Abstract
Objectives
Percutaneous ablation of breast cancer has shown promise as a treatment alternative to open lumpectomy. We hypothesized that percutaneous removal of breast cancer followed by percutaneous ablation to sterilize and widen the margins would not only provide fresh naïve tissue for tumor marker and research investigation but better achieve negative margins post ablation.
Methods
Patients diagnosed by percutaneous biopsy (ultrasound or stereotactic-guided) with breast cancer ≤1.5cm, >1cm from the skin and <5mm residual disease by MRI were accrued to this IRB-approved study. Patients were randomized to laser versus radiofrequency ablation (RFA). The US-guided ablation was performed in the OR and followed by immediate excision, whole mount pathology with PCNA staining and reconstruction.
Results
21 patients were accrued to the study. The laser arm (3 patients) pathology demonstrated unpredictability of the ablation zone and residual live tumor. Fifteen patients received RFA and all showed 100% ablation and negative margins. The mean pre-ablation estimated tumor size was O.7cm±0.2cm(range 0.3–1cm). MRI was helpful in ruling out multicentricity but less so in predicting presence or absence of residual disease. Seven of these patients showed no residual tumor and the other half showed residual dead tumor (O.5cm±0.7cm(range 0.1–2.5cm)) at the biopsy site with clear margins.
Conclusion
This pilot study represents the first report of the feasibility of the novel approach to minimally invasive therapy that is percutaneous excision and effectively cytoreduction followed by RFA of margins for the treatment of breast cancer.
INTRODUCTION
Breast cancer treatment favors breast conservation surgery, with an emphasis on improved cosmesis. However, breast conservation surgery via lumpectomy results in some deformity and often requires repeat surgery to establish clear margins.1,2,3,4,5 Radiation is then added to complete the treatment resulting in further diminution of the cosmetic result. Percutaneous treatment of breast cancer was born out of the desire for immediate and incisionless therapy and the tools now available to potentially accomplish such management.
The rationale for using radiofrequency ablation to treat breast cancer is based on promising results seen in treating several other tumor types, including liver, bone, brain, kidney, pancreas, and prostate (reviewed in 6). Several trials of percutaneous RFA in patients with breast cancer have also yielded promising results (Table 1). Complete coagulative necrosis of intact tumors was noted in 86–100% of patients. Treatment was well tolerated, with few treatment-related complications noted in these ablate and resect protocols.7,8,9,10,11,12 Several investigators have published the results of percutaneous ablation (without resection) usually in very ill patients with or without radiation and following patients with periodic biopsy of the cavity with favorable results in short-term follow-up.(Table 2) Likewise percutaneous laser ablation may be used in the same way. Clinical and basic science studies have shown that Interstitial Laser Photocoagulation (ILP) can effectively ablate cancers via a percutaneous approach.13,14,15,16,17,18,19,20,21
Table 1.
| Trials | Patients | n | Treatment | Outcome |
|---|---|---|---|---|
| Izzo et al82001 (pilot) | T1/2 | 26 | US-guided RFA(margin of ≥ 5mm) | Complete coagulative necrosis in 25/26 |
| Singletary et al 12 2002 | T1 | 30 | Intraoperative RFA→ excision | Complete ablation in 87% of patients |
| Burak et al102003 | Tumor ≤ 2 cm in diameter | 10 | RFA → surgery (1–3 wk later) | No residual lesions detected on MRI in 8/9 patients |
| Hayashi et al11 2003 | Tumor ≤ 3 cm in diameter | 10 | RFA → surgery (1–2 wk later) | Complete coagulative necrosis in 19/22 |
| Fornage et al92004 | Tumor ≤ 2 cm in diameter | 21 | RFA → surgery | Complete coagulative necrosis in 20 pts |
| Noguchi et al34 2006 | T1 | 10 | RFA → surgery | No Viable Tumor |
| Earashi et al35 2007 | T1 | 24 | RFA → surgery | No Viable Tumor |
| Manenti et al36 2009 | Tumor ≤ 2 cm in diameter | 34 | RFA → surgery (4 wks later) | Complete coagulative necrosis in 26/25 |
| Wiksel et al37 2010 | ≤1.6cm | 31 | RFA → surgery | Complete coagulative necrosis in 33/34 |
Table 2.
| Trials | RFA Treatment | n | Assessment of Ablated Lesion | Outcome |
|---|---|---|---|---|
| Oura et al38 | Cool Tip | 52 | FNA biopsy | 15 month mean F/U 1 LR, 1 burn |
| Brkljacic et al39 | 6 | Routine in very ill patients |
9–49 months, 1 infection, 2 deaths due to other disease | |
| Susini et al40 | 3 | Core | No LR 18 mo | |
| Earashi et al40 | Starburst XL | 6 | Mammotome | 4 month mean F/U No LR |
| Marcy et al41 | Elektrotom | 4 | Core Needle biopsy | 29 month mean F/U 1 LR 1 abscess |
| Yamamoto et al 42 | Cool Tip | 29 | Vacuum-assisted core biopsy | 1 month, no viable tumor in 24 of 26 |
Nonetheless, several limitations of percutaneous ablation must be addressed before this technique can be considered a definitive treatment modality in breast cancer. Percutaneous ablation does not allow for full assessment of the lesion and therefore could never be used for in situ carcinoma; lack of methodology or imaging to assure ablation of the lesion; follow-up imaging remains problematic in detecting residual or recurrent disease; ablating tumors after taken cores only for diagnosis leaves little opportunity for discovery of future treatments.
Using Image-guided Vacuum-assisted Excisional Biopsy (IVEB) for lesion removal, and interstitial laser photocoagulation (ILP) or radiofrequency ablation (RFA) for ablation of margins, we propose to combine these methods to treat patients with small breast cancers. In this way small lesions are removed or near removed and the percutaneous application of ablation treats the surrounding subcutaneous tissue in order to obtain the necessary margin. Therefore, we hypothesized that laser or radiofrequency ablation after single-insertion IVEB could be used to achieve negative margins in unicentric breast cancers ≤1.5 cm in diameter (staged as T1c or less).
Further we sought to find a means to assess the ablation zone. Thus we tested MRI as a means to evaluate the extent of residual disease after percutaneous biopsy. In addition, we sought to develop a method to image the extent of the ablation zone and the distance from the skin for safety.
METHODS
Patients
This was an IRB-approved protocol. Patients with a diagnosis of unicentric invasive breast cancer ≤1.5 cm diagnosed by IVEB who meet the eligibility criteria and had 1 cm residual disease by MRI were presented the protocol and underwent the informed consent process. (Figure 1) Those patients agreeing to participate were randomized to treatment with either radiofrequency or laser for margin ablation. Surgery was scheduled for the next available date and according to patient preference. All options for treatment of the breast were presented to the patient including lumpectomy and mastectomy with or without reconstruction.
Figure 1.
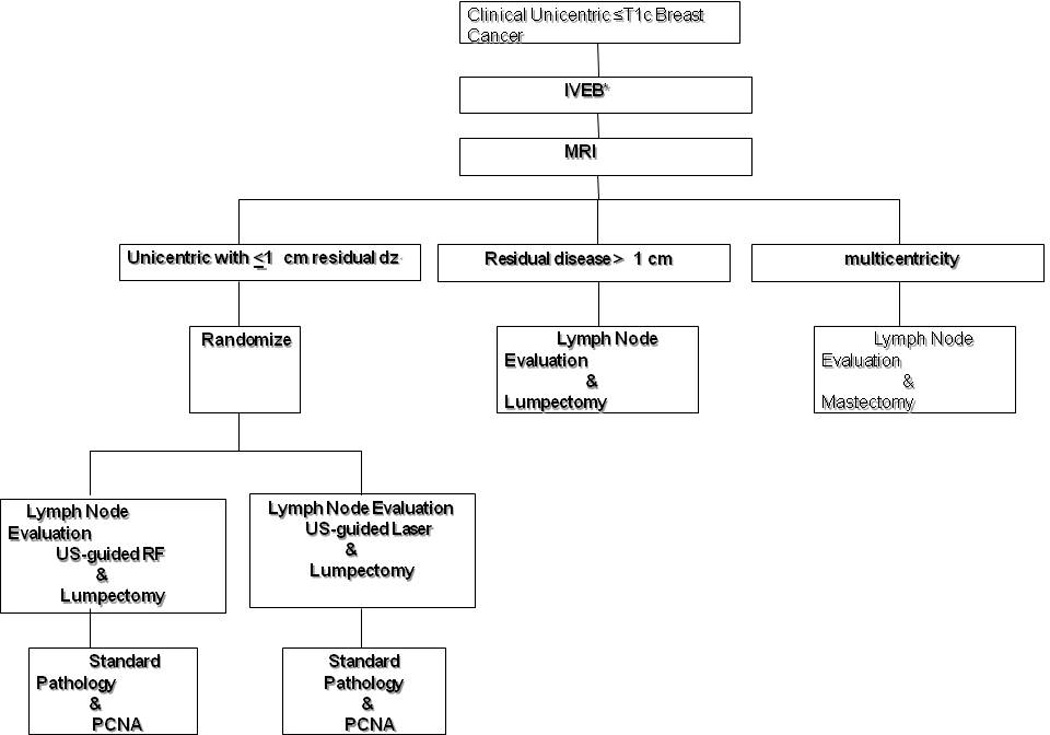
Protocol Design in Phase II with for percutaneous excision followed by radiofrequency ablation.
Study Procedure
Image-guided Vacuum-assisted Excisional Biopsy (IVEB)
Local anesthesia will be accomplished with at least 5 cc of Lidocaine HCL 1% with Epinephrine 1:100,000 and 5 cc Bupivacaine 0.5%. The breast lesion will be removed with the Mammotome Breast Biopsy System ® (Ethicon, Cincinnati, OH) using ultrasound or mammographic guidance for placement of the 8-gauge probe (as per the 501 K use statement, which specifies that after diagnosis an open procedure must be done for any malignancy). The Mammotome® is commonly used at our institution to obtain diagnosis and has been reported to completely remove cancerous lesions (pathologically) nearly 50 percent of the time.22
For lesions not visualized by US a stereotactic procedure was performed with the same system fitted for the stereotactic table.
MRI
A RODEO MRI was scheduled prior to surgery to evaluate for residual and multicentric disease, thus determining eligibility for conservative breast surgery. All images were obtained with a 1.5-Tesla MRI imager using the RODEO pulse sequence, pre- and post-gadolinium contrast (0.1 mmol/kg), high-resolution, three-dimensional images (256×256×128, 5-minute scan time). If definitive residual disease (1.0 cm) post-IVEB or multicentric disease is noted on MRI, the participant then proceeded to standard care and follow-up.
Laser Ablation
Patients randomized to undergo ablation with ILP, at the time of lumpectomy while under general anesthesia, had a Nd:YAG laser cylindrical fiber (1,064 nm) introduced through a 16-gauge needle; the shape of the fiber matched the cavity of the tumor bed. The laser tip was placed in the hematoma in the center of the lesion center of the biopsy cavity site using direct US guidance, a method that we have developed at the PI institution.23 Initial settings for the laser were based on the size of the tumor removed, as judged by the size of the original lesion (by US) and of the cavity left by the percutaneous biopsy.
The Indigo OPTIMA Laser System (Ethicon Endo-surgery Inc, a Johnson-Johnson company, Cincinatti, OH) was used in the operating room with the Laser Safety Officer (SF) present. This laser has wavelength in the range 800–850 nm, power range 2–20 W in continuous mode. This laser has continuous regime with adjustable time duration up to 30 min. The laser fiber, Diffuser-Tip, was developed specially for described laser.
The laser was operated by a laser-certified technician (SF). Laser ablation was performed by introduction of the fiber through a 12–14-gauge needle that is guided by ultrasound into the zone to be treated. After determination of the correct spatial position, the needle was removed. The parameters for laser treatment were chosen from a Table of parameters that depended on the cavity size, power and time. After treatment the laser fiber was removed. The results of the laser ablation for margins were determined by the pathology evaluation on the lumpectomy specimen. If less than the minimum measurement for success (5 mm), the dosage and time was changed for the subsequent cases according to the following approximate equation:
where T exp = the time dose used in the laser ablation for margin, dpredic = the predictable margin depth of the treated zone (1cm), dexp = the margin depth determined after laser treatment by the final pathology.
RFA
In patients randomized to undergo RFA, initial ablative settings on the RF probe were determined by the size of the ablation zone that the operator wished to achieve. The final ablation zone was determined by the size of the tumor removed, as judged by US of the cavity left by percutaneous biopsy (Figure 2), and comprised the size of the cavity plus 2 cm (1-cm margins on each side). (Figure 3a,b) On the day of the planned lumpectomy, after the patient was under anesthesia, the RFA probe (StarBurst® XL, Semi-Flex, Angiodynamics, Queensbury, NY) was inserted into the center of the cavity under US guidance, similarly to the needle-guided procedure. The electrodes were deployed, and the probe connected to the RF generator. Five temperature zones at the periphery of the ablation site are monitored through out the procedure.(Figure 3b) The temperature is gradually increased to the target 100°C over 1–2 minutes. After the 1-minute cool-down period, the probe is retracted from the breast and the tumor bed resected in standard fashion for a lumpectomy.
Figure 2.
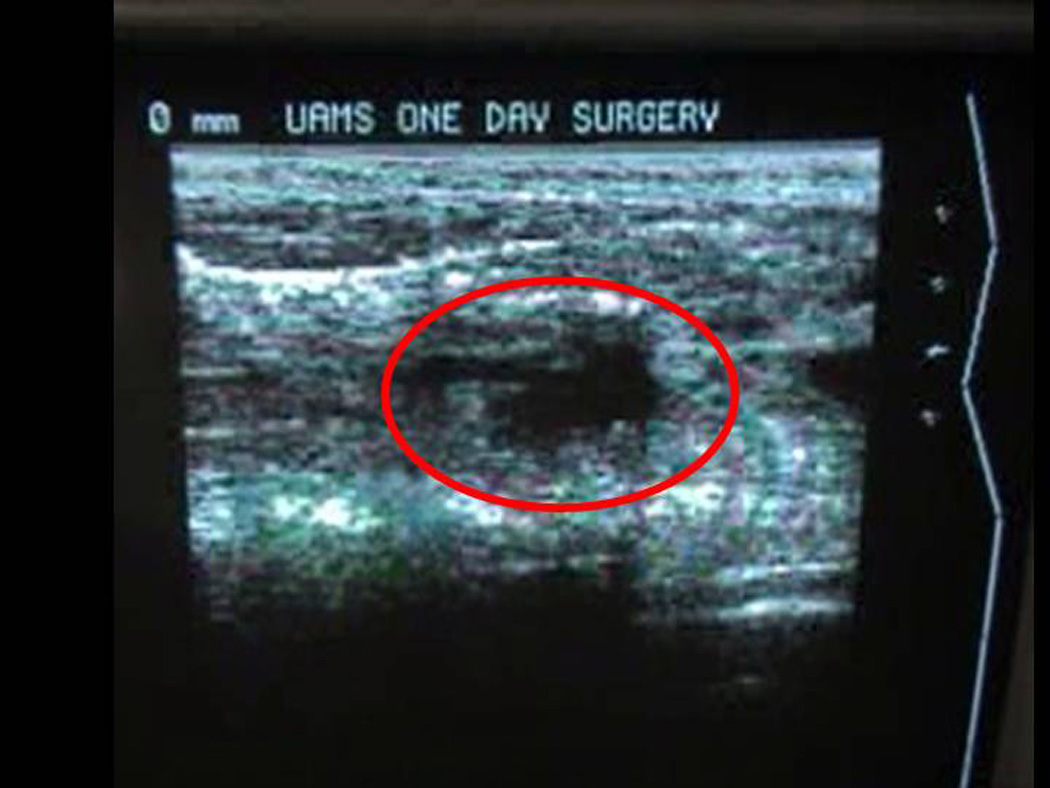
Ultrasound visualization of hematoma in cavity created by percutaneous biopsy.
Figure 3.
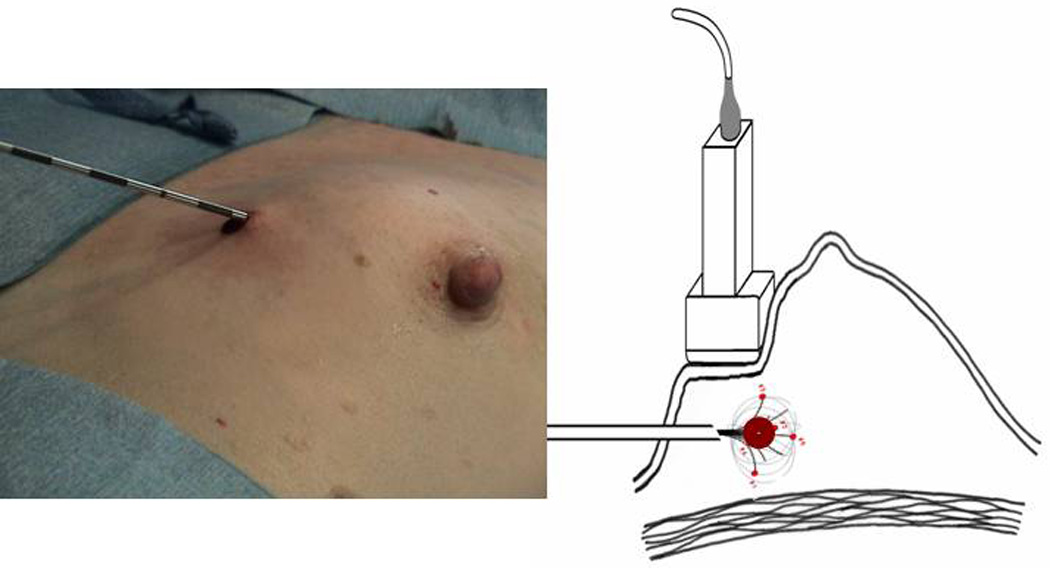
a. Percutaneous RFA deployed into patient, b. Diagram of PeRFA Procedure demonstrating RFA deployed around hematoma and showing the 5 active heating tines of the RF probe.
Standard Breast Surgery
The efficacy of RFA or laser to ablate margins after IVEB was confirmed in this protocol by immediate excisional biopsy. This procedure will be completed after the axillary staging and immediately after the ablation. The breast surgery may include lumpectomy (which will be directed with US-guidance to assure more accurate removal of the en bloc IVEB site and the margin zone of ablation). The tissue specimen was sent immediately from the operating room to Pathology x-ray and fine section processing.
US Doppler
US examination during ablation is a poor monitor of the effects of heat on breast tissue, the tissue appears more hyperechoic on US after RFA or laser and therefore does not give an indication of the size or completeness of the ablation zone. During the course of this study we developed a simple method of visualizing the ablation zone with Doppler US (Phillips Healthcare, Andover, MA). This is based on the movement caused by the off gassing of nitrogen that occurs at 100°C and thus can be visualized by Doppler.24,25 The Doppler monitoring allowed us to stay a safe distance from the skin and also assess the progress and size of the ablation zone. (Figure 4)
Figure 4.
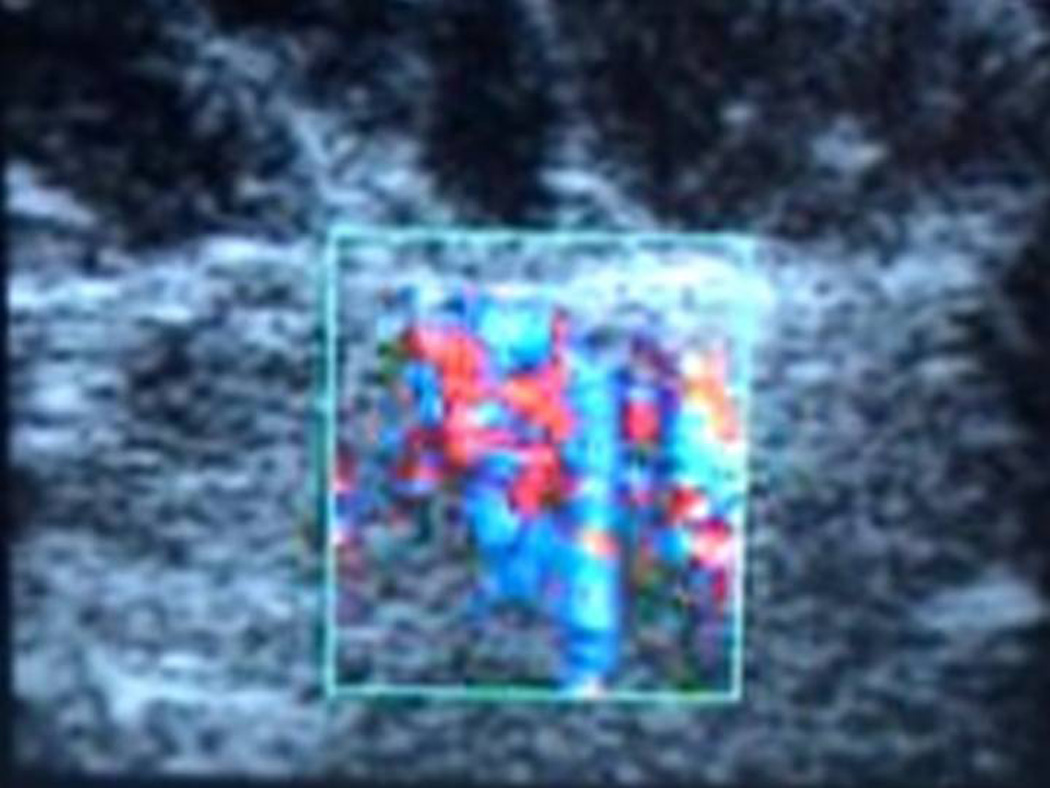
Color Doppler using a 12.5MHz ultrasound probe.
Final Pathology
The specimens were initially processed as whole mounts and blinded H&E pathology read by a single pathologist (SK) The specimens will be serially sectioned and the ablation zone measured on the gross specimen as the distance between the inner rim of the peripheral hyperthermia. (Figure 5a,b,c,d,e) PCNA stains were performed. The PCNA stain is used to determine actively replicating DNA; confirmation of cell death was noted in the absence of PCNA staining. 26
Figure 5.
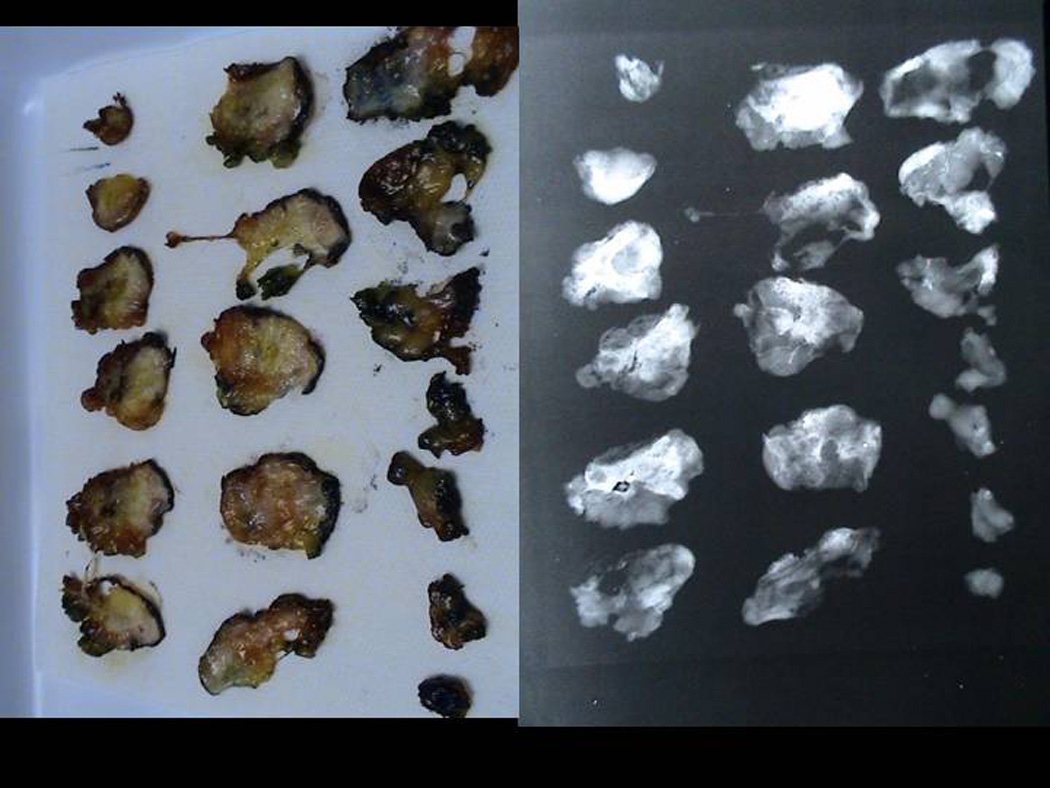
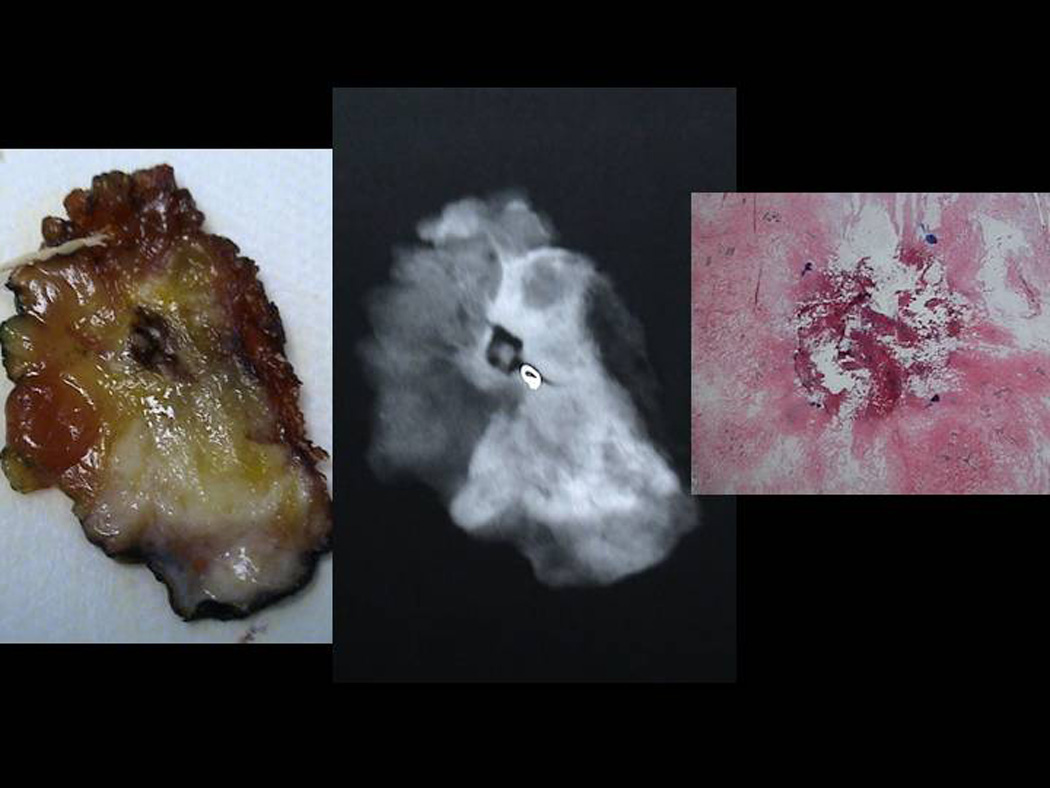
Sectioned Pathology: a. 3mm serial sections of the lumpectomy specimen post PeRFA b. Xray demonstrating clip in biopsy cavity. c. Magnified view of biopsy cavity. d. Magnified view of xray of biopsy cavity demonstrating clip. e. Pathology PeRFA cavity site demonstrating no residual tumor and pericavitary ablation.
Assessment of Complications
An assessment was documented prior to discharge from the recovery area. A 24-hour follow-up assessment will be completed by telephone interview.
RESULTS
Study Patients
Twenty-one patients were accrued to the trial. In Phase I we accrued three patients to the laser arm and three to the RFA arm. In Phase II we accrued 15 patients to the RFA arm only. (Figure 1) We had three screening failures secondary to MRI results. Therefore there were a total of 15 patients accrued to the RFA arm of this trial.
Percutaneous Excision
Nine patients entered the study after stereotactic biopsy and six patient entered after US-guided percutaneous excision.
MRI
Three patients had multicentric disease shown only by MRI and thus were excluded from the study. The mean pre-ablation zone estimated by MRI was 0.7cm±0.2cm (range 0.3 to 1 cm). Ablation was inconsistent in predicting size of or lack of residual at the tumor bed. MRI was helpful in that patients with mammographically occult multicentricity could be excluded from the trial. (Table 3)
Table 3.
| Patient # | MRI tumor size (post-biopsy) | Pathology tumor size (post-RFA) |
|---|---|---|
| 01 | 1.5 cm | No Residual |
| 02 | 2.4 cm Enhancement | 1.3 cm but non-viable |
| 03 | 0.9 cm | 0.9 cm (IDC)/1 cm DCIS but non-viable |
| 04 | - | No Residual |
| 05 | - | No Residual - atypia |
| 06 | 1.2 cm | 2.5 cm — Non-Viable |
| 07 | Possible Minimal Residual | No Residual |
| 08 | 1 cm | < 1 cm — Non-Viable |
| 09 | 0.66 cm | No residual |
| 10 | 1cm | 0.8 cm — Non-Viable |
| 11 | 1 cm | 0.5 cm — Non-Viable |
| 12 | Minimal Residual Enhancement Seen | No Residual |
| 13 | 2 cm Enhancement | Residual Focus — Non-Viable |
| 14 | Mild Enhancement Seen | 0.2 cm — Non-Viable |
| 15 | Thin Rim of Enhancement | No Residual |
Laser
The stop rule was applied in Phase I to the percutaneous laser ablation arm of this trial due to perceived imminent risk secondary to the unpredictability of the laser in even obtaining a consistent ablation zone around the IVEB cavity despite multiple modifications pre-clinical and clinical.
RFA
Fifteen patients were accrued to the RFA arm of the pilot trial. We found that RFA after percutaneous lumpectomy was successful in obtaining a negative margin and obliterating any residual disease in all but one case where a 1mm focus of atypia/DCIS was distant to the immediate ablation zone.
Intraoperative Color Doppler Monitoring Development
The RF probe was placed via ultrasound but once the ablation was started the tissues were opaque and the tines of the RF no longer visible. Based on the theory that at 100 degrees off gassing of nitrogen would create bubbles and thus movement we use Color Doppler which we found demonstrated what we estimated the zone of ablation to be. No direct correlation was made in vivo. Subsequently we took this back to the laboratory and showed that the Color Doppler zones correlate with the size of the ablation zone.25
Pathology
Tumor size was estimated to be on pre-biopsy imaging. Seven of the 15 patients showed no residual tumor on lumpectomy demonstrating the effectiveness of the percutaneous biopsy. Eight patients had non-viable tumor (no PCNA staining) present at the excision site; O.5cm±0.7cm(range 0.1–2.5cm). One patient had a viable one mm focus of DCIS/atypia outside the ablation zone. Residual tumor size (post ablation) ranged from 0 to 2.5cm. (Table 2) The average volume of ablation was 15.3±7.9cc.
Pathology
No post operative complications were seen in this study.
DISCUSSION
This study was conducted as a randomized Phase I/II design. There were two treatment arms: a laser or radiofrequency device was used to ablate margins after tumor removal via an image-guided vacuum-assisted Excisional biopsy (IVEB) device. The study was conducted effectively in two stages. Phase I the patients were randomized to receive either RF or laser ablation so the effective dose of each method could be verified. Depending on whether the tumor was visible on ultrasound, either ultrasound or stereotactic imaging was used to guide the IVEB procedure. The primary objective of the Phase I portion of the study was to confirm the energy dose of the laser method or the width deployment for the RF device required to attain a consistent ablation margin of 1 cm. For each ablation method, a standard 3+3 Phase I design was implemented so that energy dose was modified to attain the desired ablation margins, should they initially proved to be inadequate. The energy doses identified in the Phase I portion was to be used as fixed energy doses in the Phase II component of the study.
In our hands the laser ablation of the hematoma-filled cavity was unpredictable in one patient having virtually no ablation and in another being much larger than anticipated. Even though prior work by Dowlatshahi and others27 showed single-insertion ablation to be successful in whole tumor we were not able to reproduce his results even with kind assistance of Dr. Dowlatshahi during this study. The dynamics of the hematoma/cavity may have caused less reliability of the laser tip heating. This was all prior to the development of the Doppler imaging. In the future Doppler may be helpful in predicting the temperature zone and thus the success of laser.
The initial 3 patients randomized to RF in Phase I did well with targeting a 1cm margin beyond the cavity site thus RF went on to Phase II immediately. The 1cm margin around the hematoma/cavity was adopted for the rest of the cases. The main objective of the Phase II portion of the study was to characterize the tumor free zone achieved by ablation to 100 degrees followed by immediate resection. It has been shown previously that temperatures above 55 degrees result in tumor necrosis. The higher temperature we used in this study as opposed to that of other studies was based on pre-clinical and clinical work that we have published on open excision followed by ablation (eRFA) that have shown very predictable tissue necrosis at this sustained temperature.28
MRI was done prior to accrual onto the trial and only seemed to be helpful for ruling out multicentric disease which occurred in 3 of 21 patients in this study. MRI both overestimated and underestimated residual tumor size after percutaneous biopsy/excision. (Table 3) The zone of ablation was large enough however it covered even the largest of the residual tumors (2.5cm) in this series successfully.
The series that attempt complete ablation of intact tumors are divided between immediate and delayed resection. (Table 1) They almost uniformly utilize ultrasound for localization and have focused primarily on T1 disease as did we. PCNA of whole mount reconstruction was used in this study as opposed to a vital dye29 to reconstruct the ablation zone. 26,28 Others have utilized CK8/18 to assess viability as it was suggested in earlier studies that it is degraded earlier during apoptosis and therefore nonviable tissues would not stain.30 Korourian and colleagues have shown that PCNA correlates well with the amount of ablation achieved as it determines cell viability by detecting the presence of actively dividing DNA.26,28 It is felt that immediate resection as was performed in this study can underestimate assessment of cell death and the complete ablation zone. In only one case did we see a 1mm focus of atypia/DCIS away from the cavity site which had no residual tumor. Incomplete ablations have been attributed to technical difficulties such as improper placement of the probe, equipment malfunction or underestimation of tumor size by imaging.31,32
The major change in direction in this study is an attempt to percutaneously remove all evidence of tumor prior to the ablation. The potentially success of percutaneous excision of tumor by ultrasound was based on our extensive experience with percutaneous excision of benign tumors.33 Stereotactic biopsy of cancer has likewise been very successful with most series showing an ~50 percent rate of residual tumor when all calcification have been removed.25 We know that as the impedance of tumor versus fatty fibroglandular tissue of the breast is often quite different. We hypothesized that if most of the tumor was removed then ablation could proceed smoothly through similar tissue.
One of the here to for unresolved issues of RFA use in breast cancer treatment is real time visual monitoring of the tumor for evidence of ablation. With ultrasound (US) monitoring of ablation, a very hyperechoic image develops with significant shadowing that obscures visualization of the entire tumor.8,10,11 In a case report, Fornage et al reported on two tumors that showed a decrease in pre- versus post-ablation peripheral vascularity.9 In this study we were able to follow ablation zone in real time in simple Doppler mode of our ultrasound with the main purpose of avoiding skin burn that has been seen in small numbers in other studies. Based on our experience we went back to the laboratory using an in vitro model found that in deed the Doppler imaging seen with high temperature ablation (>98 degrees) correlates with the zone of ablation.25 This easy and inexpensive way to follow the ablation zone in real time overcomes many of the hurdles of percutaneous ablation.
Because RFA is best suited for deep localized, discretely visualized disease, patients that have an extensive intraductal component or invasive lobular carcinoma are not ideal candidates for this procedure. Although with PeRFA a patient with DCIS is potentially a candidate as most if not all the tumor is removed thus ruling out an invasive component. Although ablation zones have been reported as large as 4.5 cm, the tumor size in this study was limited in order to ablate the tumor and an additional 1 cm margin of surrounding normal tissue. Thus the group of patients who would be candidates for this procedure is relatively limited. For those who don’t qualify for PeRFA, lumpectomy followed by intraoperative pericavitary ablation (eRFA) is available. eRFA can prevent re-excision through extending the margin.28 We have recently opened a multicenter registry in eRFA.
Although RFA shows significant promise as an effective means of tumor destruction, there is only limited data demonstrating long term effects of ablation without subsequent excision. (Table 2) In these trials most participants were elderly, infirmed or elderly. Most but not all patients received radiation therapy. With appropriate wide ablation the potential to use PeRFA with or without radiation is a real possibility as most recurrences are at the tumor bed. Trials are planned using the methodology developed in this study for percutaneous excision followed by biopsy and/or imaging surveillance.
CONCLUSION
The paradigm of lumpectomy followed by XRT was a major advance in the treatment of breast cancer, offering better cosmesis while maintaining local and systemic recurrence equivalent to that of modified radical and radical mastectomy.34 Recently introduced ablative techniques seek to further improve cosmesis via percutaneous needle introduction.35 These ablative techniques are predicated on extirpation of the primary tumor and an additional margin of normal tissue surrounding it. The proven ability of these techniques to ablate the tumor margin would mark a therapeutic improvement because multiple studies have demonstrated the superiority of negative margins in maintaining local control of breast cancer.1,36,37,38 Ablative techniques carry several associated problems, however, including the degree of ablation necessary to kill both the tumor and a sufficient margin of tissue, the lack of complete tumor histopathology, and the loss of additional tumor tissue for clinical decision-making or for research. The experiments completed in our pilot study tested a new paradigm, based on a combination of new technologies for the percutaneous removal and margin ablation of to sterilize the remaining cavity. Unlike other percutaneous ablation techniques, this treatment protocol allows removal of the lesion, full histopathology, and margin ablation under imaging guidance. We evaluated both percutaneous laser ablation and RFA procedures because we recognized that each may best be suited for different clinical applications. The results of this pilot study indicate that RFA may be useful to sterilize the tumor bed margins after percutaneous cytoreduction or complete excision.
Footnotes
Supported by the Tenenbaum Breast Cancer Research Fund, Little Rock, Arkansas
Supported by the Fashion Footwear Association of New York (FFANY/QVC)
REFERENCES
- 1.Toi M, Winer EP, Inamoto T, Benson JR, Forbes JF, Mitsumori M, Robertson JF, Sasano H, von Minckwitz G, Yamauchi A, Klimberg VS. Identifying Gaps in the Locoregional Management of Early Breast Cancer: Highlights from the Kyoto Consensus Conference. Ann Surg Oncol. 2011 Mar 23; doi: 10.1245/s10434-011-1666-7. epub. [DOI] [PubMed] [Google Scholar]
- 2.Schnitt SJ, Abner A, Gelman R, Connolly JL, Recht A, Duda RB, Eberlein TJ, Mayzel K, Silver B, Harris JR. The relationship between microscopic margins of resection and the risk of local recurrence in patients with breast cancer treated with breast-conserving surgery and radiation therapy. Cancer. 1994;74:1746–1751. doi: 10.1002/1097-0142(19940915)74:6<1746::aid-cncr2820740617>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 3.Spivak B, Khanna MM, Tafra L, Juillard G, Giulano AB. Margin status and local recurrence after breast-conserving surgery. Arch Surg. 1994;129:952–957. doi: 10.1001/archsurg.1994.01420330066013. [DOI] [PubMed] [Google Scholar]
- 4.Pezner RD, Lipsett JA, Desai K, et al. To boost or not to boost: decreasing radiation therapy in conservative breast cancer treatment when "inked" tumor resection margins are pathologically free of cancer. Int J Radiat Oncol Biol Phys. 1988;14:873–877. doi: 10.1016/0360-3016(88)90008-9. [DOI] [PubMed] [Google Scholar]
- 5.Fourquet A, Campana F, Zafrani B, et al. Prognostic factors of breast recurrence in the conservative management of early breast cancer: a 25 year follow-up. lnt J Radiat Oncol Biol Phys. 1989;17:719–725. doi: 10.1016/0360-3016(89)90057-6. [DOI] [PubMed] [Google Scholar]
- 6.Wood BJ, Ramkaransingh JR, Fojo T, Walther MM, Libutti SK. Percutaneous tumor ablation with radiofrequency. Cancer. 2002;94(2):443–451. doi: 10.1002/cncr.10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jeffrey SS, Birdwell RL, Ikeda DM, et al. Radiofrequency ablation of breast cancer: first report of an emerging technology. Arch Surg. 1999;134:1064–1068. doi: 10.1001/archsurg.134.10.1064. [DOI] [PubMed] [Google Scholar]
- 8.Izzo F, Thomas R, Delrio P, et al. Radiofrequency ablation in patients with primary breast carcinoma: a pilot study in 26 patients. Cancer. 2001;92:2036–2044. doi: 10.1002/1097-0142(20011015)92:8<2036::aid-cncr1542>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 9.Fornage BD, Sneige N, Ross MI, et al. Small (2-cm) breast cancer treated with US-guided radiofrequency ablation: feasibility study. Radiology. 2004;231:215–224. doi: 10.1148/radiol.2311030651. [DOI] [PubMed] [Google Scholar]
- 10.Burack WE, Jr, Agnese DM, Povoski SP, et al. Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer. 2003;98:1369–1376. doi: 10.1002/cncr.11642. [DOI] [PubMed] [Google Scholar]
- 11.Hayashi AH, Silver SF, van der Westhuizen NG, et al. Treatment of invasive breast carcinoma with ultra-sound-guided radiofrequency ablation. Am J Surg. 2003;185:429–435. doi: 10.1016/s0002-9610(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 12.Singletary SE, Fornage BD, Sneige N, et al. Radiofrequency ablation of early-stage invasive breast tumors: an overview. Cancer. 2002;8:177–180. doi: 10.1097/00130404-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 13.Bown SG. Phototherapy of tumours. World J Surg. 1983;7:700–709. doi: 10.1007/BF01655209. [DOI] [PubMed] [Google Scholar]
- 14.Steger AC, Lees WR, Walmsley K, Bown SG. lnterstitiallaser hyperthermia: anew approach to local destruction of tumors. BMJ. 1989;299:362–365. doi: 10.1136/bmj.299.6695.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masters A, Bown SG. lnterstitiallaser hyperthermia in tumour therapy. Ann Chir Gynaecol. 1990;79:244–251. [PubMed] [Google Scholar]
- 16.Stonn FK, Silbennan AW, Ramming KR, et al. Clinical thennochemotherapy: a controlled trial in advanced cancer patients. Cancer. 1984;53:863–868. doi: 10.1002/1097-0142(19840215)53:4<863::aid-cncr2820530408>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 17.Matthewson D, Coleridge-Smith P, O'Sullivan IP, Northfield TC, Bown SG. Biological effects of intrahepatic neodymium:yttrium-aluminum-garnet laser photocoagulation in rats. Gastroenterology. 1987;93:550–557. doi: 10.1016/0016-5085(87)90918-8. [DOI] [PubMed] [Google Scholar]
- 18.Nolsoe CP, Torp-Pedersen S, Burcharth F, et al. Interstitial hyperthennia of colorectal liver metastases with a US-guided Nd- Y AG laser with a diffuser tip: a pilot clinical study. Radiology. 1993;187:333–337. doi: 10.1148/radiology.187.2.8475269. [DOI] [PubMed] [Google Scholar]
- 19.Amin Z, Donald II, Masters A, et al. Hepatic metastases: interstitial laser photocoagulation with real-time US monitoring and dynamic CT evaluation of treatment. Radiology. 1993;187:339–347. doi: 10.1148/radiology.187.2.8475270. [DOI] [PubMed] [Google Scholar]
- 20.Jacques SL, Rastegar S, Motamedi M, et al. Liver photocoagulation with diode laser (805 nm) vs. Nd:YAG laser (1064 nm) Proceedings SPIE. 1992;1646:107–117. [Google Scholar]
- 21.Schatz SW, Bown SG, Wyman DR, Groves IT, Wilson BC. Low-power interstitial Nd- YAG laser photocoagulation in normal rabbit brain. Lasers Med Sci. 1992;7:433–439. [Google Scholar]
- 22.Hung WK, Ying M, Chan CM, Lam HS, Mak L. Minimally invasive technology in the management of breast disease. Breast Cancer. 2009;16:23–29. doi: 10.1007/s12282-008-0072-x. [DOI] [PubMed] [Google Scholar]
- 23.Arentz C, Baxter K, Boneti C, et al. Ten-year experience with hematoma-directed ultrasound-guided (HUG) breast lumpectomy. Ann Surg Oncol. 2010 Oct;17 Suppl 3:378–383. doi: 10.1245/s10434-010-1230-x. [DOI] [PubMed] [Google Scholar]
- 24.Bland KL, Gass J, Klimberg VS. Radiofrequency, cryoablation, and other modalities for breast cancer ablation. Surg Clin North Am. 2007 Apr;87(2):539–550. doi: 10.1016/j.suc.2007.02.003. xii. [DOI] [PubMed] [Google Scholar]
- 25.Nahirnyak VM, Moros EG, Novák P, Suzanne Klimberg V, Shafirstein G. Doppler signals observed during high temperature thermal ablation are the result of boiling. Int J Hyperthermia. 2010;26(6):586–593. doi: 10.3109/02656731003801469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korourian S, Klimberg S, Henry-Tillman R, et al. Assessment of proliferating cell nuclear antigen activity using digital image analysis in breast carcinoma following magnetic resonance-guided interstitial laser photocoagulation. Breast J. 2003 Sep–Oct;9(5):409–513. doi: 10.1046/j.1524-4741.2003.09509.x. [DOI] [PubMed] [Google Scholar]
- 27.Dowlatshahi K, Dieschbourg JJ, Bloom KJ. Laser therapy of breast cancer with 3-year follow-up. Breast J. 2004 May–Jun;10(3):240–243. doi: 10.1111/j.1075-122X.2004.21436.x. [DOI] [PubMed] [Google Scholar]
- 28.Klimberg VS, Kepple J, Shafirstein G, et al. eRFA: excision followed by RFA-a new technique to improve local control in breast cancer. Ann Surg Oncol. 2006 Nov;13(11):1422–1433. doi: 10.1245/s10434-006-9151-4. [DOI] [PubMed] [Google Scholar]
- 29.Fishbein MC, Meerbaum S, Rit J, et al. Early phase acute myocardial infarct size quantification: validation of the triphenyl tetrazolium chloride tissue enzyme staining technique. Amer Heart J. 1981;101:593. doi: 10.1016/0002-8703(81)90226-x. [DOI] [PubMed] [Google Scholar]
- 30.Bloom KJ, Dowlat K, Assad L. Pathologic changes after interstitial laser therapy of infiltrating breast carcinoma. Am J Surg. 2001;182:384–388. doi: 10.1016/s0002-9610(01)00732-2. [DOI] [PubMed] [Google Scholar]
- 31.Burak WE, Jr, Agnese DM, Povoski SP, et al. Radiofrequency ablation of invasive breast carcinoma followed by delayed surgical excision. Cancer. 2003 Oct 1;98(7):1369–1376. doi: 10.1002/cncr.11642. [DOI] [PubMed] [Google Scholar]
- 32.Hayashi AH, Silver SF, van der Weshuizen NG, et al. Treatment of invasive breast carcinoma with ultrasound-guided radiofrequency ablation. Am J Surg. 2003;185(5):429–435. doi: 10.1016/s0002-9610(03)00061-8. [DOI] [PubMed] [Google Scholar]
- 33.Johnson AT, Henry-Tillman RS, Smith LF, Harshfield D, Korourian S, Brown H, Lane S, Colvert M, Klimberg VS. Percutaneous excisional breast biopsy. Am J Surg. 2002;184(6):550–554. doi: 10.1016/s0002-9610(02)01099-1. discussion 554. [DOI] [PubMed] [Google Scholar]
- 34.Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. NEJM. 2002;347(16):1270–1271. doi: 10.1056/NEJMoa022152. [DOI] [PubMed] [Google Scholar]
- 35.Singletary SE. Minimally Invasive Techniques in Breast Cancer Treatment. Semin Surg Oncol. 2001;20(3):246–250. doi: 10.1002/ssu.1040. [DOI] [PubMed] [Google Scholar]
- 36.Polgar C, Sulyok Z, Fodor J, et al. Sole brachytherapy of the tumor bed after conservation surgery for T1 breast cancer: five-year results of a phase I–II study and initial findings of a randomized phase III trial. J Surg Oncol. 2002;80:121–128. doi: 10.1002/jso.10110. [DOI] [PubMed] [Google Scholar]
- 37.Klimberg VS, Harms S, Korourian S. Assessing Margin Status. Surgical Oncology. 1999;8(2):77–84. doi: 10.1016/s0960-7404(99)00031-6. [DOI] [PubMed] [Google Scholar]
- 38.Johnson AT, Henry-Tillman RS, Klimberg VS. Breast conserving surgery: Optimizing local control in the breast with the assessment of margins. The Breast Journal. 2001;12:1–7. doi: 10.3233/bd-2001-12105. [DOI] [PubMed] [Google Scholar]
- 39.Noguchi M, Earashi M, Fujii H, Yokoyama K, Harada K, Tsuneyama K. Radiofrequency ablation of small breast cancer followed by surgical resection. J Surg Oncol. 2006 Feb 1;93(2):120–128. doi: 10.1002/jso.20398. [DOI] [PubMed] [Google Scholar]
- 40.Earashi M, Noguchi M, Motoyoshi A, Fujii H. Radiofrequency ablation therapy for small breast cancer followed by immediate surgical resection or delayed mammotome excision. Breast Cancer. 2007;14(1):39–47. doi: 10.2325/jbcs.14.39. [DOI] [PubMed] [Google Scholar]
- 41.Manenti G, Bolacchi F, Perretta T. Small breast cancers: in vivo percutaneous US-guided radiofrequency ablation with dedicated cool-tip radiofrequency system. Radiology. 2009 May;251(2):339–346. doi: 10.1148/radiol.2512080905. el. [DOI] [PubMed] [Google Scholar]
- 42.Wiksell H, Löfgren L, Schässburger KU, et al. Feasibility study on the treatment of small breast carcinoma using percutaneous US-guided preferential radiofrequency ablation (PRFA) Breast. 2010 Jun;19(3):219–225. doi: 10.1016/j.breast.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 43.Oura S, Tamaki T, Hirai I, et al. Radiofrequency ablation therapy in patients with breast cancers two centimeters or less in size. Breast Cancer. 2007;14(1):48–54. doi: 10.2325/jbcs.14.48. [DOI] [PubMed] [Google Scholar]
- 44.Brkljacic B, Cikara I, Ivanac G, et al. Ultrasound-guided bipolar radiofrequency ablation of breast cancer in inoperable patients: a pilot study. Ultraschall Med. 2010 Apr;31(2):156–162. doi: 10.1055/s-0028-1109898. [DOI] [PubMed] [Google Scholar]
- 45.Susini T, Nori J, Olivieri S, et al. Radiofrequency ablation for minimally invasive treatment of breast carcinoma. A pilot study in elderly inoperable patients. Gynecol Oncol. 2007;104(2):304–310. doi: 10.1016/j.ygyno.2006.08.049. [DOI] [PubMed] [Google Scholar]
- 46.Marcy PY, Magné N, Castadot P, Bailet C, Namer M. Ultrasound-guided percutaneous radiofrequency ablation in elderly breast cancer patients: preliminary institutional experience. Br J Radiol. 2007;80(952):267–273. doi: 10.1259/bjr/91383984. [DOI] [PubMed] [Google Scholar]
- 47.Yamamoto N, Fujimoto H, Nakamura R, et al. Pilot study of radiofrequency ablation therapy without surgical excision for T1 breast cancer: evaluation with MRI and vacuum-assisted core needle biopsy and safety management. Breast Cancer. 2011;18(1):3–9. doi: 10.1007/s12282-010-0197-6. [DOI] [PubMed] [Google Scholar]


