Abstract
Cognitive difficulties after surgery are a common complication and are associated with significant morbidity and mortality, which is concerning as the number of geriatric patients undergoing major procedures continues to rise. Postoperative cognitive impairment encompasses both postoperative delirium and postoperative cognitive decline. Delirium is a formal diagnosis and presents acutely while postoperative cognitive decline has yet to be defined and has a subtle presentation. Postoperative cognitive decline is a decline in cognitive function from baseline and has been described in elderly patients after cardiac surgery, such as coronary artery bypass graft. Although the etiology of postoperative cognitive decline has yet to be elucidated, it is most likely multifactorial with potential causes being surgery type, sleep disturbances, neuroinflammation, cerebral hypoperfusion, anesthesia, metabolic syndrome, and decreased cognitive reserve. In this case report, we present the first case of postoperative cognitive decline in a middle-age patient after left ventricular assist device placement.
Keywords: Postoperative cognitive decline, left ventricular assist device, neuroinflammation
Introduction
Heart failure is a chronic, progressive condition that affects an estimated 5.8 million Americans.1 Although cardiac transplantation is the only definitive cure for refractory heart failure, available donor organs continue to remain in short supply.2 Employing left ventricular assist devices (LVADs) as a bridge to transplantation has become a widespread practice worldwide and has reduced the mortality in this patient population.3 Furthermore, long-term LVAD therapy has demonstrated improved survival in end-stage heart failure patients who were unsuitable candidates for transplantation, thus leading to an additional role as destination therapy.4 As the number of patients diagnosed with heart failure continues to grow and the implantation of LVADs consequently rises, the neurocognitive complications of this therapy become increasingly important as they are associated with significant morbidity and death.5,6 Neurological events are one of the most common late complications of LVADs7,8 and include, among others, cerebral embolism.8 Although the long-term neurological complications of LVADs have been reported5,8–11 and the majority of postoperative neurologic complications occur in the first few days to weeks postimplantation,12 little is known about the cognitive sequelae in the postoperative period soon after implantation.
Postoperative cognitive impairment is a frequent phenomenon and comprises postoperative delirium (POD), postoperative cognitive dysfunction (POCD), and dementia,13 with POD and POCD being the most common following surgery.14–16 Both POD and POCD denote a post-surgical cognitive decline, deficits in attention is a shared feature,16 advancing age is a common risk factor, and the two result in increased morbidity and mortality.15 Also, an increased incidence in POCD during Postoperative Week 1 was observed in patients with POD.16 However, each possesses unique characteristics (Table 1). The incidence of POD is estimated to be at least 20 percent in patients 65 years of age or older, while that of POCD is about 25 percent in patients 60 years of age or older,15 though one should note that the incidence of POCD varies depending on which tests are used and when the patients are evaluated.14 Although POCD has been studied extensively in cardiac procedures, such as coronary artery bypass graft surgery (CABG) (incidence of 43%), it is also a complication of noncardiac surgeries, such as total hip joint replacement (incidence of 17%).17 Additionally, POCD has been observed following minor/outpatient surgeries with an incidence of 6.8 percent.18
TABLE 1.
| FEATURES | POSTOPERATIVE DELIRIUM | POSTOPERATIVE COGNITIVE DECLINE |
|---|---|---|
| Onset | Acute | Subtle |
| Presentation | Usually Postoperative Days 1–3; rarely after Postoperative Day 6 | Usually not prior to Postoperative Day 5–10 |
| Duration | Days to weeks | Weeks to months |
| Attention | Impaired | Impaired |
| Oriented | Often disoriented | Normal |
| Reversible | Usually | Usually, but can be long-lasting |
| Formal diagnosis? | Defined in DSM-IV-TR and WHO’s classification of diseases | No formal definition; diagnosis required pre- and postoperative neuropsychological testing |
| Bedside screening tools? | CAM, CAM-ICU | None; commonly tested cognitive domains are memory, executive function, processing speed, and attention |
- CAM
Confusion Assessment Method;
- ICU
intensive care unit;
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision;
- WHO
World Health Organization
POCD is poorly understood and has no standardized screening tools yet, though the most frequently tested cognitive domains are attention and memory.19 Diagnosis requires comparison of preoperative and postoperative neuropsychological testing with a battery of tests to determine if cognitive decline has occurred.14,15 POCD generally refers to a subtle impairment in memory, executive functioning, processing speed, and concentration following a major surgery.13,16,20,21 POCD typically does not present prior to Postoperative Days 5 to 1015 and can be categorized as acute/early (detected within 1 week and more likely in middle-aged patients22), intermediate (detected within 3 months), or long-term (detected 1–2 years postoperatively).16 POCD is usually transient, and younger patients tend to recover more quickly from POCD than their elderly counterparts (>60 years in age) in whom the condition may last months.20 POCD is a common complication following CABG,21 yet to the authors’ knowledge, no cases of POCD post-LVAD have been reported. Here we present the case of a patient with POCD after LVAD implantation.
Case Report
Our patient was a 44-year-old African-American man with a two-year history of progressive congestive heart failure for which he had been hospitalized multiple times in the past year. Also, he was status post-biventricular implantable cardiac defibrillator implantation two years prior. He was admitted at an outside facility for decompensated heart failure where he received a diagnosis of end-stage dilated nonischemic cardiomyopathy, which resulted in his transfer to our hospital. He was deemed to be an appropriate transplant candidate, and had a HeartMate II left ventricular assist device (LVAD) placed. His medications included warfarin, carvedilol, epoetin alpha, furosemide, heparin drip, insulin glargine, and lisinopril.
Complete blood count was remarkable for hemoglobin/hematocrit of 8.3g/dL/36g/dL, with a mildly elevated white blood cell count. Complete metabolic profile was significant for albumin of 2.8g/dL, total protein of 5.7g/dL, and blood urea nitrogen/creatinine of 25mg/dL/1.4mg/dL. Urine, blood, and stool cultures all demonstrated no growth of any pathogens. His activated partial thromboplastin time was 56 seconds, while chest x-ray showed stable cardiomegaly.
A psychiatry consult was called on Postoperative Day 12 (Hospital Day 32) for possible mood and/or cognitive problems. In the postoperative period, the patient was noted to have poor motivation, decreased sleep, difficulty following directions, irritable mood, and had forgotten his VAD on several instances. On initial evaluation, the patient denied dysphoria, auditory/visual hallucinations, delusions, or lethality. The Vigilant A section of the Confusion Assessment Method-Intensive Care Unit23 was intact and the Richmond Agitation Sedation Scale score was zero. Cognitively, he was oriented times 3, without waxing or waning of alertness and with a Mini Mental State Examination (MMSE) score of 27.24 On the MMSE, short-term memory was significant for being unable to remember three items after a few minutes but able to recall two items with cues, and he lost two points for attention and concentration (able to list days of week in reverse order but with several repetitions and inversions and unable to do months of year in reverse). For semantic verbal fluency, the patient was able to list eight four-legged animals in one minute, while on test of phonemic fluency, he was only able to state seven words that began with the letter F. On test of digit span, our patient could repeat three, four, and five digits forwards and was only able to list three digits backwards (even after 2 trials). His insight and judgment into his illness was limited. He endorsed an irritable mood, which he attributed to a long hospitalization and poor sleep quality. Also, he demonstrated a full range of moods with mood-congruent affect. The patient’s physical exam revealed no significant findings. His labs were unremarkable aside from an elevated C-reactive protein (>20mg/dL), and no pre- or postoperative neuroimaging was performed. On review of his medical history, the patient denied any history of alcohol or illicit drug abuse as well as any psychiatric illnesses or cognitive difficulties.
During his hospital course, the patient’s short-term and working memory showed gradual improvement. By time of discharge on Postoperative Day 20, he was able to recall two items without cues and three items with prompting. Also, he was able to list the days of the week in reverse without repetition or inversions, but still unable to list months of the year in reverse. His tests of verbal and phonemic fluency improved by one and two words, respectively, while digit span testing improved in listing four but not five digits, backwards.
Discussion
The symptoms of POCD include memory loss, decreased concentration, and impaired executive and abstract functioning.21 Our patient presented with several of these deficits, including short-term memory impairment, impaired concentration (vis-à-vis, his difficulty listing days of the week and months of the year in reverse), and executive function deficits. Furthermore, both on Postoperative Day 1 and on our evaluation, CAM-ICU was negative for delirium. Additionally, since this patient presented on Postoperative Day 12 with symptoms of subtle onset, the timing and nature of his symptoms fits POCD. POD is less likely since the patient did not exhibit any decreased awareness, disorientation, or waxing and waning of symptoms that characterize this condition.
Increasing age is an unequivocal risk factor in POCD;15,20 however, it can affect patients of any age as shown by our patient. Additional risk factors include incipient neurodegenerative disease and anticholinergic drug treatment, both of which can increase the likelihood of uncontrolled neuroinflammation.25
The pathogenesis of POCD is probably multifactorial in origin.13 Possible causes include anesthesia, surgery type, sleep disturbances, metabolic syndrome, and neuroinflammation.20,26
Given the ability to evaluate neuroinflammation through serum markers of inflammation, we have chosen to review the role of neuroinflammation in the development of POCD (please see Table 2 for a summary of potential pathogenic mechanisms).
TABLE 2.
| ETIOLOGY | PROPOSED MECHANISM |
|---|---|
| Neuroinflammation | Activation of the systemic inflammatory response is a feature of several acute medical or surgical disorders, particularly when involving tissue destruction and/or infection. In such cases, pro-inflammatory cytokines circulating in the bloodstream elicit a cascade of functional and structural changes within the neurovascular unit leading to the activation of microglial cells and astrocytes. The acute neuroinflammatory reaction affects physiological processes implicated in neuronal and synaptic function with consequent neurochemical disturbances and functional disconnection between different anatomical structures. |
| General anesthesia | In-vitro studies have shown that inhaled anesthetics result in amyloid aggregates and neurofibrillary tangles, which are known to result in neurodegenerative changes. Such findings appear to support the hypothesis that general anesthesia is neurotoxic yet no significant difference in postsurgical cognition has been observed between general and regional anesthetics in humans. |
| Surgery type, specifically cardiac surgery (e.g., coronary artery bypass graft [CABG] and left ventricular assist devices [LVAD]) | This has been attributed to the use of cardiopulmonary bypass (CPB), which is used during the implantation of certain LVADs (as in our patient) and CABG. CPB can also produce a systemic inflammatory response, making the blood-brain barrier more permeable, and can produce microemboli. Postmortem exams of patients who had cardiac procedures with CPB have revealed multiple small capillary arteriolar dilatations in the brain, which are caused by microemboli. |
| Metabolic syndrome | Lack of cognitive reserve is accelerated by aging-induced frontal-subcortical syndrome. Many of the diagnostic characteristics that define metabolic syndrome are individually related to cognitive impairment; hypertension, obesity, elevated serum triglyceride concentrations, and hyperglycemia are all known risk factors for postoperative cognitive dysfunction. |
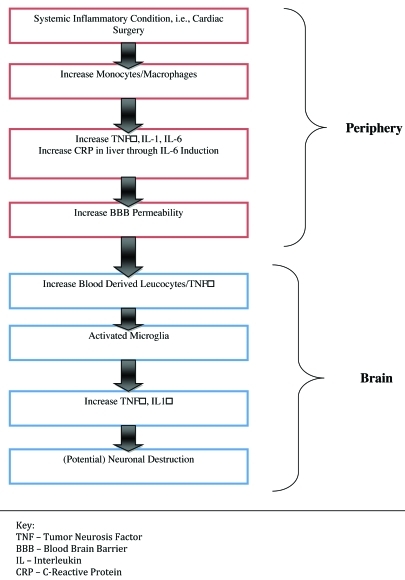
Cardiac surgery is associated with a systemic inflammatory response, resulting in the production of proinflammatory cytokines (i.e., interleukin 1-beta, tumor necrosis factor-alpha, and interleukin 6), especially in procedures using CPB.27 This may occur as a result of surgical trauma, blood contact with the CPB circuit, and/or lung reperfusion injury.28 Peripheral proinflammatory cytokines can communicate with the brain via several means, including as a result of decreased permeability of the blood-brain barrier (BBB). The latter could develop due to altered expression of tight-junctional proteins. Regardless, it has been presumed that cardiac surgery, with resulting acute systemic inflammation, is associated with BBB dysfunction.29 In the central nervous system, microglia are usually in a quiescent state; their activation seems to be pivotal in the innate immune response of the brain. Because of their monocyte and macrophage lineage, microglia can secrete a range of inflammatory mediators (e.g., cytokines, chemokines, and proteases). These locally produced inflammatory mediators not only modulate further immunological actions but also weaken astrocytic tight junctions and affect neuronal function. This effect on neuronal function has been posited to be the final step that causes clinically manifest behavioral/cognitive changes as a result of a systemic inflammation.25 Cytotoxic substances released by activated microglia might not only cause acute, reversible, behavioral/cognitive effects (as can occur in POD) but also lead to medium-longer lasting detrimental effects through bystander damage to neighboring neurons, as in POCD (Figure 1).25
FIGURE 1.
Systemic inflammatory insults are associated not only with full-blown delirium but they are also implicated in more subtle neuropsychiatric symptoms. Animal experiments using several learning and memory paradigms have consistently shown that peripheral immune system activation has a significant impact on cognition. For example, impairments in memory consolidation of previously learned tasks and disruption of working memory were reported in animals following LPS-induced immune stimulation. Cognitive changes following acute systemic inflammation are thought to result from cellular and molecular synergic interactions in different brain regions and particularly in the hippocampus. Pro-inflammatory IL-1 has long been recognized to impair hippocampal-dependent fear conditioning and to have an important role in neurophysiological processes of memory consolidation, possibly modulating synaptic plasticity. IL-6 has also been implicated in hippocampal dysfunction and thus affect central nervous system function, including memory and cognition.29
Multiple elevated inflammatory markers are especially indicative of systemic inflammation and are predictive of subsequent cognitive dysfunction. Elevated serum IL-6 and C-reactive protein (CRP) concentrations are associated with reduced cognition and contribute to accelerated functional decline in the elderly. An association between CRP concentration during middle age and subsequent risk of dementia has also been demonstrated.28 Patients with postsurgical neurocognitive decline after cardiac surgery were observed to have a significant increase in the proinflammatory markers CRP and IL-1 compared to patients without cognitive decline. Such a response has not been observed in patients with POD.14 Elevated postoperative IL-6 and CRP concentrations have been associated with short- and medium-term cognitive dysfunction after coronary artery surgery; however, in our hospital, there is no availability to IL-6 levels, thus we focus, in particular, on our patient’s elevated CRP.
Our patient’s most notable cognitive deficits were in short-term memory and executive functions. The former was noted on MMSE, and the latter by tests of verbal and phonemic fluency and digit span. One study has already reported that performance on immediate word list recall and delayed word list recall showed at least a 2-standard deviation (SD) decrease from baseline and performance on five additional tests, including digit span, and demonstrated a 1-SD decrease one week after surgery. After three months, performance on six tests, including immediate word list recall, delayed word list recall, and digit span, continued to show at least a 1-SD decrease from baseline. These data suggest slightly improved but still impaired cognitive functions at three months compared with one week after surgery.28
This same study divided the CRP scores into high versus low CRP levels. Each cognitive domain was more adversely affected by higher CRP levels; however, recent nonverbal and verbal memory performance was at least 1-SD different between the high and low CRP groups at both one week and three months. Additionally, overall cognitive function (all tests in battery) was significantly different between the high and low CRP concentration groups (P=0.04 and P=0.01, respectively, when CRP assayed at baseline and on the first postoperative day) at both 1 week and 3 months.28
Neuropathologically, dysfunction in recent memory may be caused by impairments in hippocampi, postulated to be affected by proinflammatory cytokines, including CRP30 (as previously discussed); however, executive functions depend on prefrontal cortices including dorsolateral white matter tracts. Difficulty in completing executive function tasks suggests an impairment in the frontal lobe functions of POCD patients. Interestingly, one study showed that higher CRP levels have been associated with higher semiquantitative scores of white matter hyperintensities. These findings suggest the role of CRP as a very sensitive and early marker of cerebral small vessel disease. More specifically, these same data suggest that low-grade inflammation as assessed by high-sensitivity C-reactive protein is associated with cerebral microstructural disintegration that predominantly affects frontal pathways and corresponding executive function.31
The greatest limitation of our case report is that the cognitive assessment performed was not a cognitive battery, but rather individual cognitive tests; thus, it is truly difficult to draw any functionally specific conclusions about the nature of our patient’s cognitive deficits or draw any firm conclusions about degree of cognitive recovery and to what extent he showed continued deficits. Another challenge is there is no clear information about how he functioned cognitively prior to his surgery(ies). Nonetheless, this limitation could exemplify how better neuropsychological assessments are needed to enable clinicians to begin addressing such questions. More specifically, with electronic medical record mandates, a new evidence base can be accumulated advancing the understanding of the neuropsychological morbidity associated with cardiac procedures, such as LVAD insertion. A practical consideration is that whereas baseline pre-implant assessments would be preferred, due to overall medical instability and severity of illness prior to LVAD insertion, obtaining measurements/data collection could be challenging, potentially limiting this to the least sick and most able end-stage heart failure population.32
In summary, our patient exhibited symptoms consistent with POCD with slight improvement in cognitive function on discharge. His POCD symptoms could have been due to any of the possible causes discussed previously. He had a past medical history significant for metabolic syndrome, which may have predisposed him to developing POCD. He underwent cardiac surgery with CPB and general anesthesia was used, both of which have been associated with POCD, yet the role of surgery and anesthesia type still remains inconclusive.16 Lastly, his labs were remarkable for an elevated CRP, which may indicate the presence of postsurgical neuroinflammation. Phenomenologically, he exhibited impairment in verbal memory (short-term recall) and executive functions, the domains most commonly affected in studies of POCD. Thus, most likely, our patient’s POCD was due to a combination of the aforementioned factors. The absence of a standard definition and cognitive tests for POCD makes comparing the findings of studies difficult,19 and we feel that additional study and research are warranted to establish formal diagnostic criteria and bedside screening tools.
Footnotes
FUNDING: No funding was received for the preparation of this article.
FINANCIAL DISCLOSURES: Dr. Spiegel is on the speaker’s bureau for Forest Pharmaceuticals, Sunovion Pharmaceuticals, and Merck Pharmaceuticals. Dr. Chen has not conflicts of interest relevant to the content of this article.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121(7):e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Taylor DO, Stehlik J, Edwards LB, et al. Registry of the International Society for Heart and Lung Transplantation: twenty-sixth official adult heart transplant report-2009. J Heart Lung Transplant. 2009;28(10):1007–1022. doi: 10.1016/j.healun.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 3.Aaronson KD, Eppinger MJ, Dyke DB, et al. Left ventricular assist device therapy improves utilization of donor hearts. J Am Coll Cardiol. 2002;39(8):1247–1254. doi: 10.1016/s0735-1097(02)01751-5. [DOI] [PubMed] [Google Scholar]
- 4.Rose EA, Gelijns AC, Moskowitz AJ, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Eng J Med. 2001;345(20):1435–443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 5.Slaughter MS, Sobieski MA, Gallagher C, et al. Low incidence of neurologic events during long-term support with the HeartMate XVE left ventricular assist device. Tex Heart Inst J. 2008;35(3):245–249. [PMC free article] [PubMed] [Google Scholar]
- 6.Zimpfer D, Wieselthaler G, Czerny M, et al. Neurocognitive function in patients with ventricular assist devices: a comparison of pulsatile and continuous flow devices. ASAIO J. 2006;52(1):24–27. doi: 10.1097/01.mat.0000191334.51375.7e. [DOI] [PubMed] [Google Scholar]
- 7.Lazar RM, Shapiro PA, Jaski BE, et al. Neurological events during long-term mechanical circulatory support for heart failure: the Randomized Evaluation of Mechanical Assistance for the Treatment of Congestive Heart Failure (REMATCH) experience. Circulation. 2004;109(20):2423–2427. doi: 10.1161/01.CIR.0000129414.95137.CD. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SR, Givertz MM, Stewart GC, et al. Ventricular assist devices the challenges of outpatient management. J Am Coll Cardiol. 2009;54(18):1647–1659. doi: 10.1016/j.jacc.2009.06.035. [DOI] [PubMed] [Google Scholar]
- 9.Komoda T, Drews T, Sakuraba S, et al. Executive cognitive dysfunction without stroke after long-term mechanical circulatory support. ASAIO J. 2005 Nov-Dec;51(6):764–768. doi: 10.1097/01.mat.0000183685.81983.5d. [DOI] [PubMed] [Google Scholar]
- 10.Thoennissen NH, Schneider M, Allroggen A, et al. High level of cerebral microembolization in patients supported with the DeBakey left ventricular assist device. J Thorac Cardiovasc Surg. 2005;130(4):1159–1166. doi: 10.1016/j.jtcvs.2005.02.068. [DOI] [PubMed] [Google Scholar]
- 11.Thomas CE, Jichichi D, Petrucci R, et al. Neurologic complications of the Novacor left ventricular assist device. Ann Thorac Surg. 2001;72(4):1311–1315. doi: 10.1016/s0003-4975(01)03004-1. [DOI] [PubMed] [Google Scholar]
- 12.Lietz K, Brown K, Ali SS, et al. The role of cerebral hyperperfusion in postoperative neurologic dysfunction after left ventricular assist device implantation for end-stage heart failure. J Thorac Cardiovasc Surg. 2009;137(4):1012–1019. doi: 10.1016/j.jtcvs.2008.11.034. [DOI] [PubMed] [Google Scholar]
- 13.Monk TG, Price CC. Postoperative cognitive disorders. Curr Opin Crit Care. 2011;17(4):376–381. doi: 10.1097/MCC.0b013e328348bece. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deiner S, Silverstein JH. Postoperative delirium and cognitive dysfunction. Br J Anaesth. 2009;103(Suppl 1):i41–146. doi: 10.1093/bja/aep291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krenk L, Rasmussen LS. Postoperative delirium and postoperative cognitive dysfunction in the elderly - what are the differences? Minerva Anestesiol. 2011 July;77(7):742–749. [PubMed] [Google Scholar]
- 16.Tsai TL, Sands LP, Leung JM. An update on postoperative cognitive dysfunction. Adv Anesth. 2010;28(1):269–284. doi: 10.1016/j.aan.2010.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Evered L, Scott DA, Silbert B, et al. Postoperative cognitive dysfunction is independent of type of surgery and anesthetic. Anesth Analg. 2011;112(5):1179–1185. doi: 10.1213/ANE.0b013e318215217e. [DOI] [PubMed] [Google Scholar]
- 18.Canet J, Raeder J, Rasmussen LS, et al. Cognitive dysfunction after minor surgery in the elderly. Acta Anaesthesiol Scand. 2003;47(10):1204–1210. doi: 10.1046/j.1399-6576.2003.00238.x. [DOI] [PubMed] [Google Scholar]
- 19.Rudolph JL, Schreiber KA, Culley DJ, et al. Measurement of post-operative cognitive dysfunction after cardiac surgery: a systematic review. Acta Anaesthesiol Scand. 2010;54(6):663–677. doi: 10.1111/j.1399-6576.2010.02236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krenk L, Rasmussen LS, Kehlet H. New insights into the pathophysiology of postoperative cognitive dysfunction. Acta Anaesthesiol Scand. 2010;54(8):951–956. doi: 10.1111/j.1399-6576.2010.02268.x. [DOI] [PubMed] [Google Scholar]
- 21.Sauër AM, Kalkman C, van Dijk D. Postoperative cognitive decline. J Anesth. 2009;23(2):256–259. doi: 10.1007/s00540-009-0744-5. [DOI] [PubMed] [Google Scholar]
- 22.Johnson T, Monk T, Rasmussen LS, et al. Postoperative cognitive dysfunction in middle-aged patients. Anesthesiology. 2002;96(6):1351–1357. doi: 10.1097/00000542-200206000-00014. [DOI] [PubMed] [Google Scholar]
- 23.Ely EW, Inouye S, Bernard G, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU) JAMA. 2001;286:2703–2710. doi: 10.1001/jama.286.21.2703. [DOI] [PubMed] [Google Scholar]
- 24.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 25.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375(9716):773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 26.Hudetz JA, Patterson KM, Amole O, et al. Postoperative cognitive dysfunction after noncardiac surgery: effects of metabolic syndrome. J Anesth. 2011;25(3):337–344. doi: 10.1007/s00540-011-1137-0. [DOI] [PubMed] [Google Scholar]
- 27.Newman MF, Matthew JP, Grocott HP, et al. Central nervous system injury associated with cardiac surgery. Lancet. 2006;368(9536):694–703. doi: 10.1016/S0140-6736(06)69254-4. [DOI] [PubMed] [Google Scholar]
- 28.Hudetz JA, Gandhi SD, Iqbal Z, et al. Elevated postoperative inflammatory biomarkers are associated with short- and medium-term cognitive dysfunction after coronary artery surgery. J Anesth. 2011;25(1):1–9. doi: 10.1007/s00540-010-1042-y. [DOI] [PubMed] [Google Scholar]
- 29.Cerejeira J, Firmino H, Vaz-Serra A, et al. The neuroinflammatory hypothesis of delirium. Acta Neuropathol. 2010;119:737–754. doi: 10.1007/s00401-010-0674-1. [DOI] [PubMed] [Google Scholar]
- 30.Lin HB, Yang XM, Li TJ, et al. Memory deficits and neurochemical changes induced by C-reactive protein in rats: implication in Alzheimer's disease. Psychopharmacology (Berl) 2009;204(4):705–714. doi: 10.1007/s00213-009-1499-2. [DOI] [PubMed] [Google Scholar]
- 31.Wersching H, Duning T, Lohmann H, et al. Serum C-reactive protein is linked to cerebral microstructural integrity and cognitive function. Neurology. 2010;74(13):1022–1029. doi: 10.1212/WNL.0b013e3181d7b45b. [DOI] [PubMed] [Google Scholar]
- 32.Petrucci RJ, Wright S, Naka Y, et al. Neurocognitive assessments in advanced heart failure patients receiving continuous-flow left ventricular assist devices. J Heart Lung Transplant. 2009;28:542–549. doi: 10.1016/j.healun.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 33.Van Dijk D, Jansen EW, Hijman R, et al. Cognitive outcome after off-pump and on-pump coronary artery bypass graft surgery: a randomized trial. JAMA. 2002;287(11):1405–1412. doi: 10.1001/jama.287.11.1405. [DOI] [PubMed] [Google Scholar]
- 34.Hudetz JA, Patterson KM, Iqbal Z, et al. Metabolic syndrome exacerbates short-term postoperative cognitive dysfunction in patients undergoing cardiac surgery: results of a pilot study. J Cardiothorac Vasc Anesth. 2011;25(2):282–287. doi: 10.1053/j.jvca.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 35.Marnell L, Mold C, Du Clos TW. C-reactive protein: ligands, receptors and role in inflammation. Clin Immunol. 2005;117(2):104–111. doi: 10.1016/j.clim.2005.08.004. [DOI] [PubMed] [Google Scholar]