Summary
Recent clinical trials comparing the use of erythropoiesis-stimulating agents targeting low (generally a hemoglobin of 90–115 g/L) and near-normal hemoglobin targets (generally a hemoglobin >130 g/L) in patients with chronic kidney disease have shown no improvements in clinical outcomes (aside from a small reduction in transfusion) and potential harm for erythropoiesis-stimulating agents use targeting near-normal hemoglobin targets. Based on these results, the US Food and Drug Administration recently released modified recommendations for more conservative dosing of erythropoiesis-stimulating agents in patients with CKD. These recommendations now stress individualizing therapy for each patient and using the lowest possible erythropoiesis-stimulating agents dose required to reduce the need for transfusions. The evolution in the management of anemia associated with chronic kidney disease over time and the recent evidence that has stimulated these labeling changes is discussed. Also, the US Food and Drug Administration labeling changes are discussed, and areas of controversy are highlighted. Although the US Food and Drug Administration labeling changes are based on the results of recent large randomized trials testing ESAs targeting near-normal hemoglobin levels, more specific guidance to clinicians would have been helpful.
Introduction
The US Food and Drug Administration (FDA) recently released modified recommendations for more conservative dosing of erythropoiesis-stimulating Agents (ESA) in patients with CKD (1). This release led to revisions of the boxed warning, warnings and precautions, and dosage and administration sections of the ESA labels, which now stress individualizing therapy for each patient and using the lowest possible ESA dose required to reduce the need for transfusions (Table 1). The new labels recommend reducing the ESA dose when hemoglobin exceeds 100 and 110 g/L in nondialysis and dialysis CKD patients, respectively. The labels also note that, for patients who do not respond adequately over a 12-wk escalation period, increasing the ESA dose more is unlikely to improve response and may increase risks—acknowledging the difficulty in managing patients with ESA resistance.
Table 1.
ESA label changes for the safe use of ESAs (1)
| The ESA labels now warn: |
| In controlled trials with CKD patients, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered ESA to target a hemoglobin level of greater than 11 g/dl. |
| No trial has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks. |
| ESA labels now recommend: |
| For patients with CKD, consider starting ESA treatment when the hemoglobin level is less than 10 g/dl. This advice does not define how far below 10 g/dl is appropriate for an individual to initiate. This advice also does not recommend that the goal is to achieve a hemoglobin of 10 g/dl or a hemoglobin above 10 g/dl. Individualize dosing, and use the lowest dose of ESA sufficient to reduce the need for red blood cell transfusions. Adjust dosing as appropriate. |
| The drug label previously recommended that ESA be dosed to achieve and maintain hemoglobin levels within the target range of 10–12 g/dl in CKD patients. This target concept has been removed from the label. |
| Additional information for healthcare professionals who treat patients with CKD: |
| Using ESAs to target a hemoglobin level of greater than 11 g/dl increases the risk of serious adverse cardiovascular events and has not been shown to provide additional patient benefit. |
| No clinical trial to date has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks. |
| The ESA Medication Guide [Epogen (6)/Procrit (7) or Aranesp (8)] should be provided to each patient or their representative when an ESA is dispensed. |
| The lowest ESA dose sufficient to reduce the need for red blood cell transfusions should be used. |
| For patients with CKD not on dialysis, consider initiating ESA treatment only when the hemoglobin level is less than 10 g/dl and the following considerations apply. |
| The rate of hemoglobin decline indicates the likelihood of requiring a red blood cell transfusion. |
| Reducing the risk of alloimmunization and/or other red blood cell transfusion-related risks is a goal. |
| If the hemoglobin level exceeds 10 g/dl, reduce or interrupt the dose of ESA and use the lowest dose of ESA sufficient to reduce the need for red blood cell transfusions. |
| For patients with CKD on dialysis |
| Initiate ESA treatment when the hemoglobin level is <10 g/dl. |
| If the hemoglobin level approaches or exceeds 11 g/dl, reduce or interrupt the dose of ESA. |
| When initiating or adjusting therapy, monitor hemoglobin levels at least weekly until stable, and then monitor at least monthly. |
| For patients who do not respond adequately over a 12-wk escalation period, increasing the ESA dose further is unlikely to improve response and may increase risks. |
| Adverse events involving ESAs should be reported to the US Food and Drug Administration MedWatch program. |
ESA, erythropoiesis-stimulating agent.
These changes are the latest in the evolution of anemia management for patients with CKD. Erythropoietin was the first biologic drug licensed for use in the area of end-stage renal disease, and by many accounts, it revolutionized the management of anemia in ESRD, which at the time, was primarily based on the use of red cell transfusions (with associated risks of viral infections and alloimmunization).
Initial trials of ESA usually aimed for a target hemoglobin of <120 g/L and compared this therapy with placebo (2). Results uniformly found an increase in hemoglobin and a reduction in the need for transfusion (3,4). Most early studies were not designed or powered to examine clinical outcomes other than quality of life, and no differences were apparent in such other outcomes. Several of the studies showed improvements in certain domains of quality of life compared with placebo (4).
After ESAs were approved by the FDA, their widespread use in ESRD patients led to interest in evaluating the association between achieved hemoglobin level and outcomes. Such observational studies suggested that patients with the highest hemoglobin had the best outcomes, with lower adjusted mortality rates and cardiovascular events (5). Although several studies also reported that patients with the highest hemoglobin level required the lowest ESA doses (suggesting that patients with lower hemoglobin had relative ESA resistance, likely because of more comorbid illnesses), these observational studies led to randomized trials comparing lower (generally in the range of 90–115 g/L) and near-normal hemoglobin targets (typically exceeding 130 g/L) on clinical outcomes.
Outcomes Relevant to the Management of Anemia in CKD
Randomized trials of ESA have evolved from studying hemoglobin as a primary outcome to focusing on clinically relevant outcomes such as mortality and cardiovascular outcomes. This development has been critical to our understanding of the role of ESA in the management of patients with CKD. Accordingly, the focus of contemporary trials has moved from putative predictors of good outcomes (i.e., higher hemoglobin, with associated reduction in the risk of transfusions) to more patient-relevant clinical outcomes (Figure 1). In some ways, this move has been a difficult transition for clinicians, many of whom have encountered patients who feel better at higher hemoglobin levels. The challenge for clinical trials has been to determine whether such patients feel better because higher ESA doses have led to the higher hemoglobin, because of placebo effects resulting from knowledge of the higher hemoglobin, or because better underlying health (without intercurrent illness or major comorbidity) has enabled higher hemoglobin levels.
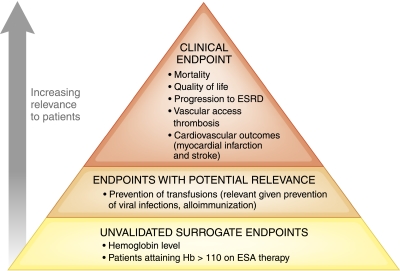
Figure 1.
Hierarchy of outcomes that are relevant to erythropoiesis-stimulating agent (ESA) management for patients with anemia related to CKD.
Figure 1 highlights a hierarchy of outcomes that is useful to consider when evaluating any intervention. For the evaluation of ESA, the most relevant clinical outcomes are mortality, cardiovascular events, vascular access thrombosis, and quality of life. Moving down the hierarchy, outcomes that are also relevant to the assessment of ESA (although less so than the outcomes noted above) include receipt of red cell transfusion. Farther down the hierarchy of outcomes are those outcomes that are less relevant to patients (but on which the nephrology community has traditionally based much of our clinical decision making), including unvalidated surrogate endpoints such as achieved hemoglobin level (6,7).
Although many nephrologists would agree with the hierarchy of outcomes presented in Figure 1, it is perhaps unsurprising that our community has traditionally focused on hemoglobin as an outcome or quality of care marker. Clinical trials have typically compared different hemoglobin targets, making these values almost synonymous with clinical outcomes themselves. However, more importantly, prescribing guidelines (or restrictions) in many countries have focused on hemoglobin targets (8) rather than clinical outcomes, ESA dose restrictions, or healthcare costs (2).
Overview of Trials Comparing Low and Near-Normal Hemoglobin Targets
Although many small studies have compared the impact of different ESA dosing or hemoglobin targets on nonclinical outcomes (including hemoglobin and need for transfusion) (2), four large studies have tested the effect of different target hemoglobin levels on clinical outcomes: one study in hemodialysis patients (9) and more recently, three studies in nondialysis CKD patients (10–12). In the study by Besarab et al. (9), 1233 hemodialysis patients with clinical evidence of congestive heart failure or ischemic heart disease were assigned to receive increasing doses of ESA to target a hemoglobin of 140 g/L or receive doses of ESA targeting a hemoglobin of ∼100 g/L throughout the study. The study was stopped early because of the unexpected excess risk of death in the near-normal target group. There were 183 deaths and 19 nonfatal myocardial infarctions among the patients in the normal hemoglobin group and 150 deaths and 14 nonfatal myocardial infarctions among those people in the low hemoglobin group [risk ratio for the normal hemoglobin group compared with the low hemoglobin group was 1.3; 95% confidence interval (CI)=0.9–1.9]. Patients in the near-normal hemoglobin arm required more iron supplements and nearly three times the dose of ESA (9).
In the Correction of Hemoglobin and Outcomes in Renal Insufficiency study (11), 1432 patients with nondialysis CKD were assigned to receive either an ESA dose targeted to achieve a hemoglobin level of 135 g/L or a dose targeted to achieve a level of 113 g/L. The risk of the primary endpoint (a composite of death and cardiovascular endpoints) was higher in the near-normal hemoglobin group (hazard ratio=1.34; 95% CI=1.03–1.74; P=0.03). The hazard ratio for death comparing near-normal versus low hemoglobin target was 1.48 (P=0.07). In the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin β study (12), 603 patients with nondialysis CKD were randomly assigned to a target hemoglobin level range of 130–150 or 105–115 g/L. The primary endpoint, a composite of eight cardiovascular events, was similar between groups. Although dialysis was required in more patients in the near-normal hemoglobin group (127 versus 111 patients, P=0.03), rates of change in estimated GFR were similar.
In the Trial to Reduce cardiovascular Events (TREAT) trial (10), patients with diabetes and nondialysis CKD were randomized to ESA with a target hemoglobin >130 g/L or placebo, with rescue ESA therapy allowed for sustained hemoglobin <90 g/L. The risk of death or a composite cardiovascular outcome did not differ across the treatment groups ((hazard ratio for darbepoetin versus placebo=1.05; 95% CI=0.94–1.17; P=0.41), although the risk of stroke was higher in people randomized to darbepoetin (hazard ratio=1.92; 95% CI=1.38–2.68; P<0.001). Additionally, Pfeffer et al. (10) noted that, among patients with a history of cancer at baseline, there were 60 deaths in 188 patients assigned to darbepoetin and 37 deaths in 160 patients assigned to placebo (P=0.13), raising concern about the use of ESA in patients with a history of cancer.
A meta-analysis of trials comparing near-normal and low hemoglobin targets in CKD patients treated with ESA (including the trials above except for the TREAT study) identified nine randomized trials that enrolled 5143 patients (13). There was a significantly higher risk of all-cause mortality (risk ratio=1.17, 95% CI=1.01–1.35; P=0.031) and vascular access thrombosis (risk ratio=1.34; 95% CI=1.16–1.54; P=0.0001) in the near-normal hemoglobin target compared with the low hemoglobin target.
In summary, these recent trials indicate a consistent trend to increased risk for the use of ESA to target near-normal hemoglobin levels in dialysis and nondialysis CKD patients with anemia, but they offer no specific information on the risk of targeting hemoglobin between 115 and 130 g/L (compared with the comparator level used in most of these randomized controlled trials (hemoglobin of 90–115 g/L). It remains unknown at what hemoglobin level the risk of adverse events begins to increase.
Concerns and Controversy Associated with the New FDA Labeling for ESA
An important issue requiring clarification relates to the first FDA warning: “In controlled trials with CKD patients, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered ESA to target a hemoglobin level of greater than 11 g/dL.” This statement does not seem to be factually correct, because trials found higher risks in patients randomized to near-normal hemoglobin targets (i.e., >130 g/L and not >110 g/L). Information from clinical trials is not available to inform the selection of a hemoglobin target between 115 and 130 g/L.
Moreover, although the proportion of ESA recipients with hemoglobin levels below 100 g/L and above 120 g/L was previously included as part of the Centers for Medicare and Medicaid Services (CMS) Quality Incentive Program (QIP) for ESRD care, CMS has announced that they will remove the lower limit performance measure from the QIP in response to the change in the FDA labeling. Some have opposed the new FDA labels, arguing that removing this lower target level would put patients at higher risk of red cell transfusions and lower quality of life.
Regarding the risk of transfusion, it is important to note that the FDA is not mandating that ESA therapy be withdrawn. It is true that early placebo-controlled trials showed that ESA therapy led to a significant 85% reduction in the use of transfusion [i.e., relative risk, 0.15; 95% CI=0.05–0.47] (2). However, in trials comparing near-normal and low hemoglobin targets, the risk of receiving transfusions (among trials reporting this finding as an outcome) was 13.7% and 19.6% in near-normal and low hemoglobin arms, respectively (relative risk, 0.69; 95% CI=0.57–0.82; I2=0%) (2). In the TREAT trial, CKD patients in the control arm were not given darbepoetin unless hemoglobin was persistently <90 g/L, and blood transfusions were administered to 24.5% of patients assigned to placebo versus 14.8% of patients assigned to darbepoetin (P<0.001) (10). It is possible that rescue ESA therapy for patients with persistent hemoglobin levels between 90 and 100 g/L would have further reduced the difference in use of transfusions. Although blood transfusion is certainly associated with certain adverse effects (including alloimmunization), the systematic review suggests that the reduced risk of transfusion associated with near-normal hemoglobin targets is offset by the 17% higher risk of mortality and 34% higher risk of access thrombosis (13).
Another systematic review examined the impact of hemoglobin target on quality of life in patients treated with low (90–120 g/L) and near-normal hemoglobin target levels (>120 g/L) (14). The review found that there was incomplete reporting of data by randomized trials, and many of the studies were unblinded—both of which are likely to exaggerate the quality of life impact noted for near-normal targets. Available data suggested statistically significant changes in four of eight domains of the SF-36. Statistically significant changes were noted in the physical function [weighted mean difference (WMD)=2.9; 95% CI=1.3–4.5), general health (WMD=2.7; 95% CI=1.3–4.2), social function (WMD=1.3; 95% CI= −0.8–3.4), and mental health (WMD=0.4; 95% CI=0.1–0.8) domains. Of note, the changes were well below the threshold for a minimal clinically important difference, either when defined as a five-point absolute change in an SF-36 domain (15) or when based on the effect size (the ratio of observed mean change to baseline SD)—where a change of >0.5 SD has been found to be a remarkably good predictor of a minimally important difference (16). The placebo controlled TREAT study reported quality of life outcomes for all domains that were measured, identifying the Functional Assessment of Cancer Therapy (FACT)-fatigue score as the primary quality of life outcome. In TREAT, mean changes of 4.2 and 2.8 respectively were observed in the FACT-fatigue scale for the darbepoetin and placebo groups after 6 months (P<0.001). When considering that the baseline standard deviation was 12 (implying effect sizes of 0.35 and 0.23 respectively for the darbepoetin and placebo group), the difference in mean FACT-fatigue scores across the groups was clinically very small. Expressing this metric in a different fashion, the TREAT investigators comment that an additional 6% of patients in the darbepoetin group experienced a clinically detectable change in FACT fatigue scores (P=0.002), suggesting that 17 additional patients would need to be treated to near-normal levels with darbepoetin to have one experience a clinically detectable change in FACT fatigue scores.
In summary, the results of these analyses suggest that a large component of the apparent clinical link between ESA use and better quality of life relates to a placebo or healthy patient effect.
Managing Patients with ESA Resistance
The new FDA labels indirectly address the issue of ESA resistance, because they state that, for patients who do not respond adequately over 12 wk of dose escalation, increasing the ESA dose further is unlikely to improve response and may increase risk. Although no clinical trials have investigated different ESA dosing protocols in patients with ESA resistance, this concern seems appropriate given that these patients are at exceedingly high risk of cardiovascular events and mortality—even after adjustment for patient comorbidity (17). Given that ∼15% of ESRD patients have ESA resistance (18), trials are urgently required to determine the optimal strategy for anemia management in this population.
The New FDA Labeling: Additional Guidance to Providers Needed
The new FDA label also falls short of providing guidance to ESA prescribers about appropriate dosing. “Individualizing therapy to reduce the risk of transfusion” (Table 1) is somewhat vague and may prove difficult to apply in practice. In fact, individualizing therapy (based on a patient’s hemoglobin and their clinical status) may have contributed to the fact that 34% of US hemodialysis patients had hemoglobin >120 g/L, despite reimbursement regulations requiring a reduction in ESA dosing when hemoglobin exceeded 125 g/L (19). Many centers have now initiated nurse or pharmacist-led anemia protocols, where ESA dosing is based on hemoglobin levels (and trends in hemoglobin levels), current ESA dosing, and indices of iron storage. Although the evidence in support of these protocols comes mostly from observational studies (20), one randomized trial suggested that such protocols may increase the proportion of patients achieving the target hemoglobin (21).
Despite the problems associated with target hemoglobin ranges, the increasing use of anemia protocols, the difficulties in individualizing therapy based on patient symptoms, and the current lack of a suitable alternative suggest that anemia protocols and target hemoglobin ranges are here to stay. Although the new labels avoid recommending a target range, they do recommend reducing ESA doses when hemoglobin exceeds 110 g/L in dialysis patients (100 g/L in non-dialysis CKD) (Table 1). Given the stated goal of minimizing use of transfusions, perhaps the FDA should simply have recommended targeting hemoglobin ranges of 90–110 and 90–100 gL, respectively, in these two populations. These ranges are lower than the comfort zone for many nephrologists but would be largely consistent with the control arms of the near-normal hemoglobin trials. Adopting these lower ranges will be associated with a small increase in the use of transfusions (and their associated risks), which are often triggered by precipitous drops in hemoglobin associated with bleeding or acute inflammatory states. To reduce this risk, protocols might consider judiciously increasing the dose of ESA (and searching for an underlying cause, if appropriate) in outpatients with declining hemoglobin, even when within the range of 90–100 g/L.
Given that the primary goal of ESA use now seems to be preventing exposure to blood products, the frequency of red cell transfusions would seem to be a reasonable clinical performance measure to track by CMS. It is likely that this measure would correlate with the proportion of patients with hemoglobin persistently below 90 g/L.
Summary
Clinical trials have consistently shown that targeting hemoglobin of >130 g/L leads to important harms, including cardiovascular events, access thrombosis, and possibly mortality. Moreover, because the target hemoglobin level in the low hemoglobin groups in the largest randomized controlled trials generally ranged from 90 to 115 g/L, it is unknown at what target hemoglobin level above this range the incidence of adverse events increases. Given that the evidence in support of quality of life benefits is inconsistent and of very small magnitude, aiming for targets above this level is not justifiable. When one considers that the goal of ESA treatment is to improve clinical outcomes and reduce the need for transfusions as much as possible rather than achieve a particular hemoglobin target, the recent FDA labeling update seems balanced, although more specific guidance to clinicians would have been helpful.
Disclosures
M.T. holds a peer-reviewed research grant from the Canadian Institutes of Health Research that is partially funded by Abbott Laboratories.
Acknowledgments
M.T. and B.J.M. are supported by Alberta Innovates—Health Solutions (formerly Alberta Heritage Foundation for Medical Research) Health Scholar Awards. M.T. was also supported by a Government of Canada Research Chair in the optimal care of people with chronic kidney disease. M.T. and B.J.M. were supported by an alternative funding plan from the Government of Alberta and the Universities of Alberta and Calgary.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.FDA: FDA Drug Safety Communication: Modified Dosing Recommendations to Improve the Safe Use of Erythropoiesis-Stimulating Agents in Chronic Kidney Disease, 2011. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm259639.htm. Accessed November 15, 2011
- 2.Tonelli MKS, Wiebe N, Shrive F, Hemmelgarn B, Manns B: Erythropoiesis-Stimulating Agents for Anemia of Chronic Kidney Disease: Systematic Review and Economic Evaluation. Technology Report Number 106, Ottawa, Canada, Canadian Agency for Drugs and Technologies in Health, 2008 [Google Scholar]
- 3.Bahlmann J, Schöter KH, Scigalla P, Gurland HJ, Hilfenhaus M, Koch KM, Muthny FA, Neumayer HH, Pommer W, Quelhorst E: Morbidity and mortality in hemodialysis patients with and without erythropoietin treatment: A controlled study. Contrib Nephrol 88: 90–106, 1991 [DOI] [PubMed] [Google Scholar]
- 4.Canadian Erythropoietin Study Group: Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. BMJ 300: 573–578, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins AJ, Ma JZ, Xia A, Ebben J: Trends in anemia treatment with erythropoietin usage and patient outcomes. Am J Kidney Dis 32[Suppl 4]: S133–S141, 1998 [DOI] [PubMed] [Google Scholar]
- 6.Fleming TR, DeMets DL: Surrogate end points in clinical trials: Are we being misled? Ann Intern Med 125: 605–613, 1996 [DOI] [PubMed] [Google Scholar]
- 7.Manns B, Owen WF, Jr., Winkelmayer WC, Devereaux PJ, Tonelli M: Surrogate markers in clinical studies: Problems solved or created? Am J Kidney Dis 48: 159–166, 2006 [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation: KDOQI: Clinical practice guidelines and clinical practice recommendations for anemia in chronic kidney disease. Am J Kidney Dis 47[Suppl 3]: S1–S146, 2006 [DOI] [PubMed] [Google Scholar]
- 9.Besarab A, Bolton WK, Browne JK, Egrie JC, Nissenson AR, Okamoto DM, Schwab SJ, Goodkin DA: The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med 339: 584–590, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Pfeffer MA, Burdmann EA, Chen CY, Cooper ME, de Zeeuw D, Eckardt KU, Feyzi JM, Ivanovich P, Kewalramani R, Levey AS, Lewis EF, McGill JB, McMurray JJ, Parfrey P, Parving HH, Remuzzi G, Singh AK, Solomon SD, Toto RTREAT Investigators: A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med 361: 2019–2032, 2009 [DOI] [PubMed] [Google Scholar]
- 11.Singh AK, Szczech L, Tang KL, Barnhart H, Sapp S, Wolfson M, Reddan DCHOIR Investigators: Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med 355: 2085–2098, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Drüeke TB, Locatelli F, Clyne N, Eckardt KU, Macdougall IC, Tsakiris D, Burger HU, Scherhag ACREATE Investigators: Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med 355: 2071–2084, 2006 [DOI] [PubMed] [Google Scholar]
- 13.Phrommintikul A, Haas SJ, Elsik M, Krum H: Mortality and target hemoglobin concentrations in anaemic patients with chronic kidney disease treated with erythropoietin: a meta-analysis. Lancet 369: 381–388, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Clement FM, Klarenbach S, Tonelli M, Johnson JA, Manns BJ: The impact of selecting a high hemoglobin target level on health-related quality of life for patients with chronic kidney disease: A systematic review and meta-analysis. Arch Intern Med 169: 1104–1112, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Wyrwich KW, Tierney WM, Babu AN, Kroenke K, Wolinsky FD: A comparison of clinically important differences in health-related quality of life for patients with chronic lung disease, asthma, or heart disease. Health Serv Res 40: 577–591, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norman GR, Sloan JA, Wyrwich KW: Interpretation of changes in health-related quality of life: The remarkable universality of half a standard deviation. Med Care 41: 582–592, 2003 [DOI] [PubMed] [Google Scholar]
- 17.Panichi V, Rosati A, Bigazzi R, Paoletti S, Mantuano E, Beati S, Marchetti V, Bernabini G, Grazi G, Rizza GM, Migliori M, Giusti R, Lippi A, Casani A, Barsotti G, Tetta CRISCAVID Study Group: Anaemia and resistance to erythropoiesis-stimulating agents as prognostic factors in haemodialysis patients: Results from the RISCAVID study. Nephrol Dial Transplant 26: 2641–2648, 2011 [DOI] [PubMed] [Google Scholar]
- 18.Klarenbach SW, Moist LM, Foley RN, Barrett BJ, Madore F, White CT, Culleton BF, Tonelli M, Manns BJCanadian Society of Nephrology: Clinical practice guidelines for supplemental therapies and issues. Kidney Int Suppl 110: S19–S24, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Collins AJ, Brenner RM, Ofman JJ, Chi EM, Stuccio-White N, Krishnan M, Solid C, Ofsthun NJ, Lazarus JM: Epoetin alfa use in patients with ESRD: An analysis of recent US prescribing patterns and hemoglobin outcomes. Am J Kidney Dis 46: 481–488, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Moist LM, Foley RN, Barrett BJ, Madore F, White CT, Klarenbach SW, Culleton BF, Tonelli M, Manns BJCanadian Society of Nephrology: Clinical practice guidelines for evidence-based use of erythropoietic-stimulating agents. Kidney Int Suppl 110: S12–S18, 2008 [DOI] [PubMed] [Google Scholar]
- 21.Brimble KS, Rabbat CG, McKenna P, Lambert K, Carlisle EJ: Protocolized anemia management with erythropoietin in hemodialysis patients: a randomized controlled trial. J Am Soc Nephrol 14: 2654–2661, 2003 [DOI] [PubMed] [Google Scholar]