Summary
Background and objectives
Previous studies reporting an association between high BP and high sodium and low potassium intake or urinary sodium/potassium ratio (U[Na+]/[K+]) primarily included white men and did not control for cardiovascular risk factors.
Design, setting, participants, & measurements
This cross-sectional study investigated the association of U[Na+]/[K+] with BP in 3303 participants using robust linear regression.
Results
Mean age was 43±10 years, 56% of participants were women, and 52% were African American. BP was higher in African Americans than in non–African Americans, 131/81±20/11 versus 120/76±16/9 mmHg (P<0.001). Mean U[Na+]/[K+] was 4.4±3.0 in African Americans and 4.1±2.5 in non–African Americans (P=0.002), with medians (interquartile ranges) of 3.7 (3.2) and 3.6 (2.8). Systolic BP increased by 1.6 mmHg (95% confidence interval, 1.0, 2.2) and diastolic BP by 1.0 mmHg (95% confidence interval, 0.6, 1.4) for each 3-unit increase in U[Na+]/[K+] (P<0.001 for both). This association remained significant after adjusting for diabetes mellitus, smoking, body mass index, total cholesterol, GFR, and urine albumin/creatinine ratio. There was no interaction between African-American race and U[Na+]/[K+], but for any given value of U[Na+]/[K+], both systolic BP and diastolic BP were higher in African Americans than in non–African Americans. The diastolic BP increase was higher in men than in women per 3-unit increase in U[Na+]/[K+] (1.6 versus 0.9 mmHg, interaction P=0.03).
Conclusions
Dietary Na+ excess and K+ deficiency may play an important role in the pathogenesis of hypertension independent of cardiovascular risk factors. This association may be more pronounced in men than in women.
Introduction
Hypertension affects 50 million people in the United States and 1 billion worldwide and is associated with an increased risk of ESRD and significant morbidity and mortality (1–4). The cardiovascular risk associated with BP is, in fact, continuous, independent of other risk factors, and increases beginning at a BP as low as 115/75 mmHg (4). Although the pathogenesis of primary hypertension is still not well understood, a variety of modifiable factors such as diet, especially one high in sodium (Na+) and low in potassium (K+) content, have been implicated as mechanisms. Classic studies and observational data implicate that the combined effect of low K+ and high Na+ on BP seems greater than either alone, especially in blacks or African Americans (5–13). We suggested a role for WNK1 (with no lysine [K]) as a mediator of renal Na+ retention and salt-sensitive hypertension in a state of dietary K+ deficiency (12,13). However, observational data indicating an association between high Na+ and low K+ intake or a high urinary Na+/K+ ratio and high BP or cardiovascular disease were limited by the use of imperfect measures of Na+ and K+ intake, such as dietary recall questionnaires (14,15), lack of control for confounding variables such as cardiovascular risk factors (16–19), and inclusion of predominantly white male participants (16,17,20).
The purpose of our study was to investigate the association of urinary Na+/K+ ratio (U[Na+]/[K+]) with increased systolic and diastolic BP independent of cardiovascular risk factors, albuminuria, and estimated GFR (eGFR), and to further delineate whether an interaction by race or sex exists. We examined a multiethnic sample of community-dwelling participants oversampled for African Americans, with approximately equal numbers of men and women.
Materials and Methods
Study Participants
The Dallas Heart Study is a cross-sectional population-based, probability sample of Dallas county residents aged 30–65 years (21). This study was approved by the institutional review board and participants gave informed consent. An initial in-home visit was made to 6101 participants to obtain demographic and health-related data. A second in-home visit was performed for fasting venous blood and first-void morning urine samples in 3557 participants. Our analyses include 3303 participants with data for estimations of serum and urine creatinine, urine electrolytes, urine microalbumin, and eGFR.
Clinical Variables
Race was dichotomized as African American or non–African American on the basis of self-report. Five BP measurements were taken by trained personnel with the participant in the seated position using an automatic oscillometric device previously validated against direct catheter-based intra-arterial pressure measurements (21). The mean of the third, fourth, and fifth measurements was used for analysis. Hypertension was defined as follows: (1) self-reported diagnosis, (2) treatment for hypertension, (3) systolic BP ≥140 mmHg, or (4) diastolic BP ≥90 mmHg. Diabetes mellitus was defined on the basis of the following: (1) self-report combined with insulin or oral hypoglycemic use, (2) fasting glucose ≥126 mg/dl, or (3) nonfasting glucose ≥200 mg/dl. The definition of hypercholesterolemia was based on a fasting calculated LDL cholesterol ≥160 mg/dl, nonfasting direct LDL ≥160 mg/dl, total cholesterol ≥240 mg/dl, or the use of statin medication. Participants who smoked at least 100 cigarettes in their lifetime or who had smoked in the previous 30 days were classified as smokers.
Urinary and Kidney Function Measurements
A first-void morning urine sample was used for urinary parameters. Urinary potassium concentration U[K+] and sodium concentration U[Na+] were analyzed and expressed as milliequivalents per liter. U[Na+]/[K+] was calculated by dividing U[Na+] by U[K+]. The urine albumin/creatinine ratio (UACR) was calculated by dividing urine albumin by urine creatinine and was expressed as milligrams per gram. Albuminuria was defined as an UACR of ≥17 mg/g in men and ≥25 mg/g in women (22). The four-variable Modification of Diet in Renal Disease study formula was used to estimate GFR (23).
Statistical Analyses
For bivariate comparisons, categorical variables were compared using the chi-squared test, and continuous variables were compared using the t test. Multivariable robust linear regression was used to evaluate the association of U[Na+]/[K+] with systolic and diastolic BP. To minimize any potential effect of the right-skewed U[Na+]/[K+] distribution on the regression fit, robust linear regression estimation was used instead of classic linear regression. Robust regression is less sensitive to the extreme values of predictors and thus the regression fit is less affected by the skewed U[Na+]/[K+] distribution. Regression diagnostics were used to explore the presence and effect of potential outliers (influence points), and <2.5% of the sample was classified as outliers, which is considered acceptable.
The association between U[Na+]/[K+] as an independent variable and the presence of hypertension as the dependent variable was explored using logistic regression. Other candidate covariates were included in the multivariable models if clinically relevant and/or statistically significant in univariate analyses. The interactions of race × U[Na+]/[K+] and sex × U[Na+]/[K+] were also tested.
To test that the potential effect of diuretics, angiotensin-converting enzyme (ACE) inhibitors, and angiotensin receptor blockers (ARBs) on urinary Na+ and K+ excretion did not significantly affect the results, sensitivity analyses with the exclusion of participants receiving treatment with these medications were performed. All statistical tests were two-sided, were conducted at the 0.05 significance level, and were reported using P values. Interaction term P values <0.1 were considered statistically significant. All analyses were performed using SAS Enterprise Guide software (version 3.0; SAS, Cary, NC) and SAS software (version 9.1.3; SAS).
Results
Characteristics of the Cohort
Among the 3303 participants, the mean age was 43.0±10.1 years and 55.9% were women (Table 1). Fifty-two percent were African Americans, 28.9% were Caucasians, 17.1% were Hispanics, and 2.1% were of other races. Thirty-six percent had hypertension and 21% were being treated with antihypertensive medications (Table 1). Mean systolic BP and diastolic BP were 125.2±19.0 mmHg and 78.5±10.3 mmHg, respectively.
Table 1.
Clinical characteristics of the cohort
| Variable | Entire Cohort (N=3303) | African Americans (n=1713) | Non–African Americans (n=1590) | P Valuea |
|---|---|---|---|---|
| Age (yr) | 43.0±10.1; 43.0 (16.0) | 44.7±10.3; 44.0 (16.0) | 43.0±9.9; 42.0 (15.0) | <0.001 |
| Sex | 0.06 | |||
| men | 44.1 | 42.5 | 45.8 | |
| women | 55.9 | 57.5 | 54.2 | |
| Race | ||||
| African American | 51.9 | |||
| Caucasian | 28.9 | |||
| Hispanic | 17.1 | |||
| other | 2.1 | |||
| Smoker | 45.5 | 47.1 | 43.9 | 0.07 |
| Hypertension | 36.0 | 47.1 | 24.0 | <0.001 |
| Diabetes mellitus | 12.0 | 14.5 | 9.3 | <0.001 |
| Hypercholesterolemia | 13.0 | 12.7 | 13.3 | 0.59 |
| Medications | ||||
| all antihypertensives | 20.9 | 26.1 | 15.3 | <0.001 |
| all diuretics | 9.0 | 12.8 | 4.9 | <0.001b |
| loop diuretics | 2.5 | 3.7 | 1.2 | <0.001b |
| thiazide diuretics | 6.7 | 9.2 | 3.7 | <0.001b |
| potassium-sparing diuretics | 3.9 | 5.2 | 1.6 | <0.001b |
| ACE inhibitors | 10.1 | 12.4 | 7.6 | <0.001 |
| ARBs | 1.7 | 2.0 | 1.3 | 0.13b |
| Body mass index (kg/m2) | 29.6±7.0; 28.5 (8.7) | 30.5 ±7.5; 29.5 (9.7) | 28.7±6.3; 27.7 (7.6) | <0.001 |
| Systolic BP (mmHg) | 125.2±19.0; 124.7 (21.0) | 130.5±20.0; 128.0 (24.3) | 119.5±16.1; 121.7 (19.0) | <0.001 |
| Diastolic BP (mmHg) | 78.5±10.3; 77.3 (13.0) | 81.2± 10.7; 80.0 (13.6) | 75.5±9.1; 75.0 (12.0) | <0.001 |
| Serum glucose (mg/dl) | 103.5±44.8; 93.0 (18.0) | 105.7±49.7; 92.0 (20.0) | 101.1±38.7; 93.0 (15.0) | 0.003 |
| Serum cholesterol (mg/dl) | 180.4±39.8; 177.0 (50.0) | 177.8±40.3; 174.0 (49.0) | 183.2±39.1; 179.0 (49.0) | <0.001 |
| GFR (ml/min per 1.73 m2)c | 100.3±24.7; 104.8 (40.7) | 103.2±26.1; 98.8 (36.6) | 97.1±22.7; 109.2 (41.0) | <0.001 |
| UACR (mg/g) | 21.8±191.0; 2.9 (3.5) | 32.4±250.1; 3.1 (4.9) | 10.4±90.4; 2.8 (2.7) | <0.001 |
Data are expressed as mean ± SD, median (IQR), or percentage. IQR, interquartile range; ACE, angiotensin-converting enzyme; ARB, angiotensin receptor blocker; UACR, urine albumin/creatinine ratio.
P value is for the comparison of variables between African-American and non–African-American racial groups using a two-sample t test for continuous variables and chi-squared test for categorical variables.
Fisher’s exact test was used for the comparison.
GFR was estimated using the four-variable Modification of Diet in Renal Disease Study formula.
The African-American group had a higher mean age than the non–African-American group, but the proportions of men and women did not significantly differ (Table 1). A higher proportion of African Americans was diabetic, hypertensive, and treated with antihypertensive medications. Mean systolic BP and diastolic BP were higher among African Americans versus non–African Americans, at 130.5/81.2±20.0/10.7 mmHg versus 119.5/75.5±16.1/9.1 mmHg, respectively (P<0.001). The mean UACR was 32.4 mg/g (in the range of microalbuminuria) in African Americans and 10.4 mg/g in non–African Americans (P<0.001) (Table 1). U[Na+]/[K+] was 4.4±3.0 and 4.1±2.5 in the two racial groups, respectively (P=0.002). Corresponding median (interquartile range) values were 3.7 (3.2) for African Americans and 3.6 (2.8) for non–African Americans.
Univariate Associations between Risk Factors and U[Na+]/[K+]
Systolic and diastolic BP, body mass index (BMI), and serum glucose were significantly and directly associated with U[Na+]/[K+], and UACR was indirectly correlated. Categorical variables significantly associated with higher U[Na+]/[K+] in univariate models included African-American racial group and presence of hypertension and nonsmoker status (data not shown).
Relationships between U[Na+]/[K+] and BP
Systolic BP increased by 1.58 mmHg (95% confidence interval [95% CI], 0.9, 2.2) per each 3-unit increase in U[Na+]/[K+], and diastolic BP increased by 1.02 mmHg (95% CI, 0.6, 1.4) (P<0.001 for both correlations) (Table 2). In adjusted models, systolic BP was 1.16 mmHg (95% CI, 0.6, 1.7) and diastolic BP was 0.84 mmHg (95% CI, 0.5, 1.2) higher for each 3-unit increase in U[Na+]/[K+] (P<0.001 for both associations) (Table 2). Other factors associated with both systolic and diastolic BP in multivariable models included increased age, male sex, African-American race, and presence of diabetes mellitus, as well as higher BMI, total cholesterol, and UACR (Table 3). Smoker status was also associated with diastolic BP, and there was also a small but statistically significant correlation between eGFR and systolic BP.
Table 2.
Robust linear regression for systolic and diastolic BPs
| Independent Variable, U[Na+]/[K+] | Systolic BP | Diastolic BP | ||
|---|---|---|---|---|
| Change (95% CI)a | P Value | Change (95% CI)a | P Value | |
| Model 1 | 1.58 (0.93, 2.23) | <0.001 | 1.02 (0.64, 1.40) | <0.001 |
| Model 2 | 1.16 (0.63, 1.70) | <0.001 | 0.84 (0.50, 1.18) | <0.001 |
| Model 3 | 1.36 (0.82, 1.90) | <0.001 | 0.95 (0.95, 1.30) | <0.001 |
Model 1: univariable model. Model 2: multivariable model, adjusted for age, sex, race, diabetes mellitus, smoking, body mass index, total cholesterol, estimated GFR, and urine albumin/creatinine ratio. Model 3: Sensitivity analysis excluding participants receiving treatment with diuretic, angiotensin-converting enzyme inhibitors, and angiotensin receptor blocker medication. U[Na+]/[K+], urinary sodium/potassium ratio; 95% CI, 95% confidence interval.
Unit for change in BP is mmHg per each 3-unit increase in U[Na+]/[K+].
Table 3.
Multivariable robust linear regression for systolic BP and diastolic BP
| Independent Variable | Change (95% CI) | P Value |
|---|---|---|
| Systolic BP | ||
| urine [Na+]/[K+]a | 1.16 (0.62, 1.70) | <0.001 |
| ageb | 2.33 (2.06, 2.60) | <0.001 |
| male sex | 8.10 (6.90, 9.30) | <0.001 |
| African-American race | 7.37 (6.36, 8.39) | <0.001 |
| diabetes mellitus | 4.27 (2.64, 5.90) | <0.001 |
| smoker status | 1.01 (0.01, 2.01) | 0.05 |
| body mass index (kg/m2)b | 2.81 (2.43, 3.18) | <0.001 |
| total cholesterol (mg/dl)b | 0.13 (0.07, 0.19) | <0.001 |
| GFR (ml/min per 1.73 m2)b | 0.10 (0.05, 0.20) | 0.04 |
| UACR (mg/g)b | 0.21 (0.18, 0.25) | <0.001 |
| Diastolic BP | ||
| urine [Na+]/[K+]a | 0.84 (0.50, 1.18) | <0.001 |
| ageb | 0.89 (0.72, 1.06) | <0.001 |
| male sex | 2.96 (2.20, 3.72) | <0.001 |
| African-American race | 4.12 (3.48, 4.76) | <0.001 |
| diabetes mellitus | 1.07 (0.06, 2.09) | 0.04 |
| smoker status | 0.66 (0.03, 1.29) | 0.04 |
| body mass index (kg/m2)b | 1.59 (1.36, 1.83) | <0.001 |
| total cholesterol (mg/dl)b | 0.12 (0.08, 0.16) | <0.001 |
| GFR (ml/min per 1.73 m2)b | 0.04 (−0.02, 0.10) | 0.19 |
| UACR (mg/g)b | 0.02 (0.01, 0.03) | 0.001 |
95% CI, 95% confidence interval; U[Na+]/[K+], urinary sodium/potassium ratio; UACR, urine albumin/creatinine ratio.
Per 3-unit increase.
Per 5-unit increase.
Of the 3303 participants, 519 (15.7%) were receiving treatment with one or more diuretic, ACE inhibitor, or ARB medication classes that could potentially affect urinary Na+ or K+. Sensitivity analyses by the exclusion of participants receiving treatment with these medications did not significantly affect the results (Tables 2, 4, and 5). Finally, U[Na+]/[K+] was significantly associated with the presence of hypertension using logistic regression with an odds ratio of 1.13 (95% CI, 1.05, 1.22) in the univariable model and 1.12 (95% CI, 1.02, 1.22) in the multivariable model, for each 3-unit increase in U[Na+]/[K+] (data not shown).
Table 4.
Robust linear regression analysis for BP stratified by race
| African American | Non–African American | |||
|---|---|---|---|---|
| Change (95% Confidence Interval)a | P Value | Change (95% Confidence Interval)a | P Value | |
| Systolic BP, U[Na+]/[K+] | ||||
| model 1 | 1.23 (0.30, 2.17) | 0.01 | 1.39 (0.51, 2.26) | 0.002 |
| model 2 | 1.22 (0.42, 2.01) | 0.003 | 1.09 (0.35, 1.82) | 0.004 |
| model 3 | 1.53 (0.71, 2.36) | <0.001 | 1.01 (0.27, 1.75) | 0.008 |
| Diastolic BP, U[Na+]/[K+] | ||||
| model 1 | 0.77 (0.25, 1.29) | 0.004 | 1.11 (0.56, 1.66) | <0.001 |
| model 2 | 0.75 (0.27, 1.23) | 0.002 | 0.91 (0.43, 1.40) | <0.001 |
| model 3 | 1.00 (0.48, 1.52) | <0.001 | 0.81 (0.32, 1.31) | 0.001 |
Model 1: univariable model. Model 2: multivariable model, adjusted for age, sex, race, diabetes mellitus, smoking, body mass index, total cholesterol, estimated GFR, and urine albumin/creatinine ratio. Model 3: sensitivity analysis excluding participants receiving treatment with diuretic, angiotensin-converting enzyme inhibitors, and angiotensin receptor blocker medication. U[Na+]/[K+], urinary sodium/potassium ratio.
Unit for change in BP is mmHg per each 3-unit increase in U[Na+]/[K+].
Table 5.
Robust linear regression analysis for BP stratified by sex
| Female | Male | |||
|---|---|---|---|---|
| Change (95% CI)a | P Value | Change (95% CI)a | P Value | |
| Systolic BP, U[Na+]/[K+] | ||||
| model 1 | 1.26 (0.36, 2.17) | 0.006 | 1.86 (0.98, 2.74) | <0.001 |
| model 2 | 0.89 (0.18, 1.61) | 0.01 | 1.57 (0.78, 2.36) | <0.001 |
| model 3 | 1.00 (0.24, 1.75) | 0.01 | 1.85 (1.07, 2.62) | <0.001 |
| Diastolic BP, U[Na+]/[K+] | ||||
| model 1 | 0.75 (0.23, 1.28) | 0.005 | 1.30 (0.74, 1.86) | <0.001 |
| model 2 | 0.51 (0.06, 0.96) | 0.03 | 1.27 (0.75, 1.78) | <0.001 |
| model 3 | 0.63 (0.14, 1.12) | 0.01 | 1.31 (0.79, 1.83) | <0.001 |
Model 1: univariable model. Model 2: multivariable model, adjusted for age, sex, race, diabetes mellitus, smoking, body mass index, total cholesterol, estimated GFR, and urine albumin/creatinine ratio. Model 3: sensitivity analysis excluding participants receiving treatment with diuretic, angiotensin-converting enzyme inhibitors, and angiotensin receptor blocker medication. 95% CI, 95% confidence interval; U[Na+]/[K+], urinary sodium/potassium ratio.
Unit for change in BP is mmHg per each 3-unit increase in U[Na+]/[K+].
Race and Sex Interactions
There were 1713 African-American and 1590 non–African-American participants. After stratification by race, in multivariable models systolic BP increased by 1.22 mmHg (95% CI, 0.42, 2.01) for each 3-unit increase in U[Na+]/[K+] (P=0.003), and diastolic BP increased by 0.75 mmHg (95% CI, 0.27, 1.24) (P=0.002) in the African-American racial group (Table 4). Corresponding values in non–African Americans were 1.09 mmHg (95% CI, 0.35, 1.82) for systolic BP and 0.91 mmHg (95% CI, 0.43, 1.40) for diastolic BP. For any value of U[Na+]/[K+], on average, African Americans had an 11.0 mmHg higher systolic BP and 5.4 mm Hg higher diastolic BP than their non–African-American counterparts (data not shown). In addition, the presence of African-American race (versus non–African-American race) increased the odds of having hypertension by 2.5 (multivariable odds ratio, 2.51; 95% CI, 2.11, 3.00) (data not shown).
However, there was no statistically significant interaction detected between African-American race and U[Na+]/[K+] in models of systolic or diastolic BP (interaction P values 0.7 and 0.4, respectively), or in models in which hypertension was the outcome measure. To investigate whether the absence of an effect of race on the correlation between U[Na+]/[K+] and BP was not diluted by the mixed racial composition of the non–African-American group, we performed an exploratory analysis in which three racial groups were considered separately: African Americans (n=1713), Caucasians (n=956), and others (n=634). In this exploratory analysis, we did not find a significant U[Na+]/[K+] × race interaction either (P=0.38; data not shown). Sensitivity analysis by the exclusion of participants receiving diuretics, ACE inhibitors, or ARBs attenuated the magnitude of the association between U[Na+]/[K+] and both systolic and diastolic BP in African Americans but not in non–African Americans (Table 4).
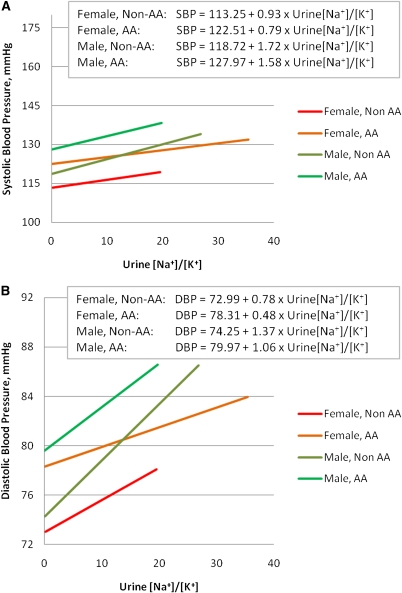
There were 1847 female and 1456 male participants in this study. Systolic BP increased to a greater degree in men compared with women for every 3-unit increase in U[Na+]/[K+], 1.57 mmHg (95% CI, 0.78, 2.36) versus 0.89 mmHg (95% CI, 0.18, 1.61) in the adjusted models (Table 5, model 2). The magnitude of the increase in diastolic BP in men was more than two-fold that in women (1.27 mmHg [95% CI, 0.75, 1.78] versus 0.51 mmHg [95% CI, 0.06, 0.96]), with an interaction P value that was statistically significant (P=0.03 for the multivariable model). However, the U[Na+]/[K+] × sex interaction P value for systolic BP was 0.1 and did not reach statistical significance. The U[Na+]/[K+] × sex interaction terms were also statistically significant after participants receiving treatment with diuretics, ACE inhibitors, or ARBs were excluded (P=0.09 for systolic and P=0.06 for diastolic BP) (Table 5, model 3). Figure 1 illustrates the linear regression of U[Na+]/[K+] on systolic and diastolic BP and reveals that for each value of U[Na+]/[K+], African Americans had a higher BP than non–African Americans. Similar results were observed for men compared with women.
Figure 1.
Association of urinary sodium/potassium ratio with blood pressure using linear regression: sex and race interactions. Linear regression of urine [Na+]/[K+] on (A) systolic BP and (B) diastolic BP. AA, African American; Non-AA, non–African American; SBP, systolic BP; DBP, diastolic BP.
Discussion
Our findings indicate a robust positive correlation between U[Na+]/[K+] and prevalent BP independently of most relevant clinical covariates. The unique advantage of this study is the concurrent availability of data on kidney function and albuminuria. Our results confirm the findings reported by previous observational studies and extend these findings utilizing a large multiethnic community-dwelling group of participants, in which nonwhite racial and ethnic groups were well represented, showing that the ratio of Na+/K+ intake may play a significant role in the pathogenesis of primary hypertension. Although the majority of participants were not hypertensive, our analysis extends previous findings to nonhypertensive individuals who may be at risk for the future development of hypertension, and thus increases generalizability. In addition, we used direct measurements of U[Na+]/[K+], rather than indirect measures such as dietary recall. We further substantiate that this association is independent of other cardiovascular risk factors and is intensified in men compared with women. There was also a trend toward a larger BP effect in African-American versus non–African-American participants.
Classic studies by Krishna et al. (6) and Morris et al. (7) implicate that a combined effect of dietary Na+ excess and K+ deficit is greater than either alone as a mediator of high BP. Decreased efficacy of K+ intake in lowering BP when accompanied by low Na+ intake has also been reported in larger trials such as the Dietary Approaches to Stop Hypertension (DASH) trial (9,24). Previous studies reported an association between urinary Na+/K+ ratio and BP but had several limitations. For example, INTERSALT, a large epidemiologic study, reported a positive correlation between urinary Na+/K+ ratio and BP, which after adjustment for age, sex, BMI, and alcohol consumption was statistically significant in only 8 of the 52 centers for systolic BP and 3 centers for diastolic BP (17). No adjustment was made for eGFR and other cardiovascular risk factors and <10% of participants were black (16,17). Similarly, no adjustment was made for eGFR or cardiovascular risk factors in analyses done by Hsiao et al. (18) and Kwok et al. (19). Cook et al. (20) reported an association between urinary Na+/K+ ratio and subsequent cardiovascular risk among 2306 participants of the Trials of Hypertension Prevention study (TOHP), only 14% of which were African American. Fifty percent of our cohort was African American, with equal percentages of each sex. Inclusion of a large proportion of African Americans is particularly important because this racial group not only has higher hypertension prevalence, but also has higher salt sensitivity (7,8) and lower dietary K+ intake (25). African Americans develop complications of hypertension, such as stroke, left ventricular hypertrophy, and ESRD, at disproportionately higher rates (26–28). Therefore, insight into mechanisms that prevent or treat hypertension in this group could provide significant benefits.
Previous data raise the interesting question of whether differences in intake between races are primary, or secondary and subsequent to primary differences in renal tubular handling and excretion, or due to differences in gastrointestinal absorption. Turban et al. (11) reported that baseline urinary K+ excretion was significantly lower in blacks compared with whites, which was maintained after an 8-week consumption of the DASH diet. One proposed mechanism is augmented furosemide-sensitive Na+-K+-2Cl− co-transporter activity in the thick ascending limb of Henle’s loop in blacks, which leads to increased fractional Na+ reabsorption upstream to the location of the epithelium-like sodium channel, reducing tubular flow and Na+ delivery and resulting in less K+ excretion (8). The observation that furosemide inhibition of Na+-K+-2Cl− co-transporter results in a more significant increase in K+ excretion in blacks compared with whites supports this hypothesis (8,29). Another potential mechanism as suggested by Lazrak et al. (12) and Huang et al. (13), could be the increased ratio of long WNK1 kinase to kidney-specific WNK1, resulting in renal Na+ retention and salt-sensitive hypertension in those with low dietary K+ intake, such as African Americans.
We found a significant interaction between U[Na+]/[K+] and sex, such that men had a higher diastolic BP compared with women for each unit of increase in U[Na+]/[K+]. Sex differences in salt sensitivity were not as extensively studied as racial differences. There were no significant sex interactions reported in the DASH trial (10), but in the DASH-Sodium trial, the effects of sodium intake were significantly greater in women than men on the DASH diet (9). Similarly, in phase I of the TOHP study, an intervention to reduce dietary Na+ intake resulted in a larger systolic BP effect in women than in men. However, women had lower Na+ intake at baseline and were more likely to decrease urinary Na+ to target, suggesting that better adherence to the low-salt diet may have resulted in lower BP in response to intervention (30). In 87 Japanese hypertensive patients, salt sensitivity did not differ between sexes, but the decrease in plasma renin activity after NaCl loading was significantly smaller in women than in men, suggesting that the mechanism of BP elevation after NaCl loading may differ between sexes (31). It should be noted that the TOHP I and DASH-Sodium studies only included individuals with prehypertension and stage I hypertension, whereas a large proportion of our sample did not have hypertension.
Although our population-based study allowed the evaluation of U[Na+]/[K+] and BP in a large multiethnic sample, participants were not on a prespecified controlled diet, and 24-hour urine collection data were not available for analysis. We could not, therefore, examine the dietary contributions of Na+ alone or K+ alone. To reduce this potential limitation, we analyzed U[Na+]/[K+] as a measure of the ratio of dietary Na+/K+ intake. Importantly, the positive correlation of BP with U[Na+]/[K+] is consistent with the INTERSALT study results (16), in which 24-hour urine collection data were used as indices of intake. Given the cross-sectional nature of this analysis, future studies need to determine whether these relationships would hold longitudinally, both by virtue of changes in diet (repeatability of the measurements) and the variability of BP over time.
Furthermore, although the correlation that we report between U[Na+]/[K+] and BP is modest, it is clinically relevant from a population-based perspective given the implications of small reductions in BP for primary prevention of cardiovascular disease. Similarly to our results, the correlation coefficients between urinary Na+/K+ ratio and BP were also modest in larger studies (16,32). Long-term data from large observational and randomized trials suggest that even a small 2-mmHg reduction in diastolic BP would result in a 17% decrease in the prevalence of hypertension, a 6% reduction in coronary heart disease, and a 15% decrease in stroke and transient ischemic attacks (33). In addition, some data suggested that low Na+ and high K+ dietary content may have potential benefits beyond direct BP effects, such as long-term favorable effects on cardiovascular outcomes (20,34,35).
In summary, this analysis supports the hypothesis that dietary Na+ excess and K+ deficiency may play an important role in hypertension pathogenesis and extends these findings across both sexes and racial groups in a multiethnic sample. It further demonstrates that this association may be independent of other traditional cardiovascular risk factors and measures of kidney function. Future studies need to elucidate whether differences in urinary Na+/K+ ratio can be primarily explained by dietary Na+ and K+ intake or whether sex and racial differences in renal Na+ and K+ handling are also implicated. Importantly, larger trials are necessary to evaluate whether long-term dietary modifications in prehypertensive participants would decrease progression to hypertension and diminish long-term cardiovascular events.
Disclosures
None.
Acknowledgments
This work was supported by Grant P30DK079328 from the University of Texas Southwestern Medical Center O’Brien Kidney Research Core Center and by seed funding from the Charles and Jane Pak Center for Mineral Metabolism (S.S.H.). CL.H. was supported by Grant DK59530. O.W.M. was supported by Grants DK48482 and DK54392, as well as the Simmons Family Foundation. The Dallas Heart Study was supported by a grant from the Donald W. Reynolds Foundation and USPHS GCRC Grant #M01-RR00633 from the National Center for Research Resources of the National Institutes of Health.
This study was presented in abstract form at the 41st Annual Meeting of the American Society of Nephrology in Philadelphia, Pennsylvania, November 2008.
The views expressed in this article are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Ostchega Y, Yoon SS, Hughes J, Louis T: Hypertension Awareness, Treatment, and Control—Continued Disparities in Adults: United States, 2005–2006. NCHS Data Brief No. 3. Hyattsville, MD, National Center for Health Statistics; 2008. Available at: http://www.cdc.gov/nchs/data/databriefs/db03.pdf Accessed January 4, 2010 [PubMed]
- 2.Vasan RS, Larson MG, Leip EP, Evans JC, O’Donnell CJ, Kannel WB, Levy D: Impact of high-normal blood pressure on the risk of cardiovascular disease. N Engl J Med 345: 1291–1297, 2001 [DOI] [PubMed] [Google Scholar]
- 3.Hsu CY, McCulloch CE, Darbinian J, Go AS, Iribarren C: Elevated blood pressure and risk of end-stage renal disease in subjects without baseline kidney disease. Arch Intern Med 165: 923–928, 2005 [DOI] [PubMed] [Google Scholar]
- 4.Lewington S, Clarke R, Qizilbash N, Peto R, Collins R, Prospective Studies Collaboration: Age-specific relevance of usual blood pressure to vascular mortality: A meta-analysis of individual data for one million adults in 61 prospective studies. Lancet 360: 1903–1913, 2002 [DOI] [PubMed] [Google Scholar]
- 5.Adrogué HJ, Madias NE: Sodium and potassium in the pathogenesis of hypertension. N Engl J Med 356: 1966–1978, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Krishna GG, Miller E, Kapoor S: Increased blood pressure during potassium depletion in normotensive men. N Engl J Med 320: 1177–1182, 1989 [DOI] [PubMed] [Google Scholar]
- 7.Morris RC, Jr, Sebastian A, Forman A, Tanaka M, Schmidlin O: Normotensive salt sensitivity: Effects of race and dietary potassium. Hypertension 33: 18–23, 1999 [DOI] [PubMed] [Google Scholar]
- 8.Aviv A, Hollenberg NK, Weder A: Urinary potassium excretion and sodium sensitivity in blacks. Hypertension 43: 707–713, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER, 3rd, Simons-Morton DG, Karanja N, Lin PH. DASH-Sodium Collaborative Research Group: Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. N Engl J Med 344: 3–10, 2001 [DOI] [PubMed] [Google Scholar]
- 10.Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. DASH Collaborative Research Group: A clinical trial of the effects of dietary patterns on blood pressure. N Engl J Med 336: 1117–1124, 1997 [DOI] [PubMed] [Google Scholar]
- 11.Turban S, Miller ER, 3rd, Ange B, Appel LJ: Racial differences in urinary potassium excretion. J Am Soc Nephrol 19: 1396–1402, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lazrak A, Liu Z, Huang CL: Antagonistic regulation of ROMK by long and kidney-specific WNK1 isoforms. Proc Natl Acad Sci USA 103: 1615–1620, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang CL, Kuo E: Mechanisms of disease: WNK-ing at the mechanism of salt-sensitive hypertension. Nat Clin Pract Nephrol 3: 623–630, 2007 [DOI] [PubMed] [Google Scholar]
- 14.Ascherio A, Rimm EB, Hernán MA, Giovannucci EL, Kawachi I, Stampfer MJ, Willett WC: Intake of potassium, magnesium, calcium, and fiber and risk of stroke among US men. Circulation 98: 1198–1204, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Alderman MH, Cohen H, Madhavan S: Dietary sodium intake and mortality: The National Health and Nutrition Examination Survey (NHANES I). Lancet 351: 781–785, 1998 [DOI] [PubMed] [Google Scholar]
- 16.INTERSALT Cooperative Research Group: INTERSALT: An international study of electrolyte excretion and blood pressure. Results for 24 hour urinary sodium and potassium excretion. BMJ 297: 319–328, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dyer AR, Elliott P, Shipley M, Stamler R, Stamler J: Body mass index and associations of sodium and potassium with blood pressure in INTERSALT. Hypertension 23: 729–736, 1994 [DOI] [PubMed] [Google Scholar]
- 18.Hsiao ZK, Wang SY, Hong ZG, Liu K, Cheng TY, Stamler J, Tao SC: Timed overnight sodium and potassium excretion and blood pressure in steel workers in North China. J Hypertens 4: 345–350, 1986 [DOI] [PubMed] [Google Scholar]
- 19.Kwok TCY, Chan TYK, Woo J: Relationship of urinary sodium/potassium excretion and calcium intake to blood pressure and prevalence of hypertension among older Chinese vegetarians. Eur J Clin Nutr 57: 299–304, 2003 [DOI] [PubMed] [Google Scholar]
- 20.Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK. Trials of Hypertension Prevention Collaborative Research Group: Joint effects of sodium and potassium intake on subsequent cardiovascular disease: The Trials of Hypertension Prevention follow-up study. Arch Intern Med 169: 32–40, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Victor RG, Haley RW, Willett DL, Peshock RM, Vaeth PC, Leonard D, Basit M, Cooper RS, Iannacchione VG, Visscher WA, Staab JM, Hobbs HH. Dallas Heart Study Investigators: The Dallas Heart Study: A population-based probability sample for the multidisciplinary study of ethnic differences in cardiovascular health. Am J Cardiol 93: 1473–1480, 2004 [DOI] [PubMed] [Google Scholar]
- 22.National Kidney Foundation: K/DOQI Clinical Practice Guidelines for Chronic Kidney Disease: Evaluation, classification and stratification. Am J Kidney Dis 39[Suppl 1]: S46–S103, 2002 [PubMed] [Google Scholar]
- 23.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. Modification of Diet in Renal Disease Study Group: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 24.Grimm RH, Jr, Neaton JD, Elmer PJ, Svendsen KH, Levin J, Segal M, Holland L, Witte LJ, Clearman DR, Kofron P, LaBounty RK, Crow R, Prineas RJ: The influence of oral potassium chloride on blood pressure in hypertensive men on a low-sodium diet. N Engl J Med 322: 569–574, 1990 [DOI] [PubMed] [Google Scholar]
- 25.Panel on Dietary Reference Intakes for Electrolytes and Water, Standing Committee on the Scientific Evaluation of Dietary References Intakes; Institutes of Medicine of the National Academies: Dietary References Intakes for Water, Potassium, Sodium, Chloride, and Sulfate Washington, DC, National Academies Press, 2004, pp 186–268
- 26.Kizer JR, Arnett DK, Bella JN, Paranicas M, Rao DC, Province MA, Oberman A, Kitzman DW, Hopkins PN, Liu JE, Devereux RB: Differences in left ventricular structure between black and white hypertensive adults: The Hypertension Genetic Epidemiology Network study. Hypertension 43: 1182–1188, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Klag MJ, Whelton PK, Randall BL, Neaton JD, Brancati FL, Stamler J: End-stage renal disease in African-American and white men. 16-year MRFIT findings. JAMA 277: 1293–1298, 1997 [PubMed] [Google Scholar]
- 28.Kittner SJ, White LR, Losonczy KG, Wolf PA, Hebel JR: Black-white differences in stroke incidence in a national sample. The contribution of hypertension and diabetes mellitus. JAMA 264: 1267–1270, 1990 [PubMed] [Google Scholar]
- 29.Luft FC, Grim CE, Fineberg N, Weinberger MC: Effects of volume expansion and contraction in normotensive whites, blacks, and subjects of different ages. Circulation 59: 643–650, 1979 [DOI] [PubMed] [Google Scholar]
- 30.Kumanyika SK, Hebert PR, Cutler JA, Lasser VI, Sugars CP, Steffen-Batey L, Brewer AA, Cameron M, Shepek LD, Cook NR. Trials of Hypertension Prevention Collaborative Research Group: Feasibility and efficacy of sodium reduction in the Trials of Hypertension Prevention, phase I. Hypertension 22: 502–512, 1993 [DOI] [PubMed] [Google Scholar]
- 31.Ishibashi K, Oshima T, Matsuura H, Watanabe M, Ishida M, Ishida T, Ozono R, Kajiyama G, Kanbe M: Effects of age and sex on sodium chloride sensitivity: Association with plasma renin activity. Clin Nephrol 42: 376–380, 1994 [PubMed] [Google Scholar]
- 32.Whelton PK, Buring J, Borhani NO, Cohen JD, Cook N, Cutler JA, Kiley JE, Kuller LH, Satterfield S, Sacks FM, Taylor JO. Trials of Hypertension Prevention (TOHP) Collaborative Research Group: The effect of potassium supplementation in persons with a high-normal blood pressure. Results from phase I of the Trials of Hypertension Prevention (TOHP). Ann Epidemiol 5: 85–95, 1995 [DOI] [PubMed] [Google Scholar]
- 33.Cook NR, Cohen J, Hebert PR, Taylor JO, Hennekens CH: Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med 155: 701–709, 1995 [PubMed] [Google Scholar]
- 34.Tobian L, Hanlon S: High sodium chloride diets injure arteries and raise mortality without changing blood pressure. Hypertension 15: 900–903, 1990 [DOI] [PubMed] [Google Scholar]
- 35.Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, Tsai SY, Pan WH: Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr 83: 1289–1296, 2006 [DOI] [PubMed] [Google Scholar]