Abstract
Objective
This study focused on comparing the expression levels of p16, Ki-67, and minichromosome maintenance 7 (MCM7) protein in normal and affected cervical epithelium to ascertain the biological significance of these markers in detecting progressive cervical disease.
Methods
A quantitative and based on-scanning-microscopy analysis of the three markers expression was performed in normal and cervical intraepithelial neoplasia (CIN) I, II, and III tissues. p16 area as well as p16, Ki-67, and MCM7 positive cells or nuclei were evaluated according to their distribution and extent through the cervical epithelium.
Results
A clear p16 over-expression was observed in all the dysplastic epithelium tissue samples. The quantitative analysis of p16 area as well as the number of p16 positive cells was able to better discriminate the CIN lesions grades than the usual semi-quantitative analysis. The average Ki-67 labeling indexes for the normal epithelium, CIN I, CIN II, and CIN III groups were 19.8%, 27.3%, 32.8%, and 37.1%, respectively, whereas the mean MCM7 labeling indexes for the correspondent grades were 27.0%, 30.4%, 50.5%, and 67.2%. The Ki-67 and MCM7 labeling indexes were closely correlated with the CIN histological grade, with higher labeling indexe values obtained from the more severe lesions (p<0.05), being the MCM7 labeling indexes the highest values in all the CIN categories (p<0.05).
Conclusion
We observed a good correlation among the p16, Ki-67, and MCM7 data. In addition, MCM7 demonstrated to be a more efficient and sensitive marker to assess disease progression in the uterine cervix.
Keywords: Cervical intraepithelial neoplasia, Genes p16, Immuno-fluorescence analysis, Ki-67 antigen, MCM7 protein
INTRODUCTION
Screening programs based on cytological staining techniques (Pap test) has led to a remarkable decline in incidence and mortality from invasive cervical cancer. However the Pap test efficacy is hampered by the high inter-observer variability and false negative/positive rates [1]. Even the "gold standard" histological assessment of cervical biopsies can be significantly affected by the intra- and inter-observer inconsistencies. Novel markers potentially applied on cytological/histological specimens could improve the identification of lesions with increased risk for progression. p16INK4a (p16) is a tumor suppressor protein typically over-expressed in dysplastic and neoplastic cervix epithelium [1]. By means of a semi-quantitative analysis, several studies showed that high-grade lesions (cervical intraepithelial neoplasia [CIN] II to invasive cancer) exhibited a full-thickness and strong p16 immuno-staining [1-3]. However, a quantitative evaluation of p16 positive areas and cells in normal and affected cervical epithelium has not been performed.
The proliferative capacity of tumor cells is a fundamental feature of growing tumors. Ki-67 is a general proliferation marker [4] widely used to characterize malignant lesions including those of the cervix. The Ki-67 staining into the upper epithelium layers accomplishes the severity of cervical lesions [2,5], but the exact nuclear function of Ki-67 antigen is largely unknown [4]. Thus, detecting markers directly involved in DNA replication might be a more precise way to evaluate proliferative behavior in dysplastic and neoplastic processes.
Minichromosome maintenance (MCM) proteins (2-7) complex consists of six subunits with essential functions in initiation and elongation of DNA replication [6]. Additionally, it has only been demonstrated in replicating cells [7].
MCM7 was reported to be associated with tumor formation, progression and malignant conversion [8]. It has also been considered a valuable proliferation marker in several cancer types, such as oral, prostatic, colonic [9], lung-adenocarcinoma and cervical [8]. Few studies investigated the MCM7 expression in human cervix tissues [7,8], and there were no reports that comparatively evaluated the MCM7 expression with those originating from the most commonly used p16 and Ki-67 markers. The purpose of this study was to compare the potential of these three nuclear proteins as biomarkers for cervical disease progression.
MATERIALS AND METHODS
1. Tissue samples
Twenty paraffin-embedded cervical samples (5 normal epithelium, 5 CIN I, 5 CIN II, and 5 CIN III cases) were obtained from the archive of a large histopathological diagnostic service laboratory. This study was approved by the National Council of Ethics in Research.
2. Indirect immuno-fluorescence
Serial sections (5 µM-thick) were immersed in xylene, re-hydrated, heated in water-bath for 30 minutes for antigen retrieval, and blocked with 2% phosphate-buffered saline and bovine serum albumin (PBS/BSA) for 60 minutes at room temperature (RT). Sections were incubated overnight at 4℃ with the primary antibodies: p16 mouse-monoclonal antibody-clone JC8 (1:800, LabVision, Fremont, CA, USA), Ki-67 rabbit-polyclonal antibody-clone H-300 (1:100, Santa Cruz Biotechnology, Santa Cruz, CA, USA) and MCM7 mouse-monoclonal antibody, clone DCS-141 (1:1000, Abcam, Cambridge, MA, USA). Sections were next incubated (1 hour-RT) with secondary antibodies: Cy2-Streptavidin-conjugated anti-mouse antibody (Molecular-Probes Inc., Eugene, OR, USA) and Cy5-conjugated anti-rabbit antibody (Molecular-Probes Inc.), washed, mounted (Hydramount, National Diagnostics, Atlanta, GA, USA) and examined at high-magnification fields (40x) by scanning confocal microscopy (Carl Zeiss LSM/510Meta, Carl Zeiss Jena, Germany).
Strong nuclear and cytoplasmic labeling was considered a p16 positive-reaction; the Ki-67/MCM7 markers' positivity was based on strong nuclear labeling detection. Quantitative determination of p16 immuno-positive areas and the number of positive cells and/or nuclei for all markers was performed by the KS-300/Image Analyzer Carl Zeiss. Spearman's correlation statistical-technique was used for analyzing the association between the biomarker's expression and the cervical-epithelium histological grade. Pearson's correlation statistical-technique was used for comparative-evaluation between p16 positive cells/nuclei and Ki-67/MCM7 positive nuclei. A p<0.05 was considered statistically significant. The quantitative results were also expressed in a semi-quantitative scale, by using previously published scoring-criteria [2]. The Ki-67 and MCM7 labeling indexes (LIs) were calculated by dividing the number of positive nuclei by the total nuclei number [9].
RESULTS
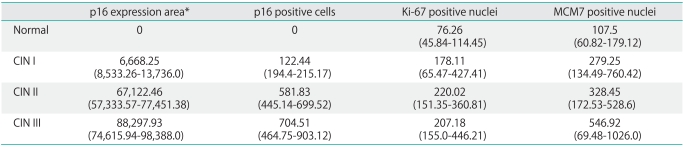
Normal tissues did not show any positive areas or immuno-reactive cells for p16 (Table 1). However an increasing number of p16 immuno-labeled areas was observed along with the severity of cervical disease (Table 1). A similar result was verified when these values were expressed in a semi-quantitative scale: the majority of CIN I, II, and III samples exhibited a growing pattern of p16 positive areas (Table 2). In accordance with the quantitative p16 area analysis, an increase was observed in p16 positive cells according to the CIN severity degree (Table 1).
Table 1.
Quantitative mean values of p16 immuno-expression area and the number of p16, Ki-67, and MCM7 positive cells/nuclei according to the histological diagnosis
Values are presented as number (range).
MCM, minichromosome maintenance; CIN, cervical intraepithelial neoplasia.
*p16 immuno-positive areas values were expressed in square micrometers (µm2).
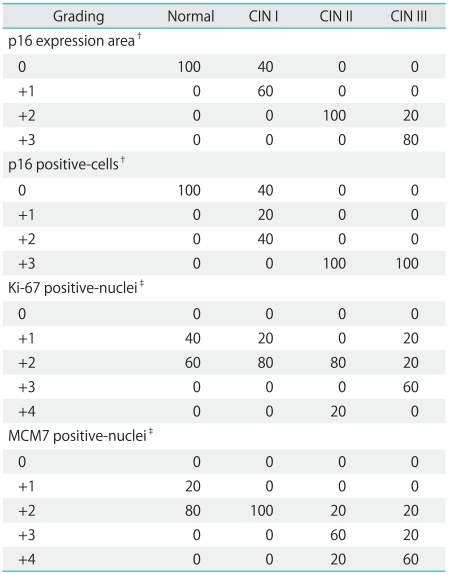
Table 2.
Semi-quantitative values of p16 area and the p16, Ki-67, and MCM7 cells/nuclei immuno-labeling according to the histological diagnosis*
MCM, minichromosome maintenance; CIN, cervical intraepithelial neoplasia.
*Percentage of samples from each histological diagnosis group exhibiting the considered marker expression. †Samples were classified as 0, +1, +2, +3 according to the absence (0) or presence of 5-25%, 25-75%, and >75% of p16 positive areas and cells. ‡Samples were scored as 0, +1, +2, +3, and +4 when exhibited none, 1-20%, 20-40%, 40-60%, and >60% of Ki-67/MCM7 positive nuclei [2].
However, when these results were semi-quantitatively expressed the p16 immuno-labeling analysis within the CIN groups showed greater variability (Table 2). Regarding the Ki-67 results, the number of Ki-67 positive nuclei increased from normal to CIN II epithelium, but no notable differences were observed between CIN II and III values (Table 1). Most normal, CIN I and II tissue samples, showed a similar Ki-67 labeling pattern (+2 score), whereas the majority of CIN III samples scored as +3. A growing expression of MCM7 was observed from normal to CIN III samples, with the highest MCM7 expression values detected in CIN III cases (Table 1).
Accordingly, most of normal and all the CIN I samples were MCM7 scored as +2, while the majority (60%) of CIN II and III samples were scored as +3 and +4 (Table 2). The MCM7 LIs exhibited a good correlation with the Ki-67 LIs (p<0.05), and the MCM7 labeling-index for each case was considerably higher than the corresponding Ki-67 LIs.
The obtained Ki-67 LIs mean values were 19.8 (with samples/values ranging from 15.8 to 22.5) for the normal epithelium, 27.31 (range, 8.4 to 33.75) for CIN I-group, 32.84 (range, 22.0 to 66.2) for CIN II-category, and 37.16 (range, 19.25 to 49.1) for CIN III-group (p<0.05). The average MCM7 LIs values for the corresponding groups were 27.06 (with values ranging from 15.1 to 37.6), normal epithelium; 30.36 (range, 20.2 to 36.5), CIN I; 50.54 (range, 37.7 to 70.4), CIN II; and 67.26 (range, 39.0 to 100.0), CIN III; p<0.05.
According to the biomarkers' expression distribution through the cervical epithelium, all the normal cervical tissue samples were negative for p16 and showed Ki-67 and MCM7 labeling in the lower (1/3) basal epithelium (Fig. 1). Forty percent of CIN I samples exhibited p16 immuno-expression in the basal layer, while 100% of CIN II and III samples showed labeling of 2/3 and 3/3 of the epithelium. Concerning the Ki-67 expression topology, 60% of CIN I and 100% of CIN II samples showed 2/3 of the epithelium staining while 60% of CIN III exhibited labeling in 3/3 of the epithelium. Most of the normal and CIN I tissue samples expressed MCM7 in the 1/3 basal-epithelium, whereas the majority of CIN II and III cases showed MCM labeling in 2/3 of the epithelium.
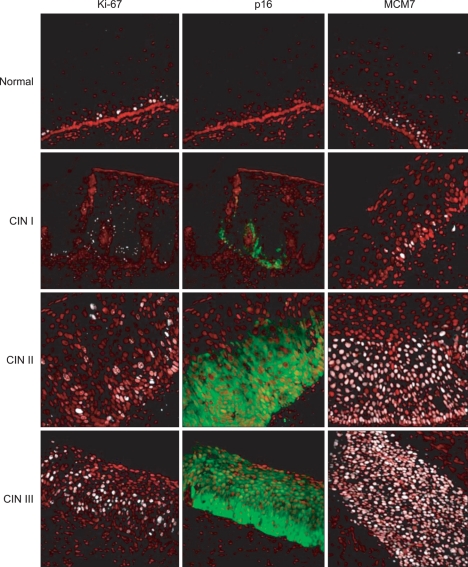
Fig. 1.
Comparative immuno-fluorescent analysis of Ki-67, p16, and minichromosome maintenance 7 (MCM7) markers expression in human-cervical tissue-samples obtained by loop electrosurgical excision procedure (LEEP). The diagnoses were independently reviewed/confirmed by three certified pathologists. Positive Ki-67 and MCM7 cells show fluorescence-labeling confined to the nucleus (white), whereas p16-positive cells show nuclear/and/cytoplasmic labeling (green). Nuclei were immuno-labeled with propidium-iodide (red). CIN, cervical intraepithelial neoplasia.
DISCUSSION
In this study we have shown, using a quantitative-evaluation approach, that p16, Ki-67, and MCM7 markers are significantly correlated with cervical lesion progression. Several studies confirmed negative to minimal p16-staining in normal/reactive cervical biopsies, with considerable variability in the CIN grade samples presenting strong p16 immuno-reactivity [1,3]. Therefore, quantitative analysis of p16 areas and/or cells seemed to be a better approach when performing the categorization of CIN groups than conventional semi-quantitative evaluation. Additionally, we performed a comparative analysis between Ki-67 and MCM7 proliferation-related proteins as CIN progression markers. Ki-67 positivity extending to 2/3 of the epithelium was reported to be associated with increasing cervical dysplasia degree [2,5]. However, this marker specificity is low [2], Ki-67 expression-levels can be altered by external factors and there is no evidence that this protein is essential for cell proliferation [4]. Here, we observed a higher expression of both Ki-67 and MCM7 in high-grade when compared to low-grade lesions or normal cervical-epithelium. Few studies have examined MCMs as proliferation markers in cervical disease and have focused mostly in analyzing the MCM2/5 expression. In only one study the MCM7 staining topology-pattern was evaluated in cervical, normal and affected tissues [8]. Our results are concordant with this report, since a significant association between the p16, Ki-67, and MCM7 topology-patterns expression and human-cervical disease progression was observed. Other reports showed that MCM7 labeling-indexes were consistently higher than Ki-67 LIs in several cancer types [8,9]. Previous studies have shown that, additionally to proliferative cells, MCM proteins were expressed in potent or early-proliferating cells [6]. Thus, these observations may imply that, Ki-67 does not label all proliferating cells of some tumors [4].
We believe that MCM7 offers a great advantage over other proliferating biomarkers, since it is not expressed in cells undergoing DNA repair [6] and exhibits a higher sensitivity to identify abnormal precursor cells than Ki-67/PCNA [8]. Consequently, MCM7 is regarded an excellent candidate marker for cancer screening, surveillance, and prognosis [7,9]. In a prospective study involving the analysis of cervical smear samples from 455 Indian women, the combined use of MCM2/5-staining and Pap-counterstaining disclosed 10 previously missed cases of biopsy-proven cervical cancer or pre-cancer when using the standard Pap-staining [10]. Although this study was focused on the evaluation of MCM7 as an indicator of progressive-cervical disease in tissue samples, the biomarker expression demonstrated a satisfactory correlation with CIN progression, suggesting that MCM7 could be used as a valuable tool for improving the diagnosis and screening of pre-malignant lesions and cervical cancer.
ACKNOWLEDGMENTS
This work was supported by grants from the Conselho Nacional de Pesquisas (CNPq) and from the Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Tsoumpou I, Arbyn M, Kyrgiou M, Wentzensen N, Koliopoulos G, Martin-Hirsch P, et al. p16(INK4a) immunostaining in cytological and histological specimens from the uterine cervix: a systematic review and meta-analysis. Cancer Treat Rev. 2009;35:210–220. doi: 10.1016/j.ctrv.2008.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agoff SN, Lin P, Morihara J, Mao C, Kiviat NB, Koutsky LA. p16(INK4a) expression correlates with degree of cervical neoplasia: a comparison with Ki-67 expression and detection of high-risk HPV types. Mod Pathol. 2003;16:665–673. doi: 10.1097/01.MP.0000077518.78046.0C. [DOI] [PubMed] [Google Scholar]
- 3.Kalof AN, Cooper K. p16INK4a immunoexpression: surrogate marker of high-risk HPV and high-grade cervical intraepithelial neoplasia. Adv Anat Pathol. 2006;13:190–194. doi: 10.1097/00125480-200607000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Scholzen T, Gerdes J. The Ki-67 protein: from the known and the unknown. J Cell Physiol. 2000;182:311–322. doi: 10.1002/(SICI)1097-4652(200003)182:3<311::AID-JCP1>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 5.Keating JT, Cviko A, Riethdorf S, Riethdorf L, Quade BJ, Sun D, et al. Ki-67, cyclin E, and p16INK4 are complimentary surrogate biomarkers for human papilloma virus-related cervical neoplasia. Am J Surg Pathol. 2001;25:884–891. doi: 10.1097/00000478-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Bell SP, Dutta A. DNA replication in eukaryotic cells. Annu Rev Biochem. 2002;71:333–374. doi: 10.1146/annurev.biochem.71.110601.135425. [DOI] [PubMed] [Google Scholar]
- 7.Freeman A, Morris LS, Mills AD, Stoeber K, Laskey RA, Williams GH, et al. Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin Cancer Res. 1999;5:2121–2132. [PubMed] [Google Scholar]
- 8.Brake T, Connor JP, Petereit DG, Lambert PF. Comparative analysis of cervical cancer in women and in a human papillomavirus-transgenic mouse model: identification of minichromosome maintenance protein 7 as an informative biomarker for human cervical cancer. Cancer Res. 2003;63:8173–8180. [PubMed] [Google Scholar]
- 9.Nishihara K, Shomori K, Fujioka S, Tokuyasu N, Inaba A, Osaki M, et al. Minichromosome maintenance protein 7 in colorectal cancer: implication of prognostic significance. Int J Oncol. 2008;33:245–251. [PubMed] [Google Scholar]
- 10.Mukherjee G, Muralidhar B, Bafna UD, Laskey RA, Coleman N. MCM immunocytochemistry as a first line cervical screening test in developing countries: a prospective cohort study in a regional cancer centre in India. Br J Cancer. 2007;96:1107–1111. doi: 10.1038/sj.bjc.6603679. [DOI] [PMC free article] [PubMed] [Google Scholar]