Abstract
Background
In patients with diabetes mellitus (DM) and upper GI symptoms, a diagnosis of diabetic gastroparesis is often considered, but population-based data on the epidemiology of diabetic gastroparesis are lacking. We aimed to estimate the frequency of and risk factors for gastroparesis among community subjects with DM
Methods
In this population-based, historical cohort study, the medical records linkage system of the Rochester Epidemiology Project was used to identify 227 Olmsted County, MN residents with type 1 DM in 1995, a random sample of 360 residents with type 2 DM, and an age- and sex-stratified random sample of 639 non-diabetic residents. Using defined diagnostic criteria, we estimated the subsequent risk of developing gastroparesis in each group through 2006. The risk in DM, compared to frequency matched community controls, was assessed by Cox proportional hazards modelling.
Results
The cumulative proportions developing gastroparesis over a 10-year time period were 5.2% in type 1 DM, 1.0% in type 2 DM, and 0.2% in controls. The age- and gender-adjusted hazard ratios (HR) for gastroparesis (relative to controls) was 33 (95% CI: 4.0–274) in type 1 DM and 7.5 (95% CI: 0.8–68) in type 2 DM. The risk of gastroparesis in type 1 DM was significantly greater than in type 2 DM (HR: 4.4 [1.1, 17]). Heartburn (HR: 6.6 [1.7, 25]) at baseline was associated with diabetic gastroparesis in type 1 DM.
Conclusions
Gastroparesis is relatively uncommon in patients with DM, although an increased risk for gastroparesis was observed in type 1 DM.
INTRODUCTION
Gastroparesis is a clinical syndrome characterized by delayed gastric emptying in the absence of mechanical obstruction of the stomach.1 Symptoms of gastroparesis are variable but may include early satiety, nausea, vomiting, bloating, and upper abdominal pain. In several studies based on patients seen at tertiary centers, diabetes mellitus (DM) accounted for almost one third of gastroparesis cases.2–4 Diabetic gastroparesis can result in nutritional compromise, impaired glucose control, and a poorer quality of life, independent of other factors such as age, tobacco and alcohol use, or type of diabetes.4–6
The epidemiology of diabetic gastroparesis is largely unknown. The prevalence of gastroparesis is difficult to estimate due to the poor correlation of symptoms with gastric emptying.3, 7 Cross-sectional studies,8–10 in most cases using radionuclide techniques to measure gastric emptying, have established that gastric emptying of a solid or nutrient liquid meal is abnormally slow in up to 30–50% of outpatients with long standing type 1 or type 2 DM. However, these studies were all undertaken in referral centers and almost certainly overestimate the prevalence of the condition because of referral and selection bias.8–10 The incidence (new onset) of diabetic gastroparesis in the general population has not to our knowledge been reported.
In considering the mechanisms responsible for abnormal gastric motor function in DM, two major factors have been suggested, namely autonomic neuropathy and sustained hyperglycemia.11–15 Traditionally, GI symptoms in diabetic patients have been attributed to disordered motor function as a result of the irreversible autonomic neuropathy that frequently accompanies long-standing disease.15 However, in a longitudinal study, progression of autonomic dysfunction and long standing disease were not associated with any slowing of gastric emptying.6
Thus, we aimed to evaluate the risk of gastroparesis among un-selected diabetic subjects in the general community and to evaluate potential risk factors for developing gastroparesis. We hypothesized that long disease duration and autonomic neuropathy would be independent risk factors for developing diabetic gastroparesis.
METHODS
Study Setting
The Olmsted County, MN population comprises 124,277 persons (2000 US Census data), of whom 89% are white; socio-demographically, the community is very similar to the US white population except for higher education and income levels.16 Over 95% of County residents receive their medical care from one of the two group practices (Mayo Medical Center and Olmsted Medical Center). The Mayo Clinic has maintained a common medical record system with its two affiliated hospitals (Saint Marys’ and Rochester Methodist) for over 100 years. Recorded diagnoses and surgical procedures are indexed, including the diagnoses made for outpatients seen in office or clinic consultations, emergency room visits or nursing home care, as well as the diagnoses recorded for hospital inpatients. Under the auspices of the Rochester Epidemiology Project (REP), this indexing and records-linkage system was expanded to non-Mayo providers of care, including the Olmsted Medical Center. Annually, over 80% of the entire population is attended by one or both of these two practices, and 96% of the entire population is seen at least once during any given 3-year period.16 Consequently, this medical records linkage system provides what is essentially an enumeration of the population from which random samples can be drawn.
Subjects
The present investigation took advantage of a previous study that used REP resources to construct a diabetes prevalence cohort comprising all Olmsted County, MN residents who met National Diabetes Data Group (NDDG) criteria as of January 1, 1995,17 created utilizing a 1990 Rochester diabetes prevalence cohort18 and persons assigned a diagnosis of DM or DM-like condition in the REP diagnostic index from 1945 through 1995. 19 Person known to be deceased or who were younger than 18 years (because gastroparesis is uncommon in this age group21) or older than 75 years as of January 1, 1995, (because follow-up would be limited) were excluded. For the current study, we used the existing constructed population-based DM prevalence cohort consisting of 269 residents with type 1 DM and 409 residents with type 2 DM for GI symptom survey at 1995.17
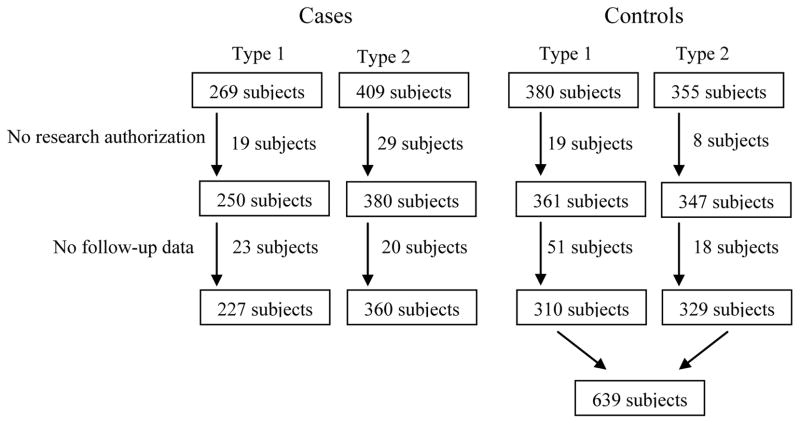
Using the REP database, Olmsted County residents, who were age-and sex-stratified to approximate the age and sex distributions of the residents with type 1 DM and type 2 DM, respectively, were sampled at random. The complete (inpatient and outpatient) medical record of each potential control was reviewed to confirm that they did not meet criteria for DM prior to 1995 and that they resided in Olmsted County in 1995. A total of 1374 subjects (269 with type 1 DM, 409 with type 2 DM, 380 controls for type 1 DM, and 355 controls for type 2 DM) were potentially eligible for the current study, but 73 subjects who refused to provide research authorization based on Minnesota statute19 and 112 subjects who did not have follow-up data after 1995 were excluded (Figure 1). Thus, our final study cohort totalled 1226 subjects, including 227 patients with type 1 DM, 360 with type 2 DM, 310 controls for type 1 DM, and 329 controls for type 2 DM.
Figure 1.
Flow diagram for ascertainment of diabetic cases and controls. Among 269 subjects with type 1 DM, 19 subjects refused to provide medical information and 23 subjects did not have follow up data. Among 406 subjects with type 2 DM, 29 subjects refused to provide research authorization and 20 subjects did not have follow up data. Among 380 controls (for type 1 DM) and 365 controls (for type 2 DM), 25 subjects refused to provide research authorization and 69 subjects did not have follow up data. Finally 227 subjects with type 1 DM, 360 with type 2 DM, and 639 controls were included in this study.
Definitions
Diabetes Mellitus
Diagnostic criteria for DM closely followed NDDG recommendations20 and consisted of two consecutive fasting glucose levels ≥ 140 mg/dl or 1 - and 2-h levels ≥ 200 mg/dl obtained during a standard oral glucose tolerance test. Persons who failed to meet these criteria but for whom oral agents or insulin were used for at least 2 weeks also qualified as having diabetes.18
Type 1 or 2 DM.17, 21
Persons were classified as having type 1 DM if age at diagnosis of DM (i.e., fulfillment of National Diabetes Data Group Investigators’ criteria) is younger than 20 years, or if they met the following 3 criteria: (1) body mass index at diagnosis for men less than 27.8 kg/m2 and for women less than 27.3 kg/m2; (2) insulin therapy within 2 weeks of diagnosis and continued for at least 1 year or until death; and (3) evidence of ketones in serum or urine samples. Conversely, persons who were older than 20 years at diagnosis and did not meet all criteria for type 1 DM were classified as having type 2 DM.
Gastroparesis
Gastroparesis was defined as meeting at least one of the following criteria as recorded in the complete (inpatient and outpatient) community medical records21: 1) Delayed gastric emptying by standard scintigraphy; or 2) Symptoms of nausea and/or vomiting, postprandial fullness, early satiety, bloating, or epigastric pain for more than 3 months plus a physician diagnosis of gastroparesis; or 3) Symptoms of nausea and/or vomiting, postprandial fullness, early satiety, bloating, or epigastric pain for more than 3 months, and food retention on endoscopy or upper GI study.
Ascertainment of Gastroparesis
As described in detail elsewhere,22 the REP diagnostic index and the database of gastric emptying were used to identify subjects with a clinical diagnosis of gastroparesis or any possible gastroparesis condition during the study period (between January 1, 1996 and December 31, 2006). Out of the total 1226 subjects (227 patients with type 1 DM, 360 with type 2 DM, and 639 controls ([310 for type 1 DM and 329 for type 2 DM]), 57 potential gastroparesis cases were identified by either the REP diagnostic index (n = 57), the gastric emptying test database (n = 3) or both (n =3). For confirming the presence of gastroparesis, one author (R.S.C.) conducted a thorough review of physicians’ notes, medication history, past medical and surgical history, radiologic studies (including double contrast upper GI X-ray, small bowel and colon studies, abdominal-pelvic CT scan, and gastric scintigraphy), as well as endoscopy and pathology reports. Based on this detailed review, 3 subjects with a history of gastroparesis before 1995 were excluded. Moreover, 39 additional patients were excluded because of enteric tube feeding (9), spinal cord problems (5), neurologic disease (2), complications after gastric surgery (5), other motility problems (5), gastroesophageal reflux symptoms (3), mechanical obstruction (4), or malignancy (3, esophagus, pancreas, and small bowel); 3 patients had other reasons. Thus, we identified 15 incident gastroparesis cases between 1996 and 2006 (figure 2 shows the flow sheet for case identification).
Figure 2.
Flow diagram for assessment of diabetic gastroparesis. In 227 cases with type 1 DM, we identified 20 potential cases from the Rochester Epidemiology Project (REP) who may have had gastroparesis, and 10 subjects were confirmed to have gastroparesis. In 360 cases with type 2 DM, 17 potential gastroparesis cases were identified and 4 cases were confirmed. Among 639 controls, 20 potential gastroparesis cases were identified but only 1 subject had confirmed gastroparesis.
Assessment of Risk Factors
As described in detail elsewhere,17 a validated GI questionnaire (BDQ) was mailed to all 250 subjects with type 1 DM who had provided the research authorization, with a response rate of 55% (138/250). Although the survey was also mailed to the other groups, including those with type 2 DM and controls, their data could not be used for assessing risk factors because so few gastroparesis cases developed among those subjects.
The survey included questions covering GI tract symptoms, the Somatic Symptom checklist (non-GI symptoms), and symptoms of autonomic and peripheral neuropathy derived from the Neuropathy Symptom Profile developed by Dyck et al.23 As described in detail elsewhere,23 this instrument has been shown to have adequate content, predictive and construct validity in subjects with health, motor neuron disease, diabetic neuropathy, and amyloidosis. Numbness, heat sensation, or a prickly feeling in any part of the body, or weakness in arms or legs were considered indicative of peripheral neuropathy.23 Insufficient sweating and sweating on the face after eating cheese (or gustatory sweating) were suggestive of autonomic dysfunction.23 Postural dizziness was not used as an index of autonomic dysfunction because very few subjects (1%) reported this, and the rates were comparable between subjects with DM and controls.17
Statistical Analysis
The subjects with type 1 or 2 DM and controls were considered at risk for gastroparesis from the entry year to diagnosis of gastroparesis, date of death, end of follow-up (Dec 31, 2006), or the date on which participants moved out of the registry ascertainment area. Hazard ratios (HR) for developing gastroparesis (and 95% confidence intervals [CI]) were calculated from the estimated coefficients in Cox proportional hazards regression models. The cumulative incidence of gastroparesis in patients with type 1 diabetes was estimated via the Kaplan Meier method. We could not evaluate risk factors for gastroparesis in patients with type 2 DM vs corresponding controls due to the small number of gastroparesis “events”. All models were adjusted for potential confounders: age, gender, and/or DM duration. Analyses were done with SAS version 9.1 (SAS Institute, Cary, NC). A significance level of less than 0.05 was used, and all tests were two-sided. A total of 15 subjects developed gastroparesis through the last follow-up date of December 2006. With 15 events there was approximately 80% power (2-sided alpha =0.05) to detect a hazard ratio of 4.2, or greater, for overall diabetics relative to controls. Among the Type 1 diabetics with survey response data, there were 4 events, and assuming roughly 50% of these subjects reported a particular (discrete) risk factor (e.g., nausea), there would be approximately 80% power (2-sided alpha =0.05) to detect a hazard ratio of 16, or greater.
RESULTS
Gastroparesis in Subjects with Type 1 or 2 DM versus Controls
Table 1 shows the demographic features of 227 subjects with type 1 DM, 360 with type 2 DM, and 639 non-diabetic controls (310 for type 1 DM and 329 for type 2 DM) at baseline in 1995. Duration of disease was associated with type of diabetes (p<0.001). Overall, 14 of 587 subjects with type 1 or 2 DM had developed gastroparesis. Over a 10-year follow-up, 10 of 227 subjects with type 1 DM had developed gastroparesis, compared to four among 360 subjects with type 2 DM; only one subject developed gastroparesis among 639 controls between 1/1/1996 and 12/31/2006. The KM estimate of the cumulative proportions (95% CI) developing gastroparesis by 10 years was 5.2% (95% CI: 2.0–8.3) for Type 1 diabetics, 1.0% (95% CI: 0–2.1) for Type II diabetics and 0.2% (95% CI: 0–0.5) for controls. In the combined group of diabetics, the Kaplan Meier estimate for cumulative proportion developing gastroparesis by 10 years was 2.6% (95% CI: 1.2–4.0).
Table 1.
Clinical characteristics of Olmsted County, MN residents with type 1 and 2 diabetes mellitus (DM) and non-diabetic controls.
| Type 1 DM (n=227) | Type 2 DM (n=360) | Controls (n=639) | |
|---|---|---|---|
| Age, median years (range) | 37 (18, 73) | 59 (28, 76) | 50 (19, 77) |
| Male | 110 (48.5%) | 190 (52.8%) | 323 (50.6%) |
| Female | 117 (51.5%) | 170 (47.2%) | 316 (49.4%) |
| Disease duration, median years (range)* | 21.0 (3.9,52.9) | 8.8 (0.1,45.4) | |
| Development of gastroparesis (n=15) | 10 (4.4%) | 4 (1.1%) | 1 (0.2%) |
| No gastroparesis (n=1211) | 217 (95.6%) | 356 (98.9%) | 638 (99.8%) |
p<0.0001, rank sum test for difference of disease duration between type 1 and type 2 DM
The age- and gender-adjusted HR (relative to controls) for gastroparesis was 33 (95% CI: 4.0 – 274; p=0.001) in patients with type 1 DM, while in patients with type 2 DM the HR was 7.5 (95% CI: 0.8–68; p=0.073). In addition, the risk of developing gastroparesis was significantly greater among patients with type 1 DM compared to type 2 DM (HR: 4.4; 95% CI: 1.1–17; p=0.032).
Predictors for Gastroparesis in Type 1 DM
Among a total of 138 responders with type 1 DM on the survey, only 4 subjects subsequently developed gastroparesis between 1996 and 2006. Table 2 shows the HRs for gastroparesis according to demographics, gastrointestinal symptoms, or autonomic/peripheral neuropathy in these subjects. The cumulative proportion of gastroparesis at 10 years in younger subjects (< 40 years) with type 1 DM was 8.5% (95% CI: 1.1–15.4) but in older subjects was 7.2 % (95% CI: 0.1–13,8). The cumulative proportion with gastroparesis was higher in women compared to men, but the difference was not statistically significant (Table 2). In addition, the cumulative proportion with gastroparesis was higher in subjects with longer disease duration (more than 20 years) compared to subjects with less disease duration, but this association was not statistically significant either. Adjusting for age, gender and duration of disease, a significantly increased risk of gastroparesis was observed in subjects with heartburn at baseline (HR: 6.6; 95% CI: 1.7–25; p=0.006), relative to the subjects with no heartburn. However, no other GI symptom including nausea, vomiting, abdominal discomfort, was significantly associated with gastroparesis in subjects with type 1 DM. Autonomic neuropathy and symptoms of peripheral neuropathy were also not significantly associated with developing gastroparesis in subjects with type 1 DM (Table 2).
Table 2.
Cumulative incidence and predictors of developing gastroparesis in patients with type 1 DM
| Cumulative % (95% CI) | HR (95% CI)§ | |
|---|---|---|
| Age < 40 (n=71) | 8.5 (1.1,15) | 1.2 (0.7,2.2)+ |
| Age >= 40 (n=62) | 7.2 (0.1,14) | 1.0 (ref) |
| Male (n=59) | 2.1 (0,6.2) | 0.2 (0.02,1.2) |
| Female (n=74) | 12.2 (3.9,20) | 1.0 (ref) |
| Disease duration <= 20 years (n=56) | 11.1 (1.4,20) | 0.9 (0.6,1.3)* |
| Disease duration > 20 years (n=77) | 6.0 (0.1,12) | 1.0 (ref) |
| Disease duration (per year) | 0.98 (0.92,1.05) | |
|
| ||
| Heartburn (n=15) | 26.7 (0.5,46) | 6.6 (1.7,25) |
| no heartburn (n=118) | 5.2 (0.6,9.6) | 1.0 (ref) |
|
| ||
| Acid regurgitation(n=38) | 14.4 (0,27) | 2.5 (0.6,9.6) |
| no acid regurgitation (n=90) | 6.2 (0.8,11) | 1.0 (ref) |
|
| ||
| Nausea (n=15) | 14.4 (0,31) | 1.9 (0.4,9.5) |
| no nausea (n=118) | 7.0 (1.8,12) | 1.0 (ref) |
|
| ||
| Vomiting (n=5) | 0 | NE |
| no vomiting (n=124) | 8.5 (3.0,14) | |
|
| ||
| Dyspepsia (n=25) | 9.1 (0,20) | 1.0 (0.2,5.0) |
| no dyspepsia (n=108) | 7.6 (2.0,13) | 1.0 (ref) |
|
| ||
| Straining (N=6) | 0 | NE |
| No straining (n=127) | 8.3 (2.9,13) | |
|
| ||
| Hard or lumpy stools (n=21) | 4.8 (0,13) | 0.8 (0.1,6.5) |
| no hard or lumpy stools (n=112) | 8.5 (2.7,14) | 1.0 (ref) |
|
| ||
| Loose stools (n=8) | 16.7 (0,42) | 1.5 (0.2,12) |
| no loose stools (n=125) | 7.4 (2.3,12) | 1.0 (ref) |
|
| ||
| Abdominal pain (n=25) | 9.1 (0,20) | 1.0 (0.2,5.0) |
| no abdominal pain (n=108) | 7.6 (2.0,13) | 1.0 (ref) |
|
| ||
| Peripheral neuropathy (overall) (n=66) | 11.9 (3.7,20) | 3.1 (0.6,15) |
| no peripheral neuropathy (n=62) | 3.9 (0,9.0) | 1.0 (ref) |
| Numbness (n=46) | 14.0 (2.9,24) | 2.6 (0.6,11) |
| no numbness (n=82) | 4.5 (0,9.3) | 1.0 (ref) |
| Muscular weakness (n=44) | 15.9 (3.2,27) | 4.1 (0.99,17) |
| no muscular weakness (n=84) | 4.1 (0,8.6) | 1.0 (ref) |
|
| ||
| Autonomic neuropathy (overall) (n=13) | 16.7 (0,35) | 2.6 (0.5,14) |
| no autonomic neuropathy (n=115) | 7.3 (1.9,12) | 1.0 (ref) |
| Insufficient sweating(n=9) | 22.2 (0,45) | 3.3 (0.6,19) |
| no insufficient sweating (n=119) | 7.1 (1.9,12) | 1.0 (ref) |
| Facial sweating (n=5) | 0 | NE |
| no facial sweating (n=123) | 8.6 (3.0,14) | |
Adjusted for age, gender and disease duration
HR per 10 years of age
HR per 5 years of disease duration
NE = not estimable due to zero category counts
DISCUSSION
Gastroparesis is relatively rare in the general population, and we have previously calculated an age-adjusted annual incidence of just 2.4 per 100,000 for men and 9.8 per 100,000 for women in a US population.22 However, DM does appear to increase this risk substantially. In our study, the risk of developing gastroparesis among subjects with type 1 DM was elevated over 30-fold, whereas the risk in subjects with type 2 DM was increased almost 8-fold, relative to age- and sex-matched controls. Moreover, we observed that subjects with type 1 DM were four times more likely to develop gastroparesis than those with type 2 DM, consistent with poorer diabetic control and high rates of autonomic neuropathy that occur in patients with type 1 DM.24, 25 A remarkable finding of the current study is that the incidence of clinically-evident gastroparesis among those with diabetes is still rare.
The current population-based epidemiological study of diabetic gastroparesis suggests that the impact of diabetes on the likelihood of gastroparesis among community patients may have been previously overestimated because previous work on gastroparesis in subjects with DM were mainly based on upper GI symptoms by surveys, not on actual diagnosis or tests.11, 22, 25 The reported prevalence of delayed gastric emptying in patients with longstanding diabetes in tertiary hospital settings has ranged from 28% to 65%,10, 26–30 but, to our knowledge, there are no other true population-based studies describing the risk of gastroparesis in unselected diabetic subjects. In the present study, we observed that the cumulative incidence of developing gastroparesis in type 1 DM was 5.2% over 10 years, while the overall cumulative risk in type 2 DM was 1.0%; only 0.2% of controls developed gastroparesis during the follow-up. In a small study from a tertiary hospital setting, Jones et al.6 observed that gastric emptying of the solid component was delayed in 10 of 20 patients with DM at baseline, and was delayed in an additional 6 patients at follow-up. By contrast, our study demonstrated a very low incidence of gastroparesis in a relatively large community-based DM population.
In a recent population-based study, Jung et al.22 found a distinct female predominance of gastroparesis: The incidence and prevalence of gastroparesis in women were four times higher than in men. Our study also showed a female predominance of gastroparesis in subjects with type 1 DM, although this was not statistically significant possibly due to the small sample size and limited statistical power. Other studies have also reported that female gender was associated with delayed gastric emptying in patients with diabetes.3, 29 Female hormonal changes might explain this gender bias as premenopausal women on oral contraceptives, pregnant women during labor, and postmenopausal women receiving hormone therapy have developed delayed gastric emptying for both solids and liquids that appears to be reversible.31–33 Another possible explanation is a difference in health-care seeking behavior. It is generally accepted that most functional gastrointestinal disorders are more common in women than in men, and females with dyspepsia seek health care more frequently than males.34
Other risk factors for diabetic gastroparesis remain poorly defined,4, 6, 30 although duration of disease and the presence of complications such as retinopathy, neuropathy, and nephropathy are thought to be potentially important.4, 29, 30 We did not find a significant association between DM duration and the development of gastroparesis, and the hazard ratio was 0.9 (95% CI: 0.6–1.3). In a larger cross-sectional questionnaire study of subjects with DM recruited from outpatient clinics (n = 209) and the community (n = 892), Bytzer et al.25 showed that co-existing peripheral neuropathy was significantly associated with the development of gastroparesis, but autonomic neuropathy was not. In addition, they showed that poor glycemic control measured both by self-report and Hb A1c was an independent risk factor for upper GI symptoms, but the duration and type of diabetes was not significant.25 In a 2-year prospective follow-up study, Quan et al.35 observed no clear association between gastrointestinal symptoms and autonomic neuropathy or glycemic control. The current study did not look at whether long-term poor glycemic control associated with the development of complications such as neuropathy contributes to the risk of gastroparesis. Further studies on the link between glycemic control and developing the gastroparesis in subjects with diabetes are warranted. However, we did not find any significant association between neuropathy and the development of gastroparesis, although heartburn was a predictor.
The current study has several strengths. We undertook a population-based approach which should have minimized the effects of referral bias and improved the generalizability of our results, at least to the US white population, although non-whites are underrepresented in this population.18 The population at risk was clearly defined because they were selected from well-established community cohorts with and without documented DM.17 Notably, ascertainment of gastroparesis (gastric emptying database with confirmed with record review) was based on a community-wide diagnostic index and test results with record review, that has an established track record of accuracy.16 There are also some potential limitations of this study, most importantly the limited sample size. This was particularly problematic in evaluating potential risk factors for gastroparesis among the subjects with type 1 DM, but we believe these results to be of use in designing future studies of the problem. Also, this was a historical cohort study, and a key issue may be underreporting or under-recognition of gastroparesis because we only identified those who visited a physician for symptoms of gastroparesis. However, our community-wide diagnostic index was designed to be highly sensitive,16 and we did undertake a detailed medical record review to confirm the diagnosis of gastroparesis by an expert. In addition, although validated diagnostic criteria for gastroparesis are lacking, we relied on previously published criteria21 but these may have overcalled true gastroparesis and the incidence may be even lower.
In summary, this is to our knowledge the first population-based cohort study to describe the incidence and risk factors for gastroparesis in subjects with diabetes mellitus in the community. An increased risk for gastroparesis in Type 1 DM, and possibly Type 2 DM, was observed. However, gastroparesis occurs in fewer than 5% of people with Type 1 DM in the community. Thus, we conclude that gastroparesis is a relatively uncommon complication of diabetes. Consequently, other causes of upper GI symptoms in diabetic patients need to be considered first in clinical practice.
Study Highlights.
What is current knowledge
Diabetes is said to be one of the most common etiologies of gastroparesis
The incidence (new onset) of gastroparesis in diabetic patients is unknown
30–50% of outpatients with long standing type 1 or type 2 DM have slow gastric emptying
What is new here
The risk of developing gastroparesis among subjects with type 1 DM was elevated over 30-fold, whereas the risk in subjects with type 2 DM was increased almost 8-fold, relative to age- and sex-matched controls
Subjects with type 1 DM were four times more likely to develop gastroparesis than those with type 2 DM
The incidence of gastroparesis among those with diabetes is still rare
Acknowledgments
This study was supported by American College of Gastroenterology, and made possible by the Rochester Epidemiology Project (Grant R01- AG034676 from the National Institute on Aging).
The authors wish to thank Lori R. Anderson for her assistance in the preparation of the manuscript.
Footnotes
Specific author contributions: Design, analysis, writing and revising of this paper: Rok Seon Choung, G. Richard Locke III, Cynthia L. Leibson, Alan R. Zinsmeister, L. Joseph Melton III, and Nicholas J. Talley; statistical analysis: Alan R. Zinsmeister and Cathy D. Schleck.
Conflict of Interest
Dr. Talley and Mayo Clinic have licensed the Talley Bowel Disease Questionnaire.
References
- 1.Parkman HP, Hasler WL, Fisher RS. American Gastroenterological Association medical position statement: diagnosis and treatment of gastroparesis. Gastroenterology. 2004;127:1589–91. doi: 10.1053/j.gastro.2004.09.054. [DOI] [PubMed] [Google Scholar]
- 2.Park MI, Camilleri M. Gastroparesis: clinical update. Am J Gastroenterol. 2006;101:1129–39. doi: 10.1111/j.1572-0241.2006.00640.x. [DOI] [PubMed] [Google Scholar]
- 3.Horowitz M, Wishart JM, Jones KL, Hebbard GS. Gastric emptying in diabetes: an overview. Diabet Med. 1996;13:S16–22. [PubMed] [Google Scholar]
- 4.Camilleri M. Clinical practice. Diabetic gastroparesis. N Engl J Med. 2007;356:820–9. doi: 10.1056/NEJMcp062614. [DOI] [PubMed] [Google Scholar]
- 5.Kong MF, Horowitz M, Jones KL, Wishart JM, Harding PE. Natural history of diabetic gastroparesis. Diabetes Care. 1999;22:503–7. doi: 10.2337/diacare.22.3.503. [DOI] [PubMed] [Google Scholar]
- 6.Jones KL, Russo A, Berry MK, Stevens JE, Wishart JM, Horowitz M. A longitudinal study of gastric emptying and upper gastrointestinal symptoms in patients with diabetes mellitus. Am J Med. 2002;113:449–55. doi: 10.1016/s0002-9343(02)01228-7. [DOI] [PubMed] [Google Scholar]
- 7.Abell TL, Bernstein VK, Cutts T, Farrugia G, Forster J, Hasler WL, McCallum RW, Olden KW, Parkman HP, Parrish CR, Pasricha PJ, Prather CM, Soffer EE, Twillman R, Vinik AI. Treatment of gastroparesis: a multidisciplinary clinical review. Neurogastroenterol Motil. 2006;18:263–83. doi: 10.1111/j.1365-2982.2006.00760.x. [DOI] [PubMed] [Google Scholar]
- 8.Meier M, Linke R, Tatsch K, Standl E, Schnell O. An advanced approach for the assessment of gastric motor function in long-term type 1 diabetes mellitus with and without autonomic neuropathy. Clin Auton Res. 2002;12:197–202. doi: 10.1007/s10286-002-0023-0. [DOI] [PubMed] [Google Scholar]
- 9.Iber FL, Parveen S, Vandrunen M, Sood KB, Reza F, Serlovsky R, Reddy S. Relation of symptoms to impaired stomach, small bowel, and colon motility in long-standing diabetes. Dig Dis Sci. 1993;38:45–50. doi: 10.1007/BF01296772. [DOI] [PubMed] [Google Scholar]
- 10.Horowitz M, Harding PE, Maddox AF, Wishart JM, Akkermans LM, Chatterton BE, Shearman DJ. Gastric and oesophageal emptying in patients with type 2 (non-insulin-dependent) diabetes mellitus. Diabetologia. 1989;32:151–9. doi: 10.1007/BF00265086. [DOI] [PubMed] [Google Scholar]
- 11.Bytzer P, Talley NJ, Leemon M, Young LJ, Jones MP, Horowitz M. Prevalence of gastrointestinal symptoms associated with diabetes mellitus: a population-based survey of 15,000 adults. Arch Intern Med. 2001;161:1989–96. doi: 10.1001/archinte.161.16.1989. [DOI] [PubMed] [Google Scholar]
- 12.Samsom M, Akkermans LM, Jebbink RJ, van Isselt H, vanBerge-Henegouwen GP, Smout AJ. Gastrointestinal motor mechanisms in hyperglycaemia induced delayed gastric emptying in type I diabetes mellitus. Gut. 1997;40:641–6. doi: 10.1136/gut.40.5.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jones KL, Kong MF, Berry MK, Rayner CK, Adamson U, Horowitz M. The effect of erythromycin on gastric emptying is modified by physiological changes in the blood glucose concentration. Am J Gastroenterol. 1999;94:2074–9. doi: 10.1111/j.1572-0241.1999.01280.x. [DOI] [PubMed] [Google Scholar]
- 14.Ko GT, Chan WB, Chan JC, Tsang LW, Cockram CS. Gastrointestinal symptoms in Chinese patients with Type 2 diabetes mellitus. Diabet Med. 1999;16:670–4. doi: 10.1046/j.1464-5491.1999.00135.x. [DOI] [PubMed] [Google Scholar]
- 15.Locke GR., 3rd Epidemiology of gastrointestinal complications of diabetes mellitus. Eur J Gastroenterol Hepatol. 1995;7:711–6. [PubMed] [Google Scholar]
- 16.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 17.Maleki D, Locke GR, 3rd, Camilleri M, Zinsmeister AR, Yawn BP, Leibson C, Melton LJ., 3rd Gastrointestinal tract symptoms among persons with diabetes mellitus in the community. Arch Intern Med. 2000;160:2808–16. doi: 10.1001/archinte.160.18.2808. [DOI] [PubMed] [Google Scholar]
- 18.Leibson CL, O’Brien PC, Atkinson E, Palumbo PJ, Melton LJ., 3rd Relative contributions of incidence and survival to increasing prevalence of adult-onset diabetes mellitus: a population-based study. Am J Epidemiol. 1997;146:12–22. doi: 10.1093/oxfordjournals.aje.a009187. [DOI] [PubMed] [Google Scholar]
- 19.Melton LJ., 3rd The threat to medical-records research. N Engl J Med. 1997;337:1466–70. doi: 10.1056/NEJM199711133372012. [DOI] [PubMed] [Google Scholar]
- 20.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–57. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 21.Melton LJ, 3rd, Palumbo PJ. Empirical determination of diabetes clinical type. Diabetes Care. 1985;8:205–6. doi: 10.2337/diacare.8.2.205. [DOI] [PubMed] [Google Scholar]
- 22.Jung HK, Choung RS, Locke GR, 3rd, Schleck CD, Zinsmeister AR, Szarka LA, Mullan B, Talley NJ. The incidence, prevalence, and outcomes of patients with gastroparesis in Olmsted County, Minnesota, from 1996 to 2006. Gastroenterology. 2009;136:1225–33. doi: 10.1053/j.gastro.2008.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dyck PJ, Karnes J, O’Brien PC, Swanson CJ. Neuropathy Symptom Profile in health, motor neuron disease, diabetic neuropathy, and amyloidosis. Neurology. 1986;36:1300–8. doi: 10.1212/wnl.36.10.1300. [DOI] [PubMed] [Google Scholar]
- 24.Merio R, Festa A, Bergmann H, Eder T, Eibl N, Stacher-Janotta G, Weber U, Budka C, Heckenberg A, Bauer P, Francesconi M, Schernthaner G, Stacher G. Slow gastric emptying in type I diabetes: relation to autonomic and peripheral neuropathy, blood glucose, and glycemic control. Diabetes Care. 1997;20:419–23. doi: 10.2337/diacare.20.3.419. [DOI] [PubMed] [Google Scholar]
- 25.Bytzer P, Talley NJ, Hammer J, Young LJ, Jones MP, Horowitz M. GI symptoms in diabetes mellitus are associated with both poor glycemic control and diabetic complications. Am J Gastroenterol. 2002;97:604–11. doi: 10.1111/j.1572-0241.2002.05537.x. [DOI] [PubMed] [Google Scholar]
- 26.Jones KL, Horowitz M, Carney BI, Wishart JM, Guha S, Green L. Gastric emptying in early noninsulin-dependent diabetes mellitus. J Nucl Med. 1996;37:1643–8. [PubMed] [Google Scholar]
- 27.Koch KL, Stern RM, Stewart WR, Vasey MW. Gastric emptying and gastric myoelectrical activity in patients with diabetic gastroparesis: effect of long-term domperidone treatment. Am J Gastroenterol. 1989;84:1069–75. [PubMed] [Google Scholar]
- 28.Samsom M, Vermeijden JR, Smout AJ, Van Doorn E, Roelofs J, Van Dam PS, Martens EP, Eelkman-Rooda SJ, Van Berge-Henegouwen GP. Prevalence of delayed gastric emptying in diabetic patients and relationship to dyspeptic symptoms: a prospective study in unselected diabetic patients. Diabetes Care. 2003;26:3116–22. doi: 10.2337/diacare.26.11.3116. [DOI] [PubMed] [Google Scholar]
- 29.Jones KL, Russo A, Stevens JE, Wishart JM, Berry MK, Horowitz M. Predictors of delayed gastric emptying in diabetes. Diabetes Care. 2001;24:1264–9. doi: 10.2337/diacare.24.7.1264. [DOI] [PubMed] [Google Scholar]
- 30.Samsom M, Bharucha A, Gerich JE, Herrmann K, Limmer J, Linke R, Maggs D, Schirra J, Vella A, Worle HJ, Goke B. Diabetes mellitus and gastric emptying: questions and issues in clinical practice. Diabetes Metab Res Rev. 2009;25:502–14. doi: 10.1002/dmrr.974. [DOI] [PubMed] [Google Scholar]
- 31.Baron TH, Ramirez B, Richter JE. Gastrointestinal motility disorders during pregnancy. Ann Intern Med. 1993;118:366–75. doi: 10.7326/0003-4819-118-5-199303010-00008. [DOI] [PubMed] [Google Scholar]
- 32.Baschetti R. Gastric emptying: gender differences. N Z Med J. 1997;110:238. [PubMed] [Google Scholar]
- 33.Knight LC, Parkman HP, Brown KL, Miller MA, Trate DM, Maurer AH, Fisher RS. Delayed gastric emptying and decreased antral contractility in normal premenopausal women compared with men. Am J Gastroenterol. 1997;92:968–75. [PubMed] [Google Scholar]
- 34.Stanghellini V, Tosetti C, Paternico A, Barbara G, Morselli-Labate AM, Monetti N, Marengo M, Corinaldesi R. Risk indicators of delayed gastric emptying of solids in patients with functional dyspepsia. Gastroenterology. 1996;110:1036–42. doi: 10.1053/gast.1996.v110.pm8612991. [DOI] [PubMed] [Google Scholar]
- 35.Quan C, Talley NJ, Jones MP, Spies J, Horowitz M. Gain and loss of gastrointestinal symptoms in diabetes mellitus: associations with psychiatric disease, glycemic control, and autonomic neuropathy over 2 years of follow-up. Am J Gastroenterol. 2008;103:2023–30. doi: 10.1111/j.1572-0241.2008.01943.x. [DOI] [PubMed] [Google Scholar]