Abstract
Objective:
To determine the relationship between β-amyloid (Aβ) load as measured by [11C]–Pittsburgh compound B (PiB) PET and cognitive function in cognitively normal older adults.
Methods:
We studied 408 cognitively normal older adults who participated in the population-based Mayo Clinic Study of Aging (MCSA) from January 2009 through March 2011. The participants underwent PiB PET and neuropsychometric testing within 6 months. The association between PiB retention and cognitive function was measured by partial correlation and an interaction with APOE status was tested using linear regression after adjusting for age, sex, and education.
Results:
Higher PiB retention was associated with cognitive performance (Spearman partial r = −0.18; p < 0.01), specifically the memory, language, attention/executive, and visual-spatial processing domains in the whole group of participants. The association between PiB retention and cognition was modified by the APOE status on linear regression analysis even after controlling for the differences in the distribution of PiB values among APOE ϵ4 carriers and noncarriers (p = 0.02). Cognitive performance was associated with the Aβ deposition in the frontal, temporal, and parietal lobe association cortices in APOE ϵ4 carriers on SPM analysis (p < 0.001).
Conclusion:
There is a modest association between PiB retention and cognitive function in cognitively normal older adults and this relationship between Aβ load and cognitive function is modified by APOE status. Whereas Aβ load is associated with greater cognitive impairment in APOE ϵ4 carriers, the cognitive function in APOE ϵ4 noncarriers is influenced less by the Aβ load, suggesting that APOE isoforms modulate the harmful effects of Aβ on cognitive function. Neurology® 2012;78:232–240
Identifying individuals with preclinical Alzheimer disease (AD) is critical for preventive clinical trials.1 While 30% of cognitively normal older adults have increased β-amyloid (Aβ) deposition on PET imaging with amyloid ligand [11C]–Pittsburgh Compound B (PiB),2,3 the effects of Aβ load on cognitive function in cognitively normal individuals has been equivocal.4
The APOE ϵ4 allele increases the risk for AD and lowers the age at onset in a gene-dose-dependent manner.5 APOE isoforms differentially regulate Aβ clearance with APOE ϵ4 having a greater disruptive effect on Aβ clearance than either APOE ϵ3 or APOE ϵ2.6,7 In line with these observations, cognitively normal carriers of APOE ϵ4 have greater Aβ load than noncarriers at a given age,3,8–11 and Aβ load increases the risk of cognitive decline in cognitively normal individuals3 or individuals without dementia.12 Because both Aβ load and APOE ϵ4 increase the risk of AD, we hypothesized that APOE status modifies the relationship between Aβ load and cognitive performance in the early stages of Aβ pathology in older adults.
Our primary objective was to determine the association between Aβ load and cognitive function in a population-based sample of cognitively normal older adults. We further investigated the effects of APOE isoforms on the association between Aβ load and cognitive performance. Finally, we performed voxel-based analysis to determine the regional pattern of Aβ deposition that is associated with cognitive performance in cognitively normal older adults.
METHODS
Participants.
We studied 408 cognitively normal older adults who participated in the Mayo Clinic Study of Aging (MCSA) from January 2009 through March 2011. MCSA is a prospective population-based study of older adults without dementia.13 Individuals participating in the MCSA undergo clinical examinations, APOE genotyping, a battery of neuropsychological tests, and MRI examinations every 15 months. After completion of each evaluation, a consensus committee meeting is held involving the behavioral neurologists, neuropsychologists, and nurses who evaluated the subjects to assign a clinical diagnosis to the participant. PET studies have been offered to all MCSA participants since January 2009 and are performed within 6 months of MRI and cognitive testing (figure e-1 on the Neurology® Web site at www.neurology.org).
Inclusion criterion was normal cognitive function which was judged according to the published criteria from normative data.14 Subjects who had a contraindication for MRI such as a pacemaker, or who were unable to participate in imaging studies because of severe illness, were excluded. Subjects were not excluded due to neurologic, psychiatric, or systemic illnesses to preserve the representativeness of the study sample as much as possible.
Participants with APOE genotype 2/2, 2/3 were labeled ϵ2 carriers, genotype 3/3 was labeled ϵ3 homozygote, genotypes 3/4 and 4/4 were labeled ϵ4 carriers. Since the impact of ϵ2/4 on AD risk remains unclear, data for this genotype were treated as a separate group.
Standard protocol approvals, registrations, and patient consents.
This study was approved by the Mayo Clinic Institutional Review Board, and informed consent for participation was obtained from every participant.
Neuropsychological testing.
Memory was evaluated by free recall retention scores computed after a 30-minute delay for the Wechsler Memory Scale—Revised Logical Memory and Visual Reproduction subtests and the Rey Auditory Verbal Learning Test. Language tests measured naming to confrontation (i.e., the Boston Naming Test) and category fluency (i.e., naming animals, fruits, and vegetables). The attention/executive measures included the Trail Making Test part B, and the Wechsler Adult Intelligence Scale—Revised (WAIS-R) Digit Symbol subtest. Visual-spatial processing was examined by the WAIS-R Picture Completion and Block Design subtests. All tests were administered by experienced psychometrists and supervised by a clinical neuropsychologist (R.J.I.). All raw neuropsychological test scores were standardized in the entire MCSA sample.14 We obtained individual domain standard (Z) scores by averaging the z scores of the individual tests included in each domain. A global cognitive function Z score was derived by averaging the 4 standardized cognitive domain scores.
MRI and PET acquisition.
MRIs were performed at 3 Tesla using an 8-channel phased array coil (GE, Milwaukee, WI). A 3-dimensional high-resolution magnetization-prepared rapid gradient echo (MPRAGE) acquisition was performed for anatomic segmentation and labeling of PiB PET scans. PET images were acquired using a PET/CT scanner (DRX; GE Healthcare) operating in 3-dimensional mode. The subjects were injected with 292–729 MBq [11C] PiB. After a 40-minute uptake period, a 20-minute PiB scan was obtained. The PiB-PET acquisition consisted of 4 5-minute dynamic frames, acquired from 40 to 60 minutes after injection. The pixel size for PET images was 1.0 mm and the slice thickness was 3.3 mm.
Analysis of PiB PET images.
PiB-PET quantitative image analysis was performed using the fully automated image processing pipeline which has previously been described in detail.15 Briefly, the method includes registering PET images to 3-dimensional MPRAGE for gray matter (GM) sharpening on SPM5. PiB-PET cortical ratio images are calculated by dividing each PiB-PET GM voxel value by the median value in the cerebellar GM region in the subject's MRI space. The global cortical PiB retention was determined by calculating the median value of the PiB-PET GM ratio from all voxels in the bilateral parietal, posterior cingulate, precuneus, temporal, prefrontal, orbitofrontal, and anterior cingulate GM regions as defined in the in-house modified anatomic labeling atlas where the average is weighted by region of interest size.15
Voxel-based analysis was performed in order to determine the topographic pattern of correlations between PiB retention and global cognitive function in cognitively normal older adults. To do so, each subject's MRI scan was spatially normalized to a custom template16 using the unified segmentation model of SPM5. The resulting deformation was applied to the PiB retention ratio images in native space in order to warp the segmented native PiB retention ratio images to the customized template. Voxel-based correlations between PiB retention ratio and global cognitive performance were assessed in SPM5. Statistical maps were displayed at a significance value of p < 0.001, uncorrected for multiple comparisons.
Statistical analysis.
For our primary analysis, we summarize associations between cognition and PiB using Spearman partial rank-order correlations which we denote by “partial rs.”17 This statistic can be thought of as a nonparametric correlation between 2 variables (e.g., PiB retention and cognitive performance) after “partialling out,” or controlling for, possible confounders. We report partial Spearman rank-order correlations adjusting for age, sex, and education among all subjects. Because the PiB distributions were highly skewed, we used partial rs to quantify associations since this method does not assume normally distributed data.17 The non-normal distribution of PiB also motivated our use of the Kruskal-Wallis test to perform nonparametric analysis of variance (ANOVA) to test for differences by APOE status.
Next, the effect of APOE genotype on the association between PiB retention and cognitive function was evaluated using linear regression models in which we included an interaction between APOE genotype and the natural log of PiB and adjust linearly for age, sex, and education. We test for associations using so-called sequential ANOVA in which we report 3 p values: 1) the significance of the log-transformed PiB variable given that age, sex, and education are in the model; 2) whether adding APOE to this model is significant; and 3) whether adding an interaction between log-transformed PiB and APOE adds significantly to the model.
As a secondary analysis intended to isolate the effect of carrying an APOE ϵ4 allele, we performed 1:1 matching of APOE ϵ4 carriers to noncarriers matching on global cortical PiB, age, sex, and education. We then performed the regression analysis and sequential ANOVA as described above. We chose APOE ϵ4 carriers as the reference group for the matched analysis because they manifest the full dynamic range of PiB retention in cognitively normal older adults and it is widely accepted that APOE ϵ4 carriers are at a higher risk for AD. We grouped the APOE ϵ2 carriers and ϵ3 homozygotes for this analysis because APOE ϵ2 carriers did not represent the full dynamic range of PiB retention in cognitively normal older adults; therefore matching APOE ϵ2 carriers to ϵ4 carriers was not possible. By performing the matched analysis we isolate the effect of having an APOE ϵ4 allele for a given level of global cortical PiB.
RESULTS
Study sample.
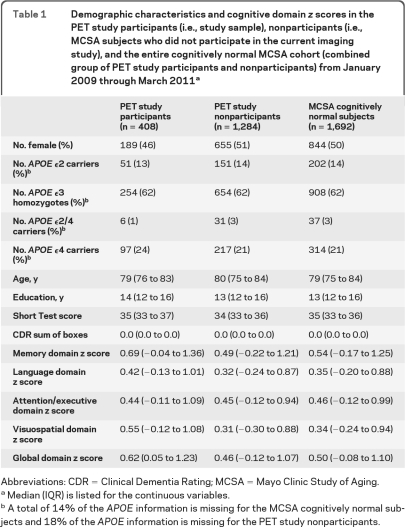
Characteristics of the study sample are described in table 1. The demographic features of the study sample (participants) were on average similar to the MCSA subjects who were evaluated from January 2009 through March 2011 but did not participate in the MRI and PET studies or did not undergo genetic testing (nonparticipants). The only exception was that the fraction of women participants was less than the fraction of women nonparticipants. The cognitive performance of the participants was slightly better than the nonparticipants specifically in memory, visual-spatial processing, and language function.
Table 1.
Demographic characteristics and cognitive domain z scores in the PET study participants (i.e., study sample), nonparticipants (i.e., MCSA subjects who did not participate in the current imaging study), and the entire cognitively normal MCSA cohort (combined group of PET study participants and nonparticipants) from January 2009 through March 2011a
Abbreviations: CDR = Clinical Dementia Rating; MCSA = Mayo Clinic Study of Aging.
Median (IQR) is listed for the continuous variables.
A total of 14% of the APOE information is missing for the MCSA cognitively normal subjects and 18% of the APOE information is missing for the PET study nonparticipants.
Correlations between Aβ load and cognitive function within the entire cohort.
In this population-based sample of cognitively normal older adults, the median (interquartile range) of the global cortical PiB retention ratio was 1.39 (1.32 to 1.67). According to the typically used cutoff of 1.50,18 139 out of 408 (34%; 95% confidence interval = 29%–39%) of the subjects were classified as PiB positive. We did not dichotomize the subjects into high or low PiB retention groups and treated cortical PiB retention ratio as a continuous variable in all analysis. Higher global cortical PiB retention ratio was associated with worse overall cognitive performance (partial rs = −0.18; p < 0.01) specifically in the memory (partial rs = −0.14; p < 0.01), attention/executive (partial rs = −0.12; p = 0.02), language (partial rs = −0.13; p = 0.01), and visual-spatial processing (partial rs = 0.13; p < 0.01) functions after adjusting for age, sex, and education.
Effect of APOE ϵ4 allele on the association between Aβ load and cognitive function.
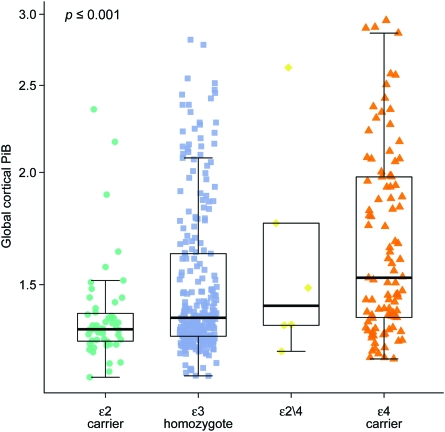
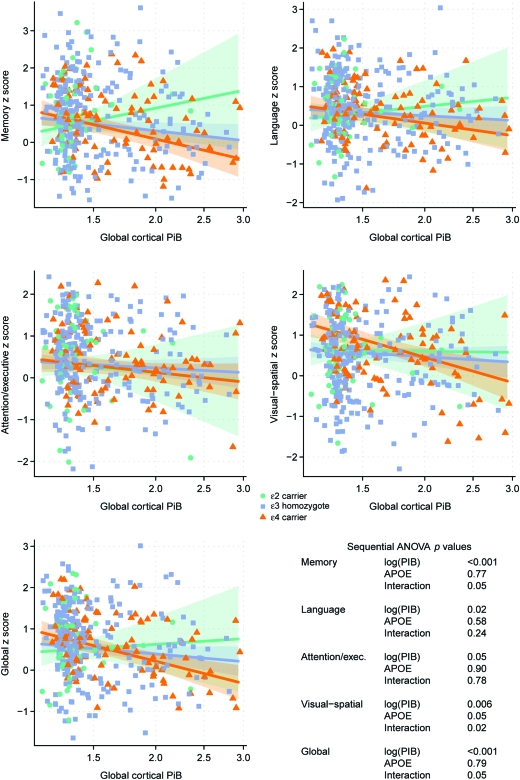
Global cortical PiB retention ratio increased from APOE ϵ2 carriers to APOE ϵ3 homozygotes to ϵ2/4 genotype, with APOE ϵ4 carriers having the highest levels of PiB retention (p < 0.001) (figure 1). APOE ϵ2/4 genotype was excluded from the 3-level linear regression analysis. In the linear regression analysis, there was an interaction with APOE status for the associations between global cortical PiB retention and global cognition (p = 0.05), memory (p = 0.05), and visual-spatial processing (p = 0.02). The association between higher PiB retention and lower cognitive performance was strongest in the APOE ϵ4 carriers and weakest in APOE ϵ2 carriers with APOE ϵ3 homozygotes being in-between the 2 groups (figure 2). We found no relationship between APOE status and cognitive function after adjusting for age, sex, and education (p > 0.09) (table e-1).
Figure 1. Global cortical Pittsburgh compound B (PiB) retention by APOE status.
Figure 2. Associations between global cortical Pittsburgh compound B (PiB) retention and standardized cognitive scores according to APOE status.
The trend lines indicate estimated mean cognition as a function of log-transformed PiB with green representing APOE ϵ2 carriers (n = 51), blue representing APOE ϵ3 homozygotes (n = 254), and orange representing APOE ϵ4 carriers (n = 97). Shaded regions indicate 95% confidence intervals. The p values from the sequential analysis of variance (ANOVA) are listed for each cognitive domain.
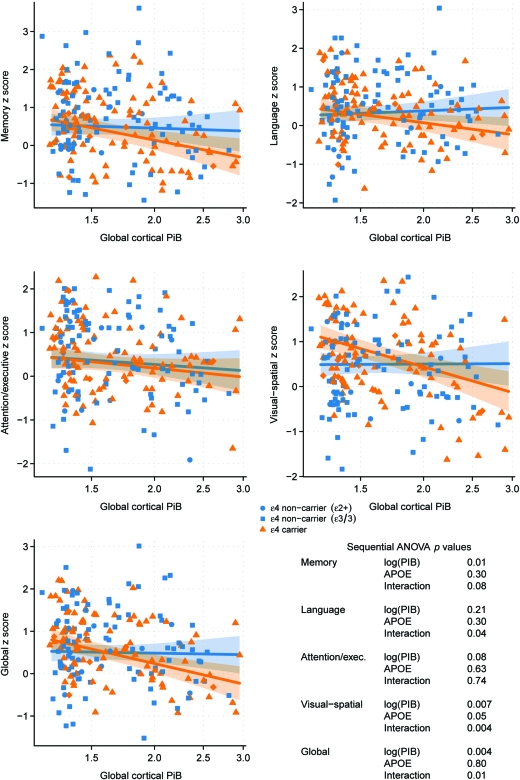
In our secondary analysis, we matched 97 ϵ4 carriers to ϵ4 noncarriers using global cortical PiB, age, sex, and education as matching criteria in order to isolate the APOE effect. A significant interaction with APOE ϵ4 carrier status was found for the associations between global cortical PiB retention and global cognition (p = 0.01), language (p = 0.04), visual-spatial processing (p < 0.01), and a trend with memory function (p = 0.08), but not with attention/executive function (p = 0.74) (figure 3). Overall, the linear regression analysis both in the whole group and in the matched group on PiB retention showed that APOE genotype modified the relationship between cortical PiB retention and global cognition.
Figure 3. Associations between global cortical Pittsburgh compound B (PiB) retention and standardized cognitive scores in APOE ϵ4 carriers and a matched group of APOE ϵ4 noncarriers.
The trend lines indicate estimated mean cognition as a function of log-transformed PiB with orange representing APOE ϵ4 carriers (n = 97) and blue representing APOE ϵ4 noncarriers (n = 97) matched to the APOE ϵ4 carriers on age, sex, education, and PiB retention ratio. Shaded regions indicate 95% confidence intervals. The p values from the sequential analysis of variance (ANOVA) are listed for each cognitive domain.
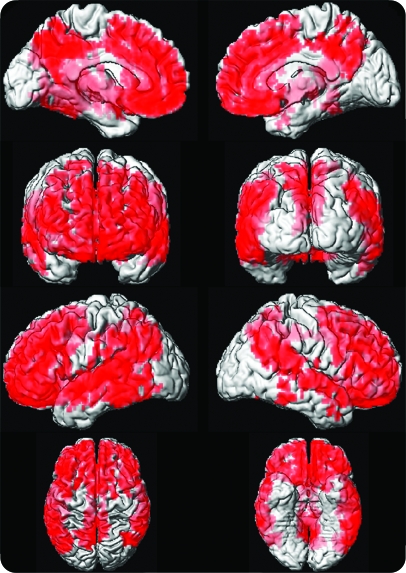
Voxel-based analysis in APOE ϵ4 carriers demonstrated that lower global cognitive performance was associated with higher PiB retention in the frontal, temporal, and parietal association cortices (p < 0.001) (figure 4). When we correlated PiB retention in specific cortical atlas regions and cognitive Z scores, we found correlations between frontal, temporal, parietal, and posterior cingulate/precuneus PiB retention ratio and global cognition, memory, language, and visual-spatial processing (p < 0.01) in APOE ϵ4 carriers. A weaker correlation between higher cingulate/precuneus PiB retention and lower memory function (p = 0.05) and global cognition (p = 0.03) was present in APOE ϵ4 noncarriers.
Figure 4. Correlations between cortical Pittsburgh compound B (PiB) retention and global cognitive performance score in APOE ϵ4 carriers.
Voxelwise analysis demonstrates that global cognitive performance is associated with PiB retention in the frontal, temporal, and parietal lobe association cortices (p < 0.001).
DISCUSSION
The current study showed that greater PiB retention on PET is associated with worse cognitive function in a population-based sample of cognitively normal older adults. The decline in cognition was observed to be steepest in APOE ϵ4 carriers and least steep in APOE ϵ2 carriers. When we stratified the cognitively normal group into APOE ϵ4 carriers and noncarriers, it was apparent that the association between PiB retention and cognitive function was primarily driven by the APOE ϵ4 carriers on matched analysis in which the dynamic range of PiB retention ratio was similar by design.
We found modest but significant associations between higher cortical PiB retention and cognitive domain functions in the whole group of participants. Similar trends of associations between Aβ load and cognitive function specifically in the memory and visual-spatial domains have been observed in smaller samples of cognitively normal older adults19,20 or older adults without dementia.21 Aβ load was treated as a continuous variable in these studies. Others that dichotomized the cognitively normal subjects into high and low PiB retention groups did not find any differences in cognitive function between the 2 groups.15,18,22,23 However, we note that even with high levels of PiB retention typically seen in subjects with AD, there was relatively little cognitive disturbance, suggesting the influence of additional mediators such as cognitive reserve, which was previously investigated,24,25 and the APOE genotype that we investigated in the current study. To our knowledge the current study demonstrated for the first time that the associations between Aβ load and cognitive function in cognitively normal older adults is modified by the APOE status. The associations between cognition and PiB retention found in cognitively normal older adults appeared to be primarily driven by the 25% of the participants who were APOE ϵ4 carriers. Conversely, we did not find any relationship between cognitive function and APOE status most likely due to the older age of our sample. AD risk in APOE ϵ4 carriers decreases after roughly age 80,26 and the influence of APOE genotype on cognitive performance dissipates after age 80.27 Hence, absence of a relationship between APOE genotype and cognition may be expected in this sample with a median age of 79.
The effects of APOE isoforms on AD pathology appear to be multifactorial. There is evidence that APOE ϵ4 may influence AD pathology by interacting with Aβ clearance thereby increasing the concentrations of toxic oligomeric Aβ,6,7,28 enhancing hyperphosphorylation of tau protein29 and reducing choline acetyltransferase activity.30 Cognitively normal young-adult APOE ϵ4 carriers show glucose metabolic reductions in regions similar to the metabolic reductions typically observed in AD,31 even in the absence of neurofibrillary tangles and Aβ plaques.32 Furthermore, the resting state fMRI connectivity is reduced in PiB-negative cognitively normal APOE ϵ4 carriers in regions that show reduced connectivity in AD.33 Overall, these data suggest that APOE ϵ4 genotype not only increases the risk for Aβ deposition but also influences AD pathology by modulating the harmful effects of Aβ on cognitive function through synergistic mechanisms.34
On voxel-based analysis we found that lower global cognitive function (derived from the 4 cognitive domain scores) correlated with greater PiB retention in the frontal, temporal, and parietal unimodal and heteromodal association cortices but not the primary visual and sensory-motor cortices and the medial temporal limbic cortex. The absence of an association between medial temporal cortex Aβ load and memory function argues against a direct functional-anatomic relationship between localized cognitive function and localized Aβ load. Associations were present in regions where there was significant Aβ deposition but not in regions with lower Aβ load. In fact, the voxel-based map of the correlation between PiB retention and cognitive performance revealed the topography of Aβ deposition that is confined to the association cortices early in the disease process in AD, as described by Braak and Braak.35
A strength of the current study is its sample. Participants were randomly selected from the Olmsted County population, in contrast to the previously studied cohorts of cognitively normal volunteers consecutively recruited from the community through advertisements or memory clinics, thereby increasing the potential for selection or volunteer bias.3,15,21,22 A high level of PiB retention (>1.5 global cortical retention ratio) was observed in 34% of the cognitively normal individuals in the current study. This is slightly higher than the 20%–30% rate of high cortical PiB retention ratio that we and others have reported in the literature, possibly because of the older age range of our sample compared to the others,3,15,21,22 but lower than the 47% rate reported in the Alzheimer's Disease Neuroimaging Initiative (ADNI) cognitively normal cohort. This was unexpected given that the ADNI cognitively normal cohort is relatively younger than the MCSA sample.36 The differences are most likely associated with the ascertainment of participants as we did not exclude subjects due to neurologic, psychiatric, or systemic illnesses in order to study a representative sample of the population. However, it is possible that people with poor general health would be less likely to participate in imaging studies. The participants performed slightly better in memory, visual-spatial function, and attention/executive function, suggesting minimal nonparticipation bias.
Although PiB retention is modestly associated with cognitive function, the presence of an APOE ϵ4 allele significantly increases Aβ load and influences the relationship between Aβ load and cognitive function in cognitively normal older adults. Conversely, Aβ load decreases with the presence of an APOE ϵ2 allele and PiB retention does not appear to influence cognitive function in APOE ϵ2 carriers.37 Whereas high Aβ load pushes APOE ϵ4 carriers closer to cognitive impairment, the cognitive function in APOE ϵ3 homozygotes and APOE ϵ2 carriers are incrementally influenced less by the Aβ load. This finding agrees with the hypothesis that APOE ϵ4 shifts the “AD biomarker cascade model”38 toward younger age, which results in an earlier onset of AD and more severe AD pathology at a given age in APOE ϵ4 carriers compared to noncarriers.3,10 A limitation of our study is the insufficient follow-up on the cohort we scanned during the last 2 years, precluding our ability to assess longitudinal associations. PiB predicted future cognitive decline in visual-spatial performance, semantic and episodic memory function in 2 independent cohorts of cognitively normal older adults or older adults without dementia12,39 and progression to AD in patients with mild cognitive impairment, indicating that PiB retention in a patient with no dementia is associated with an increased risk of cognitive dysfunction in the future.40 The effects of APOE status on Aβ-associated cognitive decline in cognitively normal older adults is subject to future investigations.
Supplementary Material
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- ANOVA
analysis of variance
- GM
gray matter
- MCSA
Mayo Clinic Study of Aging
- MPRAGE
magnetization-prepared rapid gradient echo
- PiB
Pittsburgh compound B
- WAIS-R
Wechsler Adult Intelligence Scale—Revised
Footnotes
Editorial, page 228
Supplemental data at www.neurology.org.
AUTHOR CONTRIBUTIONS
Dr. Kantarci: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, statistical analysis, study supervision, obtaining funding. Dr. Lowe: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, acquisition of data, study supervision, obtaining funding. S. Przybelski: analysis or interpretation of data, acquisition of data, statistical analysis. S. Weigand: drafting/revising the manuscript, analysis or interpretation of data, statistical analysis. M. Senjem: analysis or interpretation of data, contribution of vital reagents/tools/patients, statistical analysis. Dr. Ivnik: drafting/revising the manuscript, study concept or design, acquisition of data, study supervision. G. Preboske: analysis or interpretation of data, acquisition of data. Dr. Roberts: drafting/revising the manuscript, analysis or interpretation of data, acquisition of data, study supervision. Dr. Geda: drafting/revising the manuscript, acquisition of data. Dr. Boeve: analysis or interpretation of data, study supervision. Dr. Knopman: drafting/revising the manuscript, study concept or design, analysis or interpretation of data. Dr. Petersen: drafting/revising the manuscript, obtaining funding. Dr. Jack: drafting/revising the manuscript, study concept or design, analysis or interpretation of data, contribution of vital reagents/tools/patients, acquisition of data, study supervision, obtaining funding.
DISCLOSURE
Dr. Kantarci receives research support from the NIH. Dr. Lowe serves on scientific advisory boards for Bayer Schering Pharma and GE Healthcare and receives research support from GE Healthcare, Siemens Molecular Imaging, the NIH (NIA, NCI), the MN Partnership for Biotechnology and Medical Genomics, and the Leukemia & Lymphoma Society. S. Przybelski and S. Weigand report no disclosures. M.L. Senjem has received research support from Pfizer Inc. Dr. Ivnik serves on the editorial boards of The Clinical Neuropsychologist and Aging, Neuropsychology, and Cognition; receives publishing royalties for Clinical Interpretation of the WAIS-III and WMS-III (Academic Press, 2003); and receives research support from the NIH/NIA. G. Preboske reports no disclosures. Dr. Roberts serves as an Associate Editor for the Journal of Alzheimer's Disease and receives research support from Abbott and the NIH. Dr. Geda receives research support from the NIH and the RWJ Foundation. Dr. Boeve receives publishing royalties for The Behavioral Neurology of Dementia (Cambridge University Press, 2009) and receives research support from Cephalon, Inc., Allon Therapeutics, Inc., the NIH/NIA, the Alzheimer's Association, and the Mangurian Foundation. Dr. Knopman serves as Deputy Editor for Neurology®; serves on a data safety monitoring board for Eli Lilly and Company; has served as a consultant for Elan/Janssen AI; is an investigator in clinical trials sponsored by Elan/Janssen AI, Baxter International Inc., and Forest Laboratories, Inc.; and receives research support from the NIH. Dr. Petersen serves on scientific advisory boards for the Alzheimer's Association, the National Advisory Council on Aging (NIA), Elan/Janssen AI, Pfizer Inc (Wyeth), and GE Healthcare; receives publishing royalties from Mild Cognitive Impairment (Oxford University Press, 2003); serves as a consultant for Elan/Janssen AI and GE Healthcare; and receives research support from the NIH/NIA. Dr. Jack serves on scientific advisory boards for Elan/Janssen AI, Eli Lilly & Company, GE Healthcare, and Eisai Inc.; receives research support from Baxter International Inc., Allon Therapeutics, Inc., Pfizer Inc, the NIH/NIA, and the Alexander Family Alzheimer's Disease Research Professorship of the Mayo Foundation; and holds stock/stock options in Johnson & Johnson.
REFERENCES
- 1. Thal LJ, Kantarci K, Reiman EM, et al. The role of biomarkers in clinical trials for Alzheimer disease. Alzheimer Dis Assoc Disord 2006;20:6–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer's disease with Pittsburgh compound B. Ann Neurol 2004;55:306–319 [DOI] [PubMed] [Google Scholar]
- 3. Morris JC, Roe CM, Xiong C, et al. APOE predicts amyloid-beta but not tau Alzheimer pathology in cognitively normal aging. Ann Neurol 2010;67:122–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rabinovici GD, Jagust WJ. Amyloid imaging in aging and dementia: testing the amyloid hypothesis in vivo. Behav Neurol 2009;21:117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Corder EH, Saunders AM, Strittmatter WJ, et al. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science 1993;261:921–923 [DOI] [PubMed] [Google Scholar]
- 6. Deane R, Sagare A, Hamm K, et al. apoE isoform-specific disruption of amyloid beta peptide clearance from mouse brain. J Clin Invest 2008;118:4002–4013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Shibata M, Yamada S, Kumar SR, et al. Clearance of Alzheimer's amyloid-ss(1-40) peptide from brain by LDL receptor-related protein-1 at the blood-brain barrier. J Clin Invest 2000;106:1489–1499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Peskind ER, Li G, Shofer J, et al. Age and apolipoprotein E*4 allele effects on cerebrospinal fluid beta-amyloid 42 in adults with normal cognition. Arch Neurol 2006;63:936–939 [DOI] [PubMed] [Google Scholar]
- 9. Reiman EM, Chen K, Liu X, et al. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proc Natl Acad Sci USA 2009;106:6820–6825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vemuri P, Wiste HJ, Weigand SD, et al. Effect of apolipoprotein E on biomarkers of amyloid load and neuronal pathology in Alzheimer disease. Ann Neurol 2010;67:308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer's disease and other neurological disorders. Lancet Neurol 2011;10:241–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Villemagne VL, Pike KE, Darby D, et al. Abeta deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer's disease. Neuropsychologia 2008;46:1688–1697 [DOI] [PubMed] [Google Scholar]
- 13. Roberts RO, Geda YE, Knopman DS, et al. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology 2008;30:58–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Petersen RC, Roberts RO, Knopman DS, et al. Prevalence of mild cognitive impairment is higher in men: The Mayo Clinic Study of Aging. Neurology 2010;75:889–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jack CR, Jr, Lowe VJ, Senjem ML, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer's disease and amnestic mild cognitive impairment. Brain 2008;131:665–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vemuri P, Whitwell JL, Kantarci K, et al. Antemortem MRI based STructural Abnormality iNDex (STAND) scores correlate with postmortem Braak neurofibrillary tangle stage. Neuroimage 2008;42:559–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schemper M. Non-parametric partial association revisited. J R Stat Soc Ser D 1991;40:73–76 [Google Scholar]
- 18. Rowe CC, Ng S, Ackermann U, et al. Imaging beta-amyloid burden in aging and dementia. Neurology 2007;68:1718–1725 [DOI] [PubMed] [Google Scholar]
- 19. Fagan AM, Mintun MA, Shah AR, et al. Cerebrospinal fluid tau and ptau(181) increase with cortical amyloid deposition in cognitively normal individuals: implications for future clinical trials of Alzheimer's disease. EMBO Mol Med 2009;1:371–380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Mormino EC, Kluth JT, Madison CM, et al. Episodic memory loss is related to hippocampal-mediated beta-amyloid deposition in elderly subjects. Brain 2009;132:1310–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Pike KE, Savage G, Villemagne VL, et al. Beta-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer's disease. Brain 2007;130:2837–2844 [DOI] [PubMed] [Google Scholar]
- 22. Aizenstein HJ, Nebes RD, Saxton JA, et al. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008;65:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mintun MA, Larossa GN, Sheline YI, et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 2006;67:446–452 [DOI] [PubMed] [Google Scholar]
- 24. Vemuri P, Weigand SD, Przybelski SA, et al. Cognitive reserve and Alzheimer's disease biomarkers are independent determinants of cognition. Brain 2011;134:1479–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roe CM, Mintun MA, D'Angelo G, Xiong C, Grant EA, Morris JC. Alzheimer disease and cognitive reserve: variation of education effect with carbon 11-labeled Pittsburgh Compound B uptake. Arch Neurol 2008;65:1467–1471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Breitner JC, Wyse BW, Anthony JC, et al. APOE-epsilon4 count predicts age when prevalence of AD increases, then declines: the Cache County Study. Neurology 1999;53:321–331 [DOI] [PubMed] [Google Scholar]
- 27. Negash S, Greenwood PM, Sunderland T, et al. The influence of apolipoprotein E genotype on visuospatial attention dissipates after age 80. Neuropsychology 2009;23:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lesne S, Kotilinek L, Ashe KH. Plaque-bearing mice with reduced levels of oligomeric amyloid-beta assemblies have intact memory function. Neuroscience 2008;151:745–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ledesma MD, Medina M, Avila J. The in vitro formation of recombinant tau polymers: effect of phosphorylation and glycation. Mol Chem Neuropathol 1996;27:249–258 [DOI] [PubMed] [Google Scholar]
- 30. Dubelaar EJ, Verwer RW, Hofman MA, Van Heerikhuize JJ, Ravid R, Swaab DE. ApoE epsilon4 genotype is accompanied by lower metabolic activity in nucleus basalis of Meynert neurons in Alzheimer patients and controls as indicated by the size of the Golgi apparatus. J Neuropathol Exp Neurol 2004;63:159–169 [DOI] [PubMed] [Google Scholar]
- 31. Reiman EM, Chen K, Alexander GE, et al. Functional brain abnormalities in young adults at genetic risk for late-onset Alzheimer's dementia. Proc Natl Acad Sci USA 2004;101:284–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Valla J, Yaari R, Wolf AB, et al. Reduced posterior cingulate mitochondrial activity in expired young adult carriers of the APOE epsilon4 allele, the major late-onset Alzheimer's susceptibility gene. J Alzheimers Dis 2010;22:307–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sheline YI, Morris JC, Snyder AZ, et al. APOE4 allele disrupts resting state fMRI connectivity in the absence of amyloid plaques or decreased CSF Abeta42. J Neurosci 2011;30:17035–17040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bu G. Apolipoprotein E and its receptors in Alzheimer's disease: pathways, pathogenesis and therapy. Nat Rev Neurosci 2009;10:333–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 1991;82:239–259 [DOI] [PubMed] [Google Scholar]
- 36. Jagust WJ, Bandy D, Chen K, et al. The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement 2010;6:221–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Berlau DJ, Corrada MM, Head E, Kawas CH. APOE epsilon2 is associated with intact cognition but increased Alzheimer pathology in the oldest old. Neurology 2009;72:829–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jack CR, Jr, Knopman DS, Jagust WJ, et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol 2010;9:119–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morris JC, Roe CM, Grant EA, et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch Neurol 2009;66:1469–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Jack CR, Jr, Wiste HJ, Vemuri P, et al. Brain beta-amyloid measures and magnetic resonance imaging atrophy both predict time-to-progression from mild cognitive impairment to Alzheimer's disease. Brain 2010;133:3336–3348 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.