Abstract
An association between oral disease/periodontitis and rheumatoid arthritis (RA) has been considered since the early 1820s. The early treatment was tooth eradication. Epidemiological studies suggest that the prevalence of RA and periodontitis may be similar and about 5% of the population are aged 50 years or older. RA is considered as an autoimmune disease whereas periodontitis has an infectious etiology with a complex inflammatory response. Both diseases are chronic and may present with bursts of disease activity. Association studies have suggested odds ratios of having RA and periodontitis varying from 1.8:1 (95% CI: 1.0–3.2, NS) to 8:1 (95% CI: 2.9–22.1, p<0.001). Genetic factors are driving the host responses in both RA and periodontitis. Tumor necrosis factor-α, a proinflammatory cytokine, regulates a cascade of inflammatory events in both RA and periodontitis. Porphyromonas gingivalis is a common pathogen in periodontal infection. P. gingivalis has also been identified in synovial fluid. The specific abilities of P. gingivalis to citrullinate host peptides by proteolytic cleavage at Arg-X peptide bonds by arginine gingipains can induce autoimmune responses in RA through development of anticyclic citrullinated peptide antibodies. In addition, P. gingivalis carries heat shock proteins (HSPs) that may also trigger autoimmune responses in subjects with RA. Data suggest that periodontal therapies combined with routine RA treatments further improve RA status.
Conclusions
Periodontal infection (P. gingivalis) carries a unique risk for development of autoimmune antibodies associated with RA. Patients with RA have either lost many teeth or usually have severe periodontitis. Additional research, both in regards to basic mechanisms as well as clinical studies, are necessary before it can be said that there are causative links between RA and periodontitis. Cross-disciplinary research in well-defined populations should be performed to further enhance knowledge and develop clinical strategies how to coordinate therapy and risk assessments of RA and periodontitis.
Keywords: rheumatoid arthritis, periodontitis, bacteria, inflammation, Porphyromonas gingivalis, citrullination, genetics, review
During the last 200 years, a possible association between rheumatoid arthritis (RA) and oral disease (periodontitis) has been debated. Recent research with focus on inflammation in relation to infection by Porphyromonas gingivalis has identified an infection-immune response as one explanatory factor to why subjects with periodontitis may develop RA. Experiences from anti-inflammatory therapies in the management of RA may be useful also in the management of periodontitis. In the present review, studies on the associations, etiological co-factors, and effects of therapy in patients with RA and periodontitis will be discussed.
Approximately 1% of the total world population suffers from RA. The prevalence of RA increases with age and is three times more prevalent in women with 5% of women aged older than 55 years being affected (1, 2). RA is an autoimmune condition and diagnosed as chronic inflammatory polyarthritis when five or more joints are affected (3). The progression of RA can be (1) monocyclic (one single episode with or without therapy ending within 5 years and not reoccurring), (2) polycyclic (fluctuating with several episodes of disease activity, and (3) progressive (continuing to increase in severity and unremitting). Clinically, RA is currently diagnosed according to the 2010 American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) classification that includes nine criteria (4) [http://www.rheumatology.org/practice/clinical/classification/ra/ra_2010.asp].
The diagnosis of RA is based on the clinical history, physical examination, blood count [i.e. eosinophil sedimentation rate, serum C-reactive protein (CRP), and immunoglobulin rheumatoid factor (RF)]. In addition, imaging methods [radiographs, magnetic resonance imaging (MRI), computer imaging methods (CAT and bone scans)] are used to assess various joints. RA typically manifests with signs of inflammation, with the affected joints being swollen, warm, painful, and stiff. The inflammatory activity leads to tendon tethering, erosion, and destruction of the joint surface. This results in joint deformity impairing the range of movement and is often associated with pain.
The etiology of RA is not well understood. Most likely, there is a combination of genetic (including epigenetics – changes in gene expression or cellular phenotype caused by other mechanisms than changes in the underlying DNA sequence), environmental, hormonal, and infectious co-factors. RA has, in addition to periodontitis, also been associated with other diseases such as heart diseases, lung diseases, gastrointestinal diseases (including Morbus Crohn), osteoporosis, and skin diseases.
Periodontitis is a common disease affecting between 3 and 60% (depending on the criteria used to define periodontitis) (5). The current differential criteria for periodontal diseases include gingivitis, chronic periodontitis, aggressive periodontitis, periodontitis as a manifestation of systemic diseases, necrotizing periodontal diseases, abscesses of the periodontium, and periodontitis associated with endodontic lesions (6). Since the late 1990s, there has been an increasing interest in other diseases that can be associated with periodontitis. From a historical perspective, RA has been associated with periodontitis since the early 19th century when Benjamin Rush (American physician and politician) identified that total tooth eradication was a cure for ‘rheuma’. Therapy with extraction of all teeth was common in the early 20th century, promoting the focal infection theory and the values of full mouth tooth eradication. This continued to be the praxis in many countries until Cecil and coworkers in the late 1930s concluded that tooth eradication was not a solution to the treatment of RA. In 1952, the American Medical Association clarified that this practice was not based on scientific evidence and that tooth eradication should not be considered as a treatment approach to reduce the severity, or symptoms of RA (7).
To what extent the eradication of all teeth in subjects with advanced periodontitis has a systemic positive impact continues to be an open question. The local infection-driven inflammatory process of the periodontium has, in general, been proposed as one possible mechanism triggering systemic inflammatory processes or spread of infection (8, 9). Taylor et al. (10) identified that if non-smoking subjects with severe periodontitis had all their teeth extracted, a significant decrease in serum CRP, plasminogen activator inhibitor-1, fibrinogen, WBC, and platelet counts would occur. Thus, it appears that eradication of an oral source of infection has systemic effects resulting in a reduction of systemic inflammation. In addition, others have shown that non-surgical periodontal therapy can result in not only an immediate severe increase in serum CRP levels but also that CRP values decrease to pretreatment values within 30 days (11). Bacteremia with an oral bacterial origin may, in part, explain the increase in serum markers of inflammation. Both observations can be used to accept the proof of principle that periodontitis is not an isolated local disease. Periodontitis is a disease with involvement of soft and hard tissues, a complex infectious etiology with significant inflammatory ‘systemic’ consequences.
The periodontium, a bacterial habitat and a systemic disease in the susceptible host
Consistent with RA, a cyclic nature of chronic periodontitis has been identified (12, 13). The infectious etiology of periodontitis is well established (not referenced here). The subgingival biofilm may be in flux and constantly respond to nutritional and other challenges. Data suggest that the subgingival microbiological profile is also dependent on gene clusters, and it is not necessarily the periodontal probing pocket depth that defines the microbiological profile. Data suggest an important genetic impact on the infectious susceptibility in subjects with periodontitis (14). The periodontal ‘niche’ harbors a uniquely broad spectrum of bacteria (15, 16).
A single pathogen fulfilling the DNA sequence-based identification of pathogens as required by the modified Henle-Koch postulate (17) has not been identified for the periodontal infection. In part, the criteria have been fulfilled in that it is possible to infect the periodontium with P. gingivalis (18) and that immunization against this pathogen can control the infection in the same animal model (19, 20). Data have also shown that purified porphypains from P. gingivalis and used as a vaccine provides protection against periodontitis in a non-human primate model (21). This may be an important observation, especially in the relation to citrullination (see below) and a possibility to control P. gingivalis-induced citrullination. Many of the bacterial species considered as putative pathogens and identified in the oral cavity can also be found at other locations. This may be a consequence of host susceptibility, a natural habitat for such bacteria, or infectious etiology of disease (i.e. (22–25)). The effects of bacteremia from oral sources have been difficult to study.
Genetics in periodontitis and rheumatoid arthritis
Genome-wide association studies have identified replicable, genetic associations between common single nucleotide polymorphisms (SNPs) and risk of common autoimmune and inflammatory (immune-mediated) diseases such as RA (26). In recent years, several studies have also been published assessing genetic factors in periodontitis. Due to the lack of power and study design flaws almost, all genetic variants associated with periodontitis that have been published are, therefore, questionable (27). Notwithstanding, genetic factors are driving immune responses in general, and there can be no doubt that the susceptibility to periodontitis can be derived from genetic mechanisms. The most appropriate approach to studies of genetic factors in periodontitis should be a cross-disciplinary medical study approach.
The literature on the relationship between genetic factors and RA is extensive. Studies have yielded novel genetic loci underlying several common diseases, including RA. Thus, ‘leukocyte activation and differentiation’, ‘pattern-recognition receptor signaling pathway’, and ‘chemokines and their receptors’ can explain mutation-induced RA (28). Research investigating the relationship between polymorphisms and disease has demonstrated strong links between susceptibility to RA and genetic factors. Different genetic markers have found human leukocyte antigen (HLA) genetic factors as explanatory to early onset of RA (29). In addition, and due to the aging process and immunosenescence, telomere erosion appears to proceed more rapidly in patients with RA than in healthy control subjects, resulting in an early onset of the disease in RA susceptible individuals (30).
Environmental factors can cause reversible and non-reversible genetic changes. Heritable changes in gene expression or cellular phenotype caused by mechanisms other than changes in the underlying DNA sequence are studied through epigenetics. Epigenetic changes occur without a direct change in the genetic sequence and may be reversible. Epigenetic alterations are sources of potential genetic defects resulting in gene malfunctions and may be linked to both RA (31) and periodontitis (32). Reduced synovial expression of histone deacetylases (HDACs) is proposed to contribute to pathology in RA (33). Epigenetic changes have been linked to the X chromosome. This may, partly, explain the gender difference in RA prevalence. Not only gender, viral infection, hormones, and geography but also nutrition and chemicals have been identified through epigenetics. Both intrinsic to the DNA sequence (polymorphism and mutations) and extrinsic to the DNA sequence (DNA methylation) stable and heritable changes in gene expression without changes in the genetic code can occur. There are merging data suggesting that dietary factors, such as micro-nutrients and non-nutrient dietary components, can modify epigenetic marks (34).
Rheumatoid arthritis and infection in the susceptible host
A combination of environmental and genetic factors with antibodies directed against cyclic citrullinated peptide (anti-CCP) has been associated with the onset of RA (29).
Citrullination or deamination is the term used for a genetic modification of the amino acid arginine in a protein into the amino acid citrulline and caused by enzymatic activity through peptidylarginine deaminases (PADs). Data have shown that anti-CCP antibody in addition to the RF predate the onset of RA with anti-CCP antibody levels having the highest predictive value (35).
In 2004, Rosenstein et al. (36) introduced the hypothesis that P. gingivalis, which is the sole microorganism documented to express PAD, would allow individuals with periodontitis to be exposed to citrullinated antigens, predisposing to development of anti-cyclic citrullinated peptide (CCP) antibodies and to be at risk for RA. Thus, P. gingivalis rapidly generates citrullinated host peptides by proteolytic cleavage at Arg-X peptide bonds by arginine gingipains, followed by citrullination of carboxy-terminal arginines by bacterial peptidylarginine deiminase (37). Studies have shown that P. gingivalis contains a range of endogenous citrullinated proteins that are not present in other common oral pathogens (38). The expression of citrullinated autoantigens in synovial fluid indicates the important role of citrullination in RA (39). Oral bacterial infection (P. gingivalis) may, therefore, play a role in peptide citrullination and involved in loss of self-tolerance and development of RA (40). Data suggest that citrullinated proteins are also present in the gingiva of patients with periodontitis (41). Immunization with cysteine proteases purified from P. gingivalis against periodontitis may, therefore, have significance also in the prevention and management of RA through humoral factors and with an impact on cytokine production and control of infection and inflammation (21). There are few, if any, other studies that have linked specific bacteria as potentially causative bacteria in RA. Thus, P. gingivalis may directly be linked to RA through citrullination and induction of antipeptidyl citrulline antibodies reacting to citrullinated human self-proteins (41). This may be the most convincing evidence that a pathogen associated with periodontitis has an impact in the development and progression of another disease (RA). This concept may be more plausible than what has been suggested for the impact of periodontal infection in relation to, i.e. cardiovascular diseases.
Markers of inflammation in periodontitis and rheumatoid arthritis
One of the conditions in RA has been identified as synovitis. Synovitis is characterized by leukocyte infiltration, proliferation of fibroblast-like synoviocytes, osteoclast activation, presence of mast cells, B cells, and characteristic CD4+/CD8+ ratio (42–44). The synovial fluid is rich in proinflammatory cytokines. Several interleukins (ILs) (i.e. IL-1, IL-6, IL-8, IL-15, and IL-17) (45) as well as NF-kappaB ligand (RANKL) (46) can be associated with RA. Similar proinflammatory cytokines have also been associated with inflammation in periodontitis (47).
The proinflammatory cytokine, tumor necrosis factor (TNF)-α, is of special interest for the understanding of immune responses in relation to a linkage between RA and periodontitis. The literature on the effects of elevated serum levels of TNF-α and the association between TNF-α and RA is extensive (i.e. (48)). Due to the fact that treatment with anti-TNF-α medication is commonly used to control for the inflammatory process in RA, such therapy may also be relevant for the management of periodontitis.
TNF-α is released in response to lipopolysaccharide and other bacterial byproducts. A local increase in TNF-α concentration results in heat, swelling, redness, pain, and loss of function. Elevated serum levels of TNF-α induce production of CRP and promote the expression of adhesion molecules on endothelial cells allowing neutrophil diapedesis and inducing IL-1 activation. Within the cytokine cascade, IL-1 stimulates synoviocytes to produce MMPs (stromelysin) with activation of collagenase, resulting in cartilage destruction. IL-1 is a chemoattractant facilitating the migration of polymorphonuclear cells into the synovial tissues. High levels of IL-1 also cause increased production of nitric oxide killing of chondrocytes. IL-1 regulates NFκB-osteoprotegerin-RANKL and induces osteoclast activation. These inflammatory processes result in osteolysis in both RA and periodontitis.
In regard to proinflammatory cytokines and immune responses in periodontitis, detailed information from an extensive literature review is currently available (49). TNF-α has been of interest in periodontal research as this proinflammatory cytokine while TNF-α upregulates production of prostaglandin E2 and matrix-metalloproteinases (MMPs), causing osteoclast stimulation. This results in bone resorption, a prominent pathogenic feature in periodontitis. A higher expression of protease-activated receptor-2 (PAR2), IL 1α, IL-6, IL-8, and TNF-α at periodontal site where P. gingivalis is present has been reported in cases with chronic periodontitis (50). These findings are also consistent with in vitro experiments (51).
Studies on the association and explanatory variables to support the perception of an association between rheumatoid arthritis and periodontitis
Several studies on the association between RA have been published. A summary of study designs and results obtained from such studies are presented in a time-sequential order (Table 1). There are few large cross-sectional studies assessing the associations between RA and periodontitis. Most studies have used the criteria for RA defined by the ACR (53), and the criteria for periodontitis defined by the American Association of Periodontology. The studies presented in Table 1 were identified through screening documents published in peer-reviewed journals in the English language since 2001 and avoiding other review articles. The primary focus was also on human studies.
Table 1.
Selected studies assessing the association between periodontitis and rheumatoid arthritis
| First author and reference | General study design and characteristics | Clinical data | Laboratory data | Findings | Conclusions/comments |
|---|---|---|---|---|---|
| Mercado et al. (52) | Case–control clinical study. RA defined by ACR criteria (54), visual analog scale (VAS). Periodontitis was defined as mild, moderate, or severe. 65 consecutive cases with RA and 65 matched healthy control subjects. 75% were women. | PPD, CAL BOP, panoramic radiography | CRP, ESR | ESR higher in RA+subjects with severe periodontitis (p<0.001). More tooth loss in RA+ 14 vs. 7 teeth (p<0.001). BOP and plaque index (NS), more alveolar bone loss in RA+ subjects (68% vs. 30%) (OR: 2.3, 95% CI: 1.1, 4.6, p<0.03). More severe PPD in RA+ subjects (OR: 2.2, 95% CI: 0.9, 9.4, p=0.07). Every 1 mg/l increase in CRP increased the odds of severe periodontitis (OR:1.1, 95% CI: 1.1, 1.2, p<0.001) | An association between RA and the severity of periodontitis was demonstrated in regard to bone loss. Plaque and gingival conditions were not related to dexterity loss in RA. The relationship between ESR and periodontitis was unclear. The study did not prove causality. |
| Moen et al. (54) | Case–control clinical study RA defined by ACR criteria (53) 16 subjects with RA; 14 subjects with PsA; 9 control subjects with osteoarthritis. | PPD, CAL, evidence of alveolar bone loss assessed from radiographs; HAQ; DAS28 | CRP, WBC, platelet counts, IgG conc. Synovial fluid and serum analysis: bacterial samples assessed by DNA checkerboard (40 species). | A. naeslundii, E. nodatum, P. gingivalis, P. nigrescens exclusively found in serum from RA+ subjects. Synovial fluids: oral bacteria in 14/16 RA, 17/14 PsA, and 9/9 OA subjects with highest total counts in RA (p<0.001). Bacteria in synovial fluid from RA+ and PsA+ subjects: E. saburreum, P. micra, A. israelii, S. noxia, P. acnes, C. showae, T. forsythia, C. sputigena, L. buccalis, P. intermedia No correlation between serum CRP, WBC, platelet counts, and oral bacteria. | Bacterial DNA from a variety of species can be found in synovial fluid. Higher counts of oral bacterial DNA in synovial fluid than in serum from RA+ subjects suggest that synovial fluid may capture DNA from oral bacteria. |
| Kobayashi et al. (55) | Case–control clinical study RA defined by ACR criteria (52) Periodontitis: AAP criteria (6) 100 subjects with RA, 100 subjects with periodontitis, 100 healthy subjects. | Assessment of BOP, PPD, CAL | IL 1 gene polymorphism was assessed by PCR for IL1-1A+4845, IL-1B +3954, IL-1 RN+2041, and FcyR genes. | Eighty-six per cent of RA subjects had periodontitis, but milder form of periodontitis than the periodontitis non-RA control group. The RA subjects had fewer remaining teeth. A higher prevalence of RA+ subjects with the IL-1B+3954 T allele had RA and periodontitis. | IL-1 and FcyR gene polymorphisms carry an increased risk for RA and periodontitis. This may be specific for Japanese. Anticitrullin antibodies at higher levels in subjects with RA and severe periodontitis. |
| Leksell et al. (56) | Case–control study. Subjects between 10 and 19 years 41 juvenile idiopathic arthritis (JIA) on DMARDs. 41 control subjects. | Plaque, calculus, PPD, CAL, and mucosal lesions Dental radiographs. Child Health Assessment Questionnaire Stanford HAQ disability index. | Serum RF, CRP, ESR, salivary flow rate. | 68% JIA and 12% controls had pain when opening the mouth. 12% JIA had intra-oral ulcers. 32% JIA but none in control group had increased PPD/IA+ subjects on anti-TNF-α had lower BOP scores. Medications; anti-TNF-α, DMARD, NSAIDs, and methotrexate. | Children with JIA have more oral ulcerations, more discomfort, more plaque, BOP, and gingival hyperplasia. Children with JIA on anti-TNF-α had less gingivitis. |
| De Pablo et al. (57) | Epidemiological study (NHANES III). RA defined by ACR criteria (53). Study included 103 (2.3%) subjects with RA from a total of 44,461 subjects. | Periodontal: CAL and PPD | Serum CRP | CRP values were higher in RA subjects. OR: 1.8:1; 95% CI: 1.04, 3.2 (p<0.05). Subjects with RA were more likely to be edentulous (OR: 2.27, 95% 95% CI: 1.56, 3.31, p<0.05) and to have periodontitis (OR: 1.82, 95% CI: 1.04, 3.20, p<0.05) compared with non-RA subjects. | Subjects with RA were more often edentulous and did not attend dental regularly and to have more periodontitis if dentate. |
| Nilsson et al. (58) | Case series clinical study. RA defined by ACR criteria (53). Consecutive subjects with RA. 30 healthy control subjects. | Periodontal: CAL% >3 mm, PPD% >3 mm, BOP% | Peripheral blood: ESR, CRP thrombocyte particle conc., RA factor, TNF-α, IL-Iα, IL-1 β, IL-1ra, PGE2, 5-HT serotonin | TNF-α was the only factor predictive of dental factors. TNF-α was predictive of% BOP (p<0.01).TNF-α was predictive of PPD% (p<0.05). TNF-α was predictive of% CAL (p<0.01). TNF-α differed by RA status (p<0.001). | Gingivitis and periodontitis are related to high levels of circulating TNF-α in subjects who are RA+. |
| Pischon et al. (59) | Cross sectional clinical study RA: defined by DAS28 (60), HAQ Periodontitis: mean CAL>4.0 mm. 57 RA and 52 healthy control subjects | Periodontal: gingival index, plaque index, PPD, CAL. Smoking, alcohol, BMI | ESR, CRP IgG, IgM, RF, antibodies to CCP (ELISA). | RA+ had on average 0.9 mm more CAL (p<0.001), and 0.6 mm>PPD (p<0.001) BOP and PI>(p<0.001). Ra+ subjects had a 6-folds higher risk for periodontitis (unadj.) 8.05 (95%CI: 2.9, 22.1, p<0.001) age adj. OR: 8.1 (95% CI: 2.9, 22.4, p<0.001). | Subjects with RA have significantly more CAL (periodontitis) than non-RA subjects. Oral hygiene may only partially account for this association. |
| Btytkoglu et al. (61) | Case–control clinical study. RA defined by ACR criteria (53). AAP criteria for periodontitis (6). 23 RA+ and 17 RA- but with periodontitis. 17 RA- and periodontally healthy subjects. | Periodontal: PPD, CAL, gingival index. | Serum RA factor, ESR, CRP, gingival fluid samples: ELISA: Il-1β, PGE2, plasminogen inactivator PAI-2 and plasminogen activator t-PA. | RA factor, ESR, and CRP values in RA+ subjects did not differ by periodontal diagnosis. No difference by any parameter between RA+/periodontitis and RA–/periodontitis t-PA total amounts and conc. In RA+ and periodontitis+subjects t-PA total amounts were higher than in periodontitis – subjects. PAI-2 levels, Il-1β, and PGE2 were similar between RA+and – control subjects with or without periodontitis. | The coexistence of RA and periodontitis does not seem to affect clinical periodontal findings or systemic markers of RA. The lack of difference may be the result of anti-inflammatory treatment of RA+subjects. |
| Martinez-Martinez et al. (62) | Case series clinical study. RA defined by ACR criteria (53). Subjects with refractory RA and periodontitis 19 subjects. Periodontitis defined as PPD ≥ 3 mm and CAL ≥ 2 mm at 10 sites. All subjects on DMARDs, steroids and NSAIDs. | Periodontal: PPD, CAL | Synovial fluid samples and subgingival crevicular fluid samples, serum samples, PCR analysis of A. actinomycetemcomitans, P. gingivalis, P. intermedia, P. nigrescens, T. denticola, T. forsythia. | Detected in synovial fluid: P. intermedia (89%); T. denticola (31.5%); P. gingivalis (57.8%); P. nigrescens (21%); T. forsythia (10%); A. actino. (15%). Detected in GCF: P. intermedia (100%); T. denticola (84.2%); P. gingivalis (78.9%); P. nigrescens (68%); T. forsythia (52.6%); A. actino. (21%). Detected in serum: P. intermedia (73.6%); T. denticola (21%); T. forsythia (31.5%); P. gingivalis (42.1%). | P. intermedia, P. gingivalis, and T. denticola were the most prevalent species found in synovial fluid. Possibly, a free transport of DNA to synovial fluid. The presence of P. gingivalis in synovial fluid supports the theory on anti-CCP and citrullination. |
| Dissik et al. (63) | Case–control clinical study. RA defined by MD-HAQ, DAS28, AAP classification of periodontitis (6). Subjects with RA 69 subjects or osteoarthritis, 35 subjects. RA+subjects were treated with DMARDs, NSAIDs, tetracyclines, or anti-TNF-α. | Periodontal: PPD, CAL,BOP, panoramic radiographs (bone loss). | ESR, RA factor, anti-CCP antibody titers, serum CRP | Odds ratio of an association between RA+ and having periodontitis 2.1 (95% CI: 1.1, 3.8, p<0.02). Female gender and RA+/periodontitis, OR: 2.9 (95%CI: 1.4, 36.1, p<0.02). Current smoking OR of RA+ and periodontitis 2.9 (95%CI: 1.0, 8.5, p=0.06). | Moderate to severe periodontitis was more frequently found in RA+patients and RF seropositive subjects or in subjects with the anti-CCP antibody. Female gender and smoking habits are risk factors in the RA+/periodontitis complex. |
| Trombone et al. (64) | Animal study to assess P. gingivalis, A. actinomycetemcomitans-induced periodontitis, and pristane-induced arthritis (PIA) interaction in mice. | Clinical evidence of alveolar bone loss and PPD. Inflammatory cell analysis ELISA assays, rtPCR, for bacterial assessments and RNA extractions from periodontal tissues. | Cytokines: ELISA assays: IL-1β, IFN-γ, IL-4, IL-10, IL-17, TNF-α, RANKL/OPG, MMP/s/TIMPs. Serum titers to P. gingivalis and A. actinomycetemcomitans. | Higher severity PD in the genetically inflammation prone acute inflammatory reactivity maximum (AIRmax) mice strain was associated with higher levels of TNF-α, IL-1β, IL-17, matrix MMP-13, and RANKL. Periodontitis/PIA co-induction resulted in even higher levels of IL-1β, IFN-γ, IL-17, RANKL, and MMP-13 levels. Periodontitis/PIA co-induction in AIRmin strain did not alter the course of both pathologies. P. gingivalis or A. actinomycetemcomitans infection did not further enhanced cytokine counts but may cause more bone loss in test animals. | Animal model demonstrating association between RA and periodontitis with bone loss in test animals after exp. arthritis. Amplification of innate immune responses (Th1/Th17) in RA-induced mice. The lack of added effects by P. gingivalis and A. actinomycetemcomitans infection may be explained by immune hyper reactivity by experimentally induced RA alone. |
| Mirrielees et al. (65) | Cross-sectional, Case–control study. RA defined by ACR criteria (53). 35 RA+ patients ranging in age from 22 to 64 years were enrolled and matched with 35 healthy controls and 35 subjects with periodontitis. | Routine dental (PPD, CAL, BOP) and RA criteria RA+ subjects using DMARD medication. | Concentrations of salivary IL-1β and TNF-α assessed by Luminex assay for IL-1β, and TNF-α, ELISA for MMP-8. | Salivary levels of IL-1β and TNF-α were significantly elevated in arthritis patients not receiving anti-TNF-α antibody therapy compared with arthritis patients receiving anti-TNF-α therapy and healthy controls. RA+ subjects had more >BOP than control subjects but less periodontitis. IL-1β levels are higher in RA+ subjects than in control subjects. | RA+ patients have higher levels of periodontitis than healthy controls. Anti-TNF-α antibody-based disease-modifying therapy. Significantly lowers salivary IL-1β and TNF-α levels in RA. RA in the absence of disease modifying antirheumatic drugs appears to influence levels of select salivary biomarkers of periodontitis. |
| Okada et al. (66) | Case–control clinical study RA defined by ACR criteria (53). AAP criteria periodontitis (6) Purpose of study to assess if serum levels of antibodies to bacteria associated with periodontitis may affect clinical and laboratory profiles of RA80 RA+ and 38 matched healthy control subjects. | Demographic data, smoking status, PPD, CAL, gingival index. The presence of periodontitis was defined as having ≥1 periodontitis site with PD >3 mm and CAL ≥3 mm. | Serum levels of CRP; ELISA assays: anti-citrullinated peptide, RA factor, IgG titers to A.actinomycetemcomitans, E. corrodens, P. intermedia, P. gingivalis. | Sign lower titers in RA group to A. actinomycetemcomitans and E. corrodens but higher titers in RA+ subjects for P. gingivalis IgG titers. Serum levels of anti-CCP were higher in RA+ subjects. Serum anti-P. gingivalis titers were correlated with RA factor, probing depth, and clinical attachment levels but not with anti CCP Ab levels, BOP, or CRP. | All RA+ subjects were on medication that may have affected the results. Systemic administration of corticosteroids, antirheumatic drugs, NSAIDs, TNF-α antagonist reduced signs of periodontitis. Serum levels of anti-Pg IgG antibodies were associated with RA+ and may affect serum levels of RF and periodontal condition in patients with RA. |
| Ziebolz et al. (67) | Case series, clinical study. RA defined by ACR criteria (53) and by DAS28. Periodontal screening index (CPITN/PSR). To investigate periodontal and microbiologic parameters in patients with RA 66 subjects with RA were studied. | Periodontal status, periodontal screening index (PSR/PSI), DMFT, smoking habits, Rheum tx. (methotrexate, folic acid, prednisolone, leflunomide, Ca carbonate+ cholecalciferol). Non-parametric analysis controlling for age, gender, smoking, and RA medication. | RA factor, IL 1 genotype, CRP, WBC, thrombocyte, neutrophil, lymphocyte, and granulocyte counts. Microbiology by PCR: A. actinomycetemcomitans, P. gingivalis, T. forsythia, T. denticola, P. micra, P. intermedia, C. rectus, E. nodatum, E. corrodens, C. sputigena, F. nucleatum. Detection threshold >102 | E. corrodens and C. sputigena counts were related to genotype and RA medication. Correlation between RA factor and E. nodatum (p<0.05), E. corrodens (p<0.05) and Capnocytophaga sp. (p<0.05) but not with P. gingivalis, T. forsythia, T. denticola, F. nucleatum sp. P. micra, or P. intermedia. | No patients were periodontally healthy. Most patients with RA had moderate-to-severe periodontitis and presence pathogens associated with periodontitis. No association was found between RA factor and periodontal status or microbiologic parameters. |
AAP, American Academy of Periodontology, ACR=American College of Rheumatology; BMI, body mass index; BOP, bleeding on probing; CAL, clinical attachment level; CRP, C-reactive protein; DAS28, Disease Activity Score; ESR, erythrocyte sedimentation rate; OR, odds ratio; HAQ=health assessment questionnaire; PPD, probing pocket depth; PsA, psoriatic arthritis; RA, rheumatoid arthritis; RF, rheumatoid factor; WBC, white blood cell counts; and abbreviations of bacteria names, i.e. P. gingivalis.
Genetic factors have been identified for the association between RA and periodontitis (55, 63, 67). Odds ratios that subjects with RA have more frequently and with more severe periodontitis varied between OR: 2.2:1 (52) and OR: 8.1:1 (59), whereas one study found no significant association (57). Periodontal disease severity (OR: 2.1:1) was ranked as number three as a predictive factor for RA with female gender (OR: 7:1) and smoking (OR: 2.9:1) as the primary predictors (63). Other studies have not assessed the likelihood of an association but rather defined shared etiological factors or host response mechanisms between RA and periodontitis.
One of the problems in assessing proinflammatory factors in RA and periodontitis is that some studies represent convenience studies where subjects at the time of assessment were treated with a variety of common medication against RA symptoms (see below). Thus, the lack of higher TNF-α and CRP serum levels in subjects with both RA and periodontitis may be explained by the impact of anti-inflammatory routine RA medications (58, 61, 63, 67). Another problem with the studies identified can be linked to the cross-sectional nature of study design and that the chronicity of periodontitis and previous treatments of periodontitis or RA were not accounted for.
In one well-controlled clinical case–control study (54) study including hospitalized RA patients, analyzing the frequency of different oral bacterial DNA species in periodontal pocket samples, sera, and synovial fluids of patients with RA and controls, the authors identified variable bacterial DNA concentration of bacteria with (most likely) an oral origin in synovial fluids and in serum from the patients with RA. In one case series (62) including 19 subjects remaining after screening of 500 subjects with RA refractory subjects not responding to disease modifying antirheumatic drugs (DMARDS) and periodontitis assessed the presence of pathogens (by PCR method) associated with periodontitis. The study identified that, in bacterial samples from knee joints, such bacteria were found in 100% of samples including one or several species. Among the most prevalent species were Prevotella intermedia, Treponema denticola, and P. gingivalis. Aggregatibacter actinomycetemcomitans was the least commonly found microorganism. Findings from an experimental study could also identify counts of A. actinomycetemcomitans as a factor in bone loss in RA-induced mice (64). Others have shown that anticitrullin antibody levels were significantly higher in RA subjects with advanced periodontitis than in RA subjects without periodontitis (63). Thus, such studies suggest that key pathogens in periodontitis are associated with RA. It should be recognized that one study has failed to identify pathogens in periodontitis as associated with RA (67) and may be explained by confounders (i.e. smoking), medication, that all subjects had RA, or that periodontitis was defined through a screening index.
Standard therapy in the treatment of rheumatoid arthritis
This section, briefly presents medications used in the treatment of RA. (The reader is referred to the pharmacological literature for detailed information.) The primary objectives in the treatment of patients with RA are to reduce inflammation and to correct pathological abnormalities to enhance quality of life. Treatment options include a variety of medications, efforts to reduce joint stress, physical therapy, and surgical intervention. Medications that are commonly used in RA treatment include non-steroidal anti-inflammatory agents (NSAIDs – aspirin, ibuprofen, COX-2 inhibitor) corticosteroids, and disease modifying anti-rheumatic drugs (DMARDs). In addition, injectable gold therapy (until late 1990s), cyclosporine, diet, and climate-humidity change are considered.
NSAID drugs can reduce acute inflammation and decrease pain. Such medications alone do not change the course of RA. Corticosteroids have both anti-inflammatory and immune-regulatory activity but have also been associated with several side effects (i.e. weight gain and a Cushing-like appearance). Only DMARD agents can change the course of RA but have a delayed effect and differ from NSAIDs or cortisone therapy. Currently, methotrexate is considered as the primary DMARD agent in the management of RA. The effects are related to effects on TNF-α pathways and the inhibition of an enzyme involved in the metabolism of folic acid. Methotrexate in higher dosages is also used in cancer therapies. Hydroxychloroquine, a drug used in the treatment of malaria appears to involve changes in antigen presentation with effects on the innate immune system and effective against RA. Medications with anti-TNF-α inhibitors [i.e. Etanercept (Enbrel®), Infliximab (Remicade®), and Adalimumab (Humira®] are currently used in the treatment of RA. The risks of side effects are mainly related to secondary infections.
Anti-inflammatory treatment of periodontitis has also been proposed. Data suggest that lipoxin (LXA4) antagonizes P. gingivalis-induced cell activation dependent on leukocyte-platelet interaction through downregulation of CD11b/CD18 (68, 69). Animal studies have shown a protective role for lipoxin, limiting PMN recruitment and PMN-mediated tissue injury in relation to P. gingivalis-induced infection (70). Recent data suggest that administration of omega-3 polyunsaturated fatty acids plus low-dose aspirin as an adjunctive treatment to regenerative periodontal therapy provides additional clinical benefits (70). Studies have also shown that using an adjunctive subantimicrobial-dose of doxycycline in periodontal therapy suppresses proinflammatory cytokines and regulate the inflammatory response to therapy (71).
Studies on the effects of treatment of rheumatoid arthritis on periodontitis and the effects of periodontal therapy on rheumatoid arthritis conditions
There are several studies assessing the effects of RA treatment on periodontal conditions as well as on the impact of RA conditions following periodontal therapies. The studies on RA treatment are related to DMARDs whereas the periodontal intervention studies are based on routine non-surgical periodontal debridement assessing systemic effects. A summary of findings from select studies is presented (Table 2).
Table 2.
Selected studies assessing the effects of intervention in subjects with RA and/or periodontitis
| Authors | Study conditions | Treatment | Treatment outcome |
|---|---|---|---|
| Al-Katma et al. (72) | Case–control intervention study (8 weeks) RA defined by ACR criteria (53). Periodontitis defined by AAP criteria (6) To assess the effects of non-surgical periodontal therapy on RA. | Seventeen subjects with RA+ receiving periodontal treatment 12 subjects. with RA+ with no periodontal treatment. ***RA subjects on routine RA treatment (DMARDs). RA activity assessed by DASH 28 and ESR. | 58.8% in the test group and 16.7% in the periodontally untreated group demonstrated improved RA scores. |
| Pers et al. (73) | Case–control study;40 subjects with RA RA defined by ACR criteria (53). Assessment of periodontal status BOP, PPD and CAL. Study purpose to assess the role of anti-TNF-α on periodontal status. | 20 subjects received infliximab/methotrexate every 6 weeks for ≥22 months. 20 subjects (9 with periodontitis) were studied before and after receiving 9nine infusions of infliximab. No routine periodontal therapy was performed. | Plaque index and PPD were similar in both groups. BOP increased in infliximab group (p<0.001). Mean 0.4 mm decrease in CAL in infliximab-treated group. |
| Miranda et al. (74) | Case–control study of 17 RA and 17 healthy control age-, gender-, smoking-matched subjects. RA defined by ACR criteria (53). The study evaluated the effect of rheumatological treatment on periodontitis. | Clinical dental: plaque and gingival index, PPD, CAL, GCF levels of elastase, IL-1β. No dental treatment. RA subjects were treated with prednisone (88.2%), methotrexate (76.5%), NSAIDs (76.5%), and Sulfasalazine (23.5%) | Total amounts of IL-1b (p<0.01) and total elastase higher in RA+ subjects (p<0.001). Correlation between IL-1b and total elastase in the RA+ group (r=0.883; p<0.001) but not in the control group. |
| Mayer et al. (75) | Case–control study. RA defined by ACR criteria (53). Periodontitis defined by AAP classification (6). 10 RA (RA+) subjects, treated with with infliximab, 10 RA (RA−) untreated, 10 healthy ctr. subjects. To evaluate the influence of (TNF-α) therapy on the clinical and immunologic parameters. | Routine periodontal examintion PI, BOP, PPD, CAL, GCF samples for TNF-α (ELISA assay) RA: data: DASH 28, ESR, RA factor, or anti-CCP Ab, DMARDs use, number of erosive joints, time of RA, smoking status. | PI similar by groups. GI and BOP% higher in RA compared to RA+ and in controls. PPD and CAL less than in RA+ than in RA− and in controls TNF-α overall corr. with CAL. < GCF TNF-α in RA+ than in RA− groups. Suppression of proinflammatory cytokines may reduce periodontal inflammation. |
| Ribeiro et al. (76) | Case–control study. RA defined by ACR criteria (53). Periodontitis defined by AAP. Baseline to 3 months study including consecutive subjects. 16 subjects received oral hygiene instructions, supra-gingival cleaning. 26 subjects received full mouth debridement To assess the effects of non-surgical periodontal therapy on RA status. | HAQ, RF factor, ESR, drug therapy, routine periodontal assessments (PPD and CAL). | Subjects in the supra-gingival cleaning group had an increase in NSAID and prednisone compared to baseline. No correlation between periodontal parameters and RF. Trends of RF decreased in both group after periodontal intervention, ESR and HAQ improved in the subgingival debridement group, PPD and GI improved in both groups but CAL only at advanced sites in the group with subgingival debridement. |
| Pinho et al. (77) | Case–control study in 75 subjects over 6 months. RA defined by ACR criteria (53). To assess the effects of therapy in RA+ and RA− subjects with periodontitis. | (DAS28), CRP, ESR, RF, alpha-1 acid glycoprotein, PD (periodontal disease): ≥ 2 teeth with CAL ≥6 mm and ≥1 tooth with PPD ≥5 mm. RA+, PD+, and periodontal treatment (TX+). RA+, PD+, and no periodontal treatment (TX−). RA−, PD+, and periodontal treatment (TX+). | (RA+PD+TX+): PPD reduced (p<0.01),% plaque reduced (p<0.001). BOP reduction (p =0.07), acute phase laboratory data; NS. (RA+PD+TX−): NS changes in PD parameters; ESR was reduced (p<0.001). (RA−PD+TX+): PPD,% plaque, BOP reduced (p<0.001), systemic measures reduced (p<0.001). The relationship between RA and periodontitis activity is unclear. Periodontal treatment in the control of inflammation to avoid tooth extraction is important. |
| Ortiz et al. (78) | Case–control study over 6 weeks. RA defined by ACR criteria (53). 20 subjects with severe peridontitis and RA+ (10 on DMARDs and 10 on anti-TNF-α) These subjects received non-surgical periodontal treatment. 20 RA+ subjects with similar periodontal conditions (10 on DMARDs and 10 on anti-TNF-α). These subjects. did not receive periodontal treatment during the study. To assess the effect of non-surgical periodontal treatment on the signs and symptoms of RA in patients treated with or without anti-TNF-α medications. | Routine periodontal data: BOP, PI, PPD, CAL, number of teeth. RA data: VAS, ESR, DAS28, and TNF-α levels RF. | No gender effect. No changes on periodontal conditions in the two groups not receiving periodontal therapy. No difference in ESR by periodontal conditions. In both periodontal treatment groups, TNF-α decreased (p<0.001) and the number of symptomatic joints (VAS) decreased (p<0.01). Periodontal treatment groups showed sign. Decrease in BOP, PPD, and gain of attachment. Non-surgical periodontal therapy had a beneficial effect on the signs and symptoms of RA, regardless of the medications used to treat this condition. Anti-TNF-α therapy without periodontal treatment had no significant effect on the periodontal condition. |
| Queiroz-Junior et al. (79) | Case–control animal study of chronic Ag-induced arthritis (AIA) induced and treated with infliximab, 10 mg/kg, versus animals infected with A.a. JP2 clone (1×109 CFU). Chlorhexidine was applied in the mouth of mice every 2 d after AIA until day 14. Mice were killed at different time points (7, 14, 30, 45, and 60 days postinfection). | Morphometric evaluation of maxillae and histological examination. Knee joints of five mice per group were collected for histological evaluation. Quantification of a neutrophil enzyme marker and a macrophage enzyme marker. The concentrations of IL-1β, IL-6, IL-17, IL-10, IFN-γ, TNF-α, tumor growth factor-β, RANKL, osteoprotegerin (OPG), and anticollagen I total IgG in serum of mice were measured. Assessment of CRP. | Induction of AIA resulted in severe alveolar bone loss. Alveolar bone loss in animals with AIA was similar to that induced by oral infection with A.actinomycetemcomitans. Anti-TNF-α greatly improved AIA conditions but had no effects on the number of bacteria. Treatment with chlorhexidine improved periodontal conditions. |
Most studies on RA status as effects by non-surgical routine periodontal therapy have used the American Rheumatology College criteria from 1987 (51). It appears that most study samples have been derived from clinics providing care for RA subjects. History of DMARD or other medication can be difficult to assess. Current therapy, using DMARDs might have masked the impact of RA on the development of periodontal. For example, the extent of gingival inflammation and pseudo-pockets may have been managed through DMARDS or other anti-inflammatory agents used over longer periods of time. It is also highly likely that extensive tooth extractions have been performed in RA subjects participating in the reported intervention studies. For example, the NHANES III data reported by de Pablo et al. (57) identified that >50% of RA subjects were edentulous. Therefore, a large number of subjects with RA cannot be assessed in relation to the association with periodontitis. These edentulous subjects may represent severe cases with periodontitis and RA. The fact that one study has identified a high odds ratio for periodontitis and RA should be carefully considered (59). It is important to recognize that RA is not a highly prevalent disease whereas the information on the prevalence of periodontitis is highly divergent (5). This makes it difficult to interpret odds ratio calculations in different study populations.
Usually, the intervention studies have been performed in subjects who use anti-inflammatory drugs for the management of RA during the periodontal intervention. Therefore, the additional effects of periodontal therapy to reduce systemic inflammation may have been masked by the efficacy of routine systemic medication to control for inflammation (i.e. DMARDS). In one study, serum anti-P. gingivalis titers were correlated with RA factor and periodontal conditions, and treatment with DMARDS improved periodontal conditions (66). This further supports the infectious relationship between periodontitis and RA.
Two studies (70, 72) assessed the effects of anti-TNF-α in the treatment of RA demonstrated that anti-TNF-α therapy resulted in clinical benefits in regard to periodontal conditions. Presently, TNF-α therapy for the treatment of periodontitis has only been assessed in subjects with RA. Unpublished data through experiences by the author of this review suggest that TNF-α therapy in a patient with Morbus Crohn drastically improved severe periodontal conditions. Anti-TNF-α therapy is, however, also associated with serious adverse effects (80, 81). At the present time, anti-TNF-α therapy or the use of DMARDs in the sole management of periodontitis cannot be advocated. Further information about the impact of such medications could be important for the current understanding and management of periodontitis.
Alternative medicine and nutrition in modulation of inflammation in RA and periodontitis
Accumulating data suggest that epigenetic changes through the regulation proinflammatory responses through NFκB regulation affecting TNF-α may be crucially involved in the pathology of RA and other chronic inflammatory diseases (82). Data suggest that oxidative stress is profound in RA and reactive oxygen species play a causal role in RA. Medications used to manage oxidant/antioxidant imbalance may provide alternatives in the treatment of RA (34, 83, 84). There are merging data suggesting that dietary factors, such as micronutrients and non-nutrient dietary components can modify epigenetic marks (85). Thus, patients with RA benefit from a diet rich in antioxidants, lactobacilli, and fibers (86). In one pilot study, beneficial effects of an antioxidant intervention on clinical parameters for RA were identified (87). Recent data suggest that the administration of omega-3 fatty acids (omega-3 FA) reduces the extent of swollen and tender joint in patients with active RA (88). There is also emerging evidence that dietary intake of polyphenols interferes with P. gingivalis, suggesting that polyphenols in diet may be useful in the management of periodontal disease (89). At this time, there are no studies that specifically have addressed the effects of polyphenols in patients with RA and periodontitis.
Citrullination and heat chock proteins
P. gingivalis infection may through citrullination play a significant role in RA. Although P. gingivalis hitherto is the only bacterium that has been identified that expresses endogenous citrullinated proteins, other factor may also be associated with citrullination and through this mechanism associated with RA (36–40). Citrullinated proteins can be found in keratin and filagrin in skin and, i.e. oral mucosa. Citrullinated proteins are present in the gingiva of patients with periodontitis (37). Such citrullinated proteins are also involved in keratinocyte differentiation. Physiological citrullination of myelin has been linked to multiple sclerosis (90). Environmental and genetic factors may induce autoimmunity to specific citrullinated proteins (91).
The literature on the role of HSPs in autoimmune disease and in periodontitis is extensive. HSPs linked to P. gingivalis have hitherto not been considered in relation to RA. HSP60 from P. gingivalis can trigger molecule linking infectious periodontitis and autoimmune atherosclerosis (92). The progression of atherosclerosis can be explained in terms of the immune response to bacterial HSPs with bacterial HSPs are major antigenic determinants during infection (93, 94). Current data also suggest that HSPs are significant factors also in RA and related to citrullination (95, 96).
Summary of review
There is historical evidence of a link between RA and periodontitis. Although several studies have suggested an association between RA and periodontitis well-controlled large studies are needed to confirm these observations. As for many other studies assessing the relationship between periodontitis and diseases: i.e. diabetes mellitus, cardiovascular diseases, and adverse pregnancy outcomes, studies have used different criteria for the definition of periodontitis. Many studies have included few subjects. Therefore, the lack of statistical power is of concern. Most studies have included convenience cohorts and may represent special populations with RA. Gender, age, and smoking habits in subjects with RA and periodontitis are other issues that are difficult to address in small samples. This makes it almost impossible to compare and merge study results and to draw firm conclusions.
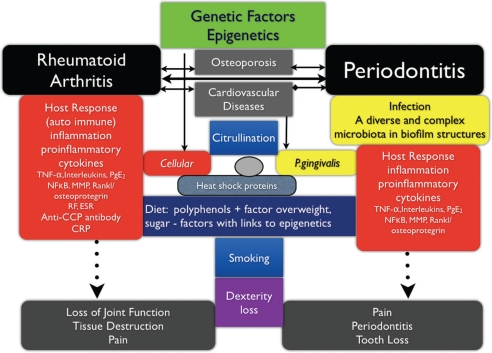
Additional research both in regards to basic mechanisms as well as clinical studies are needed before it can be said that there are causative links between RA and periodontitis. Nevertheless, some factors can be identified. In Figure 1, factors that may be proven important for further assessments of the association between RA and periodontitis are presented.
Genetic factors are driving the host responses to chronic diseases with a complex pathogenesis. Much attention has been given to TNF-α, a proinflammatory cytokine regulating a cascade of inflammatory events in many disease including RA and periodontitis. Other diseases such as osteoporosis and some cardiovascular diseases may share similar genetic tracts to disease.
Infection has been identified as a primary etiology for many diseases. The specific abilities of P. gingivalis as mentioned above through citrullination and the development of specific autoantibodies may be a primary link between RA and periodontitis and explain not only the chronicity in RA but also the chronicity of periodontitis. Whether other oral bacteria associated with periodontitis are able to induce citrullination has not yet been assessed. In addition, P. gingivalis HSPs may trigger autoimmune responses.
Fig. 1.
Illustration of pathways of genetics, inflammation, and infectious links between rheumatoid arthritis and periodontal diseases.
Acknowledgements
I appreciate the discussions and encouragement at the Meeting of the Scandinavian Society of Periodontology in Bergen, Norway, 2011, to write this review. The review is based on the background information collected in preparation for the lecture on this topic at the meeting in Bergen.
Conflict of interest and funding
The author declares no conflict of interest. No external funding.
References
- 1.Sacks JJ, Luo YH, Helmick CG. Prevalence of specific types of arthritis and other rheumatic conditions in the ambulatory health care system in the United States, 2001–2005. Arthritis Care Res. 2010;62:460–4. doi: 10.1002/acr.20041. [DOI] [PubMed] [Google Scholar]
- 2.Myasoedova E, Crowson CS, Kremers HM, Therneau TM, Gabriel SE. Is the incidence of rheumatoid arthritis rising? results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010;62:1576–82. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Silman AJ, Hochberg MC. New York: Oxford University Press; 2001. Epidemiology of the rheumatic diseases. second ed. [Google Scholar]
- 4. Available from: http://www.rheumatology.org/practice/clinical/classification/ra/ra_2010.asp [cited 14 October 2011]
- 5.Page RC, Eke PI. Case definitions for use in population-based surveillance of periodontitis. J Periodontol. 2007;78:1387–99. doi: 10.1902/jop.2007.060264. [DOI] [PubMed] [Google Scholar]
- 6.Armitage GC. Manual periodontal probing in supportive periodontal treatment 2000. Periodontol. 1996;12:33–9. doi: 10.1111/j.1600-0757.1996.tb00078.x. [DOI] [PubMed] [Google Scholar]
- 7.Anonymous. An Evaluation of the effect of dental focal infection on health. JADA. 1952;42:609–97. [Google Scholar]
- 8.Desvarieux M, Demmer RT, Rundek T, Boden-Albala B, Jacobs DR, Jr, Sacco RL, et al. Periodontal microbiota and carotid intima-media thickness: the Oral Infections and Vascular Disease Epidemiology Study (INVEST) Circulation. 2005;111:576–82. doi: 10.1161/01.CIR.0000154582.37101.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hayashi C, Gudino CV, Gibson FC, III, Genco CA. Review: pathogen-induced inflammation at sites distant from oral infection: bacterial persistence and induction of cell-specific innate immune inflammatory pathways. Mol Oral Microbiol. 2010;25:305–16. doi: 10.1111/j.2041-1014.2010.00582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor BA, Tofler GH, Carey HM, Morel-Kopp MC, Philcox S, Carter TR, et al. Full-mouth tooth extraction lowers systemic inflammatory and thrombotic markers of cardiovascular risk. J Dent Res. 2006;85:74–8. doi: 10.1177/154405910608500113. [DOI] [PubMed] [Google Scholar]
- 11.Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, et al. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–20. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- 12.Lindhe J, Okamoto H, Yoneyama T, Haffajee A, Socransky SS. Longitudinal changes in periodontal disease in untreated subjects. J Clin Periodontol. 1989;16:662–70. doi: 10.1111/j.1600-051x.1989.tb01037.x. [DOI] [PubMed] [Google Scholar]
- 13.Persson GR, DeRouen TA, Page RC. Relationship between gingival crevicular fluid levels of aspartate aminotransferase and active tissue destruction in treated chronic periodontitis patients. J Periodontal Res. 1990;25:81–7. doi: 10.1111/j.1600-0765.1990.tb00896.x. [DOI] [PubMed] [Google Scholar]
- 14.Papapanou PN, Behle JH, Kebschull M, Celenti R, Wolf DL, Handfield M, et al. Subgingival bacterial colonization profiles correlate with gingival tissue gene expression. BMC Microbiol. 2009;9:221. doi: 10.1186/1471-2180-9-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paster BJ, Olsen I, Aas JA, Dewhirst FE. The breadth of bacterial diversity in the human periodontal pocket and other oral sites 2000. Periodontol. 2006;42:80–7. doi: 10.1111/j.1600-0757.2006.00174.x. [DOI] [PubMed] [Google Scholar]
- 16.Preza D, Olsen I, Willumsen T, Grinde B, Paster BJ. Diversity and site-specificity of the oral microflora in the elderly. Eur J Clin Microbiol Infect Dis. 2009;28:33–40. doi: 10.1007/s10096-009-0743-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fredericks DN, Relman DA. Sequence-based identification of microbial pathogens: a reconsideration of Koch's postulates. Clin Microbiol Rev. 1996;9:18–33. doi: 10.1128/cmr.9.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holt SC, Ebersole J, Felton J, Brunsvold M, Kornman KS. Implantation of Bacteroides gingivalis in nonhuman primates initiates progression of periodontitis. Science. 1988;239:55–7. doi: 10.1126/science.3336774. [DOI] [PubMed] [Google Scholar]
- 19.Persson GR, Engel D, Whitney C, Darveau R, Weinberg A, Brunsvold M, et al. Immunization against Porphyromonas gingivalis inhibits progression of experimental periodontitis in nonhuman primates. Infect Immun. 1994;62:1026–31. doi: 10.1128/iai.62.3.1026-1031.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moritz AJ, Cappelli D, Lantz MS, Holt SC, Ebersole JL. Immunization with Porphyromonas gingivalis cysteine protease: effects on experimental gingivitis and ligature-induced periodontitis in Macaca fascicularis. J Periodontol. 1998;69:686–97. doi: 10.1902/jop.1998.69.6.686. [DOI] [PubMed] [Google Scholar]
- 21.Page RC, Lantz MS, Darveau R, Jeffcoat M, Mancl L, Houston L, et al. Immunization of Macaca fascicularis against experimental periodontitis using a vaccine containing cysteine proteases purified from Porphyromonas gingivalis . Oral Microbiol Immunol. 2007;22:162–8. doi: 10.1111/j.1399-302X.2007.00337.x. [DOI] [PubMed] [Google Scholar]
- 22.Strauss J, Kaplan GG, Beck PL, Rioux K, Panaccione R, Devinney R, et al. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis. 2011;17:1971–8. doi: 10.1002/ibd.21606. [DOI] [PubMed] [Google Scholar]
- 23.Brook I. Role of anaerobic bacteria in infections following tracheostomy, intubation, or the use of ventilatory tubes in children. Ann Otol Rhinol Laryngol. 2004;113:830–4. doi: 10.1177/000348940411301010. [DOI] [PubMed] [Google Scholar]
- 24.Nikolaitchouk N, Andersch B, Falsen E, Strömbeck L, Mattsby-Baltzer I. The lower genital tract microbiota in relation to cytokine-, SLPI- and endotoxin levels: application of checkerboard DNA-DNA hybridization (CDH) APMIS. 2008;116:263–77. doi: 10.1111/j.1600-0463.2008.00808.x. [DOI] [PubMed] [Google Scholar]
- 25.Persson R, Hitti J, Verhelst R, Vaneechoutte M, Persson R, Hirschi R, et al. The vaginal microflora in relation to gingivitis. BMC Infect Dis. 2009;9:6. doi: 10.1186/1471-2334-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cotsapas C, Voight BF, Rossin E, Lage K, Neale BM, Wallace C, et al. FOCiS Network of Consortia. Pervasive sharing of genetic effects in autoimmune disease. PLoS Genet. 2011;7:e1002254. doi: 10.1371/journal.pgen.1002254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schäfer AS, Jepsen S, Loos BG. Periodontal genetics: a decade of genetic association studies mandates better study designs. J Clin Periodontol. 2011;38:103–7. doi: 10.1111/j.1600-051X.2010.01653.x. [DOI] [PubMed] [Google Scholar]
- 28.Nakaoka H, Cui T, Tajima A, Oka A, Mitsunaga S, Kashiwase K, et al. A systems genetics approach provides a bridge from discovered genetic variants to biological pathways in rheumatoid arthritis. PLoS One. 2011;6:e25389. doi: 10.1371/journal.pone.0025389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez-Rodríguez L, Lamas JR, Varadé J, Tornero-Esteban P, Abasolo L, de la Concha EG, et al. Combined influence of genetic and environmental factors in age of rheumatoid arthritis onset. Rheumatol Int. 2011 doi: 10.1007/s00296-011-2090-9. [DOI] [PubMed] [Google Scholar]
- 30.Costenbader KH, Prescott J, Zee RY, De Vivo I. Immunosenescence and rheumatoid arthritis: does telomere shortening predict impending disease? Autoimmun Rev. 2011;10:569–73. doi: 10.1016/j.autrev.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ospelt C, Reedquist KA, Gay S, Tak PP. Inflammatory memories: is epigenetics the missing link to persistent stromal cell activation in rheumatoid arthritis? Autoimmun Rev. 2011;10:519–24. doi: 10.1016/j.autrev.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 32.Gomez RS, Dutra WO, Moreira PR. Epigenetics and periodontal disease: future perspectives. Inflamm Res. 2009;58:625–9. doi: 10.1007/s00011-009-0041-7. [DOI] [PubMed] [Google Scholar]
- 33.Grabiec AM, Reedquist KA. Histone deacetylases in RA: epigenetics and epiphenomena. Arthritis Res Ther. 2010;12:142. doi: 10.1186/ar3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McCall CE, Yoza B, Liu T, El Gazzar M. Gene-specific epigenetic regulation in serious infections with systemic inflammation. J Innate Immun. 2010;2:395–405. doi: 10.1159/000314077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rantapää-Dahlqvist S, de Jong BA, Berglin E, Hallmans G, Wadell G, Stenlund H, et al. Antibodies against cyclic citrullinated peptide and IgA rheumatoid factor predict the development of rheumatoid arthritis. Arthritis Rheum. 2003;48:2741–9. doi: 10.1002/art.11223. [DOI] [PubMed] [Google Scholar]
- 36.Rosenstein ED, Greenwald RA, Kushner LJ, Weissmann G. Hypothesis: the humoral immune response to oral bacteria provides a stimulus for the development of rheumatoid arthritis. Inflammation. 2004;28:311–8. doi: 10.1007/s10753-004-6641-z. [DOI] [PubMed] [Google Scholar]
- 37.Wegner N, Wait R, Sroka A, Eick S, Nguyen KA, Lundberg K, et al. Peptidylarginine deiminase from Porphyromonas gingivalis citrullinates human fibrinogen and α-enolase: implications for autoimmunity in rheumatoid arthritis. Arthritis Rheum. 2010;62:2662–72. doi: 10.1002/art.27552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lundberg K, Wegner N, Yucel-Lindberg T, Venables PJ. Periodontitis in RA—the citrullinated enolase connection. Nat Rev Rheumatol. 2010;6:727–30. doi: 10.1038/nrrheum.2010.139. [DOI] [PubMed] [Google Scholar]
- 39.Kinloch A, Lundberg K, Wait R, Wegner N, Lim NH, Zendman AJ, et al. Synovial fluid is a site of citrullination of autoantigens in inflammatory arthritis. Arthritis Rheum. 2008;58:2287–95. doi: 10.1002/art.23618. [DOI] [PubMed] [Google Scholar]
- 40.Lundberg K, Kinloch A, Fisher BA, Wegner N, Wait R, Charles P, et al. Antibodies to citrullinated alpha-enolase peptide 1 are specific for rheumatoid arthritis and cross-react with bacterial enolase. Arthritis Rheum. 2008;58:3009–19. doi: 10.1002/art.23936. [DOI] [PubMed] [Google Scholar]
- 41.Nigrovic PA, Lee DM. Synovial mast cells: role in acute and chronic arthritis. Immunol Rev. 2007;217:19–37. doi: 10.1111/j.1600-065X.2007.00506.x. [DOI] [PubMed] [Google Scholar]
- 42.Vandooren B, Cantaert T, Noordenbos T, Tak PP, Baeten D. The abundant synovial expression of the RANK/RANKL/Osteoprotegerin system in peripheral spondylarthritis is partially disconnected from inflammation. Arthritis Rheum. 2008;58:718–29. doi: 10.1002/art.23290. [DOI] [PubMed] [Google Scholar]
- 43.Wang Q, Ma Y, Liu D, Zhang L, Wei W. The roles of B cells and their interactions with fibroblast-like synoviocytes in the pathogenesis of rheumatoid arthritis. Int Arch Allergy Immunol. 2011;155:205–11. doi: 10.1159/000321185. [DOI] [PubMed] [Google Scholar]
- 44.Choy E. New biologics for rheumatoid arthritis. J R Coll Physicians Edinb. 2011;41:234–7. doi: 10.4997/JRCPE.2011.312. [DOI] [PubMed] [Google Scholar]
- 45.Astry B, Harberts E, Moudgil KD. A cytokine-centric view of the pathogenesis and treatment of autoimmune arthritis. J Interferon Cytokine Res. 2011;31:927–40. doi: 10.1089/jir.2011.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30:340–6. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- 47.Hernández M, Dutzan N, García-Sesnich J, Abusleme L, Dezerega A, Silva N, et al. Host-pathogen interactions in progressive chronic periodontitis. J Dent Res. 2011;90:1164–70. doi: 10.1177/0022034511401405. [DOI] [PubMed] [Google Scholar]
- 48.Orita S, Koshi T, Mitsuka T, Miyagi M, Inoue G, Arai G, et al. Associations between proinflammatory cytokines in the synovial fluid and radiographic grading and pain-related scores in 47 consecutive patients with osteoarthritis of the knee. BMC Musculoskelet Disord. 2011;12:144. doi: 10.1186/1471-2474-12-144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Preshaw PM, Taylor JJ. How has research into cytokine interactions and their role in driving immune responses impacted our understanding of periodontitis? J Clin Periodontol. 2011;38:60–84. doi: 10.1111/j.1600-051X.2010.01671.x. [DOI] [PubMed] [Google Scholar]
- 50.Fagundes JA, Monoo LD, Euzébio Alves VT, Pannuti CM, Cortelli SC, Cortelli JR, et al. Porphyromonas gingivalis is associated with protease-activated receptor-2 up-regulation in chronic periodontitis. J Periodontol. 2011;82:1596–601. doi: 10.1902/jop.2011.110073. [DOI] [PubMed] [Google Scholar]
- 51.Belibasakis GN, Bostanci N, Reddi D. Regulation of protease-activated receptor-2 expression in gingival fibroblasts and Jurkat T cells by Porphyromonas gingivalis . Cell Biol Int. 2010;34:287–92. doi: 10.1042/CBI20090290. [DOI] [PubMed] [Google Scholar]
- 52.Mercado RB, Marshall RI, Klestov AC, Bartold PM. Relationship between rheumatoid arthritis and periodontitis. J Periodontol. 2001;72:779–87. doi: 10.1902/jop.2001.72.6.779. [DOI] [PubMed] [Google Scholar]
- 53.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 54.Moen K, Brun JG, Valen M, Skartveit L, Eribe EK, Olsen I, et al. Synovial inflammation in active rheumatoid arthritis and psoriatic arthritis facilitates trapping of a variety of oral bacterial DNAs. Clin Exp Rheumatol. 2006;24:656–63. [PubMed] [Google Scholar]
- 55.Kobayashi T, Ito S, Kuroda T, Yamamoto K, Sugita N, Narita I, et al. The interleukin-1 and Fcgamma receptor gene polymorphisms in Japanese patients with rheumatoid arthritis and periodontitis. J Periodontol. 2007;78:2311–8. doi: 10.1902/jop.2007.070136. [DOI] [PubMed] [Google Scholar]
- 56.Leksell E, Ernberg M, Magnusson B, Hedenberg-Magnusson B. Intraoral condition in children with juvenile idiopathic arthritis compared to controls. Int J Paediatr Dent. 2008;18:423–33. doi: 10.1111/j.1365-263X.2008.00931.x. [DOI] [PubMed] [Google Scholar]
- 57.de Pablo P, Dietrich T, McAlindon TE. Association of periodontal disease and tooth loss with rheumatoid arthritis in the US population. J Rheumatol. 2008;35:70–6. [PubMed] [Google Scholar]
- 58.Nilsson M, Kopp S. Gingivitis and periodontitis are related to repeated high levels of circulating tumor necrosis factor-alpha in patients with rheumatoid arthritis. J Periodontol. 2008;79:1689–96. doi: 10.1902/jop.2008.070599. [DOI] [PubMed] [Google Scholar]
- 59.Pischon N, Pischon T, Kröger J, Gülmez E, Kleber BM, Bernimoulin JP, et al. Association among rheumatoid arthritis, oral hygiene, and periodontitis. J Periodontol. 2008;79:979–86. doi: 10.1902/jop.2008.070501. [DOI] [PubMed] [Google Scholar]
- 60.Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 61.Biyikoğlu B, Buduneli N, Kardeşler L, Aksu K, Pitkala M, Sorsa T. Gingival crevicular fluid MMP-8 and -13 and TIMP-1 levels in patients with rheumatoid arthritis and inflammatory periodontal disease. J Periodontol. 2009;80:1307–14. doi: 10.1902/jop.2009.090130. [DOI] [PubMed] [Google Scholar]
- 62.Martinez-Martinez RE, Abud-Mendoza C, Patiño-Marin N, Rizo-Rodríguez JC, Little JW, Loyola-Rodríguez JP. Detection of periodontal bacterial DNA in serum and synovial fluid in refractory rheumatoid arthritis patients. J Clin Periodontol. 2009;36:1004–10. doi: 10.1111/j.1600-051X.2009.01496.x. [DOI] [PubMed] [Google Scholar]
- 63.Dissick A, Redman RS, Jones M, Rangan BV, Reimold A, Griffiths GR, et al. Association of periodontitis with rheumatoid arthritis: a pilot study. J Periodontol. 2010;81:223–30. doi: 10.1902/jop.2009.090309. [DOI] [PubMed] [Google Scholar]
- 64.Trombone AP, Claudino M, Colavite P, de Assis GF, Avila-Campos MJ, Silva JS, et al. Periodontitis and arthritis interaction in mice involves a shared hyper-inflammatory genotype and functional immunological interferences. Genes Immun. 2010;11:479–89. doi: 10.1038/gene.2010.13. [DOI] [PubMed] [Google Scholar]
- 65.Mirrielees J, Crofford LJ, Lin Y, Kryscio RJ, Dawson DR, III, Ebersole JL, et al. Rheumatoid arthritis and salivary biomarkers of periodontal disease. J Clin Periodontol. 2010;37:1068–74. doi: 10.1111/j.1600-051X.2010.01625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Okada M, Kobayashi T, Ito S, Yokoyama T, Komatsu Y, Abe A, et al. Antibody responses to periodontopathic bacteria in relation to rheumatoid arthritis in Japanese adults. J Periodontol. 2011;82:1433–41. doi: 10.1902/jop.2011.110020. [DOI] [PubMed] [Google Scholar]
- 67.Ziebolz D, Pabel SO, Lange K, Krohn-Grimberghe B, Hornecker E, Mausberg RF. Clinical periodontal and microbiologic parameters in patients with rheumatoid arthritis. J Periodontol. 2011;82:1424–2. doi: 10.1902/jop.2011.100481. [DOI] [PubMed] [Google Scholar]
- 68.Börgeson E, Lönn J, Bergström I, Brodin VP, Ramström S, Nayeri F, et al. Lipoxin A4 inhibits Porphyromonas gingivalis-induced aggregation and reactive oxygen species production by modulating neutrophil-platelet interaction and CD11b expression. Infect Immun. 2011;79:1489–97. doi: 10.1128/IAI.00777-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pouliot M, Clish CB, Petasis NA, Van Dyke TE, Serhan CN. Lipoxin A4 analogues inhibit leukocyte recruitment to Porphyromonas gingivalis: a role for cyclooxygenase-2 and lipoxins in periodontal disease. Biochemistry. 2000;39:4761–8. doi: 10.1021/bi992551b. [DOI] [PubMed] [Google Scholar]
- 70.Elkhouli AM. The efficacy of host response modulation therapy (omega-3 plus low-dose aspirin) as an adjunctive treatment of chronic periodontitis (clinical and biochemical study) J Periodontal Res. 2011;46:261–8. doi: 10.1111/j.1600-0765.2010.01336.x. [DOI] [PubMed] [Google Scholar]
- 71.Emingil G, Gürkan A, Atilla G, Kantarci A. Subantimicrobial-dose doxycycline and cytokine-chemokine levels in gingival crevicular fluid. J Periodontol. 2011;82:452–61. doi: 10.1902/jop.2010.100036. [DOI] [PubMed] [Google Scholar]
- 72.Al-Katma MK, Bissada NF, Bordeaux JM, Sue J, Askari AD. Control of periodontal infection reduces the severity of active rheumatoid arthritis. J Clin Rheumatol. 2007;13:134–7. doi: 10.1097/RHU.0b013e3180690616. [DOI] [PubMed] [Google Scholar]
- 73.Pers JO, Saraux A, Pierre R, Youinou P. Anti-TNF-alpha immunotherapy is associated with increased gingival inflammation without clinical attachment loss in subjects with rheumatoid arthritis. J Periodontol. 2008;79:1645–51. doi: 10.1902/jop.2008.070616. [DOI] [PubMed] [Google Scholar]
- 74.Miranda LA, Islabão AG, Fischer RG, Figueredo CM, Oppermann RV, Gustafsson A. Decreased interleukin-1beta and elastase in the gingival crevicular fluid of individuals undergoing anti-inflammatory treatment for rheumatoid arthritis. J Periodontol. 2007;78:1612–9. doi: 10.1902/jop.2007.060520. [DOI] [PubMed] [Google Scholar]
- 75.Mayer Y, Balbir-Gurman A, Machtei EE. Anti-tumor necrosis factor-alpha therapy and periodontal parameters in patients with rheumatoid arthritis. J Periodontol. 2009;80:1414–20. doi: 10.1902/jop.2009.090015. [DOI] [PubMed] [Google Scholar]
- 76.Ribeiro J, Leão A, Novaes AB. Periodontal infection as a possible severity factor for rheumatoid arthritis. J Clin Periodontol. 2005;32:412–6. doi: 10.1111/j.1600-051X.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 77.Pinho Mde N, Oliveira RD, Novaes AB, Jr, Voltarelli JC. Relationship between periodontitis and rheumatoid arthritis and the effect of non-surgical periodontal treatment. Braz Dent J. 2009;20:355–64. doi: 10.1590/s0103-64402009000500001. [DOI] [PubMed] [Google Scholar]
- 78.Ortiz P, Bissada NF, Palomo L, Han YW, Al-Zahrani MS, Panneerselvam A, et al. Periodontal therapy reduces the severity of active rheumatoid arthritis in patients treated with or without tumor necrosis factor inhibitors. J Periodontol. 2009;80:535–40. doi: 10.1902/jop.2009.080447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Queiroz-Junior CM, Madeira MF, Coelho FM, Costa VV, Bessoni RL, Sousa LF, et al. Experimental arthritis triggers periodontal disease in mice: involvement of TNF-{alpha} and the oral microbiota. J Immunol. 2011;187:3821–30. doi: 10.4049/jimmunol.1101195. [DOI] [PubMed] [Google Scholar]
- 80.Strangfeld A, Eveslage M, Schneider M, Bergerhausen HJ, Klopsch T, Zink A, Listing J. Treatment benefit or survival of the fittest: what drives the time-dependent decrease in serious infection rates under TNF inhibition and what does this imply for the individual patient? Ann Rheum Dis. 2011;70:1914–20. doi: 10.1136/ard.2011.151043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Komano Y, Tanaka M, Nanki T, Koike R, Sakai R, Kameda H, et al. REAL Study Group. Incidence and risk factors for serious infection in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors: a report from the Registry of Japanese Rheumatoid Arthritis Patients for Longterm Safety. J Rheumatol. 2011;38:1258–64. doi: 10.3899/jrheum.101009. [DOI] [PubMed] [Google Scholar]
- 82.Hassan SZ, Gheita TA, Kenawy SA, Fahim AT, El-Sorougy IM, Abdou MS. Oxidative stress in systemic lupus erythematosus and rheumatoid arthritis patients: relationship to disease manifestations and activity. Int J Rheum Dis. 2011;14:325–31. doi: 10.1111/j.1756-185X.2011.01630.x. [DOI] [PubMed] [Google Scholar]
- 83.Park MK, Park JS, Cho ML, Oh HJ, Heo YJ, Woo YJ, et al. Grape seed proanthocyanidin extract (GSPE) differentially regulates Foxp3(+) regulatory and IL-17(+) pathogenic T cell in autoimmune arthritis. Immunol Lett. 2011;135:50–8. doi: 10.1016/j.imlet.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 84.Calder PC, Albers R, Antoine JM, Blum S, Bourdet-Sicard R, Ferns GA, et al. Inflammatory disease processes and interactions with nutrition. Br J Nutr. 2009;101:S1–45. doi: 10.1017/S0007114509377867. [DOI] [PubMed] [Google Scholar]
- 85.McKay JA, Mathers JC. Diet induced epigenetic changes and their implications for health. Acta Physiol (Oxf) 2011;202:103–18. doi: 10.1111/j.1748-1716.2011.02278.x. [DOI] [PubMed] [Google Scholar]
- 86.Hänninen, Kaartinen K, Rauma AL, Nenonen M, Törrönen R, Häkkinen AS, et al. Antioxidants in vegan diet and rheumatic disorders. Toxicology. 2000;155:45–53. doi: 10.1016/s0300-483x(00)00276-6. [DOI] [PubMed] [Google Scholar]
- 87.van Vugt RM, Rijken PJ, Rietveld AG, van Vugt AC, Dijkmans BA. Antioxidant intervention in rheumatoid arthritis: results of an open pilot study. Clin Rheumatol. 2008;27:771–5. doi: 10.1007/s10067-008-0848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bahadori B, Uitz E, Thonhofer R, Trummer M, Pestemer-Lach I, McCarty M, et al. omega-3 Fatty acids infusions as adjuvant therapy in rheumatoid arthritis. J Parenter Enteral Nutr. 2010;34:151–5. doi: 10.1177/0148607109342130. [DOI] [PubMed] [Google Scholar]
- 89.Bonifait L, Grenier D. Cranberry polyphenols: potential benefits for dental caries and periodontal disease. J Can Dent Assoc. 2010;76:a130. [PubMed] [Google Scholar]
- 90.Oguz KK, Kurne A, Aksu AO, Karabulut E, Serdaroglu A, Teber S, et al. Assessment of citrullinated myelin by 1H-MR spectroscopy in early-onset multiple sclerosis. Am J Neuroradiol. 2009;30:716–21. doi: 10.3174/ajnr.A1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wegner N, Lundberg K, Kinloch A, Fisher B, Malmström V, Feldmann M, et al. Autoimmunity to specific citrullinated proteins gives the first clues to the etiology of rheumatoid arthritis. Immunol Rev. 2010;233:34–54. doi: 10.1111/j.0105-2896.2009.00850.x. [DOI] [PubMed] [Google Scholar]
- 92.Sweier DG, Shelburne PS, Giannobile WV, Kinney JS, Lopatin DE, Shelburne CE. Immunoglobulin G (IgG) class, but Not IgA or IgM, antibodies to peptides of the Porphyromonas gingivalis chaperone HtpG predict health in subjects with periodontitis by a fluorescence enzyme-linked immunosorbent assay. Clin Vaccine Immunol. 2009;16:1766–73. doi: 10.1128/CVI.00272-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Choi J, Lee SY, Kim K, Choi BK. Identification of immunoreactive epitopes of the Porphyromonas gingivalis heat shock protein in periodontitis and atherosclerosis. J Periodontal Res. 2011;46:240–5. doi: 10.1111/j.1600-0765.2010.01339.x. [DOI] [PubMed] [Google Scholar]
- 94.Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect. 2007;13:3–10. doi: 10.1111/j.1469-0691.2007.01798.x. [DOI] [PubMed] [Google Scholar]
- 95.Wu CT, Ou LS, Yeh KW, Lee WI, Huang JL. Serum heat shock protein 60 can predict remission of flare-up in juvenile idiopathic arthritis. Clin Rheumatol. 2011;30:959–65. doi: 10.1007/s10067-011-1709-2. [DOI] [PubMed] [Google Scholar]
- 96.Bodnár N, Szekanecz Z, Prohászka Z, Kemény-Beke A, Némethné-Gyurcsik Z, Gulyás K, et al. Anti-mutated citrullinated vimentin (anti-MCV) and anti-65kDa heat shock protein (anti-hsp65): New biomarkers in ankylosing spondylitis. Joint Bone Spine. 2012;79:63–6. doi: 10.1016/j.jbspin.2011.03.010. [DOI] [PubMed] [Google Scholar]