Abstract
Injectable materials offer the potential for minimally invasive therapy for myocardial infarction (MI), either as an acellular scaffold or as a cell delivery vehicle. A recently developed myocardial matrix hydrogel, derived from decellularized porcine ventricular tissue, has the potential to aid in cardiac repair following an MI. Herein, we set out to study the effects of crosslinking on the cardiac hydrogel stiffness, degradation properties, cellular migration, and catheter injectability in vitro. Crosslinking increased stiffness, while slowing degradation and cellular migration through the gels. Additionally, the crosslinked material was pushed through a clinically relevant catheter. These results demonstrate that the material properties of myocardial matrix can be tuned via crosslinking, while maintaining appropriate viscosity for catheter injectability.
Keywords: catheter injectability, cardiac repair, decellularized, degradation, mechanical properties
Introduction
Injectable materials have gained recent focus for a variety of tissue engineering applications, including treatment of the heart following a myocardial infarction (MI).[1–5] Post-MI, cardiomyocyte death along with degradation of the extracellular matrix (ECM) by matrix metalloproteinases[6] leads to wall thinning and contributes to the progression of negative left ventricular (LV) remodeling and eventual heart failure (HF).[6] Injectable biomaterial scaffolds offer a means of replacing and repairing the damaged ECM, to mitigate the process of LV remodeling.[7] Additionally, injectable materials allow for the potential advantage of catheter delivery, which would enable a minimally invasive procedure, avoiding general anesthesia and invasive surgical techniques.[8, 9]
Biomaterials that have been explored as injectable scaffolds, with or without cells, for cardiac tissue engineering include natural materials, such as fibrin,[5, 10] collagen,[4, 11] Matrigel,[12, 13] alginate,[3] naturally inspired self-assembling peptide nanofibers[14, 15], and synthetic materials.[16–19] However, none of these materials have been designed specifically to mimic the cardiac ECM, and thus do not provide the appropriate biochemical composition, important in the guidance of cell adhesion, proliferation and/or maturation.[20–22]
We have recently developed an injectable hydrogel that is derived from decellularized ventricular tissue. The decellularized myocardium is processed to create a liquid, injectable matrix, thus offering a cardiac specific scaffold.[23] This material has been shown to increase maturation of human embryonic stem cell derived cardiomyocytes in vitro,[24] suggesting the importance of providing cells with a complex tissue-specific biochemical composition. Additional studies show that the injectable myocardial matrix liquid forms a hydrogel at physiologic temperature and upon injection into myocardium.[23] However, the mechanical properties and tunability of this material have not yet been studied.
A material’s composition, structure, and mechanical properties have the potential to influence and direct cell behavior.[25–29] Modulation of structure, porosity, or the incorporation of crosslinking can affect mechanical properties, such as material stiffness, and influence cell migration and stem cell differentiation.[26] While it is hypothesized that a stiffness similar to healthy myocardium would be ideal for cardiac tissue engineering,[30] modulating mechanical properties of injectable materials may affect other material properties such as degradation and the ability to support cell infiltration. The latter are both critical in the context of cardiac tissue-engineering, where endogenous cell infiltration is necessary to repair the ischemic region of the myocardium.
In this study, we assessed whether the myocardial matrix mechanical properties could be tuned via crosslinking, and further examined how this change in material properties would affect degradation and cell infiltration. Herein, the myocardial matrix is modulated through crosslinking with glutaraldehyde (GA), as is common for collagen-based materials.[28, 31–34] The effects of crosslinking on rheological properties, degradation, and cellular migration in vitro were studied as a model system. Moreover, the crosslinked myocardial matrix was tested for compatibility with catheter injection, which would be critical for minimally invasive delivery.
Materials and Methods
Preparation of myocardial matrix
The myocardial matrix was decellularized and prepared with modifications from previously published protocols.[23] Briefly, ventricular tissue was isolated from Yorkshire farm pigs after an overdose of Pentobarbital (90 mg/kg) and cut into small rectangular pieces. Tissue was rinsed in phosphate buffered saline (PBS), and decellularized using 1% sodium dodecyl sulfate (SDS), until the ECM was white. The decellularized ECM was further rinsed with DI water overnight, lyophilized, and milled into a fine powder. The powder was then solubilized by enzymatic digestion using pepsin and 0.1M HCl prior to use.[23, 35] The solubilized myocardial matrix was adjusted to pH 7.4 with NaOH on ice and the concentration was adjusted to 8 mg/mL.
Glutaraldehyde crosslinking
Crosslinking of the myocardial matrix was induced during self-assembly, through the addition of glutaraldehyde (GA), (Sigma-Aldrich, St. Louis, MO) at various concentrations. Glutaraldehyde is commonly used to crosslink collagen gels and fibers.[25, 31, 32] Glutaraldehyde and 1x PBS were added in appropriate volumes on ice, so that the final myocardial matrix solution was 6 mg/mL, with a final concentration of 0% GA, 0.05% GA, or 0.1% (wt. %) GA. Once mixed, solubilized myocardial matrix was plated as appropriate for further experiments.
Rheological measurements
For rheological assessment, 500 μL aliquots of solubilized myocardial matrix were pipetted into glass scintillation vials and allowed to gel at 37°C overnight, with or without glutaraldehyde at varied concentration (0%, 0.05%, 0.1%). A TA instruments AR-G2 rheometer was used to test matrix rheological properties after crosslinking. Myocardial matrix gels were tested according to methods modified from collagen[36, 37] rheological testing. Here, gels were tested using a 20 mm parallel plate geometry, with a 1.2 mm gap between plates, and temperature set to 37°C. Three frequency sweeps were conducted within the linear viscoelastic strain region of 2.5% (determined experimentally), throughout the range 0.1 to 50 rad/sec (0.25 through 5 is reported). Samples were run in triplicate, and an average of values was obtained for all three frequency sweeps for each condition, run in triplicate. Above 0.6 rad/sec collagen fibrils are sensitive to strain thinning effects,[37] and thus the storage modulus (G′) is reported at 0.5 rad/sec (0.08 Hz).
Catheter injectability test
To assess the potential for clinical translation, the solubilized myocardial matrix was tested for its ability to be pushed through the nitinol tubing of a 27 G Myostar Injection catheter (Biologics Delivery Systems, Irwindale, CA). Injectable therapeutics are often stored in a lyophilized or powder state and resuspended prior to injection, thus the myocardial matrix was tested for catheter injectability after lyophilization and resuspension. Solubilized myocardial matrix was aliquoted, lyophilized, and stored at −80°C. To test catheter injectability, lyophilized myocardial matrix was removed from −80°C and resuspended with Millipore water to be 8 mg/mL. GA was then added on ice and solutions mixed. The myocardial matrix solutions at 0%, 0.05%, and 0.1% GA were allowed to sit at room temperature for 20 min prior to catheter testing. After 20 min, myocardial matrix was pulled through a 20 G needle, into a 1 mL Leur Lok syringe. The syringe was then attached to the 27 G catheter, and the solubilized, resuspended material was pushed at a rate of 0.2 mL per 30 seconds, which is the clinically relevant injection rate for intramyocardial injections.
Degradation assessment
To assess the effects of crosslinking on the degradation properties, ninhydrin reactivity was evaluated at varied timepoints.[25, 38] Solubilized myocardial matrix at 6 mg/mL, with varied degrees of glutaraldehyde crosslinking (0%, 0.05%, 0.1%), was allowed to gel overnight in microcentrifuge tubes at 100 μL volumes. Bacterial collagenase (Worthington Biomedical Corporation, Lakewood, NJ) at 125 U/mL in a 0.1 M Tris-base, 0.25 M CaCl2 solution, at pH 7.4, was added to the gels. Gels were allowed to incubate with collagenase at 37°C for 5 or 24 hours, and visual observations were made throughout the incubation. At each timepoint, samples were centrifuged at 15,000 rpm for 10 min and the supernatant was allowed to react with 2% ninhydrin (Sigma-Aldrich) in a boiling water bath for 10 min. After complete reaction, samples were read on a BioTek Synergy 4 (BioTek Instruments, Winooski, TX) spectrophotometer according to ninhydrin assay instructions, at 570 nm.
Cell culture
Prior to migration experiments, cells were cultured according to standard protocols. Three cell types were tested in this study: 3T3 fibroblasts, rat aortic smooth muscle cells (RASMCs), and human coronary artery endothelial cells (HCAECs). 3T3 fibroblasts (ATCC) were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) (Mediatech, Manassas, VA) supplemented with 10% bovine calf serum (HyClone, Logan, UT) with 1% penicillin/streptomycin (P/S). 3T3 cells were used for migration studies at P9. RASMCs were isolated from 3 day old Sprague Dawley rats, according to published protocol.[23, 39] RASMCs were cultured in DMEM supplemented with 10% fetal bovine serum (FBS) and 1% P/S on collagen coated plates. HCAECs (Cell Applications Inc, San Diego, CA) were cultured in MesoEndo Endothelial cell media (Cell Applications). Both cell types were split every 2–4 days and used at P4–6.
Cellular migration through crosslinked gels
The effects of crosslinking on cell migratory ability was tested using BD Fluroblok inserts, with 8 μm pores, (Becton Dickinson, Franklin Lakes, NJ) in 24 well companion plates, a transwell insert system used for migration studies.[40] 3T3 fibroblasts, RASMCs, and HCAECs were cultured as described above, and serum starved in DMEM supplemented with 1% P/S for 18–20 hours prior to migration experiments. 100 μL of solubilized 0% GA, 0.05% GA, or 0.1% GA myocardial matrix was carefully pipetted onto Fluoroblok inserts (upper well of the transmembrane chamber) and allowed to gel overnight. After serum starving, cells were labeled with CFSE CellTrace (Molecular Probes, Eugene, Oregon). CFSE was dissolved in DMSO and diluted to create a 100 μM solution. Cells were trypsinized and incubated with CFSE at 10 μM for 15 min at 37°C, then incubated with media alone for 30 min at 37°C. Fluoroblok inserts were placed into 24 well companion plates with 1 mL of DMEM supplemented with 10% FBS and 1% P/S in the well as a chemoattract to induce migration. Cells, in serum-starved media, were added to blank inserts, or on top of the gels in the inserts. Cells were seeded to be 150,000 cells per insert, in 250 μL of media. Blank inserts (with media), without cells served as controls. Preliminary studies confirmed that gel alone (with media) read the same as blank inserts, and were thus not included here. 3T3 fibroblasts were assessed on 0%, 0.05%, and 0.1% GA gels, while RASCMs and HCAECs were assessed on 0% and 0.1% GA gels. Each cell type was tested twice (in triplicate), to confirm reproducibility, and results from one test are shown here. Fluoroblok plates were read on a BioTek Synergy 4 spectrophotometer prior to cells being plated, immediately after seeding (time 0), and at periodic time-points for 24 hours. The Fluoroblok inserts have a black bottom and thus only cells that have migrated through the gel and to the other side of the insert membrane were detected via fluorescence.
Statistical analysis
All data are presented as the mean ± standard deviation. Samples were run in triplicate, unless otherwise noted. Statistics compared degradation and migration data at each timepoint individually. Significance was determined using one-way analysis of variance (ANOVA) with a Bonferroni correction when there were more than two groups and all groups were individually compared, or a Dunnett test when comparing only to the control group. A two-tailed student’s t-test was used for all other data. Statistical significance was accepted at p < 0.05, and results were reported as: * p < 0.05 and ** p < 0.001.
Results
Rheological properties
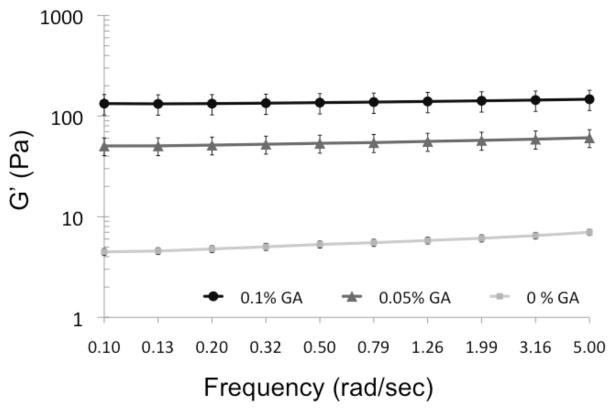
Rheological properties of myocardial matrix gels, with varying degrees of crosslinking, were assessed using a parallel plate rheometer, after overnight gelation at 37°C. A frequency sweep at a constant strain of 2.5% was conducted and shown here over the range 0.1 – 5 rad/sec. At 0.5 rad/sec, the uncrosslinked myocardial matrix gel had a storage modulus value of 5.3 ± 0.4 Pa. This value is lower than reported values for other injectable materials, such as collagen (20–80 Pa) and Matrigel (30–120 Pa).[9] However, crosslinking with glutaraldehyde was shown to increase the stiffness of myocardial matrix gels (Figure 1). Crosslinking with 0.05% GA increased G′ to 53.6 ± 10.9 Pa and crosslinking with 0.1% GA increased G′ to 136.0 ± 31.2 Pa (at 0.5 rad/sec) (Table 1). Thus, crosslinking with as little as 0.05% GA causes a 10 fold increase in storage modulus, indicating that small amounts of crosslinker can significantly affect stiffness of the myocardial matrix. The phase angle (δ), which indicates the relative elasticity or viscosity of a material, was significantly lower when myocardial matrix was crosslinked (δ0%GA = 10.5 ± 0.3°, δ0.05%GA = 5.4 ± 0.6°, δ0.1%GA = 4.0 ± 0.1°), indicating that crosslinking is able to bring the material closer to an ideal elastic material (as defined by δ = 0) (Table 1). Thus, crosslinking increases the stiffness and elasticity of the myocardial matrix, as assessed by parallel plate rheology.
Figure 1.
Rheological effects of crosslinking. Plot of rheological data of GA crosslinked myocardial matrix gels at 0%, 0.05%, and 0.1% GA. Increasing concentrations of GA, increases storage modulus (G′).
Table 1.
Rheology Results
| Storage Modulus (G′)
|
Phase Angle (δ)
|
|
|---|---|---|
| (Pa) | (°) | |
| 0% GA | 5.3 ± 0.4 | 10.5 ± 0.3 |
| 0.05% GA | 53.6 ± 10.9* | 5.4 ± 0.6** |
| 0.1% GA | 136.0 ± 31.2* | 4.0 ± 0.1** |
p<0.05,
p<0.001 indicate statistically significant difference from uncrosslinked 0% gluteraldehyde (GA) gels, as assessed by one-way ANOVA with Dunnet post-test.
Catheter injectability
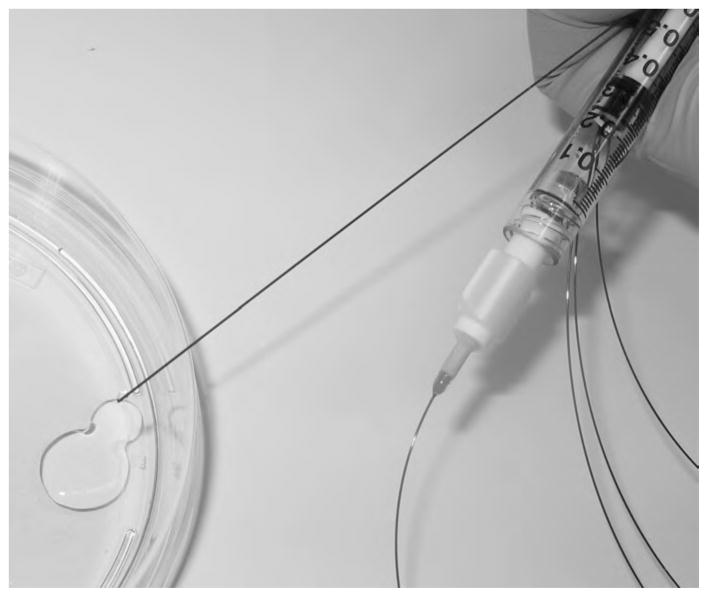
Crosslinked myocardial matrix was tested for its ability to be pushed through a 27 G Myostar Injection catheter, used in clinical trials for transendocardial delivery of cellular therapies.[41] Myocardial matrix was mixed with varying degrees of GA and allowed to sit for 20 min at room temperature prior to catheter testing. After 20 min, myocardial matrix at 0%, 0.05%, and 0.1% GA was successfully pushed through the 27 G catheter (Figure 2), suggesting catheter injectability.
Figure 2.
Catheter injectability. Despite incorporation of 0.1 % GA, the crosslinked myocardial matrix was capable of being injected through the nitinol tubing of the 27 G Myostar catheter.
Modulation of degradation
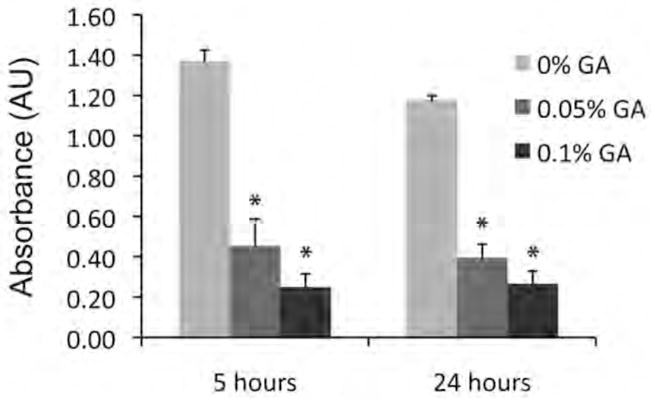
The effect of crosslinking on the rate of degradation of myocardial matrix gels was evaluated using an established ninhydrin assay. Observation of the gels at 5 hours revealed that the uncrosslinked myocardial matrix was no longer an intact gel, while crosslinked samples remained intact. At 24 hours, crosslinked samples continued to remain intact. The degradation was measured at these time points by absorbance, measure in arbitrary units (AU) (Figure 3). Bonferroni correction to compare individual groups indicated significant differences of each crosslinked condition from uncrosslinked, but no difference in degradation between 0.05% and 0.1% GA gels. Additional observation of samples at 48 and 72 hours revealed that uncrosslinked gels were still intact. At one-week, quantification showed no statistical difference among groups (0%, 0.05%, and 0.1% GA) (data not shown).
Figure 3.
Myocardial matrix degradation in vitro. Degradation of uncrosslinked and crosslinked gels at 5 and 24 hours of incubation with collagenase. Increasing AU indicates increased degradation. *p<0.001, indicates statistical difference from 0% GA at that timepoint as assessed by a Bonferroni post test after a one-way ANOVA.
Cellular migration
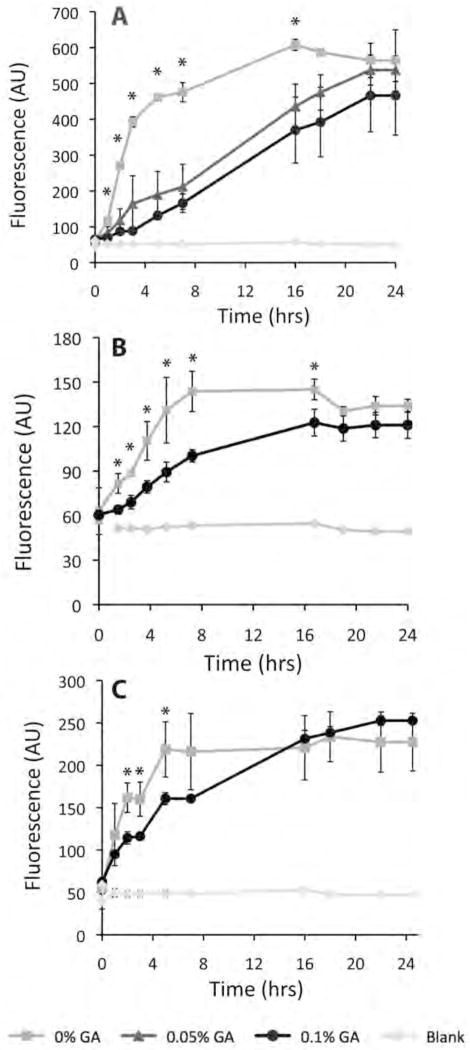
Crosslinking was shown to slow the migration of 3T3 Fibroblast cells, RASMCs, and HCAECs through the myocardial matrix gels, as assessed using a BD Fluoroblok insert setup. Myocardial matrix gels were formed in the upper well of the transwell chambers, and cell migration through the gels and to other side of the membrane was assessed via fluorescence quantification. 3T3 fibroblast migration was evaluated as proof of concept, as fibroblasts are a cell type known to be migratory, and commonly used for in vitro experiments of GA crosslinked gels.[28, 31, 32, 42] Additionally, in the heart, fibroblasts differentiate to be myofibroblasts, which aid in LV remodeling.[43] Fibroblast migration through crosslinked gels of 0.05% GA (n=3), and 0.1% GA (n=3) was slowed, as compared to uncrosslinked gels (n=2), up to 16 hours post-plating (p < 0.05). Because no statistically significant difference was seen between 0.05% GA and 0.1% GA gels at any timepoint, only 0.1% GA was used for additional cell migration studies.
RASMCs and HCAECs are cardiac specific vascular cell types, and thus were studied to understand the effects of crosslinking on the migration of cell types that would be critical for neovascularization in vivo. RASMCs and HCAECs were assessed on uncrosslinked and 0.1% GA gels. Statistically decreased fluorescent values for crosslinked gel samples indicated slowed migration of each cell type through crosslinked gels (n=3), as compared to uncrosslinked gels (n=3) at individual timepoints. RASMC migration was statistically equivalent at time 0, while fluorescence values were statistically lower over 1.5 – 17 hours when crosslinked. HCAEC migration was statistically equivalent 1 hour post plating, yet fluorescence values indicating migratory cells were statistically lower over 2 – 5 hours with crosslinked samples. Additionally, migration of 3T3 fibroblasts, RASMCs, and HCAECs on crosslinked and uncrosslinked gels was equivalent at the final timepoints, indicating migration of cells through crosslinked gels was slowed, but not inhibited (Figure 4).
Figure 4.
Cellular migration through crosslinked gels. Plots of cellular migration of (A) 3T3 fibroblasts, (B) RASMCs, (C) HCAECs on myocardial matrix gels of varied crosslinking %. *p<0.05 indicates statistical difference among groups by ANOVA for 3T3 migration, and statistical difference by t-test for RASMCs and HCAECs.
Discussion
This study demonstrates that the material properties of the myocardial matrix hydrogel can be adjusted via crosslinking. Glutaraldehyde crosslinking increased storage modulus, prolonged degradation time, and slowed cellular migration in vitro. Rheological assessment showed a >10 fold increase in storage modulus of the myocardial matrix gels upon GA crosslinking. In addition, degradation by collagenase in vitro of uncrosslinked material occurred within 24 hours, while GA crosslinked gels fully degraded after one-week. Crosslinking of myocardial matrix gels slowed the migration of 3T3 fibroblasts, RASMCs, and HCAECs. These results suggest that crosslinking of myocardial matrix may delay degradation and affect cellular migration upon use as a scaffold in vivo. Despite crosslinking, the solubilized, resuspended myocardial matrix continued to be compatible with injection through a clinically relevant catheter.
While immune response and cytotoxicity are potential concerns when using GA,[44] limited or no cytotoxicity has been reported when used at low concentrations, in vitro and in vivo.[28, 31–34] Studies have shown that gelatin and collagen materials crosslinked with up to 0.25% GA show limited or no cytotoxicity to fibroblast cultures.[28, 31, 32, 42] A comparative study demonstrated that GA crosslinked collagen showed the greatest persistence (resistance to degradation), with minimal immunogenicity, comparable to or better than Gelfoam Gelatin powder, Avitene Microfibrillar and Collastat Collagen Hemostat products.[34] More recently, collagen/chitosan scaffolds crosslinked with concentrations of up to 0.25% GA demonstrated good biocompatibility in vivo.[28] Despite the cited studies that indicate limited cytotoxicity at low concentration, additional concerns such as calcification are common with GA. GA was used here to demonstrate proof-of-concept that the myocardial matrix scaffold could be crosslinked to modulate material properties. Alternative crosslinkers, such as transglutaminase, which are known to be more biocompatible, may be more feasible for in vivo use.
Crosslinking of the myocardial matrix increased the stiffness of the hydrogels, as assessed by rheology, indicating the tunability of the naturally derived material. A recent study suggests that a stiffer version methacrylated hyaluronic acid (MeHA) hydrogel is better able to normalize wall stress upon injection in an ovine infarct model, than a weaker version of the same material;[45] however, both stiffnesses of the material resulted in a decline in cardiac function as compared to baseline. Additional materials explored as injectable scaffolds, including fibrin,[5, 10] collagen,[4, 11] Matrigel,[12, 13] alginate,[3] pNIPAAM derivatives,[16, 18, 19] and PEG[17] have a wide range of elastic or shear moduli (~ 10 Pa to 20 kPa).[9, 46] Although it is hypothesized that the mechanical properties of an injectable material contribute to effects on the myocardium, cardiac function, and transplanted cells, and despite a recent study using finite element modeling to examine the impact of injectable polymers on the heart,[46] the ideal mechanical and degradation properties of materials intended to be injectable therapies for cardiac tissue engineering remain to be elucidated. While crosslinking can be employed to increase stiffness, it also affectsother material properties.
It was also shown in this work that crosslinking of the myocardial matrix slowed degradation time, demonstrating degradation tunability of the matrix for potential in vivo application. Tunability of degradation is important when designing a scaffold to ensure that the material provides mechanical support for the appropriate amount of time, provides an extracellular milieu for transplanted cells, and degrades at a rate that is conducive to cellular infiltration and tissue repair. Herein, collagenase was used as an in vitro degradation simulation of collagenase and MMP activity within the myocardium post-MI. Complete degradation of uncrosslinked matrix is shown here to occur in as few as four hours, while it has been observed in our lab that uncrosslinked matrix remains present within infarcted rat myocardium at least one-week post-injection (data unpublished). These data indicate that the in vitro degradation profiles, under these conditions, are faster than those observed in vivo. Similar results were observed for a collagen/chitosan scaffold, where degradation of gels was complete at 12 hours in vitro and took 3 days in vivo.[28] It was also shown that degradation of crosslinked gels was slowed both in vitro and in vivo.
The rapid in vitro degradation in this study suggests that concentration of collagenase may have been too aggressive to mimic in vivo levels. However, concentrations of collagenases and MMPs are known to dynamically in a state of flux post-MI,[47] and may be patient specific, as certain drugs can inhibit collagen degradation,[48] suggesting that creating degradation conditions to appropriately mimic the dynamic in vivo environment may be difficult. While the time profile of these in vitro degradation results do not match the in vivo time profile, this data does indicate that crosslinking will likely slow degradation of the myocardial matrix gels in vivo. Delayed degradation of a material scaffold within the MI or border zone could be beneficial to prolong the positive impact on mechanical stability of the LV wall, or increase survival of transplanted cells. Additionally, degradation products of extracellular matrices are known to promote cellular recruitment,[49, 50] which could be enhanced by an extended degradation time.
The ability of cells to migrate into or through the injected scaffold material in vivo is important to allow for positive remodeling, vascular formation, and tissue repair. Herein cellular migration of three relevant cell types through uncrosslinked and crosslinked gels was tested. 3T3 fibroblasts were the first cell type tested, as fibroblasts can transdifferentiation into myofibroblasts, which play a role in remodeling.[43] Additionally, RASMCs and HCAECs were evaluated, as vascular cell types important for neovascularization in vivo. It was previously shown that RASMCs and HCAECs migrated towards soluble myocardial matrix.[23] Here, migration of each cell type through crosslinked gels was slowed, although not inhibited. Previous studies that have explored crosslinking of collagen with UV, carbodiimides, and dehydrothermal (DHT) have shown increased mechanical properties and decreased cell migration,[25] as well as the interesting result that increased stiffness or altered porosity slowed migration.[42] Cellular migration through a 3D construct is known to be affected by matrix stiffness, as well as pore size, proteolysis, and integrin expression, which affects the cell-matrix adhesion properties.[51, 52] The slowed migration seen here could be due to increased material stiffness, or in part due to porosity changes as a result of crosslinking. Additionally, cells may have to remodel the crosslinked material to travel through it. Cellular infiltration is an important component of cardiac repair, allowing for regeneration of the damaged MI region. Thus, while crosslinking allows for increased material stiffness and slowed degradation times, which are thought to be beneficial effects, slowed cellular migration may hinder regenerative capabilities. Important for translation to an in vivo model however, is the result that the myocardial matrix can be modified by crosslinking, thereby slowing, but not inhibiting cellular recruitment to the damaged ischemic tissue for cardiac repair.
Injectable materials are attractive therapeutic options for cardiac repair, because they offer the potential of minimally invasive delivery. However, many materials being explored in small animal models may not translate to catheter delivery, because of their rapid gelation times.[8] Additionally, varying the properties of a solubilized material, through the addition of a crosslinker, may alter gelation kinetics and flow properties, which could complicate or inhibit the material’s ability to be pushed through a catheter of small diameter. In this work, we demonstrated that the addition of GA to the liquid myocardial matrix solution allowed for catheter injection when tested in vitro. However, to confirm catheter injectability for clinical translation, further testing in a large animal model, with GA or other crosslinkers, will be necessary.
Conclusion
These results demonstrate the ability of crosslinking, with GA, to increase stiffness of, prolong degradation of, and alter cellular migration through the recently developed myocardial matrix hydrogel in vitro. In vivo studies will however be critical to validate these findings as well as provide the necessary evaluation of tissue-host interaction and response. The myocardial matrix, studied here, is a material derived from porcine ventricular tissue that has been shown to retain a complex biochemical composition, which thus provides a mimic of native cardiac ECM. While the optimal mechanical properties required for cardiac repair remain to be elucidated, this study demonstrates that the material properties of the myocardial matrix can be altered to meet potential tissue-engineering requirements for myocardial repair following an MI, while maintaining catheter injectability.
Acknowledgments
The authors would like to thank Amul Shah for assistance with rheometry, Todd Johnson for catheter injectability assistance, Jessica DeQuach, Joy Lin, and Nikhil Rao for cell culture assistance, as well as Dr. Airong Song, D. Adam Young, and Mike Salvatore for technical input. The authors are also grateful to Biosense Webster (Cordis) for providing the Myostar catheter.
Footnotes
Supporting information for this article is available at the bottom of the article’s abstract page, which can be accessed from the journal’s homepage at http://www.macros.wiley-vch.de, or from the author.
References
- 1.Lu WN, Lu SH, Wang HB, Li DX, Duan CM, Liu ZQ, Hao T, He WJ, Xu B, Fu Q, Song YC, Xie XH, Wang CY. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2008.0143. [DOI] [PubMed] [Google Scholar]
- 2.Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, Petnehazy O, Landa N, Feinberg MS, Konen E, Goitein O, Tsur-Gang O, Shaul M, Klapper L, Cohen S. J Am Coll Cardiol. 2009;54:1014. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. Circulation. 2008;117:1388. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 4.Dai W, Wold LE, Dow JS, Kloner RA. J Am Coll Cardiol. 2005;46:714. doi: 10.1016/j.jacc.2005.04.056. [DOI] [PubMed] [Google Scholar]
- 5.Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Tissue Eng. 2004;10:403. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- 6.Mann DL. Circulation. 1999;100:999. doi: 10.1161/01.cir.100.9.999. [DOI] [PubMed] [Google Scholar]
- 7.Christman KL, Lee RJ. J Am Coll Cardiol. 2006;48:907. doi: 10.1016/j.jacc.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 8.Singelyn JM, Christman KL. J Cardiovasc Transl Res. doi: 10.1007/s12265-010-9202-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nelson DM, Ma Z, Fujimoto KL, Hashizume R, Wagner WR. Acta Biomater. doi: 10.1016/j.actbio.2010.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. J Am Coll Cardiol. 2004;44:654. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- 11.Suuronen EJ, Veinot JP, Wong S, Kapila V, Price J, Griffith M, Mesana TG, Ruel M. Circulation. 2006;114:I138. doi: 10.1161/CIRCULATIONAHA.105.001081. [DOI] [PubMed] [Google Scholar]
- 12.Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu CH, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen YH, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Nature Biotechnology. 2007;25:1015. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- 13.Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Circulation. 2005;112:I173. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- 14.Padin-Iruegas MEMD, Misao YMD, Davis MEP, Segers VFMMDP, Esposito GP, Tokunou TMDP, Urbanek KMD, Hosoda TMDP, Rota MP, Anversa PMD, Leri AMD, Lee RTMD, Kajstura JP. Circulation. 2009;120:876. doi: 10.1161/CIRCULATIONAHA.109.852285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Circulation. 2005;111:442. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang T, Wu DQ, Jiang XJ, Zhang XZ, Li XY, Zhang JF, Zheng ZB, Zhuo R, Jiang H, Huang C. Eur J Heart Fail. 2009;11:14. doi: 10.1093/eurjhf/hfn009. [DOI] [PubMed] [Google Scholar]
- 17.Dobner S, Bezuidenhout D, Govender P, Zilla P, Davies N. J Card Fail. 2009;15:629. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 18.Li XY, Wang T, Jiang XJ, Lin T, Wu DQ, Zhang XZ, Okello E, Xu HX, Yuan MJ. Cardiology. 115:194. doi: 10.1159/000281840. [DOI] [PubMed] [Google Scholar]
- 19.Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, Wagner WR. Biomaterials. 2009;30:4357. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jawad H, Ali NN, Lyon AR, Chen QZ, Harding SE, Boccaccini AR. J Tissue Eng Regen Med. 2007;1:327. doi: 10.1002/term.46. [DOI] [PubMed] [Google Scholar]
- 21.Lutolf MP, Hubbell JA. Nat Biotechnol. 2005;23:47. doi: 10.1038/nbt1055. [DOI] [PubMed] [Google Scholar]
- 22.Leor J, Amsalem Y, Cohen S. Pharmacol Ther. 2005;105:151. doi: 10.1016/j.pharmthera.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Biomaterials. 2009;30:5409. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeQuach JA, Mezzano V, Miglani A, Lange S, Keller GM, Sheikh F, Christman KL. PLoS One. 5:e13039. doi: 10.1371/journal.pone.0013039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cornwell KG, Lei PD, Andreadis ST, Pins GD. Journal of Biomedical Materials Research. 2007;80A:362. doi: 10.1002/jbm.a.30893. [DOI] [PubMed] [Google Scholar]
- 26.Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 27.Agrawal CM, Ray RB. J Biomed Mater Res. 2001;55:141. doi: 10.1002/1097-4636(200105)55:2<141::aid-jbm1000>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 28.Ma L, Gao C, Mao Z, Zhou J, Shen J, Hu X, Han C. Biomaterials. 2003;24:4833. doi: 10.1016/s0142-9612(03)00374-0. [DOI] [PubMed] [Google Scholar]
- 29.Kuo CK, Ma PX. Biomaterials. 2001;22:511. doi: 10.1016/s0142-9612(00)00201-5. [DOI] [PubMed] [Google Scholar]
- 30.Jacot JG, McCulloch AD, Omens JH. Biophys J. 2008;95:3479. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ulubayram K, Aksu E, Gurhan SI, Serbetci K, Hasirci N. J Biomater Sci Polym Ed. 2002;13:1203. doi: 10.1163/156856202320892966. [DOI] [PubMed] [Google Scholar]
- 32.Sheu MT, Huang JC, Yeh GC, Ho HO. Biomaterials. 2001;22:1713. doi: 10.1016/s0142-9612(00)00315-x. [DOI] [PubMed] [Google Scholar]
- 33.Rault I, Frei V, Herbage D, Abdul-Malak N, Huc A. Journal of Materials Science: Materials in Medicine. 1996:215. [Google Scholar]
- 34.DeLustro F, Condell RA, Nguyen MA, McPherson JM. J Biomed Mater Res. 1986;20:109. doi: 10.1002/jbm.820200110. [DOI] [PubMed] [Google Scholar]
- 35.Freytes DO, Martin J, Velankar SS, Lee AS, Badylak SF. Biomaterials. 2008;29:1630. doi: 10.1016/j.biomaterials.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 36.Rosenblatt J, Devereux B, Wallace DG. Biomaterials. 1992;13:878. doi: 10.1016/0142-9612(92)90182-n. [DOI] [PubMed] [Google Scholar]
- 37.Rosenblatt DBJ, Wallace DG. Journal of Applied Polymer Science. 1993;50:953. [Google Scholar]
- 38.Partis MD, Griffiths DG, Roberts GC, Beechey RB. Journal of Protein Chemistry. 1983;2:263. [Google Scholar]
- 39.San Antonio JD, Karnovsky MJ, Ottlinger ME, Schillig R, Pukac LA. Arterioscler Thromb. 1993;13:748. doi: 10.1161/01.atv.13.5.748. [DOI] [PubMed] [Google Scholar]
- 40.Gobin AS, West JL. FASEB J. 2002;16:751. doi: 10.1096/fj.01-0759fje. [DOI] [PubMed] [Google Scholar]
- 41.Steendijk P, Smits PC, Valgimigli M, van der Giessen WJ, Onderwater EE, Serruys PW. Nat Clin Pract Cardiovasc Med. 2006;3(Suppl 1):S94. doi: 10.1038/ncpcardio0416. [DOI] [PubMed] [Google Scholar]
- 42.Miron-Mendoza M, Seemann J, Grinnell F. Biomaterials. 31:6425. doi: 10.1016/j.biomaterials.2010.04.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jugdutt BI. Circulation. 2003;108:1395. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 44.Huang-Lee LL, Cheung DT, Nimni ME. J Biomed Mater Res. 1990;24:1185. doi: 10.1002/jbm.820240905. [DOI] [PubMed] [Google Scholar]
- 45.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, Gorman JH, 3rd, Gorman RC, Burdick JA. Proc Natl Acad Sci U S A. 107:11507. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Circulation. 2006;114:2627. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 47.Cleutjens JP, Kandala JC, Guarda E, Guntaka RV, Weber KT. J Mol Cell Cardiol. 1995;27:1281. doi: 10.1016/s0022-2828(05)82390-9. [DOI] [PubMed] [Google Scholar]
- 48.Arora PD, Silvestri L, Ganss B, Sodek J, McCulloch CA. J Biol Chem. 2001;276:14100. doi: 10.1074/jbc.M010298200. [DOI] [PubMed] [Google Scholar]
- 49.Reing JE, Zhang L, Myers-Irvin J, Cordero KE, Freytes DO, Heber-Katz E, Bedelbaeva K, McIntosh D, Dewilde A, Braunhut SJ, Badylak SF. Tissue Eng Part A. 2008 doi: 10.1089/ten.tea.2007.0425. [DOI] [PubMed] [Google Scholar]
- 50.Brennan EP, Tang XH, Stewart-Akers AM, Gudas LJ, Badylak SF. J Tissue Eng Regen Med. 2008;2:491. doi: 10.1002/term.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zaman MH, Trapani LM, Sieminski AL, Mackellar D, Gong H, Kamm RD, Wells A, Lauffenburger DA, Matsudaira P. Proc Natl Acad Sci U S A. 2006;103:10889. doi: 10.1073/pnas.0604460103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Even-Ram S, Yamada KM. Curr Opin Cell Biol. 2005;17:524. doi: 10.1016/j.ceb.2005.08.015. [DOI] [PubMed] [Google Scholar]