Summary
Vitamin D has garnered much research and debate about supplementation in recent years, not only as it pertains to patients with kidney disease but also to those in the general population. This review discusses observational and available clinical trial evidence about the effects of both calcitriol and vitamin D analogs (active) and ergocalciferol and cholecalciferol (nutritional) vitamin D in patients with CKD and ESRD.
Introduction
Vitamin D has garnered much research and debate about supplementation not only as it pertains to patients with kidney disease but also to the general population. Patients with kidney disease have reduced activity of the enzyme 1-α hydroxylase (CYP27B1) in the kidneys, which converts 25-hydroxyvitamin D (25(OH)D) to its more active form, 1,25-dihydroxyvitamin D (1,25(OH)2D), and thus patients with kidney disease have traditionally been given vitamin D replacement with active, 1,25-dihydroxvitamin D or a related analog. Although extrarenal 1-α hydroxylation has been appreciated for some time (1,2), the location and potential use of 1-α hydroxylation for autocrine and paracrine signaling has led nephrologists to consider replacing nutritional vitamin D, the inactive form, as well as active vitamin D in patients with kidney disease (Table 1). Interestingly, some vitamin D analogs, such as paricalcitol, actually lower 1,25(OH)2D levels, thus acting as vitamin D mimetics (3). This review outlines the available evidence for multiple outcomes associated with vitamin D in patients with kidney disease.
Table 1.
Commonly available forms of active and nutritional vitamin D
| Nutritional Forms of Vitamin D | Active Forms of Vitamin D |
|---|---|
| Ergocalciferol (D2) | Calcitriol (D3) |
| Cholecalciferol (D3) | Paricalcitol (D2) |
| Doxercalciferol (D2) | |
| Maxacalcitol (D3) | |
| 1-alfa-calcidiol (D3) | |
| 22-oxacalcitriol (D3) |
D2 or D3 designate underlying structure. D2 is commonly made in plants, whereas D3 is made in animals.
Vitamin D Physiology
Individuals obtain vitamin D either through consuming vitamin D–rich foods (oily fish, dairy products) or supplements, or through the skin’s exposure to ultraviolet B radiation producing vitamin D. This vitamin D, once it enters the body, is extracted by the liver and converted to 25-hydroxyvitamin D, which circulates in the blood stream and is used to evaluate an individual’s vitamin D nutritional status because of its relatively long t1/2 (2–3 weeks). The more active form of vitamin D, 1,25-dihydroxyvitamin D, has a t1/2 of only 8–12 hours and responds dynamically to changes in calcium and phosphate metabolism. 25(OH)D circulates in nanogram per milliliter concentrations, which are 1000-fold higher than concentrations of 1,25(OH)2D. Although 25(OH)D stimulates the vitamin D receptor (at 100- to 150-fold higher concentrations than 1,25(OH)2D) in vitro (4,5), it is unclear whether it has effects in vivo.
Vitamin D and CKD: Metabolic Bone Disease
As kidney function worsens, low circulating 1,25-dihyrdoxyvitamin D levels, low calcium levels, and high serum phosphate levels lead to secondary hyperparathyroidism (SHPT). SHPT, identified by elevated parathyroid hormone (PTH) levels, is associated with both bone disease (renal osteodystrophy) and, in epidemiologic studies, poor outcomes in dialysis patients (6–8). Before the discovery of calcitriol, patients with ESRD were treated with high doses of nutritional vitamin D to treat SHPT (9). Unfortunately, most of the studies of its effects during that time are small and observational in nature. Once calcitriol was introduced and subjected to more rigorous, albeit small, randomized controlled trials, it quickly became the mainstay of therapy for SHPT (10–12). Two recent Cochrane reviews (13,14) confirm that in both dialysis and predialysis CKD patients (4 studies, 153 patients), calcitriol and vitamin D analogs decrease PTH (−196 pg/ml [95% CI, −298 to −94] in dialysis patients; −49 pg/ml [95% CI, −86 to −13] in predialysis patients) but increase serum phosphate and calcium levels. Not enough data exist from randomized clinical trials to draw conclusions about patient-level outcomes such as fractures, mortality, or need for dialysis in predialysis patients (13,14). Another meta-analysis of nutritional vitamin D compounds was recently performed and the authors found that in four randomized clinical trials (90 patients) including both dialysis and nondialysis CKD patients, PTH levels decreased significantly (−31.5 pg/ml [95% CI, −57 to −6.1]) (15). Similarly to the data about calcitriol and vitamin D analogs, there was no evidence regarding patient outcomes (15). Since the topic was last reviewed in the Clinical Journal of the American Society of Nephrology in 2009 (16), there have been several new published clinical trials of nutritional vitamin D in CKD, which are outlined in Table 2. One recently published, randomized, not-blinded study in 80 mostly white men showed that paricalcitol decreased PTH levels, whereas ergocalciferol did not (17). In summary, meta-analyses indicate that both nutritional and active vitamin D therapies have been shown to lower PTH levels, the primary indication for their use. The evidence is stronger, with larger, better designed, clinical trials for active vitamin D. Although the effect of vitamin D on PTH is clearly established, animal studies and observational studies in humans have suggested roles of vitamin D, both active and nutritional, in systems outside of bone and mineral metabolism. The remainder of this review focuses on these areas (Figure 1).
Table 2.
Recently published randomized controlled trials of nutritional vitamin D supplementation in CKD
| Study (Reference) | Study Type | Sample Size | Control Group | Randomized | Results | Limitations |
|---|---|---|---|---|---|---|
| Chandra et al. (67) | Double-blind, placebo-controlled, randomized controlled pilot study | 20 | Yes | Yes | Among cholecalciferol-treated participants, serum 25(OH)D concentration increased on average from 17.3 ng/ml (95% CI, 11.8–25.2) at baseline to 49.4 ng/ml (95% CI, 33.9–-72.0) at week 12. As-treated analysis indicated a trend toward lower PTH levels among cholecalciferol-treated participants (P=0.07) | Small study |
| Short follow-up period | ||||||
| Dogan et al. (66) | Randomized | 40 | Yes | Yes | Administration of depot oral cholecalciferol (300,000 IU vitamin D3) resulted in a significant increase in calcidiol (6.8±3.5 to 17.8±21.4 ng/ml, P<0.001), significant decrease in iPTH (368±274 to 279±179 pg/ml, P<0.001). No statistically significant change in Ca, P, Ca × P, and urinary calcium creatinine rate was observed | Small study |
| Short follow-up period | ||||||
| Methodology does not specify whether investigators were blinded to the intervention | ||||||
| Oksa et al. (68) | Randomized | 87 | No | Yes | Vitamin D insufficiency/deficiency in CKD significantly improved after the 12-mo cholecalciferol treatment, with more significant improvement with higher dose (20,000 IU/wk) being more effective and equally safe | Lack of a placebo control |
| The inclusion of a subgroup of patients who received calcium carbonate for correction of metabolic acidosis is a potential confounder | ||||||
| Kovesdy et al. (17) | Randomized, not blinded | 80 | Active | Randomized | 80 CKD patients randomized to ergocalciferol versus paricalcitol. Paricalcitol group showed lower PTH levels than ergocalciferol group | Not blinded |
| Differential initiation of phosphate binders in the two groups |
95% CI, confidence interval; PTH, parathyroid hormone.
Figure 1.
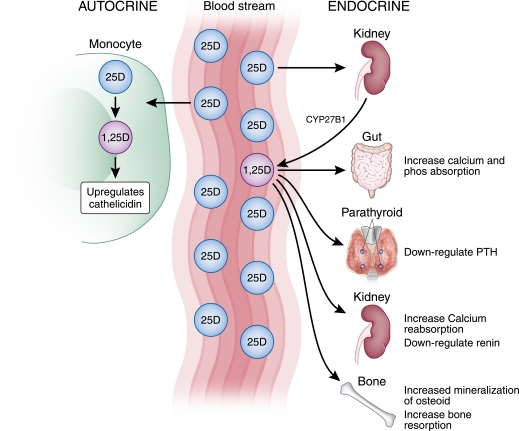
Physiology of vitamin D in the body. It circulates as 25-hydroxyvitamin D (25D), which then gets converted in the kidney to 1,25-dihydroxyvitamin D (1,25D). The 1,25D has endocrine effects, shown at the right. 25D can also be taken up by monocytes and other cells where it is converted to 1,25D acting in an autocrine fashion to upregulate cathelicidin production.
Effects of Vitamin D and Its Analogs in Animal Models
BP, Left Ventricular Hypertrophy, and Albuminuria
Complications of CKD in humans include hypertension, left ventricular hypertrophy and diastolic dysfunction, and albuminuria, and animal models suggest that vitamin D may play a role in these complications. One of the stronger pieces of evidence that vitamin D exerts an effect on the cardiovascular and renal systems comes from vitamin D receptor knockout mice. These mice develop elevated BPs and left ventricular hypertrophy (18), which occurs due to a rise in renin consequent to loss of normal suppression of the renin-angiotensin system by vitamin D (19). In rats with spontaneous hypertension, treatment with vitamin D analogs ameliorates left ventricular hypertrophy and improves left ventricular diastolic measures (20). Active vitamin D, in various forms, decreases albuminuria in multiple animal models of kidney disease, including Heymann nephritis (21), murine MRL/l lupus nephritis (22), mercuric-chloride–induced nephrotic syndrome (23), and subtotal nephrectomized rats (24). In the anti-Thy 1.1 model of GN, rats treated with 1,25(OH)2D3 had less albuminuria (25) and showed preserved slit-diaphragm protein morphology (26). Combination therapy with an angiotensin receptor blocker and a vitamin D analog abrogated the albuminuria in the streptozotocin-induced diabetic nephropathy model (27). Rats treated with 22-oxa-calcitriol, a vitamin D analog, had lower levels of TGF-β1 protein in the tubules and glomeruli compared with nontreated diseased rats (24,28). Thus, multiple animal models suggest a role for active vitamin D in cardiac structure and function, albuminuria, and kidney fibrosis.
Vascular Calcification and Infection
Vascular calcification and infection are other common complications of CKD in which vitamin D has been implicated. Interestingly, an early experimental model of atherosclerosis was the cholesterol- and vitamin D–fed rat, a model without superimposed kidney disease. These rats were given an extremely high dose of vitamin D2 (1.8 million U/kg) and developed aortic atherosclerosis (29). However, there is potentially an important difference in action depending on the dose of the vitamin D analog. For example, in a mouse model of kidney disease, low levels of vitamin D (paricalcitol or calcitriol) were protective against vascular calcification, whereas higher doses were associated with more calcification (30). Potentially, both lack of and too much vitamin D may lead to vascular calcification.
Vitamin D likely also plays a role in the innate immune response. Activation of the vitamin D receptor and the vitamin D 1-α hydroxylase gene leads to increased expression of cathelicidin, an antimicrobial peptide (31). Low levels of cathelicidin are associated with a higher risk of death from infectious disease in dialysis patients (32). In 60 participants without kidney disease, cathelicidin levels increased after vitamin D supplementation (33).
Observational Studies of Vitamin D
Low Vitamin D Levels in CKD and ESRD and Outcomes
Multiple observational studies have shown low levels of both 25(OH)D and 1,25(OH)2D in patients with CKD and ESRD (34,35). Many factors may account for low levels of 25(OH)D in kidney disease, including the loss of vitamin D binding protein in the urine (36), ineffective synthesis in the skin upon exposure to ultraviolet B radiation (37), and likely reduced nutritional intake and sun exposure.
Low 25(OH)D levels in patients with CKD and ESRD have been associated with a higher risk of all-cause mortality and a faster progression of kidney disease (34,38–40). In the general population, low 25(OH)D levels have also been associated with all-cause mortality, cardiovascular events, peripheral vascular disease, hypertension, congestive heart failure, and the later need for renal replacement therapy (41–46). Low 1,25(OH)2D levels have been associated with all-cause mortality (34,47). The studies of vitamin D levels are all potentially confounded by sicker patients having low vitamin D levels because of less sun exposure or poor nutrition. Therefore, randomized trials are required to test whether supplementation of vitamin D may affect outcomes.
Evaluation of Active Vitamin D Therapy and Outcomes
Multiple observational studies have shown an association between the use of active vitamin D therapy in patients on dialysis and with CKD and improved survival. These range from larger studies from databases of dialysis providers to smaller cohort studies (6,7,48–51). Active vitamin D therapy has also been associated with slower progression to ESRD (52). There are a few studies in the literature in which an association between activated vitamin D use and improved survival was not found (53,54). One of these studies showed a mortality benefit for vitamin D (combining both oral and intravenous vitamin D analogs) using traditional models and marginal structural models but not when using a more complicated modeling system called instrumental variable models (53). The other study did show an association with improved all-cause mortality but not with specific causes of mortality such as cardiovascular or infection (54), suggesting the possibility that perhaps all of these specific causes are influenced by vitamin D and thereby diluting the effect on any individual one. There are no published reports of associations between nutritional vitamin D supplementation and improved survival in kidney disease, and data are mixed in the general population (55,56).
Randomized Clinical Trials of Active Vitamin D Therapy in CKD
Albuminuria and BP
Several recent studies have highlighted the importance of vitamin D therapy in areas outside of traditional bone and mineral metabolism in humans. Several small, randomized clinical trials have evaluated the effect of active vitamin D therapy on albuminuria, a marker of kidney damage. A single-center study of 61 patients showed lower urine protein/creatinine ratios and lower PTH levels in patients randomized to paricalcitol compared with placebo (57). Another small single-center study of 24 patients randomized to two different doses of paricalcitol or placebo showed lower high-sensitivity C-reactive protein levels and lower rates of 24-hour albumin excretion in the paricalcitol group (58).
A large, placebo-controlled, double-blinded, randomized clinical trial of two different doses of paricalcitol in 281 participants with type 2 diabetes mellitus showed similar results (59). In this multicenter, multinational study, all patients had to have albuminuria and be taking an angiotensin-converting enzyme inhibitor or angiotensin receptor blocker at baseline. The study population in the trial had a mean age of 64 years, and 69% of participants were male, 72% were white, 14% were black, and the median urinary albumin excretion was approximately 700 mg per 24 hours. The results showed that there was a significant reduction in the urinary albumin/creatinine ratio in participants taking the 2-µg dose compared with placebo. This was associated with a lowering of estimated GFR (eGFR), as estimated from serum creatinine, which was also significant at the 1-µg dose. Twelve weeks after randomization, the eGFR was 2 ml/min per 1.73 m2 lower in the participants receiving the 1-µg dose, whereas it was 4 ml/min per 1.73 m2 lower in participants receiving the 2-µg dose. BP was also significantly lower in the participants randomized to the 2-µg dose by a mean of approximately 8 mmHg. Interestingly, a recent study showed that in a small group of patients with kidney disease, paricalcitol increased serum creatinine levels without affecting iothalamate GFR measurements (60). The multicenter paricalcitol study is important for several reasons. It shows a decrease in albuminuria and eGFR in patients with type 2 diabetes with nephropathy in a nicely designed and executed randomized trial. It does not, however, answer the more important question of whether this decrease in albuminuria will translate to better clinical outcomes, such as less rapid progression to dialysis.
Other Outcomes
A small recent trial comparing doxercalciferol and cholecalciferol showed no difference in end-of-treatment PTH levels between active and nutritional vitamin D compounds (61). This study found no effect on BP or albuminuria. Another currently ongoing placebo-controlled randomized clinical trial is evaluating treatment of 227 stage 3 and 4 CKD patients with paricalcitol with change in left ventricular mass index as the primary outcome (62). This study, the PRIMO Study (NCT00497146), is also evaluating diastolic function and cardiovascular hospitalizations as secondary outcomes.
Interventional Studies of Nutritional Vitamin D in Patients with CKD and ESRD
The fact that the 1-α hydroxylase enzyme has been found in parts of the body outside the kidney suggests that there may be a role for nutritional vitamin D in patients with kidney disease. A recent study in hemodialysis patients showed that 1,25-dihydroxvitamin D levels increased after supplementation with nutritional vitamin D, suggesting that even in ESRD there is enough extrarenal 1-α hydroxylase activity to influence serum levels (63). A cohort study of 158 hemodialysis patients who received cholecalciferol supplementation in a nonrandomized study showed higher 25(OH)D, 1,25-dihydroxyvitamin D, and albumin levels, while at the same time reducing serum calcium, PTH, brain natriuretic peptide, left ventricular mass index, and erythropoietin stimulating agent and active vitamin D doses (64). A study of seven hemodialysis patients who underwent cholecalciferol supplementation reported that after supplementation, there were lower levels of proinflammatory cytokines, IL-8, IL-6, and TNF and differences in circulating monocyte proteins (65). These studies are small and are not randomized clinical trials; however, their results suggest that nutritional vitamin D may be needed in patients with kidney disease. However, larger well designed trials are required.
Current Ongoing Studies of Nutritional Vitamin D
There are several current ongoing studies of nutritional vitamin D supplementation that are worth noting although their results are not yet available. One hundred and five dialysis patients, as part of the Dialysis Infection and Vitamin D in New England (DIVINE) study (NCT 00892099), are being randomized to high-dose ergocalciferol (50,000 IU/week), low-dose ergocalciferol (50,000 IU/mo), or placebo for 12 weeks. Primary end points include cathelicidin, cytokine, and PTH levels over follow-up and the incidence of infections. Another randomized clinical trial is currently evaluating the effect of ergocalciferol supplementation versus placebo on albuminuria and 24 hour BPs in patients with stage 3 and 4 CKD (NCT 01029002). The Vitamin D and OmegA-3 trial (VITAL; NCT01169259) will test the role of 2000 IU/d of vitamin D and ω-3 fatty acids (in a 2×2 factorial design) in the primary prevention of cancer and cardiovascular disease among 20,000 men and women throughout the United States. A recent search of clinicaltrials.gov revealed over 1000 records of ongoing or completed clinical trials of vitamin D for various health outcomes.
Judging the Evidence and Unanswered Questions
Results from randomized clinical trials, conducted in a population similar to the patient and with clinically meaningful end points, comprise the gold standard for clinical decision making. Unfortunately, this level of evidence does not exist for vitamin D therapy in CKD. We know from clinical trials that both nutritional and active vitamin D decrease PTH levels, but we do not know whether that affects fracture rates or other adverse outcomes, including mortality. We know from clinical trials that paricalcitol decreases albuminuria, but we do not know if it decreases the risk of dialysis or progression of kidney disease. Without randomized clinical trials, causation cannot be inferred from observational designs. As other examples have shown, such as hormone therapy in women and statin use in dialysis patients, observational studies or even clinical trials in other populations may not inform the clinician of the correct treatment. Because well designed clinical trials are expensive, evidence from animal studies, observational studies, and small pilot randomized trials with surrogate outcomes are needed to evaluate which therapies have the most potential for success to be tested in definitive clinical trials. We have outlined above several ongoing clinical trials; however, even with the results of these studies, many questions remain unanswered. For example, do we need to measure 25(OH)D levels in all CKD patients, or can we replete knowing most are deficient? Can we combine nutritional and active vitamin D or does this put patients at increased risk?
Multiple observational studies suggest an important role of vitamin D in patients with CKD and ESRD and potentially in the general population. There are potentially different roles for nutritional and active vitamin D compounds (Table 3). Nutritional vitamin D may play more of a role in infections, whereas active vitamin D compounds may play more of a role in albuminuria and mortality. Both nutritional and active vitamin D eventually affect the same vitamin D receptor; however, nutritional vitamin D has to undergo additional activation in the body, potentially at sites distant from the kidney. Active vitamin D has been shown to decrease albuminuria, BP, and eGFR in patients with diabetic kidney disease. There are current ongoing studies to test these outcomes with nutritional vitamin D compounds as well. It is important to mention that there are very few data about combining therapy with both nutritional and active vitamin D compounds; thus, caution should be used in clinical practice because of worry about possible vitamin D intoxication, manifested by hypercalcemia and possibly vascular calcifications. Much further work is needed in this area.
Table 3.
Table of possible effects of vitamin D supplementation in patients with kidney disease
| Outcome | Nutritional or Active Vitamin D | Proposed Mechanism | Level of Evidence | References |
|---|---|---|---|---|
| PTH suppression | A, maybe N | Direct suppression of parathyroid gland | A: RCT | 13,14 |
| N: limited RCTs | 15,66 | |||
| Reduction of albuminuria | A, maybe N | Suppression of renin-angiotensin system | A: RCT | 59 |
| N: limited RCTs | 61 | |||
| Reduced risk of infections | N | Increase in cathelicidin levels | Observational studies in general population only | 32,33 |
| Reduction of BP | A, maybe N | Suppression of renin-angiotensin system | A: RCT | 59 |
| Progression of kidney disease | A, maybe N | Suppression of renin-angiotensin system | A: Observational studies only | 51,52 |
| N: low levels associated with outcome | 38,46 | |||
| Cardiovascular effects (left ventricular hypertrophy and vascular calcification) | A | Suppression of renin-angiotensin system, possibly direct effects on myocytes | Animal studies | 20 |
| Mortality | A | Likely multiple mechanisms | Observational studies only | 6,7,48 |
PTH, parathyroid hormone; A, active; N, nutritional; RCT, randomized controlled trial.
Disclosures
R.I.T. is supported by a research grant from Abbott Laboratories and has received reimbursement for travel expenses by Abbott Laboratories.
Acknowledgments
The authors thank Judy K. Tan, MD, for assistance with the manuscript.
M.L.M. is supported by Grants K23 DK078774, R01 DK080123, and R01 DK087783, and R.I.T. is supported by Grants R21HL102882 and R01DK084974, all from the National Institutes of Health.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
References
- 1.Dusso A, Lopez-Hilker S, Rapp N, Slatopolsky E: Extra-renal production of calcitriol in chronic renal failure. Kidney Int 34: 368–375, 1988 [DOI] [PubMed] [Google Scholar]
- 2.Weisman Y, Vargas A, Duckett G, Reiter E, Root AW: Synthesis of 1,25-dihydroxyvitamin D in the nephrectomized pregnant rat. Endocrinology 103: 1992–1996, 1978 [DOI] [PubMed] [Google Scholar]
- 3.Finch JL, Tokumoto M, Nakamura H, Yao W, Shahnazari M, Lane N, Slatopolsky E: Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am J Physiol Renal Physiol 298: F1315–F1322, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raisz LG, Trummel CL, Holick MF, DeLuca HF: 1,25-dihydroxycholecalciferol: A potent stimulator of bone resorption in tissue culture. Science 175: 768–769, 1972 [DOI] [PubMed] [Google Scholar]
- 5.Brumbaugh PF, Haussler MR: 1 Alpha,25-dihydroxycholecalciferol receptors in intestine. I. Association of 1 alpha,25-dihydroxycholecalciferol with intestinal mucosa chromatin. J Biol Chem 249: 1251–1257, 1974 [PubMed] [Google Scholar]
- 6.Melamed ML, Eustace JA, Plantinga L, Jaar BG, Fink NE, Coresh J, Klag MJ, Powe NR: Changes in serum calcium, phosphate, and PTH and the risk of death in incident dialysis patients: A longitudinal study. Kidney Int 70: 351–357, 2006 [DOI] [PubMed] [Google Scholar]
- 7.Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, McAllister CJ, Budoff MJ, Salusky IB, Kopple JD: Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70: 771–780, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM: Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol 15: 2208–2218, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Recker R, Schoenfeld P, Letteri J, Slatopolsky E, Goldsmith R, Brickman A: The efficacy of calcifediol in renal osteodystrophy. Arch Intern Med 138[Spec No]: 857–863, 1978 [PubMed] [Google Scholar]
- 10.Baker LR, Muir JW, Sharman VL, Abrams SM, Greenwood RN, Cattell WR, Goodwin FJ, Marsh FP, Adami S, Hately W, et al. : Controlled trial of calcitriol in hemodialysis patients. Clin Nephrol 26: 185–191, 1986 [PubMed] [Google Scholar]
- 11.Maxwell DR, Benjamin DM, Donahay SL, Allen MK, Hamburger RJ, Luft FC: Calcitriol in dialysis patients. Clin Pharmacol Ther 23: 515–519, 1978 [DOI] [PubMed] [Google Scholar]
- 12.Berl T, Berns AS, Hufer WE, Hammill K, Alfrey AC, Arnaud CD, Schrier RW: 1,25 dihydroxycholecalciferol effects in chronic dialysis. A double-blind controlled study. Ann Intern Med 88: 774–780, 1978 [DOI] [PubMed] [Google Scholar]
- 13.Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF: Vitamin D compounds for people with chronic kidney disease requiring dialysis. Cochrane Database Syst Rev (4): CD005633, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Palmer SC, McGregor DO, Craig JC, Elder G, Macaskill P, Strippoli GF: Vitamin D compounds for people with chronic kidney disease not requiring dialysis. Cochrane Database Syst Rev (4): CD008175, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Kandula P, Dobre M, Schold JD, Schreiber MJ, Jr, Mehrotra R, Navaneethan SD: Vitamin D supplementation in chronic kidney disease: A systematic review and meta-analysis of observational studies and randomized controlled trials. Clin J Am Soc Nephrol 6: 50–62, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalantar-Zadeh K, Kovesdy CP: Clinical outcomes with active versus nutritional vitamin D compounds in chronic kidney disease. Clin J Am Soc Nephrol 4: 1529–1539, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Kovesdy CP, Lu JL, Malakauskas SM, Andress DL, Kalantar-Zadeh K, Ahmadzadeh S: Paricalcitol versus ergocalciferol for secondary hyperparathyroidism in CKD stages 3 and 4: A randomized controlled trial [published online ahead of print August 30, 2011]. Am J Kidney Dis doi:10.1053/j.ajkd.2011.06.027 [DOI] [PubMed] [Google Scholar]
- 18.Xiang W, Kong J, Chen S, Cao LP, Qiao G, Zheng W, Liu W, Li X, Gardner DG, Li YC: Cardiac hypertrophy in vitamin D receptor knockout mice: Role of the systemic and cardiac renin-angiotensin systems. Am J Physiol Endocrinol Metab 288: E125–E132, 2005 [DOI] [PubMed] [Google Scholar]
- 19.Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP: 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest 110: 229–238, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kong J, Kim GH, Wei M, Sun T, Li G, Liu SQ, Li X, Bhan I, Zhao Q, Thadhani R, Li YC: Therapeutic effects of vitamin D analogs on cardiac hypertrophy in spontaneously hypertensive rats. Am J Pathol 177: 622–631, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Branisteanu DD, Leenaerts P, van Damme B, Bouillon R: Partial prevention of active Heymann nephritis by 1 alpha, 25 dihydroxyvitamin D3. Clin Exp Immunol 94: 412–417, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lemire JM, Ince A, Takashima M: 1,25-Dihydroxyvitamin D3 attenuates the expression of experimental murine lupus of MRL/l mice. Autoimmunity 12: 143–148, 1992 [DOI] [PubMed] [Google Scholar]
- 23.Lillevang ST, Rosenkvist J, Andersen CB, Larsen S, Kemp E, Kristensen T: Single and combined effects of the vitamin D analogue KH1060 and cyclosporin A on mercuric-chloride-induced autoimmune disease in the BN rat. Clin Exp Immunol 88: 301–306, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwarz U, Amann K, Orth SR, Simonaviciene A, Wessels S, Ritz E: Effect of 1,25 (OH)2 vitamin D3 on glomerulosclerosis in subtotally nephrectomized rats. Kidney Int 53: 1696–1705, 1998 [DOI] [PubMed] [Google Scholar]
- 25.Panichi V, Migliori M, Taccola D, Filippi C, De Nisco L, Giovannini L, Palla R, Tetta C, Camussi G: Effects of 1,25(OH)2D3 in experimental mesangial proliferative nephritis in rats. Kidney Int 60: 87–95, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Migliori M, Giovannini L, Panichi V, Filippi C, Taccola D, Origlia N, Mannari C, Camussi G: Treatment with 1,25-dihydroxyvitamin D3 preserves glomerular slit diaphragm-associated protein expression in experimental glomerulonephritis. Int J Immunopathol Pharmacol 18: 779–790, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Z, Zhang Y, Ning G, Deb DK, Kong J, Li YC: Combination therapy with AT1 blocker and vitamin D analog markedly ameliorates diabetic nephropathy: Blockade of compensatory renin increase. Proc Natl Acad Sci USA 105: 15896–15901, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makibayashi K, Tatematsu M, Hirata M, Fukushima N, Kusano K, Ohashi S, Abe H, Kuze K, Fukatsu A, Kita T, Doi T: A vitamin D analog ameliorates glomerular injury on rat glomerulonephritis. Am J Pathol 158: 1733–1741, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kunitomo M, Kinoshita K, Bandô Y: Experimental atherosclerosis in rats fed a vitamin D, cholesterol-rich diet. J Pharmacobiodyn 4: 718–723, 1981 [DOI] [PubMed] [Google Scholar]
- 30.Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA: Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol 19: 1509–1519, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik SR, Ochoa MT, Schauber J, Wu K, Meinken C, Kamen DL, Wagner M, Bals R, Steinmeyer A, Zügel U, Gallo RL, Eisenberg D, Hewison M, Hollis BW, Adams JS, Bloom BR, Modlin RL: Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science 311: 1770–1773, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Gombart AF, Bhan I, Borregaard N, Tamez H, Camargo CA, Jr, Koeffler HP, Thadhani R: Low plasma level of cathelicidin antimicrobial peptide (hCAP18) predicts increased infectious disease mortality in patients undergoing hemodialysis. Clin Infect Dis 48: 418–424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bhan I, Camargo CA, Jr, Wenger J, Ricciardi C, Ye J, Borregaard N, Thadhani R: Circulating levels of 25-hydroxyvitamin D and human cathelicidin in healthy adults. J Allergy Clin Immunol 127: 1302–1304, e1, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo CA, Jr, Tonelli M, Thadhani R: Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72: 1004–1013, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Bhan I, Burnett-Bowie SA, Ye J, Tonelli M, Thadhani R: Clinical measures identify vitamin D deficiency in dialysis. Clin J Am Soc Nephrol 5: 460–467, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koenig KG, Lindberg JS, Zerwekh JE, Padalino PK, Cushner HM, Copley JB: Free and total 1,25-dihydroxyvitamin D levels in subjects with renal disease. Kidney Int 41: 161–165, 1992 [DOI] [PubMed] [Google Scholar]
- 37.Jacob AI, Sallman A, Santiz Z, Hollis BW: Defective photoproduction of cholecalciferol in normal and uremic humans. J Nutr 114: 1313–1319, 1984 [DOI] [PubMed] [Google Scholar]
- 38.Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, Mallamaci F, Zoccali C: Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int 75: 88–95, 2009 [DOI] [PubMed] [Google Scholar]
- 39.Drechsler C, Pilz S, Obermayer-Pietsch B, Verduijn M, Tomaschitz A, Krane V, Espe K, Dekker F, Brandenburg V, März W, Ritz E, Wanner C: Vitamin D deficiency is associated with sudden cardiac death, combined cardiovascular events, and mortality in haemodialysis patients. Eur Heart J 31: 2253–2261, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Drechsler C, Verduijn M, Pilz S, Dekker FW, Krediet RT, Ritz E, Wanner C, Boeschoten EW, Brandenburg V; NECOSAD Study Group: Vitamin D status and clinical outcomes in incident dialysis patients: Results from the NECOSAD study. Nephrol Dial Transplant 26: 1024–1032, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Melamed ML, Michos ED, Post W, Astor B: 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168: 1629–1637, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang TJ, Pencina MJ, Booth SL, Jacques PF, Ingelsson E, Lanier K, Benjamin EJ, D’Agostino RB, Wolf M, Vasan RS: Vitamin D deficiency and risk of cardiovascular disease. Circulation 117: 503–511, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Melamed ML, Muntner P, Michos ED, Uribarri J, Weber C, Sharma J, Raggi P: Serum 25-hydroxyvitamin D levels and the prevalence of peripheral arterial disease: Results from NHANES 2001 to 2004. Arterioscler Thromb Vasc Biol 28: 1179–1185, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Forman JP, Giovannucci E, Holmes MD, Bischoff-Ferrari HA, Tworoger SS, Willett WC, Curhan GC: Plasma 25-hydroxyvitamin D levels and risk of incident hypertension. Hypertension 49: 1063–1069, 2007 [DOI] [PubMed] [Google Scholar]
- 45.Pilz S, März W, Wellnitz B, Seelhorst U, Fahrleitner-Pammer A, Dimai HP, Boehm BO, Dobnig H: Association of vitamin D deficiency with heart failure and sudden cardiac death in a large cross-sectional study of patients referred for coronary angiography. J Clin Endocrinol Metab 93: 3927–3935, 2008 [DOI] [PubMed] [Google Scholar]
- 46.Melamed ML, Astor B, Michos ED, Hostetter TH, Powe NR, Muntner P: 25-hydroxyvitamin D levels, race, and the progression of kidney disease. J Am Soc Nephrol 20: 2631–2639, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W: Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 168: 1340–1349, 2008 [DOI] [PubMed] [Google Scholar]
- 48.Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernán MA, Camargo CA, Jr, Thadhani R: Activated injectable vitamin D and hemodialysis survival: A historical cohort study. J Am Soc Nephrol 16: 1115–1125, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Shoji T, Shinohara K, Kimoto E, Emoto M, Tahara H, Koyama H, Inaba M, Fukumoto S, Ishimura E, Miki T, Tabata T, Nishizawa Y: Lower risk for cardiovascular mortality in oral 1alpha-hydroxy vitamin D3 users in a haemodialysis population. Nephrol Dial Transplant 19: 179–184, 2004 [DOI] [PubMed] [Google Scholar]
- 50.Wolf M, Betancourt J, Chang Y, Shah A, Teng M, Tamez H, Gutierrez O, Camargo CA, Jr, Melamed M, Norris K, Stampfer MJ, Powe NR, Thadhani R: Impact of activated vitamin D and race on survival among hemodialysis patients. J Am Soc Nephrol 19: 1379–1388, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K: Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med 168: 397–403, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B: Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol 19: 1613–1619, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tentori F, Albert JM, Young EW, Blayney MJ, Robinson BM, Pisoni RL, Akiba T, Greenwood RN, Kimata N, Levin NW, Piera LM, Saran R, Wolfe RA, Port FK: The survival advantage for haemodialysis patients taking vitamin D is questioned: Findings from the Dialysis Outcomes and Practice Patterns Study. Nephrol Dial Transplant 24: 963–972, 2009 [DOI] [PubMed] [Google Scholar]
- 54.St Peter WL, Li S, Liu J, Gilbertson DT, Arneson TJ, Collins AJ: Effects of monthly dose and regular dosing of intravenous active vitamin D use on mortality among patients undergoing hemodialysis. Pharmacotherapy 29: 154–164, 2009 [DOI] [PubMed] [Google Scholar]
- 55.Autier P, Gandini S: Vitamin D supplementation and total mortality: A meta-analysis of randomized controlled trials. Arch Intern Med 167: 1730–1737, 2007 [DOI] [PubMed] [Google Scholar]
- 56.LaCroix AZ, Kotchen J, Anderson G, Brzyski R, Cauley JA, Cummings SR, Gass M, Johnson KC, Ko M, Larson J, Manson JE, Stefanick ML, Wactawski-Wende J: Calcium plus vitamin D supplementation and mortality in postmenopausal women: The Women’s Health Initiative calcium-vitamin D randomized controlled trial. J Gerontol A Biol Sci Med Sci 64: 559–567, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fishbane S, Chittineni H, Packman M, Dutka P, Ali N, Durie N: Oral paricalcitol in the treatment of patients with CKD and proteinuria: A randomized trial. Am J Kidney Dis 54: 647–652, 2009 [DOI] [PubMed] [Google Scholar]
- 58.Alborzi P, Patel NA, Peterson C, Bills JE, Bekele DM, Bunaye Z, Light RP, Agarwal R: Paricalcitol reduces albuminuria and inflammation in chronic kidney disease: A randomized double-blind pilot trial. Hypertension 52: 249–255, 2008 [DOI] [PubMed] [Google Scholar]
- 59.de Zeeuw D, Agarwal R, Amdahl M, Audhya P, Coyne D, Garimella T, Parving HH, Pritchett Y, Remuzzi G, Ritz E, Andress D: Selective vitamin D receptor activation with paricalcitol for reduction of albuminuria in patients with type 2 diabetes (VITAL study): A randomised controlled trial. Lancet 376: 1543–1551, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Agarwal R, Hynson JE, Hecht TJ, Light RP, Sinha AD: Short-term vitamin D receptor activation increases serum creatinine due to increased production with no effect on the glomerular filtration rate. Kidney Int 80: 1073–1079, 2011 [DOI] [PubMed] [Google Scholar]
- 61.Moe SM, Saifullah A, LaClair RE, Usman SA, Yu Z: A randomized trial of cholecalciferol versus doxercalciferol for lowering parathyroid hormone in chronic kidney disease. Clin J Am Soc Nephrol 5: 299–306, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thadhani R, Appelbaum E, Chang Y, Pritchett Y, Bhan I, Agarwal R, Zoccali C, Wanner C, Lloyd-Jones D, Cannata J, Thompson T, Audhya P, Andress D, Zhang W, Ye J, Packham D, Singh B, Zehnder D, Manning WJ, Pachika A, Solomon SD: Vitamin D receptor activation and left ventricular hypertrophy in advanced kidney disease. Am J Nephrol 33: 139–149, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, Chazot C: Evidence for persistent vitamin D 1-alpha-hydroxylation in hemodialysis patients: Evolution of serum 1,25-dihydroxycholecalciferol after 6 months of 25-hydroxycholecalciferol treatment. Nephron Clin Pract 110: c58–c65, 2008 [DOI] [PubMed] [Google Scholar]
- 64.Matias PJ, Jorge C, Ferreira C, Borges M, Aires I, Amaral T, Gil C, Cortez J, Ferreira A: Cholecalciferol supplementation in hemodialysis patients: Effects on mineral metabolism, inflammation, and cardiac dimension parameters. Clin J Am Soc Nephrol 5: 905–911, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD: Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol 21: 353–361, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dogan E, Erkoc R, Sayarlioglu H, Soyoral Y, Dulger H: Effect of depot oral cholecalciferol treatment on secondary hyperparathyroidism in stage 3 and stage 4 chronic kidney diseases patients. Ren Fail 30: 407–410, 2008 [DOI] [PubMed] [Google Scholar]
- 67.Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, Tangpricha V: Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: A randomized controlled pilot study. Endocr Pract 14: 10–17, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Oksa A, Spustová V, Krivosíková Z, Gazdíková K, Fedelesová V, Lajdová I, Stefíková K, Bernasovská G, Zilinská Z, Dzúrik R: Effects of long-term cholecalciferol supplementation on mineral metabolism and calciotropic hormones in chronic kidney disease. Kidney Blood Press Res 31: 322–329, 2008 [DOI] [PubMed] [Google Scholar]