Abstract
Objective:
Although incidence rates for mild cognitive impairment (MCI) have been reported, few studies were specifically designed to measure the incidence of MCI and its subtypes using published criteria. We estimated the incidence of amnestic MCI (aMCI) and nonamnestic MCI (naMCI) in men and women separately.
Methods:
A population-based prospective cohort of Olmsted County, MN, residents ages 70–89 years on October 1, 2004, underwent baseline and 15-month interval evaluations that included the Clinical Dementia Rating scale, a neurologic evaluation, and neuropsychological testing. A panel of examiners blinded to previous diagnoses reviewed data at each serial evaluation to assess cognitive status according to published criteria.
Results:
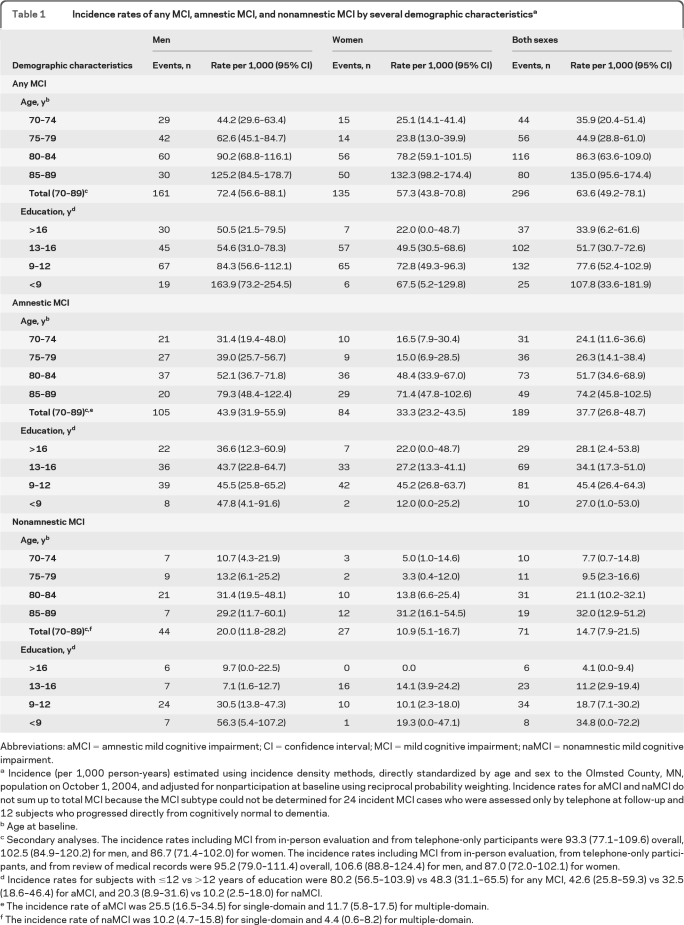
Among 1,450 subjects who were cognitively normal at baseline, 296 developed MCI. The age- and sex-standardized incidence rate of MCI was 63.6 (per 1,000 person-years) overall, and was higher in men (72.4) than women (57.3) and for aMCI (37.7) than naMCI (14.7). The incidence rate of aMCI was higher for men (43.9) than women (33.3), and for subjects with ≤12 years of education (42.6) than higher education (32.5). The risk of naMCI was also higher for men (20.0) than women (10.9) and for subjects with ≤12 years of education (20.3) than higher education (10.2).
Conclusions:
The incidence rates for MCI are substantial. Differences in incidence rates by clinical subtype and by sex suggest that risk factors for MCI should be investigated separately for aMCI and naMCI, and in men and women.
Mild cognitive impairment (MCI) can be a prodromal stage of dementia, and the distribution of incident MCI by age, sex, and other demographic variables is key to guiding etiologic research and prevention. However, incidence rates of MCI have varied across studies because of differences in study design and implementation of diagnostic criteria.1 More importantly, few studies have estimated the incidence of specific subtypes of MCI.1,2 We previously reported a higher prevalence of MCI in men than in women in Olmsted County, MN3; however, this difference has not been assessed prospectively.
Differences in risk of amnestic MCI (aMCI) and nonamnestic MCI (naMCI) in men and women may generate hypotheses about etiologic mechanisms. Thus, we estimated the incidence of MCI and its subtypes using published diagnostic criteria, and investigated some demographic risk factors in a population-based prospective cohort study, the Mayo Clinic Study of Aging (MCSA).3–5
METHODS
Study sample.
We established a population-based cohort to estimate the incidence of MCI in Olmsted County, MN. Details of the study design and participant recruitment are provided elsewhere and are only briefly summarized here.3,4 We used the medical records linkage system of the Rochester Epidemiology Project to construct a sampling frame of Olmsted County residents who were aged 70–89 years on October 1, 2004 (total population = 9,953).6,7 From an age- and sex-stratified random sample of 5,233 subjects, 4,398 subjects were eligible for the study and 2,719 (61.8%) participated in the baseline evaluation either in person (n = 2,050; 46.6%; full participants) or by telephone (n = 669; 15.2%; telephone-only participants).3,4
In-person evaluations.
Each subject underwent an interview by a nurse, a neurologic evaluation by a physician, and extensive cognitive testing by a psychometrist. The interview included questions about memory administered to the participant, and the Clinical Dementia Rating scale8 and the Functional Activities Questionnaire administered to an informant.9 The neurologic evaluation included the Short Test of Mental Status,10 a medical history review, and a complete neurologic examination. Neuropsychological testing was performed using 9 tests to assess impairment in memory, executive function, language, and visuospatial skills domains. The raw scores on each test were transformed into an age-adjusted score using normative data from Mayo's Older American Normative Studies and were scaled to have a mean of 10 and a SD of 3.11 Domain scores were computed by summing the adjusted and scaled scores within a domain, and scaling the combined scores to allow comparisons across domains.3,4 Date of birth, number of years of education, and marital status at baseline were ascertained from the nurse interview.
Diagnostic criteria.
The performance in a cognitive domain was assessed by comparing the domain score of a participant with the score in normal subjects from the Olmsted County population.11 Cognitive impairment was considered if the score was ≥1.0 SD below the mean. However, the final decision about impairment in a cognitive domain was based on a consensus agreement among the examining physician, nurse, and neuropsychologist, taking into account education, prior occupation, visual or hearing deficits, and other information.3
A diagnosis of MCI was determined according to published criteria as follows: cognitive concern by subject (from interview), informant (from the Clinical Dementia Rating scale), nurse, or physician; impairment in 1 or more of the 4 cognitive domains (from cognitive battery); essentially normal functional activities (from the Clinical Dementia Rating scale and the Functional Activities Questionnaire); and absence of dementia.3–5 Subjects with MCI were classified as having aMCI if the memory domain was impaired or naMCI if the memory domain was not impaired but at least one nonmemory domain was impaired. Subjects were also classified as single-domain vs multiple-domain MCI. A diagnosis of dementia was based on DSM-IV criteria.12 Subjects were considered to be cognitively normal if they performed within the normative range and did not meet criteria for MCI or dementia.3–5
Longitudinal follow-up.
We evaluated participants at 15-month intervals using the same protocol for in-person assessments as was used at baseline. Clinical and cognitive findings obtained from previous evaluations were not considered in making a diagnosis during follow-up. Subjects who participated in the baseline assessment but declined evaluation at follow-up were invited to participate in a telephone interview (partial participation) that included the Telephone Interview of Cognitive Status–modified (TICS-m),13,14 the Clinical Dementia Rating scale,8 and the Neuropsychiatric Inventory Questionnaire.15 The diagnosis of cognitively normal, MCI, or dementia for partial participants was made using the same criteria as were used at baseline; however, the MCI subtype could not be determined because they did not undergo the complete neuropsychological testing (n = 24).
For subjects who were examined at baseline but had no follow-up or were lost to follow-up after one or more evaluations, we reviewed the medical records contained in the records linkage system to abstract diagnoses of MCI by a neurologist or diagnoses of dementia by a physician recorded as part of routine medical practice.6,7,16
Telephone-only participants.
Subjects who participated at baseline by telephone only were interviewed using the 50-item TICS-m.4,13,14 Follow-up assessments were also performed using the TICS-m administered via telephone and these subjects were only included in secondary analyses. We used the TICS-m cutoff score ≤31 for cognitive impairment and ≤27 for dementia, based on a validation study conducted on this cohort.14
Statistical analyses.
Persons who were cognitively normal at baseline were considered at risk for incident MCI. The onset of MCI was assigned at the midpoint between the last assessment as cognitively normal and the first-ever assessment as MCI. Subjects who developed dementia without an interim diagnosis of MCI were presumed to have passed through an MCI stage with onset at the midpoint (n = 12). Subjects who refused participation, could not be contacted, or died during follow-up were censored at their last evaluation. We computed the person-years of follow-up as the time from baseline evaluation to onset of MCI, onset of dementia without interim MCI, censoring, or date of last follow-up. Our analyses included only first ever MCI diagnoses, and did not consider subjects who reverted to normal after an initial diagnosis of MCI. However, we also investigated the rate of first ever reversion from MCI back to normal.
We estimated incidence rates by age, sex, education (<9, 9–12, 13–16, and >16 years), and marital status (married, previously married, and never married) using incidence density methods (cases per 1,000 person-years). The incidence rates were directly standardized by age and sex to the Olmsted County population on October 1, 2004 (whenever applicable), and adjusted for nonparticipation at baseline using reciprocal probability weighting in Poisson regression models.3,17–19 Our primary analyses were restricted to full and partial participants only.
We used multivariable proportional hazards models with age as the time scale to assess associations of demographic factors with incident MCI (hazard ratios [HR], 95% confidence intervals [CI]). All models included sex, education (≤12 vs >12 years), and nonparticipation at baseline (using reciprocal probability weighting).3,17–19 Finally, we explored interactions between sex, age, and education. Our secondary analyses included information obtained directly from telephone-only participants and information obtained passively from medical record review for subjects who were lost to follow-up at any time after baseline.6,7,16
Standard protocol approvals, registrations, and patient consent.
The study was approved by the institutional review boards of the Mayo Clinic and of Olmsted Medical Center. Written informed consent was obtained for all participants who were examined as part of the study.
RESULTS
Study sample.
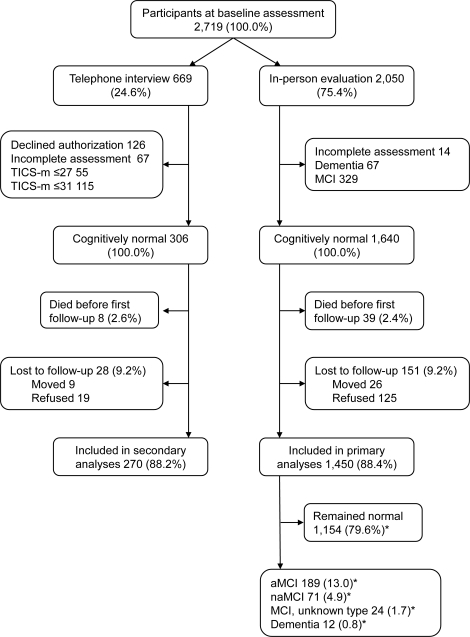
Figure 1 shows the flow chart of the study. Among 1,640 subjects evaluated in person at baseline and found to be free of dementia or MCI, 125 subjects refused follow-up and 26 subjects moved away (total = 151; 9.2%). These 151 subjects were more likely to have lower education than subjects with ≥1 follow-up (55.0% vs 43.2% with ≤12 years of education; p = 0.006); however, they were similar in regard to sex and age. Among 306 telephone-only participants who were free of dementia or cognitive impairment at baseline, 19 subjects refused follow-up and 9 subjects moved away (total = 28, 9.2%). These 28 subjects were similar to participants in regard to age, sex, and number of years of education.
Figure 1. Study flow chart.
*These percentages refer to a total of 1,450 (100.0%) subjects included in the primary analyses. aMCI = amnestic mild cognitive impairment; MCI = mild cognitive impairment; naMCI = nonamnestic mild cognitive impairment; TICS-m = Telephone Interview of Cognitive Status–modified.
Primary incidence analyses.
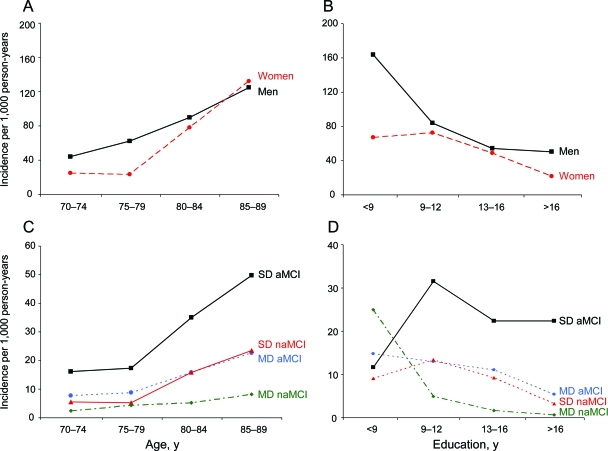
We observed 296 incident cases of MCI over a total of 4,512.9 person-years and with a median follow-up of 3.4 years (interquartile range 2.2–4.0 years). In particular, 177 (12.2%) subjects had 2 assessments, 406 (28.0%) had 3, 721 (49.7%) had 4, and 146 (10.1%) had 5 assessments. The incidence rate of MCI increased with increasing age in both men and women, and was consistently higher in men except in the age group 85–89 years (figure 2A; table 1). The incidence rate of MCI declined with increasing levels of education for both men and women (figure 2B). The incidence rate was higher for aMCI than for naMCI, and increased with age for all 4 clinical subtypes, but the increase was most marked for single-domain aMCI (figure 2C; table 1, footnotes e and f). Finally, the incidence rate declined with increasing levels of education for all 4 subtypes of MCI, but the decline was less marked for single-domain aMCI (figure 2D). The incidence rate of any MCI, aMCI, and naMCI was higher in subjects who were not married at baseline (data not shown).
Figure 2. Incidence rates of mild cognitive impairment (MCI) in Olmsted County, MN.
(A) Age- and sex-specific incidence rates of MCI. (B) Education- and sex-specific incidence rates of MCI. (C) Age- and type-specific incidence rates of MCI. (D) Education- and type-specific incidence rates of MCI. aMCI = amnestic mild cognitive impairment; MD = multiple-domain; naMCI = nonamnestic mild cognitive impairment; SD = single-domain.
Table 1.
Incidence rates of any MCI, amnestic MCI, and nonamnestic MCI by several demographic characteristicsa
Abbreviations: aMCI = amnestic mild cognitive impairment; CI = confidence interval; MCI = mild cognitive impairment; naMCI = nonamnestic mild cognitive impairment.
Incidence (per 1,000 person-years) estimated using incidence density methods, directly standardized by age and sex to the Olmsted County, MN, population on October 1, 2004, and adjusted for nonparticipation at baseline using reciprocal probability weighting. Incidence rates for aMCI and naMCI do not sum up to total MCI because the MCI subtype could not be determined for 24 incident MCI cases who were assessed only by telephone at follow-up and 12 subjects who progressed directly from cognitively normal to dementia.
Age at baseline.
Secondary analyses. The incidence rates including MCI from in-person evaluation and from telephone-only participants were 93.3 (77.1–109.6) overall, 102.5 (84.9–120.2) for men, and 86.7 (71.4–102.0) for women. The incidence rates including MCI from in-person evaluation, from telephone-only participants, and from review of medical records were 95.2 (79.0–111.4) overall, 106.6 (88.8–124.4) for men, and 87.0 (72.0–102.1) for women.
Incidence rates for subjects with ≤12 vs >12 years of education were 80.2 (56.5–103.9) vs 48.3 (31.1–65.5) for any MCI, 42.6 (25.8–59.3) vs 32.5 (18.6–46.4) for aMCI, and 20.3 (8.9–31.6) vs 10.2 (2.5–18.0) for naMCI.
The incidence rate of aMCI was 25.5 (16.5–34.5) for single-domain and 11.7 (5.8–17.5) for multiple-domain.
The incidence rate of naMCI was 10.2 (4.7–15.8) for single-domain and 4.4 (0.6–8.2) for multiple-domain.
Cohort analyses for demographic factors.
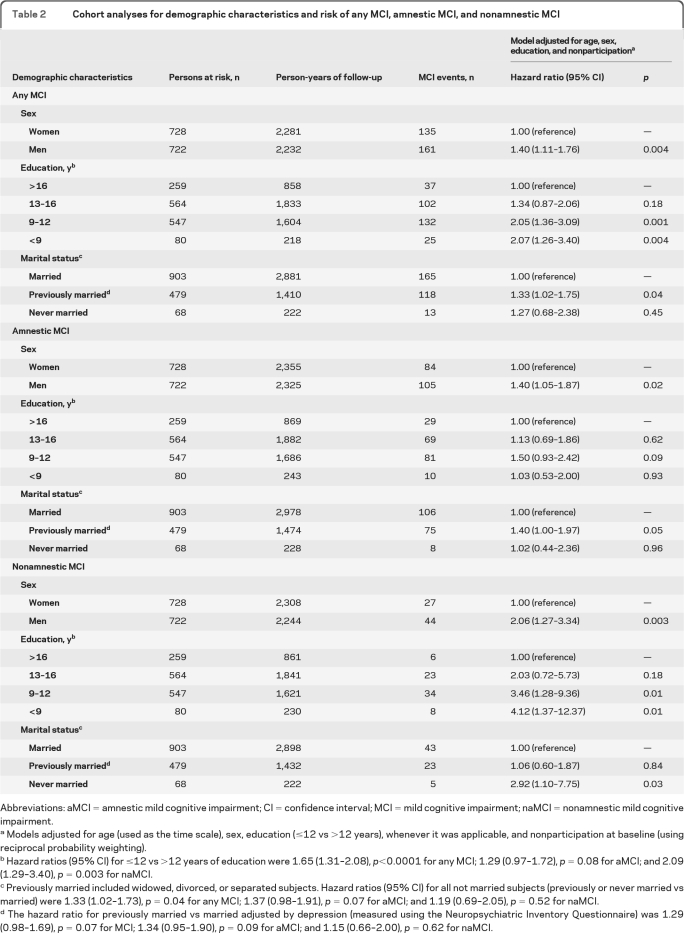
The hazard ratio of any MCI was elevated in men and for subjects with low education or previously married (table 2). The findings were similar for aMCI and naMCI considered separately (table 2). We observed a significant interaction between age and sex suggesting that the difference in risk of any MCI and aMCI between men and women attenuates with increasing age (antagonistic interaction; figure 2A and table e-1 on the Neurology® Web site at www.neurology.org). We also observed a synergistic interaction between sex and education suggesting that men with low education have an unexpectedly high risk of naMCI (figure 2B and table e-1).
Table 2.
Cohort analyses for demographic characteristics and risk of any MCI, amnestic MCI, and nonamnestic MCI
Abbreviations: aMCI = amnestic mild cognitive impairment; CI = confidence interval; MCI = mild cognitive impairment; naMCI = nonamnestic mild cognitive impairment.
Models adjusted for age (used as the time scale), sex, education (≤12 vs >12 years), whenever it was applicable, and nonparticipation at baseline (using reciprocal probability weighting).
Hazard ratios (95% CI) for ≤12 vs >12 years of education were 1.65 (1.31–2.08), p<0.0001 for any MCI; 1.29 (0.97–1.72), p = 0.08 for aMCI; and 2.09 (1.29–3.40), p = 0.003 for naMCI.
Previously married included widowed, divorced, or separated subjects. Hazard ratios (95% CI) for all not married subjects (previously or never married vs married) were 1.33 (1.02–1.73), p = 0.04 for any MCI; 1.37 (0.98–1.91), p = 0.07 for aMCI; and 1.19 (0.69–2.05), p = 0.52 for naMCI.
The hazard ratio for previously married vs married adjusted by depression (measured using the Neuropsychiatric Inventory Questionnaire) was 1.29 (0.98–1.69), p = 0.07 for MCI; 1.34 (0.95–1.90), p = 0.09 for aMCI; and 1.15 (0.66–2.00), p = 0.62 for naMCI.
Secondary analyses.
MCI incidence rates were higher when we included in our analyses data from telephone-only participants (93.3 vs 63.6; table 1, footnote c); however, they showed little incremental change when we included data from telephone-only participants plus data passively ascertained from medical records of subjects lost to follow-up (95.2 vs 93.3; table 1, footnote c). The higher incidence in men than in women persisted in both secondary analyses.
Stability of MCI diagnoses.
Of the 284 subjects who developed MCI, 148 had at least one subsequent follow-up and 98 of them remained MCI or progressed to dementia (66.2%) whereas 50 reverted to normal (33.8%; first ever reversion to normal). After accounting for death or losses to follow-up, the reversion rate from MCI to normal was 12.3% per year. The reversion rate was higher for subjects who were less impaired at the time of MCI diagnosis (as measured by global cognitive score, memory score, and Functional Activity Questionnaire).
DISCUSSION
MCI is a heterogeneous clinical entity with incidence rates that vary substantially by age, sex, and subtype. The MCSA showed a higher incidence of MCI in men compared to women that is contrary to a higher risk of dementia in women reported by some studies20 and contrary to a similar incidence rate of dementia in men and women observed in this same Olmsted County population.21 The higher risk of MCI in men was consistent for both aMCI and naMCI. In addition, our study showed higher incidence rates for aMCI than for naMCI. Finally, we observed higher rates of MCI in persons with lower education and who were not married. The observed higher incidence of MCI in men is consistent with the higher prevalence of MCI in men observed in this same Olmsted County population.3 Similarly, the increased incidence rates in subjects with lower education and in subjects who were not married were consistent with the findings for prevalence in our study.3
We were unable to directly compare our incidence rates with some of the population-based studies previously published for several reasons.1 First, unlike MCSA, age- or age- and sex-specific incidence rates were not reported for some studies.2,22,23 Second, the study participants were much younger in one study,22 or had a much different age distribution in another.23 Third, one study was conducted in a healthy cohort where MCI risk factors were an exclusion criterion.24 Finally, some studies only investigated aMCI.22–27
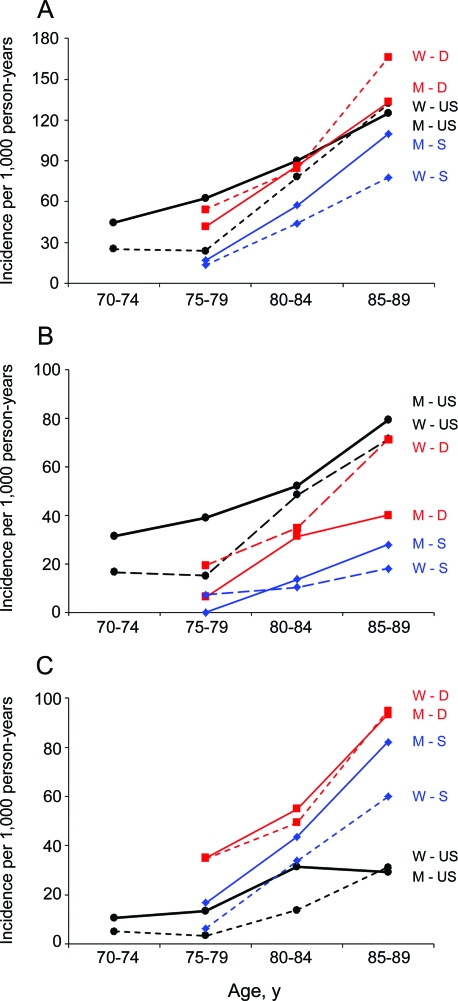
Figure 3 shows a comparison of age- and sex-specific incidence rates from our study and 2 other studies that provided rates for aMCI and naMCI separately: the Leipzig Longitudinal Study of the Aged from Germany,28 the Kungsholmen Project from Sweden,29 and the MCSA from the United States (present study). For aMCI, our incidence rates were higher than in the Swedish study but similar to the rates in the German study (figure 3B). The sex pattern in our study was opposite to the German study but was consistent with the Swedish study. For naMCI, our incidence rates were lower than the rates in both the German and the Swedish studies (figure 3C). The sex pattern was similar across the 3 studies; however, the sex differences in risk were small in the German study.
Figure 3. Comparison of age- and sex-specific incidence rates for mild cognitive impairment (MCI) across studies.
(A) Incidence rates for any MCI. (B) Incidence rates for amnestic mild cognitive impairment (aMCI). (C) Incidence rates for nonamnestic mild cognitive impairment (naMCI). The dashed lines represent rates for women (W), and the solid lines represent rates for men (M). The red lines represent the Leipzig Longitudinal Study of the Aged from Germany (D)28; the black lines represent the Mayo Clinic Study of Aging from the United States, present study (US); the blue lines represent the Kungsholmen Project from Sweden (S).29 For the Swedish study, we considered their definition of “other cognitive impairment no dementia” as equivalent to naMCI.
The variability in incidence rates across studies may, in part, be due to differences in study design and implementation of criteria for MCI. Studies that relied solely on the Mini-Mental State Examination or that used an algorithmic categorization based on neuropsychological tests, studies that required a memory complaint from the participant (as in the earlier MCI criteria),30 studies that did not collect information from an informant, studies that did not require the judgment of a clinician, or studies that applied MCI criteria retrospectively to previously collected data may have yielded different patterns. For example, the lower rates of naMCI in the MCSA may be due to the use of a consensus panel diagnosis rather than an algorithmic diagnosis. MCI diagnoses that rely solely on a neuropsychological algorithm will necessarily overestimate the frequency of deficits in several nonamnestic domains compared to a single memory domain. The variability of MCI incidence rates across studies underscores the need to standardize approaches to MCI diagnosis.1
The demographic predictors of incident MCI other than sex that were observed in the MCSA were consistent with those in some other studies. Older age was associated with an increased risk of MCI in several,2,22,28,29,31 but not in all studies.25,26,32 Low education was associated with increased risk in some studies.28,31 Amnestic MCI was associated with older age27,29 and low education.22,27 Nonamnestic MCI was associated with low education.2
Our findings for marital status are consistent with findings in the German study and do not appear to be due to a diagnostic bias.28 Subjects who had lost their spouse at baseline were expected to experience an underdiagnosis or a delayed diagnosis of MCI because of lack of an optimal informant. Indeed, previously married subjects had significantly higher scores on the Clinical Dementia Rating scale or the Functional Activity Questionnaire at the time of first diagnosis of MCI than married subjects (data not shown). The biological basis of this association remains uncertain. Subjects who had lost their spouse had a significantly higher rate of depression than married subjects at baseline15; however, adjustment for depression at baseline did not modify the association of marital status with MCI incidence noticeably (table 2, footnote d). Therefore, other mechanisms such as changes in dietary habits or lack of daily support in managing existing diseases (e.g., compliance with medications) may also be involved.
In our study, we observed 2 interesting patterns of interaction. The antagonistic interaction of sex and age was significant for aMCI but not for naMCI, whereas the synergistic interaction of sex and education was significant for naMCI but not for aMCI. One possible interpretation is that men with low education have behaviors and life events that may expose them to several risk factors leading to naMCI. Alternatively, men with low education may have been at cognitive disadvantage early in life and their low education was already a marker of limited cognitive reserve.33 These 2 patterns of interaction between sex and age and sex and education seem to differ for aMCI and naMCI, suggesting that risk factors for MCI should be investigated considering both type of MCI and sex separately. Analyses that pool men with women and aMCI with naMCI may fail to identify specific risk factors because the specific associations would be diluted away.
We observed a reversion rate from MCI to normal of approximately 12.3% per year. This rate is lower than or similar to previous estimates,2,34,35 and does not account for subjects who will revert back a second time from normal to MCI in an extended follow-up. Because our consensus diagnoses were made without knowledge of the performance at prior visits, our estimates of conversion to MCI and reversion from MCI were not biased by clinical expectations.
Our study has several strengths. First, it is a large population-based study that was specifically designed to investigate the incidence of MCI and its subtypes using published criteria.5 Second, the comprehensive clinical evaluation and the consensus approach provided reliable diagnoses of MCI and MCI subtypes; in particular, we did not use a multistage evaluation where subjects who screened negative were not fully evaluated. Third, repeated assessments of cognitive status were performed without knowledge of the cognitive status at the preceding evaluation; thus we were able to investigate the stability of MCI diagnosis over time. Finally, the passive ascertainment of incident events through the medical records linkage system for subjects who were lost to follow-up provided more accurate estimates of MCI incidence.6,7,16
Our study had some limitations. First, nonparticipants at the baseline evaluation were more likely to be men, older, or to have type 2 diabetes mellitus and a high Charlson Comorbidity Index compared to participants, as reported elsewhere.4 However, when we adjusted for potential nonparticipation bias using propensity scores, the unadjusted and adjusted estimates were similar. Second, the population of Olmsted County is primarily of European ancestry, thus the findings may not apply to other ethnic groups.
Supplementary Material
GLOSSARY
- aMCI
amnestic mild cognitive impairment
- CI
confidence interval
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- HR
hazard ratio
- MCI
mild cognitive impairment
- MCSA
Mayo Clinic Study of Aging
- naMCI
nonamnestic mild cognitive impairment
- TICS-m
Telephone Interview of Cognitive Status–modified.
Footnotes
Editorial, page 300
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Roberts: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding, administrative, technical, or material support. Dr. Geda: drafting/revising the manuscript, study concept or design, acquisition of data. Dr. Knopman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. R.H. Cha: analysis or interpretation of data, statistical analysis. Dr. Pankratz: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis, obtaining funding. Dr. Boeve: drafting/revising the manuscript, acquisition of data. Dr. Tangalos: drafting/revising the manuscript, acquisition of data. Dr. Ivnik: study concept or design, acquisition of data. Dr. Rocca: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, statistical analysis, obtaining funding, administrative, technical, or material support. Dr. Petersen: drafting/revising the manuscript, obtaining funding.
DISCLOSURE
Dr. Roberts receives research support from the NIH and Abbott. Dr. Geda receives research support from the NIH, Mayo CTSA, the RWJ Foundation (Harold Amos Scholar), and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer's Disease Research Program. Dr. Knopman serves as Deputy Editor for Neurology®; has served on a data safety monitoring board for Eli Lilly and Company; has served as a consultant for Elan/Janssen AI; is an investigator in clinical trials sponsored by Elan/Janssen AI, Baxter International Inc., and Forest Laboratories, Inc.; and receives research support from the NIH. R.H. Cha and Dr. Pankratz report no disclosures. Dr. Boeve has served as a consultant to GE Healthcare; receives publishing royalties for The Behavioral Neurology of Dementia (Cambridge University Press, 2009); and receives research support from Cephalon, Inc., Allon Therapeutics, Inc., the NIH/NIA, the Alzheimer's Association, and the Mangurian Foundation. Dr. Tangalos serves on a Data Safety Monitoring Board for Eli Lilly and Company; serves as a consultant for Purdue Pharma and Amgen; serves on the editorial boards of MD Net Guide, Journal of the American Medical Directors Association, and IM News; has received honoraria for slide development from Takeda Pharmaceutical Company Limited, Novartis, and Ortho Biotech Products, L.P.; receives research support from Baxter International, Inc. and Elan Corporation; and serves as a consultant to Novartis. Dr. Ivnik serves on the editorial boards of The Clinical Neuropsychologist and Aging, Neuropsychology, and Cognition; receives publishing royalties for Clinical Interpretation of the WAIS-III and WMS-III (Academic Press, 2003); and receives research support from the NIH/NIA. Dr. Rocca receives research support from the NIH. Dr. Petersen serves on scientific advisory boards for the Alzheimer's Association, the National Advisory Council on Aging (NIA), Elan/Janssen AI, Pfizer Inc (Wyeth), and GE Healthcare; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003); serves as a consultant for Elan/Janssen AI and GE Healthcare; and receives research support from the NIH/NIA.
REFERENCES
- 1. Luck T, Luppa M, Briel S, Riedel-Heller SG. Incidence of mild cognitive impairment: a systematic review. Dement Geriatr Cogn Disord 2010; 29: 164– 175 [DOI] [PubMed] [Google Scholar]
- 2. Manly JJ, Tang MX, Schupf N, et al. Frequency and course of mild cognitive impairment in a multiethnic community. Ann Neurol 2008; 63: 494– 506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology 2010; 75: 889– 897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008; 30: 58– 69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med 2004; 256: 183– 194 [DOI] [PubMed] [Google Scholar]
- 6. Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc 1996; 71: 266– 274 [DOI] [PubMed] [Google Scholar]
- 7. St Sauver JL, Grossardt BR, Yawn BP, et al. Use of a medical records linkage system to enumerate a dynamic population over time: The Rochester Epidemiology Project. Am J Epidemiol 2011; 173: 1059– 1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 1993; 43: 2412– 2414 [DOI] [PubMed] [Google Scholar]
- 9. Pfeffer RI, Kurosaki TT, Harrah CH, Jr, et al. Measurement of functional activities in older adults in the community. J Gerontol 1982; 37: 323– 329 [DOI] [PubMed] [Google Scholar]
- 10. Kokmen E, Smith GE, Petersen RC, et al. The Short Test of Mental Status: Correlations with standardized psychometric testing. Arch Neurol 1991; 48: 725– 728 [DOI] [PubMed] [Google Scholar]
- 11. Ivnik RJ, Malec JF, Smith GE, et al. Mayo's Older Americans Normative Studies: WAIS-R, WMS-R and AVLT norms for ages 56 through 97. Clin Neuropsychol 1992; 6 (suppl): 1– 104 [Google Scholar]
- 12. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-IV), 4th ed Washington, DC: American Psychiatric Association; 1994 [Google Scholar]
- 13. Welsh KA, Breitner JCS, Magruder-Habib KM. Detection of dementia in the elderly using telephone screening of cognitive status. Neuropsychiatry Neuropsychol Behav Neurol 1993; 6: 103– 110 [Google Scholar]
- 14. Knopman DS, Roberts RO, Geda YE, et al. Validation of the Telephone Interview for Cognitive Status-modified in subjects with normal cognition, mild cognitive impairment, or dementia. Neuroepidemiology 2010; 34: 34– 42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaufer DI, Cummings JL, Ketchel P, et al. Validation of the NPI-Q, a brief clinical form of the Neuropsychiatric Inventory. J Neuropsychiatry Clin Neurosci 2000; 12: 233– 239 [DOI] [PubMed] [Google Scholar]
- 16. Knopman DS, Petersen RC, Rocca WA, et al. Passive case-finding for Alzheimer's disease and dementia in two U.S. communities. Alzheimers Dement 2011; 7: 53– 60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kessler RC, Little RJ, Groves RM. Advances in strategies for minimizing and adjusting for survey nonresponse. Epidemiol Rev 1995; 17: 192– 204 [DOI] [PubMed] [Google Scholar]
- 18. D'Agostino RB, Jr, Rubin DB. Estimating and using propensity scores with partially missing data. J Am Stat Assoc 2000; 95: 749– 759 [Google Scholar]
- 19. Geda YE, Roberts RO, Knopman DS, et al. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and normal cognitive aging: population-based study. Arch Gen Psychiatry 2008; 65: 1193– 1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fratiglioni L, Launer LJ, Andersen K, et al. Incidence of dementia and major subtypes in Europe: a collaborative study of population-based cohorts: Neurologic Diseases in the Elderly Research Group. Neurology 2000; 54 (11 suppl 5): S10– S15 [PubMed] [Google Scholar]
- 21. Edland SD, Rocca WA, Petersen RC, et al. Dementia and Alzheimer disease incidence rates do not vary by sex in Rochester, Minn. Arch Neurol 2002; 59: 1589– 1593 [DOI] [PubMed] [Google Scholar]
- 22. Tervo S, Kivipelto M, Hanninen T, et al. Incidence and risk factors for mild cognitive impairment: a population-based three-year follow-up study of cognitively healthy elderly subjects. Dement Geriatr Cogn Disord 2004; 17: 196– 203 [DOI] [PubMed] [Google Scholar]
- 23. Verghese J, LeValley A, Derby C, et al. Leisure activities and the risk of amnestic mild cognitive impairment in the elderly. Neurology 2006; 66: 821– 827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chaves ML, Camozzato AL, Godinho C, et al. Incidence of mild cognitive impairment and Alzheimer disease in Southern Brazil. J Geriatr Psychiatry Neurol 2009; 22: 181– 187 [DOI] [PubMed] [Google Scholar]
- 25. Larrieu S, Letenneur L, Orgogozo JM, et al. Incidence and outcome of mild cognitive impairment in a population-based prospective cohort. Neurology 2002; 59: 1594– 1599 [DOI] [PubMed] [Google Scholar]
- 26. Busse A, Bischkopf J, Riedel-Heller SG, Angermeyer MC. Mild cognitive impairment: prevalence and incidence according to different diagnostic criteria: results of the Leipzig Longitudinal Study of the Aged (LEILA75+). Br J Psychiatry 2003; 182: 449– 454 [PubMed] [Google Scholar]
- 27. Solfrizzi V, Panza F, Colacicco AM, et al. Vascular risk factors, incidence of MCI, and rates of progression to dementia. Neurology 2004; 63: 1882– 1891 [DOI] [PubMed] [Google Scholar]
- 28. Luck T, Luppa M, Briel S, et al. Mild cognitive impairment: incidence and risk factors: results of the Leipzig Longitudinal Study of the Aged. J Am Geriatr Soc 2010; 58: 1903– 1910 [DOI] [PubMed] [Google Scholar]
- 29. Caracciolo B, Palmer K, Monastero R, et al. Occurrence of cognitive impairment and dementia in the community: a 9-year-long prospective study. Neurology 2008; 70: 1778– 1785 [DOI] [PubMed] [Google Scholar]
- 30. Petersen RC, Doody R, Kurz A, et al. Current concepts in mild cognitive impairment. Arch Neurol 2001; 58: 1985– 1992 [DOI] [PubMed] [Google Scholar]
- 31. Tyas SL, Salazar JC, Snowdon DA, et al. Transitions to mild cognitive impairments, dementia, and death: findings from the Nun Study. Am J Epidemiol 2007; 165: 1231– 1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ravaglia G, Forti P, Montesi F, et al. Mild cognitive impairment: epidemiology and dementia risk in an elderly Italian population. J Am Geriatr Soc 2008; 56: 51– 58 [DOI] [PubMed] [Google Scholar]
- 33. Stern Y, Gurland B, Tatemichi TK, et al. Influence of education and occupation on the incidence of Alzheimer's disease. JAMA 1994; 271: 1004– 1010 [PubMed] [Google Scholar]
- 34. Boyle PA, Buchman AS, Barnes LL, Bennett DA. Effect of a purpose in life on risk of incident Alzheimer disease and mild cognitive impairment in community-dwelling older persons. Arch Gen Psychiatry 2010; 67: 304– 310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Boyle PA, Buchman AS, Wilson RS, et al. The APOE epsilon4 allele is associated with incident mild cognitive impairment among community-dwelling older persons. Neuroepidemiology 2010; 34: 43– 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.