Abstract
Introduction
Travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops are available in Japan. We prospectively investigated the intraocular pressure (IOP)-decreasing effect of travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops and the adherence of patients to the administration protocol.
Materials and methods
We studied 43 eyes from 43 patients diagnosed with primary open- angle glaucoma, who were using prostaglandin analogs and β-blockers. The prostaglandin analogs and β-blockers were discontinued, and the treatment regimen was changed to travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops without any washout period. IOP before and at 1 month, 3 months, and 6 months after the treatment change was evaluated and compared. A questionnaire about protocol adherence was administered 1 month after the treatment change.
Results
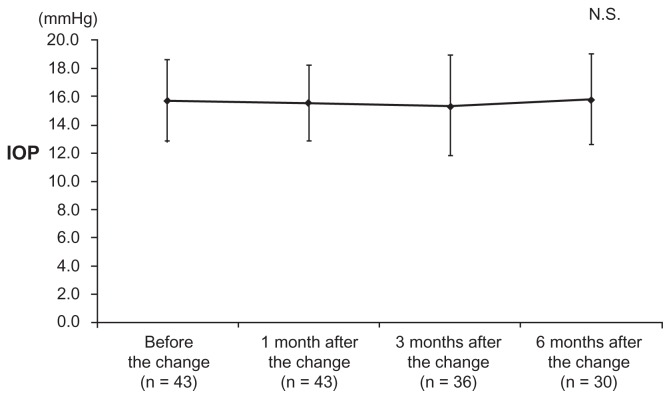
IOP was 15.7 ± 2.9 mmHg before the change, 15.5 ± 2.7 mmHg at 1 month after the change, 15.3 ± 3.6 mmHg at 3 months after the change, and 15.8 ± 3.2 mmHg at 6 months after the change, and none of the differences were significant (P = 0.191). The responses to the questionnaire showed that cases where eye drop administration was forgotten decreased after the treatment change. Moreover, because of changes in eye drops, 19.0% of patients had irritation. More than half (54.8%) of the patients preferred travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops. Seven patients (16.3%) discontinued eye drop use because of adverse reactions within 6 months after the change.
Conclusion
When the treatment regimen was changed from prostaglandin analogs and β-blockers to travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops, administration protocol adherence increased and IOP was preserved; however, adverse reactions appeared in about 16% of the cases.
Keywords: travoprost 0.004%/timolol maleate 0.5% fixed combination, β-blockers, prostaglandin analog, change, intraocular pressure
Introduction
Prostaglandin analogs are the primary treatment for glaucoma in recent years because of their strong intraocular pressure (IOP)-lowering efficacy, safety (because of few systemic adverse reactions), and convenience of once-a-day treatment. However, in cases where prostaglandin treatment does not sufficiently lower IOP, management is modified or additional administration of other eye drops is initiated. When this process is repeated, patients soon become multidrug cases, and poor protocol adherence becomes a problem. 1 In order to improve adherence to treatment protocols, combination eye drops were developed. In Japan, travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops (DuoTrav® Combination Ophthalmic Solution; Alcon Japan Ltd, Tokyo, Japan) became available in June 2010. When patients who were concomitantly using prostaglandin analogs and β-blockers switched to travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops, there were expectations of lessening of administration frequency, increase in protocol adherence, and decreases in IOP. Some studies have reported a hypotensive effect when therapy is changed from prostaglandin analogs and β-blockers to travoprost 0.004%/ timolol maleate 0.5% fixed combination eye drops.2–5 These studies show that IOP was similar2 or that IOP significantly decreased3–5 with the new treatment protocol, but no studies have included Japanese patients as subjects.
The purpose of this study was to evaluate the safety, adherence to administration protocol, and reduction in IOP when treatment is switched from concomitant use of prostaglandin analogs and β-blockers to travoprost 0.004%/ timolol maleate 0.5% fixed combination eye drops in patients diagnosed with primary open-angle glaucoma (POAG).
Materials and methods
This prospective study was conducted at the Inouye Eye Hospital from May 2010 to June 2011 and included 43 eyes from 43 patients (men: 23 patients, 23 eyes; women: 20 patients, 20 eyes) diagnosed with POAG (including normal tension glaucoma) and concomitantly using prostaglandin analogs and β-blockers. The mean patient age was 67.6 ± 11.1 years (mean age ± standard deviation) (range 40–86 years). In terms of disease type, ten cases were normal tension glaucoma and 33 cases were POAG. In terms of the number of glaucoma medications formerly used by patients, 25 patients had been using two medications, eleven patients used three medications, and seven patients used four medications. Among prostaglandin analogs, travoprost was used by 23 patients, latanoprost by 15 patients, tafluprost by four patients, and isopropyl unoprostone by one patient. β-blockers were used in conjunction with timolol in 29 cases, carteolol in eleven cases, levobunolol in two cases, and nipradilol in one case. Mean deviation by the Humphrey visual field test before the change was −9.42 ± 7.24 dB (range −24.01 to 0.41 dB). IOP before the change was 15.7 ± 2.9 mmHg (range 8–24 mmHg). If both eyes met the inclusion criteria, the eye with the higher IOP was selected. If both eyes had the same IOP, the right eye was selected. In a monocular case, the corresponding eye was selected for analysis.
Prostaglandin analogs and β-blockers were discontinued without any washout period, and treatment was changed to travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops (once a day, at night). Other eye drops were continued. The IOP before and at 1 month, 3 months, and 6 months after the switch was measured and compared by analysis of variance (ANOVA) and the Bonferroni/Dunnet test. IOP was measured by the same examiner at approximately the same time with a Goldmann applanation tonometer (Haag-Streit AG, Koeniz, Switzerland). A self-registering questionnaire about protocol adherence was conducted 1 month after the change (Chi-square test). The questionnaire included six questions:
How often does it happen that you forget to administer eye drops (before and after the change)?
-
Compared to before the change, did it sting when administering the current eye drops?
(1) stings (2) same (3) does not sting
-
Compared to before the change, did hyperemia occur after administering the current eye drops?
(1) hyperemia occurred (2) same (3) hyperemia did not occur
-
When compared to before the change, do your eyes feel dry?
(1) felt dryness (2) same (3) did not feel dry
-
Which eye drops do you prefer?
(1) former eye drops (2) neither (3) current eye drops
-
What is the reason for your answer to question 5?
(1) administration frequency (2) administration comfort (3) dryness (4) other.
Adverse reactions were examined at every check-up after the treatment change. For all cases, a P value of <0.05 was considered statistically significant. The study was reviewed and approved by the institutional review board of Inouye Eye Hospital at all participating sites. All participating subjects provided written informed consent prior to participation and the study was conducted in full compliance with all tenants of the Declaration of Helsinki.
Results
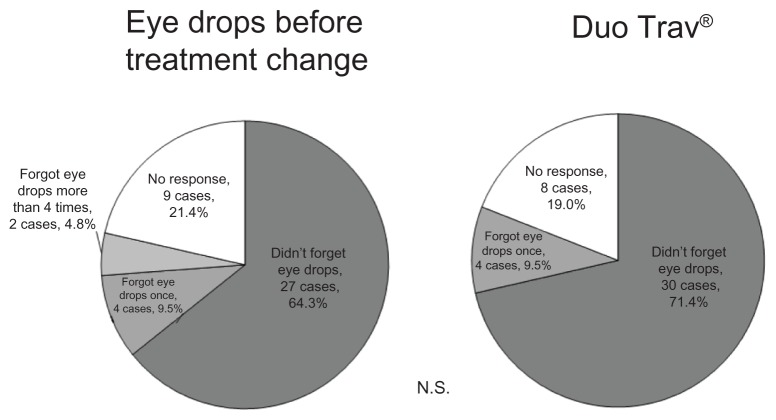
IOP was 15.5 ± 2.7 mmHg at 1 month after the change, 15.3 ± 3.6 mmHg at 3 months after the change, and 15.8 ± 3.2 mmHg at 6 months after the change; none of these values were significantly different compared to IOP before the change (15.7 ± 2.9 mmHg) (P = 0.191) (Figure 1). The alternation at 1 month, 3 months, and 6 months after the change to travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops is shown in Table 1. Among questionnaire results, before the treatment change, 27 (64.3%) patients responded that they “did not forget to administer eye drops,” four (9.5%) patients “forgot to administer once,” two (4.8%) patients “forgot to administer more than four times,” nine (21.4%) patients did not answer (Figure 2). At 1 month after the change, questionnaire results included 30 cases (71.4%) of “did not forget to administer eye drops,” four cases (9.5%) of “forgot one time,” and eight patients (19.0%) did not answer. Among the six patients who answered “forgot to administer” before the treatment change, four (66.7%) answered “did not forget to administer” and two (33.3%) answered “forgot one time” at 1 month after the change. The “did not forget to administer” group significantly increased after the change compared with that before the change (P < 0.05). Table 2 shows stinging, hyperemia, and dryness before and after treatment. In response to “which eye drops do you prefer,” five patients (11.9%) answered “former eye drops,” 23 (54.8%) answered “current eye drops,” 10 (23.8%) answered “neither,” and four patients (9.5%) did not answer. The reasons for preferring the current eye drops included “administration frequency” in 21 cases, “hyperemia lessened” in one case, and “medication expense became cheaper” in one case. Reasons for preferring the eye drops before the change included “itchiness increased” in three cases and “frequent administration is more effective” in two cases.
Figure 1.
IOP before and after changing to travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops.
Abbreviation: IOP, intraocular pressure; N.S., not significant.
Table 1.
The alternation in intraocular pressure at 1 month, 3 months, and 6 months after the change to travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops
| 1 month after the change (n = 43) | 3 months after the change (n = 36) | 6 months after the change (n = 30) | |
|---|---|---|---|
| Cases | |||
| Decreased by more than 2 mmHg | 5 (11.6%) | 9 (25.0%) | 5 (16.7%) |
| Within 1 mmHg | 35 (81.4%) | 18 (50.0%) | 16 (53.3%) |
| Increased by more than 2 mmHg | 3 (7.0%) | 9 (25.0%) | 9 (30.0%) |
Figure 2.
Results from the questionnaire on drug regimen adherence rates.
Abbreviation: N.S., not significant.
Table 2.
The results from the questionnaire on stinging, hyperemia, and dryness before and after changing treatment
| Stinging | Hyperemia | Dryness | |
|---|---|---|---|
| Cases | |||
| Yes | 8 (19.0%) | 4 (9.5%) | 3 (7.1%) |
| Same | 18 (42.9%) | 16 (38.1%) | 31 (73.8%) |
| No | 16 (38.1%) | 21 (50.0%) | 5 (11.9%) |
| No answer | – | 1 (2.4%) | 3 (7.1%) |
Thirteen patients (30.2%) discontinued the new treatment protocol within 6 months of the change. One patient stopped coming to the hospital, and in five patients, treatment was changed back to travoprost 0.004% and timolol maleate 0.5% eye drops because the IOP-decreasing effect was insufficient. Adverse reactions appeared in seven cases (16.3%) and included two cases of uneasy feeling, one case of contact dermatitis, one case of iritis onset, one case of blurred vision, one case of itchiness, and one case of ocular pain. Adverse reactions that appeared in the local part of the eye disappeared in all cases when treatment was changed back to prostaglandin analogs and β-blockers.
Discussion
Administration protocol for travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops was once a day, whereas the protocol for timolol maleate 0.5% eye drops included twice-a-day administration. Therefore, a potential reduction in IOP-decreasing efficacy was a major concern in this study. Schuman et al have reported the IOP-decreasing efficacy and safety of travoprost 0.004%/timolol maleate 0.5% fixed combination eye drop monotherapy and those of concomitantly used travoprost 0.004% eye drops and timolol maleate 0.5% eye drops.6 This randomized, double-masked study involved administration of travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops (155 cases) or travoprost 0.004% with timolol 0.5% maleate eye drops (142 cases) for patients who were diagnosed with POAG or ocular hypertension. The range of IOP decrease was 7.3–8.3 mmHg in the group using travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops and 6.8–8.5 mmHg in the group concomitantly using travoprost 0.004% eye drops with timolol maleate 0.5% eye drops; these values were not significantly different. The frequency of hyperemia appearance was significantly less in the travoprost 0.004%/timolol maleate 0.5% fixed combination eye drop group (P < 0.05). Hughes et al administered travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops (151 cases) and travoprost 0.004% eye drops with timolol maleate 0.5% eye drops (142 cases) for patients who were diagnosed with POAG or ocular hypertension in a randomized, double-masked study.7 The range of IOP decrease was 7.4–8.7 mmHg in the group using travoprost 0.004%/ timolol maleate 0.5% fixed combination eye drops and 8.5–9.0 mmHg in the group concomitantly using travoprost 0.004% eye drops with timolol maleate 0.5% eye drops; these values were not significantly different. In these past studies6,7 there was no significant difference in the IOP-decreasing efficacy of travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops and travoprost 0.004% eye drops and timolol maleate 0.5% eye drops concomitantly used.
The IOP-decreasing effects of travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops after changing the treatment regimen from prostaglandin analogs and timolol maleate 0.5% eye drops have been previously reported.2–5 Rhee et al examined and assessed the change in IOP at 8 am, 10 am, 4 pm, and 8 pm for 3 months after the treatment regimen for 73 patients who were diagnosed with POAG, exfoliation glaucoma, ocular hypertension, or pigmentary glaucoma.2 IOP was 14.4–15.4 mmHg when latanoprost 0.005% eye drops were used concomitantly with timolol maleate 0.5% eye drops and 14.7–15.5 mmHg 3 months after the change; these values were not significantly different. Arend and Raber assessed IOP 4–6 weeks after treatment change for 528 patients who were diagnosed with POAG, exfoliation glaucoma, pigmentary glaucoma, primary angle closure glaucoma, or ocular hypertension.3 Among 139 patients concomitantly using latanoprost 0.005% eye drops with timolol maleate 0.5% eye drops, IOP showed a significant decrease between the values before (19.1 ± 3.8 mmHg) and after (16.9 ± 3.4 mmHg) the change. Among 339 patients concomitantly using travoprost 0.004% eye drops with timolol maleate 0.5% eye drops, IOP showed a significant decrease between the values before (17.8 ± 3.6 mmHg) and after (16.7 ± 3.3 mmHg) treatment change. Among 50 patients concomitantly using bimatoprost 0.03% eye drops with timolol maleate 0.5% eye drops, IOP showed a significant decrease between the values before (18.2 ± 4.1 mmHg) and after (16.8 ± 4.0 mmHg) treatment change. IOP decreasing range was 1.1–2.2 mmHg. Rossi et al evaluated IOP at 1 and 6 months after treatment change in 309 patients who were diagnosed with POAG or ocular hypertension.4 Among patients concomitantly using latanoprost 0.005% eye drops with timolol maleate 0.5% eye drops, IOP significantly decreased from 18.3 ± 2.9 mmHg to 16.6 ± 2.7 mmHg at 1 month after treatment change and 16.3 ± 2.5 mmHg at 6 months after the change. Scherzer et al evaluated IOP 8 weeks after treatment change in 105 patients who were diagnosed with POAG or ocular hypertension.5 IOP was 16.4 ± 2.8 mmHg at 8 weeks after the treatment change, which was not significantly different when compared with the IOP in patients concomitantly using bimatoprost 0.03% eye drops with timolol maleate 0.5% (20.7 ± 1.2 mmHg). Because of the change in treatment, in some studies, IOP did not change2,5 and in other studies, it decreased.3,4 In the present study, there was no change in IOP after treatment change. However, because we did not set up a washout period, it is possible that the previous medication was still having an effect after the treatment change.
Adverse reactions previously reported for travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops include hyperemia, irritation, itchiness, foreign body sensation, photophobia, keratitis, blurred vision, allergic reaction, and conjunctivitis.2–8 There have been no reports of serious adverse reactions,2–8 as was also observed in the present study. In this questionnaire, 19.0% of patients felt irritation, 7.1% of patients felt dryness, and 9.5% of patients had hyperemia; therefore, it is necessary to explain the possibility of adverse reactions to the patients prior to changing treatment protocols. In the present study, the eye drops were discontinued because adverse reactions appeared in seven cases (16.3%) and IOP decrease was inefficient in five cases. The frequency of discontinuation was higher in the present study than in past reports, which included values of 0%,2 1.0%,5 and 5.1%.4 In previous studies, the reasons for discontinuation included hyperemia,4,5 pruritus,4 periocular pigmentation,4 burning,4 dyspnea,4 and allergic reaction,5 which were nearly identical to those in the present study.
Dunker et al administered a questionnaire regarding treatment protocol adherence for 1052 patients using either monotherapy or combination fixed therapy with latanoprost 0.005%/timolol maleate 0.5% fixed combination eye drops.9 For “How often does it happen that you forget to use your eye drops?” the frequency of the answer “often or almost always” significantly decreased after the change (2%) compared to that before the change (11%). For “Would you like to continue with your present eye-drops?” the frequency of the answer “probably or definitely” significantly increased after the change (92%) compared to that before the change (38%). Among cases in which latanoprost 0.005% eye drops and timolol maleate 0.5% were used concomitantly, we also compared IOP and protocol adherence before and after treatment change to latanoprost 0.005%/timolol maleate 0.5% fixed combination eye drops, using subjective judgment by a questionnaire.10 There was no change in IOP between the values observed before and after the treatment change and the frequency of forgetting eye drop administration significantly decreased; moreover, 82.1% of the patients preferred the latanoprost 0.005%/timolol maleate 0.5% fixed combination eye drops. In the present study, the frequency of forgetting eye drop administration significantly decreased; furthermore, 54.8% of the patients preferred the travoprost 0.004%/timolol maleate 0.5% fixed combination eye drops. However, because the evaluation of adherence was done by a questionnaire survey, there is a problem with reliability. If electronic adherence measurements were used, the reliability might have improved but we did not use these in the present study. Moreover, in a medication switching study (as the present study), there is the possibility that adherence will improve. The results of the present study and those of previous studies9,10 indicate that fixed combination eye drops are mostly preferred to concomitant therapy, as shown by an increase in treatment protocol adherence. However, in the present study, IOP increased by more than 2 mmHg after treatment change in three cases (7.0%) at 1 month after the change, in nine cases (25.0%) at 3 months after the change, and in nine cases (30.0%) at 6 months after the change. In these cases, the administration frequency of timolol maleate eye drops was changed from twice a day to once a day, because this was considered to decrease IOP.
In conclusion, Japanese patients diagnosed with POAG who had been formerly treated with two types of eye drops (prostaglandin analogs and β-blockers) and were subsequently treated with one type of eye drop (travoprost 0.004%/ timolol maleate 0.5% fixed combination) experienced an increase in treatment administration adherence with maintenance of IOP. However, in some cases, adverse reactions, such as stimulation, appeared and IOP increased. Therefore, patients in whom treatment is changed to fixed, combination eye drops should be carefully followed up.
Footnotes
Disclosure
The authors report no conflict of interest in this work.
References
- 1.Djafari F, Lesk MR, Harasymowycz PJ, Desjardins D, Lachaine J. Determinants of adherence to glaucoma medical therapy in long-term patient population. J Glaucoma. 2009;18(3):238–243. doi: 10.1097/IJG.0b013e3181815421. [DOI] [PubMed] [Google Scholar]
- 2.Rhee DJ, Peace JH, Mallick S, Landry TA, Bergamini MVW the study group. A study of the safety and efficacy of travoprost 0.004%/ timolol 0.5% ophthalmic solution compared to latanoprost 0.005% and timolol 0.5% dosed concomitantly in patients with open-angle glaucoma or ocular hypertension. Clin Ophthalmol. 2008;2(2):313–319. doi: 10.2147/opth.s2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arend KO, Raber T. Observational study results in glaucoma patients undergoing a regimen replacement to fixed combination travoprost 0.004%/timolol 0.5% in Germany. J Ocul Pharmacol Ther. 2008;24(4):414–420. doi: 10.1089/jop.2007.0123. [DOI] [PubMed] [Google Scholar]
- 4.Rossi GC, Pasinetti GM, Bracchino M, et al. Switching from concomitant latanoprost 0.005% and timolol 0.5% to a fixed combination of travoprost 0.004%/timolol 0.5% in patients with primary open-angle glaucoma and ocular hypertension: a 6-month, multicenter, cohort study. Expert Opin Pharmocother. 2009;10(11):1705–1711. doi: 10.1517/14656560903061283. [DOI] [PubMed] [Google Scholar]
- 5.Scherzer ML, Liehneova I, Negrete FJ, Schnober D. Travoprost 0.004%/ timolol 0.5% fixed combination in patients transitioning fixed or unifixed bimatoprost 0.03%/timolol 0.5% Adv Ther. 2011;28(8):661–670. doi: 10.1007/s12325-011-0043-z. [DOI] [PubMed] [Google Scholar]
- 6.Schuman JS, Katz GJ, Lewis RA, et al. Efficacy and safety of a fixed combination of travoprost 0.004%/timolol 0.5% ophthalmic solution once daily for open-angle glaucoma or ocular hypertension. Am J Ophthalmol. 2005;140(2):242–250. doi: 10.1016/j.ajo.2005.02.058. [DOI] [PubMed] [Google Scholar]
- 7.Hughes BA, Bacharach J, Craven ER, et al. A three-month, multicenter, double-masked study of the safety and efficacy of travoprost 0.004%/ timolol 0.5% ophthalmic solution compared to travoprost 0.004% ophthalmic solution and timolol 0.5% dosed concomitantly in subjects with open angle glaucoma or ocular hypertension. J Glaucoma. 2005;14(5):392–399. doi: 10.1097/01.ijg.0000176935.08392.14. [DOI] [PubMed] [Google Scholar]
- 8.Barnebey HS, Orengo-nania S, Flowers BE, et al. The safety and efficacy of travoprost 0.004%/timolol 0.5% fixed combination ophthalmic solution. Am J Ophthalmol. 2005;140(1):1–7. doi: 10.1016/j.ajo.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 9.Dunker S, Schmucker A, Maier H Latanoprost/timolol fixed combination study group. Tolerability, quality of life, and persistency of use in patients with glaucoma who are switched to the fixed combination of latanoprost and timolol. Adv Ther. 2007;24(2):376–386. doi: 10.1007/BF02849907. [DOI] [PubMed] [Google Scholar]
- 10.Inoue K, Okayama R, Higa R, Sawada H, Wakakura M, Tomita G. Ocular hypotensive effects and safety over 3 months of switching from an unfixed combination to latanoprost 0.005%/timolol 0.5% fixed combination. J Ocul Pharmacol Ther. 2011;27(6):581–587. doi: 10.1089/jop.2011.0057. [DOI] [PubMed] [Google Scholar]