 | −0.005 (−0.017, 0.006) | 0.39 |
| Estimated GFR (ml/min per 1.73 m2) | 0.430 | 0.015 (0.011, 0.020) | <0.001 |
| Proteinuria (g/d) | −0.029 | −0.001 (−0.001, 0.001) | 0.26 |
| C-reactive protein (mg/L) | −0.068 | −0.010 (−0.022, 0.001) | 0.07 |
| Albumin (g/dl) | 0.081 | 0.282 (0.117, 0.457) | 0.002 |
| Hemoglobin (g/dl) | −0.329 | −0.172 (−0.203, −0.142) | <0.001 |
| Ca×PO4 (mg2/dl2) | −0.125 | −0.014 (−0.022, −0.006) | <0.001 |
| Parathyroid hormone (pg/ml) | −0.120 | −0.001 (−0.003, −0.001) | 0.03 |
| Intima media thickness (mm) | −0.154 | −1.261 (−1.864, −0.631) | <0.001 |
Coefficient indicates the estimated average difference in flow-mediated dilation for 1 unit higher value in the predictor value (e.g., 1 mmHg for MAP), whereas the standardized beta indicates the average difference per higher SD in the predictor variable (e.g., 5 mmHg for MAP). Adjusted r2=0.69.
In univariate analysis, PWV strongly correlated with prolactin levels in the hemodialysis cohort (Spearman’s ρ=0.208, P=0.008) (Figure 2). Prolactin levels were not associated with MAP values in univariate analysis (Spearman’s ρ=−0.013, P=0.86). In multiple regression analysis (Table 4), adjustment for covariates that were univariately associated with PWV did not alter the statistical significance of the PWV-prolactin association.
Table 4.
Multiple regression models for pulse wave velocity (in m/s) in prevalent hemodialysis patients
| Covariate | Standardized β | Coefficient (95% Confidence Interval) | P Value |
|---|---|---|---|
| Prolactin (ng/ml) | 0.191 | 0.008 (0.002, 0.014) | 0.006 |
| Age (yr) | 0.239 | 0.030 (0.13, 0.47) | 0.001 |
| Sex (men) | 0.090 | 0.292 (−0.133, 0.716) | 0.17 |
| Mean arterial pressure (mmHg) | 0.217 | 0.028 (0.011, 0.045) | 0.002 |
| Body mass index (kg/m2) | −0.030 | −0.012 (−0.068, 0.043) | 0.65 |
| C-reactive protein (mg/l) | 0.107 | 0.136 (−0.037, 0.310) | 0.12 |
| LDL (mg/dl) | 0.229 | 0.012 (0.005, 0.019) | 0.007 |
| Parathyroid hormone (pg/ml) | 0.091 | 0.001 (0.001, 0.002) | 0.17 |
Coefficient indicates the estimated average difference in pulse wave velocity for 1 unit higher value in the predictor value (e.g., 1 mmHg for MAP), whereas the standardized β indicates the average difference per higher SD in the predictor variable (e.g., 5 mmHg for MAP). Adjusted r2=0.28.
Prolactin and Cardiovascular Events in Nondialyzed CKD Patients
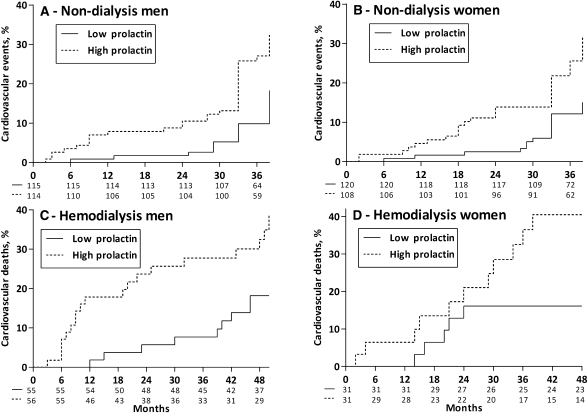
Cardiovascular outcomes were determined after a mean follow-up period of 38 months (range, 2–46). Forty-five patients died, with 40 of the deaths due to cardiovascular causes, 2 due to infectious complications, and 3 due to malignancies. Causes of cardiovascular death were coronary heart disease (n=26), sudden death (n=5), stroke (n=6), or complicated peripheral vascular disease (n=3). During the follow-up period, 106 additional nonfatal cardiovascular events were registered as follows: stroke (n=27), myocardial infarction (n=65), and peripheral vascular disease (n=14). Men and women with elevated prolactin levels (above median value) were at increased risk of cardiovascular events in Kaplan–Meier analysis (Figure 3). The association between prolactin and time-to-cardiovascular event (n=146, including a composite of fatal and nonfatal events) was studied through univariate and multivariate Cox analysis (Table 5). In crude analysis, every 10 ng/ml of increase in serum prolactin concentration increased the risk of suffering a cardiovascular event by 27% (crude HR, 1.27; 95% CI, 1.17–1.38). This elevated risk persisted after adjustment for various potential confounders, and further adjustment for FMD and IMT did not materially affect the strength of the association (HR, 1.19; 95% CI, 1.08–1.32). Exclusion of 23 patients consuming prolactin-stimulating drugs did not change the results (not shown).
Figure 3.
Kaplan-Meier curves and patients at risk for cardiovascular events and cardiovascular death. Cardiovascular events in (A) male and (B) female nondialyzed CKD patients. Cardiovascular death in (C) male and (D) female hemodialysis patients.
Table 5.
Crude and adjusted hazard ratios of serum prolactin (per 10 ng/ml) for prediction of cardiovascular outcomes (fatal and nonfatal) in nondialyzed CKD patients
| Model | Covariates | Hazard Ratio (95% Confidence Interval) | P Value |
|---|---|---|---|
| 1 | Crude risk of prolactin (per 10 ng/ml) | 1.27 (1.17–1.38) | <0.001 |
| 2 | 1+ age, sex, smoking, eGFR, diabetes, CVD, MAP | 1.20 (1.09–1.33) | <0.001 |
| 3 | 2+ C-reactive protein, serum albumin | 1.22 (1.10–1.35) | <0.001 |
| 4 | 3+ flow-mediated dilation | 1.19 (1.07–1.32) | <0.001 |
| 5 | 4+ intima-media thickness | 1.19 (1.08–1.32) | <0.001 |
Prolactin and Mortality in Prevalent Hemodialysis Patients
Fatal events were registered with a mean follow-up period of 49 months (range, 29–58). Seventy-nine patients died, 47 of them by causes attributed to CVD, including stroke (n=15), coronary artery disease (n=13), complicated peripheral vascular disease (n=9), and sudden death (n=10). Men and women with elevated prolactin levels (above median value) were at increased risk of cardiovascular mortality in Kaplan–Meier analysis (Figure 3). The association between prolactin and all-cause or CVD mortality was studied through univariate and multivariate Cox analysis (Table 6). In crude analysis, every 10 ng/ml of increase in serum prolactin concentration increased the all-cause mortality risk by 12% (crude HR, 1.12; 95% CI, 1.06–1.17) and the risk of CVD mortality by 15% (crude HR, 1.15; 95% CI, 1.08–1.21). This elevated risk persisted after adjustment for various potential confounders, and further adjustment for PWV did not materially affect the strength of the associations (HR, 1.10; 95% CI, 1.04–1.17 for all-cause mortality and HR, 1.13; 95% CI, 1.05–1.21 for CVD mortality).
Table 6.
Crude and adjusted hazard ratios of serum prolactin (per 10 ng/ml) for prediction of all-cause and cardiovascular mortality in prevalent hemodialysis patients
| Model | Covariates | All-Cause Mortality | Cardiovascular Mortality | ||
|---|---|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | P Value | Hazard Ratio (95% Confidence Interval) | P Value | ||
| 1 | Crude risk of prolactin (per 10 ng/ml) | 1.12 (1.06–1.17) | 0.001 | 1.15 (1.08–1.21) | 0.001 |
| 2 | 1+ age, sex, smoking, dialysis vintage, diabetes, CVD, MAP | 1.10 (1.04–1.17) | 0.001 | 1.13 (1.06–1.21) | <0.001 |
| 3 | 2+ C-reactive protein, serum albumin | 1.09 (1.03–1.16) | 0.002 | 1.12 (1.05–1.20) | 0.001 |
| 4 | 3+ pulse wave velocity | 1.10 (1.04–1.17) | 0.002 | 1.13 (1.05–1.21) | 0.001 |
Discussion
Our chief finding is the direct association of prolactinemia with mortality and cardiovascular events. The mechanisms by which prolactin plasma levels in CKD are associated with cardiovascular risk are still unclear. In vitro, prolactin was able to modulate the inflammatory response, to stimulate the adhesion of mononuclear cells to endothelium, and to enhance vascular smooth muscle cell proliferation (28–31). In accordance with a previous study in postmenopausal women with normal prolactinemia (32), our data showed that prolactin was an independent contributor to the variance of both FMD and PWV levels. A recent study in hypertensive men showed that diurnal fluctuations of prolactin levels associated with decreased FMD that occurs early in the morning (33). This has been suggested as a potential explanation of prolactin increasing cardiovascular risk and is supported by previous studies in pigs demonstrating regional vasoconstrictive effects for prolactin through the β2-adrenergic and nitric oxide mechanisms (34). However, adjustment within the causal pathway for FMD or for PWV in the Cox analyses did not fully explain the associations between prolactinemia and (cardiovascular) outcomes in our study. We thus speculate that endothelial dysfunction and/or arterial stiffness are not the sole mechanisms of action mediating this effect. Adequately designed mechanistic studies should clarify this issue. We also observed an association between prolactin and IMT, but this was dependent of adjustment for GFR, perhaps altogether implying a rather selective deteriorating effect of prolactin toward arteriosclerosis instead of atheromatosis. The cross-sectional nature of our design allows a double interpretation in this regard. First, prolactin is not as strong a risk factor for worse IMT as it is for eGFR. Second, with a decline in GFR, prolactin increases and prolactin is a risk factor for worsening IMT. Nevertheless, a lack of independent association between prolactin and IMT was also reported in a community-based study (18).
The main progress in our understanding of the atherogenic effects of prolactin comes from the field of peri/postpartum cardiomyopathy, a condition characterized by inflammation, autoimmunity, apoptosis, and endothelial dysfunction. Unbalanced peri/postpartum oxidative stress is linked to proteolytic cleavage of prolactin into a potent antiangiogenic, proapoptotic and proinflammatory 16-kD subform that may initiate the atherosclerotic-related complications (16). Given the similarities of this phenotype with that of uremia, it would be intriguing to study whether similar processes occur in CKD patients. Although we relate our findings to studies demonstrating a causative role for prolactin, we acknowledge that our observational design cannot determine whether prolactin is a risk factor per se or an intermediate of a larger pathophysiological pathway. For instance, prolactin retention leads to inhibition of gonadotropic hormone production, and testosterone deficiency in male CKD patients has indeed been linked to increased IMT, atherosclerotic plaque occurrence, reduced FMD, systemic inflammation, cardiovascular risk, and mortality (19–24). Conversely, increased prolactinemia could also be a consequence of decreased dopaminergic activity (8), which would in turn imply an increment in norepinephrine release and that may have adverse effects on endothelial function and on other organs, perhaps favoring myocardial hypertrophy, hypertension, and other cardiovascular comorbidities. Ultimately, this may also reflect the importance of alteration at the hypothalamic-pituitary axis, because other hormonal derangements in this axis, such as thyroid disturbances (35), have also been linked with the pathophysiology of CKD.
Our findings, if reaffirmed in subsequent studies, raise the hypothesis that prolactin-lowering therapies may improve patients’ risk in uremia. Indeed, blockade of prolactin by bromocriptine appeared successful in human pilot trials to treat peri/postpartum cardiomyopathy (36,37). Previous studies in fact report successful prolactin reduction in CKD patients after bromocriptine treatment, with no reported side effects (38–40). However, it is unknown whether prolactin normalization improved any atherosclerotic/cardiovascular-related outcomes. Intriguingly, some small studies have evaluated the effects of bromocriptine therapy in CKD patients on the basis of its dopamine D2 receptor agonist properties, reporting a reduction in BP and regression of left ventricular hypertrophy in dialysis patients (41–43). Whether this effect was mediated by prolactin reduction, at least partly, is unknown.
This study should be interpreted in light of the following strengths and limitations. A strength of this study is the confirmation of results in two independent cohorts. In the nondialyzed material, the exclusion of drugs that may confound the interpretation of the eGFR–vascular health axis is a strength but at the same time renders our data as not representative of the normal CKD population. Limitations of this study are the use of frozen samples, the existence of a single prolactin determination, and the use of different prolactin commercial methods in each of the cohorts. In the hemodialysis cohort, we should further acknowledge the lack of information on drugs potentially affecting prolactin concentration, despite that these medications (27) are seldom prescribed to dialysis patients, and the fact that routine biomarkers, but not prolactin, were the average value of the registered measures in the preceding 6 months. Cardiovascular events and deaths were retrieved from medical records, and we cannot exclude the possibility of unreported events and misclassifications. These limitations would result, however, in underestimation of the observed associations.
In conclusion, our study shows novel and intriguing links between endothelial function, arterial stiffness, cardiovascular outcomes, and prolactinemia. These findings, together with preceding experimental results indicating that prolactin-mediated mechanisms alter vascular integrity, may trigger further research to confirm or reject the hypothesis of a causative relationship between this hormonal derangement and adverse outcomes in CKD patients.
Disclosures
None.
Acknowledgments
We acknowledge support from the Gülhane School of Medicine in Turkey, the Swedish Medical Research Council (Vetenskapsrådet), the Westman’s and Osterman’s Foundations, and the University of Crete in Greece-Secretariat of the Research Committee (Code 2976).
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.de Jager DJ, Grootendorst DC, Jager KJ, van Dijk PC, Tomas LM, Ansell D, Collart F, Finne P, Heaf JG, De Meester J, Wetzels JF, Rosendaal FR, Dekker FW: Cardiovascular and noncardiovascular mortality among patients starting dialysis. JAMA 302: 1782–1789, 2009 [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Zoccali C: Endothelial dysfunction and the kidney: Emerging risk factors for renal insufficiency and cardiovascular outcomes in essential hypertension. J Am Soc Nephrol 17[Suppl 2]: S61–S63, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Carrero JJ, Stenvinkel P: Inflammation in end-stage renal disease—What have we learned in 10 years? Semin Dial 23: 498–509, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Hou SH, Grossman S, Molitch ME: Hyperprolactinemia in patients with renal insufficiency and chronic renal failure requiring hemodialysis or chronic ambulatory peritoneal dialysis. Am J Kidney Dis 6: 245–249, 1985 [DOI] [PubMed] [Google Scholar]
- 6.Cowden EA, Ratcliffe WA, Ratcliffe JG, Dobbie JW, Kennedy AC: Hyperprolactinaemia in renal disease. Clin Endocrinol (Oxf) 9: 241–248, 1978 [DOI] [PubMed] [Google Scholar]
- 7.Yavuz D, Topçu G, Ozener C, Akalin S, Sirikçi O: Macroprolactin does not contribute to elevated levels of prolactin in patients on renal replacement therapy. Clin Endocrinol (Oxf) 63: 520–524, 2005 [DOI] [PubMed] [Google Scholar]
- 8.McKenna TM, Woolf PD: Prolactin metabolic clearance and resistance to dopaminergic suppression in acute uremia. Endocrinology 116: 2003–2007, 1985 [DOI] [PubMed] [Google Scholar]
- 9.Caticha O, Norato DY, Tambascia MA, Santana A, Stephanou A, Sarlis NJ: Total body zinc depletion and its relationship to the development of hyperprolactinemia in chronic renal insufficiency. J Endocrinol Invest 19: 441–448, 1996 [DOI] [PubMed] [Google Scholar]
- 10.Stumpe KO, Kolloch R, Higuchi M, Krück F, Vetter H: Hyperprolactinaemia and antihypertensive effect of bromocriptine in essential hypertension. Identification of abnormal central dopamine control. Lancet 2: 211–214, 1977 [DOI] [PubMed] [Google Scholar]
- 11.Raaz D, Wallaschofski H, Stumpf C, Yilmaz A, Cicha I, Klinghammer L, Daniel WG, Lohmann T, Garlichs CD: Increased prolactin in acute coronary syndromes as putative co-activator of ADP-stimulated P-selectin expression. Horm Metab Res 38: 767–772, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Wallaschofski H, Lohmann T, Hild E, Kobsar A, Siegemund A, Spilcke-Liss E, Hentschel B, Stumpf C, Daniel WG, Garlichs CD, Eigenthaler M: Enhanced platelet activation by prolactin in patients with ischemic stroke. Thromb Haemost 96: 38–44, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Wallaschofski H, Eigenthaler M, Kiefer M, Donné M, Hentschel B, Gertz HJ, Lohmann T: Hyperprolactinemia in patients on antipsychotic drugs causes ADP-stimulated platelet activation that might explain the increased risk for venous thromboembolism: Pilot study. J Clin Psychopharmacol 23: 479–483, 2003 [DOI] [PubMed] [Google Scholar]
- 14.Marlettini MG, Cassani A, Morselli Labate AM, Rusticali AG, Crippa S, Trabatti M, Miniero R, Plate L, Orlandi C: Role of prolactin in pregnancy hypertension. Clin Exp Hypertens A 9: 1099–1119, 1987 [DOI] [PubMed] [Google Scholar]
- 15.Leaños-Miranda A, Márquez-Acosta J, Cárdenas-Mondragón GM, Chinolla-Arellano ZL, Rivera-Leaños R, Bermejo-Huerta S, Romero-Arauz JF, Alvarez-Jiménez G, Ramos-León JC, Ulloa-Aguirre A: Urinary prolactin as a reliable marker for preeclampsia, its severity, and the occurrence of adverse pregnancy outcomes. J Clin Endocrinol Metab 93: 2492–2499, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Hilfiker-Kleiner D, Kaminski K, Podewski E, Bonda T, Schaefer A, Sliwa K, Forster O, Quint A, Landmesser U, Doerries C, Luchtefeld M, Poli V, Schneider MD, Balligand JL, Desjardins F, Ansari A, Struman I, Nguyen NQ, Zschemisch NH, Klein G, Heusch G, Schulz R, Hilfiker A, Drexler H: A cathepsin D-cleaved 16 kDa form of prolactin mediates postpartum cardiomyopathy. Cell 128: 589–600, 2007 [DOI] [PubMed] [Google Scholar]
- 17.Corona G, Rastrelli G, Boddi V, Monami M, Melani C, Balzi D, Sforza A, Forti G, Mannucci E, Maggi M: Prolactin levels independently predict major cardiovascular events in patients with erectile dysfunction. Int J Androl 34: 217–224, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Reuwer AQ, Twickler MT, Hutten BA, Molema FW, Wareham NJ, Dallinga-Thie GM, Bogorad RL, Goffin V, Smink-Bol M, Kastelein JJ, Boekholdt SM, Khaw KT: Prolactin levels and the risk of future coronary artery disease in apparently healthy men and women. Circ Cardiovasc Genet 2: 389–395, 2009 [DOI] [PubMed] [Google Scholar]
- 19.Gungor O, Kircelli F, Carrero JJ, Asci G, Toz H, Tatar E, Hur E, Sever MS, Arinsoy T, Ok E: Endogenous testosterone and mortality in male hemodialysis patients: Is it the result of aging? Clin J Am Soc Nephrol 5: 2018–2023, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carrero JJ, Qureshi AR, Parini P, Arver S, Lindholm B, Bárány P, Heimbürger O, Stenvinkel P: Low serum testosterone increases mortality risk among male dialysis patients. J Am Soc Nephrol 20: 613–620, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karakitsos D, Patrianakos AP, De Groot E, Boletis J, Karabinis A, Kyriazis J, Samonis G, Parthenakis FI, Vardas PE, Daphnis E: Androgen deficiency and endothelial dysfunction in men with end-stage kidney disease receiving maintenance hemodialysis. Am J Nephrol 26: 536–543, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Carrero JJ, Qureshi AR, Nakashima A, Arver S, Parini P, Lindholm B, Bárány P, Heimbürger O, Stenvinkel P: Prevalence and clinical implications of testosterone deficiency in men with end-stage renal disease. Nephrol Dial Transplant 26: 184–190, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Yilmaz MI, Sonmez A, Qureshi AR, Saglam M, Stenvinkel P, Yaman H, Eyileten T, Caglar K, Oguz Y, Taslipinar A, Vural A, Gok M, Unal HU, Yenicesu M, Carrero JJ: Endogenous testosterone, endothelial dysfunction, and cardiovascular events in men with nondialysis chronic kidney disease. Clin J Am Soc Nephrol 6: 1617–1625, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Kyriazis J, Tzanakis I, Stylianou K, Katsipi I, Moisiadis D, Papadaki A, Mavroeidi V, Kagia S, Karkavitsas N, Daphnis E: Low serum testosterone, arterial stiffness and mortality in male haemodialysis patients. Nephrol Dial Transplant 26: 2971–2977, 2011 [DOI] [PubMed] [Google Scholar]
- 25.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. Modification of Diet in Renal Disease Study Group: A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz MI, Stenvinkel P, Sonmez A, Saglam M, Yaman H, Kilic S, Eyileten T, Caglar K, Oguz Y, Vural A, Cakar M, Altun B, Yenicesu M, Carrero JJ: Vascular health, systemic inflammation and progressive reduction in kidney function: Clinical determinants and impact on cardiovascular outcomes. Nephrol Dial Transplant 6: 3537–3543, 2011 [DOI] [PubMed] [Google Scholar]
- 27.Snyder PJ, Cooper DS, Martin KI: Causes of hyperprolactinemia. Version 18.2, October 2010. Available at: http://www.uptodate.com/contents/causes-of-hyperprolactinemia. Accessed December 2, 2011
- 28.Sauro MD, Buckley AR, Russell DH, Fitzpatrick DF: Prolactin stimulation of protein kinase C activity in rat aortic smooth muscle. Life Sci 44: 1787–1792, 1989 [DOI] [PubMed] [Google Scholar]
- 29.Aziz MM, Ishihara S, Rumi MA, Mishima Y, Oshima N, Kadota C, Moriyama I, Li YY, Rahman FB, Otani A, Oka A, Ishimura N, Kadowaki Y, Amano Y, Kinoshita Y: Prolactin induces MFG-E8 production in macrophages via transcription factor C/EBPbeta-dependent pathway. Apoptosis 13: 609–620, 2008 [DOI] [PubMed] [Google Scholar]
- 30.Bresson JL, Jeay S, Gagnerault MC, Kayser C, Beressi N, Wu Z, Kinet S, Dardenne M, Postel-Vinay MC: Growth hormone (GH) and prolactin receptors in human peripheral blood mononuclear cells: Relation with age and GH-binding protein. Endocrinology 140: 3203–3209, 1999 [DOI] [PubMed] [Google Scholar]
- 31.Sun R, Li AL, Wei HM, Tian ZG: Expression of prolactin receptor and response to prolactin stimulation of human NK cell lines. Cell Res 14: 67–73, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Georgiopoulos GA, Stamatelopoulos KS, Lambrinoudaki I, Lykka M, Kyrkou K, Rizos D, Creatsa M, Christodoulakos G, Alevizaki M, Sfikakis PP, Papamichael C: Prolactin and preclinical atherosclerosis in menopausal women with cardiovascular risk factors. Hypertension 54: 98–105, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Stamatelopoulos KS, Georgiopoulos GA, Sfikakis PP, Kollias G, Manios E, Mantzou E, Kyrkou K, Zakopoulos N, Papamichael CM, Alevizaki M: Pilot study of circulating prolactin levels and endothelial function in men with hypertension. Am J Hypertens 24: 569–573, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Molinari C, Grossini E, Mary DA, Uberti F, Ghigo E, Ribichini F, Surico N, Vacca G: Prolactin induces regional vasoconstriction through the beta2-adrenergic and nitric oxide mechanisms. Endocrinology 148: 4080–4090, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Carrero JJ, Qureshi AR, Axelsson J, Yilmaz MI, Rehnmark S, Witt MR, Bárány P, Heimbürger O, Suliman ME, Alvestrand A, Lindholm B, Stenvinkel P: Clinical and biochemical implications of low thyroid hormone levels (total and free forms) in euthyroid patients with chronic kidney disease. J Intern Med 262: 690–701, 2007 [DOI] [PubMed] [Google Scholar]
- 36.Sliwa K, Blauwet L, Tibazarwa K, Libhaber E, Smedema JP, Becker A, McMurray J, Yamac H, Labidi S, Struman I, Hilfiker-Kleiner D: Evaluation of bromocriptine in the treatment of acute severe peripartum cardiomyopathy: A proof-of-concept pilot study. Circulation 121: 1465–1473, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Hilfiker-Kleiner D, Meyer GP, Schieffer E, Goldmann B, Podewski E, Struman I, Fischer P, Drexler H: Recovery from postpartum cardiomyopathy in 2 patients by blocking prolactin release with bromocriptine. J Am Coll Cardiol 50: 2354–2355, 2007 [DOI] [PubMed] [Google Scholar]
- 38.Vircburger MI, Prelević GM, Perić LA, Knezević J, Djukanović L: Testosterone levels after bromocriptine treatment in patients undergoing long-term hemodialysis. J Androl 6: 113–116, 1985 [DOI] [PubMed] [Google Scholar]
- 39.Ramirez G, Butcher DE, Newton JL, Brueggemeyer CD, Moon J, Gomez-Sanchez C: Bromocriptine and the hypothalamic hypophyseal function in patients with chronic renal failure on chronic hemodialysis. Am J Kidney Dis 6: 111–118, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Bommer J, Ritz E, del Pozo E, Bommer G: Improved sexual function in male haemodialysis patients on bromocriptine. Lancet 2: 496–497, 1979 [DOI] [PubMed] [Google Scholar]
- 41.Mejía-Rodríguez O, Alvarez-Aguilar C, Vega-Gómez HE, Belio-Caro F, Vargas-Espinosa JM, Paniagua-Sierra JR: Bromocriptine induces regression of left ventricular hypertrophy in peritoneal dialysis patients. Proc West Pharmacol Soc 48: 122–125, 2005 [PubMed] [Google Scholar]
- 42.Mejía-Rodríguez O, Alvarez-Aguilar C, Ledesma-Ramírez M, Paniagua-Sierra R: Therapeutic effect of bromocriptine together with the established treatment for hypertension in patients undergoing peritoneal dialysis. Proc West Pharmacol Soc 47: 122–124, 2004 [PubMed] [Google Scholar]
- 43.Degli Esposti E, Sturani A, Santoro A, Zuccalà A, Chiarini C, Zucchelli P: Effect of bromocriptine treatment on prolactin, noradrenaline and blood pressure in hypertensive haemodialysis patients. Clin Sci (Lond) 69: 51–56, 1985 [DOI] [PubMed] [Google Scholar]