Abstract
Polyhydroxylated steroids are regulators of body shape and size in higher organisms. In metazoans intracellular receptors recognise these molecules. Plants however perceive steroids at membranes, using the membrane-integral receptor kinase BRASSINOSTEROID INSENSITIVE 1 (BRI1). The BRI1 ectodomain structure at 2.5 angstrom resolution reveals a superhelix of 25 twisted leucine-rich repeats (LRRs), an architecture that is strikingly different from the assembly of LRRs in animal Toll-like receptors. A 70 amino-acid island domain between LRRs 21 and 22 folds back into the interior of the superhelix to create a surface pocket for binding the plant hormone brassinolide. Known loss- and gain-of-function mutations closely map to the hormone-binding site. We propose that steroid binding to BRI1 generates a docking platform for a co-receptor that is required for receptor activation. Our findings have mechanistic implications for hormone, developmental and innate immunity signaling pathways in plants that use similar receptors.
Signal perception at the cell surface and transduction of this signal to the cell's interior is essential to all life forms. Plants have met this challenge in part by evolving membrane-integral receptor kinases (RKs). Many RKs are comprised of an extracellular Leucine-Rich Repeat (LRR) module and a cytoplasmic kinase domain, connected by a single membrane-spanning helix1. Receptors with this architecture (LRR-RKs) for example regulate plant growth2, development3,4 and interactions with the environment5,6. Their corresponding ligands range from small molecules7 and peptides8 to entire proteins5.
The LRR-RK BRI12,9 controls a steroid signalling pathway essential for plant growth10. While animal steroid receptors are found predominantly in the nucleus11, BRI1 is localised at the plasma-membrane and in endosomes12. The following model for BRI1 activation has been proposed: In the absence of brassinosteroid, BRI1’s kinase domain is kept in a basal state by its auto-inhibitory C-terminal tail13, as well as by interaction with the inhibitor protein BKI114. Hormone binding to the extracellular domain of BRI17,15 in a region that includes a ~70 amino acid 'island' domain16, causes a change in the receptor (a conformational change in a preformed homodimer13 or receptor dimerisation), leading to autophosphorylation of the BRI1 kinase domain17, release of its C-terminal tail13, and trans-phosphorylation of the inhibitor BKI114,18. BKI1 then dissociates from the membrane, allowing BRI1 to interact with a family of smaller LRR-RKs19, including the BRI1 ASSOCIATED KINASE 1 (BAK1)20,21. The kinase domains of BRI1 and BAK1 trans-phosphorylate each other on multiple sites22, and the fully activated receptor triggers downstream signalling events23, resulting in major changes in nuclear gene expression10.
BRI1's architecture is reminiscent of animal Toll-like innate immunity receptors (TLRs), and notably several plant LRR-RKs are immunity receptors5. It was, thus, reasonable to assume that the BRI1 ectodomain would form a TLR-like horseshoe structure24 that binds its ligand along a dimer interface, as observed in several TLRs25,26. Here, we report the structure of the ligand binding domain of Arabidopsis BRI1 in its free form and bound to the steroid brassinolide, and show that BRI1 folds into a superhelical assembly, whose interior provides the hormone-binding site. Comparison of the free and hormone-bound structures, combined with genetic data, suggests a novel activation mechanism for BRI1 that is distinct from TLRs.
Overall structure of the BRI1 ectodomain
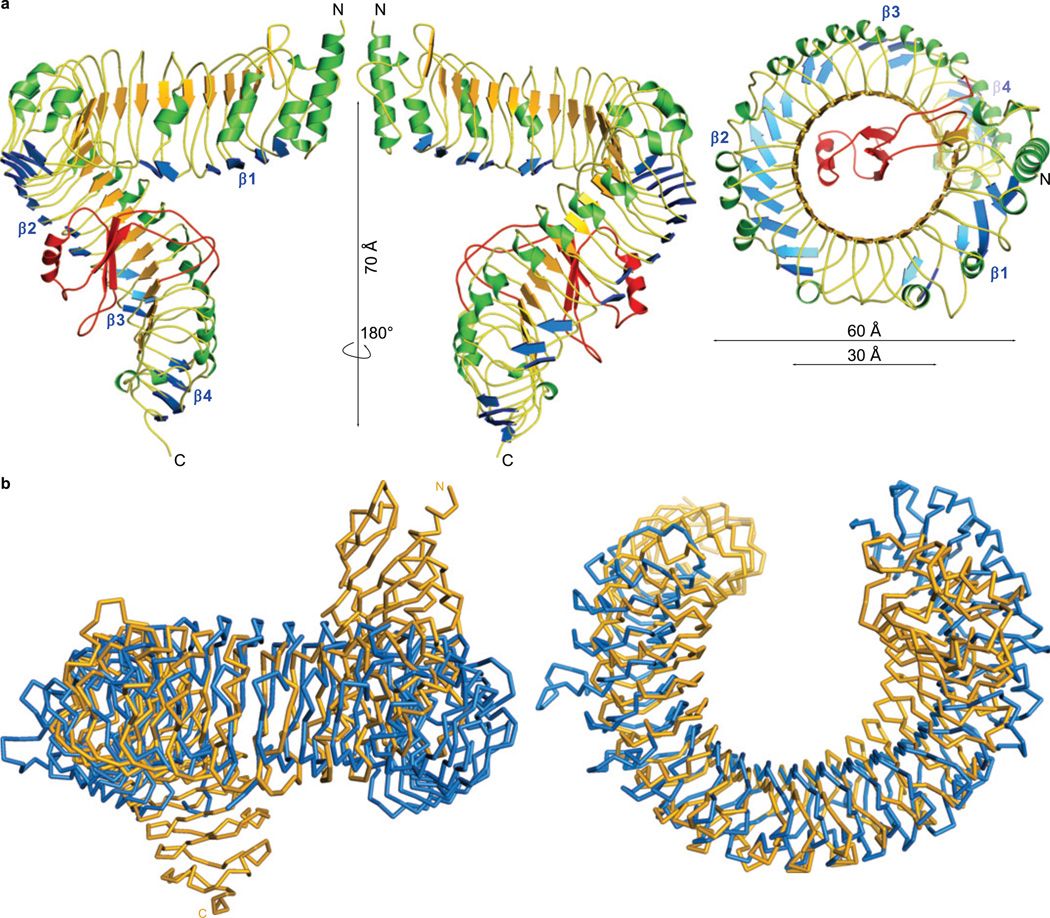
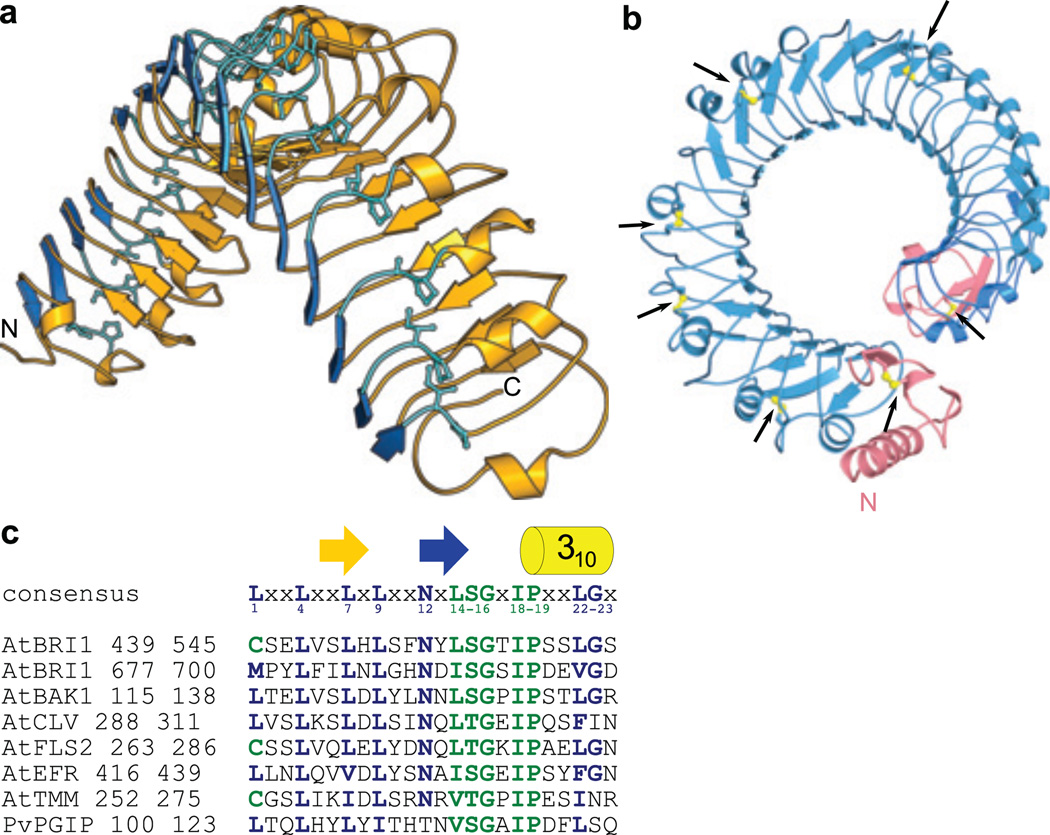
BRI1 was expressed in baculovirus-infected insect cells and the secreted ectodomain was purified by tandem-affinity and size-exclusion chromatography. The crystal structure was solved to 2.5 Å resolution by single isomorphous replacement (see Methods, Supplementary Table 1 and Fig. 1). BRI1 does not adopt the anticipated TLR-horseshoe structure but forms a right-handed superhelix composed of 25 LRRs (Fig. 1a). The helix completes one full turn, with a rise of ~70 Å. The concave surface, that determines the curvature of the solenoid27, is formed by α- and 310 helices (green in Fig. 1a) that cause inner and outer diameters of ~30 and ~60 Å, respectively. The overall curvature of BRI is similar to TLR324 (Fig. 1b), but, while the TLR3 ectodomain is essentially flat, BRI1 is highly twisted (Fig. 1b). Such twisted assemblies of LRRs have been observed previously with bacterial effector28 and adhesion proteins29, and with the plant defence protein PGIP30 (Supplementary Fig. 2). The twist of PGIP's LRR domain is caused by a non-canonical, second β-sheet that is oriented perpendicular to the central β-sheet forming the inner surface of the solenoid30. Additional β-sheets are also present in our structure (blue in Fig. 1a, Supplementary Fig. 3), but in the case of the much larger BRI1 ectodomain result in a superhelical assembly (Fig. 1a). The second β–strand in PGIP and in BRI1 is followed by an Ile-Pro spine that runs along the outer surface of the helix and provides packing interactions between consecutive LRRs (Fig. 2a and ref.30). Both structural features are directly linked to the Lt/sGxIP consensus sequence of the plant-specific LRR subfamily31 (Fig. 2c, Supplementary Fig. 4 and Table 2). Because this consensus sequence is found in other plant RKs, these receptors may also harbour twisted LRR domains (Fig. 2c), making BRI1 the primary template for the study of diverse signalling pathways in plants3–5.
Figure 1. The BRI1 ectodomain forms a superhelical assembly.
a, Ribbon diagram of the BRI1 LRR domain (front, back and top). The canonical LRR β-sheet is shown in orange, and the additional plant-specific β-sheets in blue. Helices are shown in green, and the island domain is depicted in red. b, Structural comparison of the BRI1 (shown as yellow Cα trace) and TLR3 (in blue, pdb-id: lziw)27 ectodomains. The structures superimpose with an r.m.s.d. of 4.2 Å between 341 corresponding Cα atoms. Side and top views are shown. The island domain has been omitted for clarity.
Figure 2. Plant-specific sequence fingerprints result in a superhelical BRI1 ectodomain.
a, Ribbon diagram of the convex side of BRI1 LRRs 9–25 (in yellow). The non-canonical β-strands and the Ile-Pro spine are shown in dark and light blue, respectively, b, Top view of the BRI1 ectodomain (in blue) with disulfide bridges shown in yellow. The N- and C-terminal caps are highlighted in pink, c, Sequence alignment of LRRs in BRI1, other plant receptor kinases4–7,23,24 and PGEP33. The canonical LRR consensus sequences are highlighted in blue, and plant-specific motifs are in green.
N- and C-terminal flanking regions that cap the hydrophobic core of the BRI1 solenoid are similar to caps previously described for PGIP30 (Supplementary Figure. 5). Notably, not are only these caps stabilised by disulfide bridges, but 5 additional disulfide bonds link consecutive LRR segments in the N-terminal half of the BRI1 ectodomain (Fig. 2b, Supplementary Fig. 4 and Table 2).
The island domain
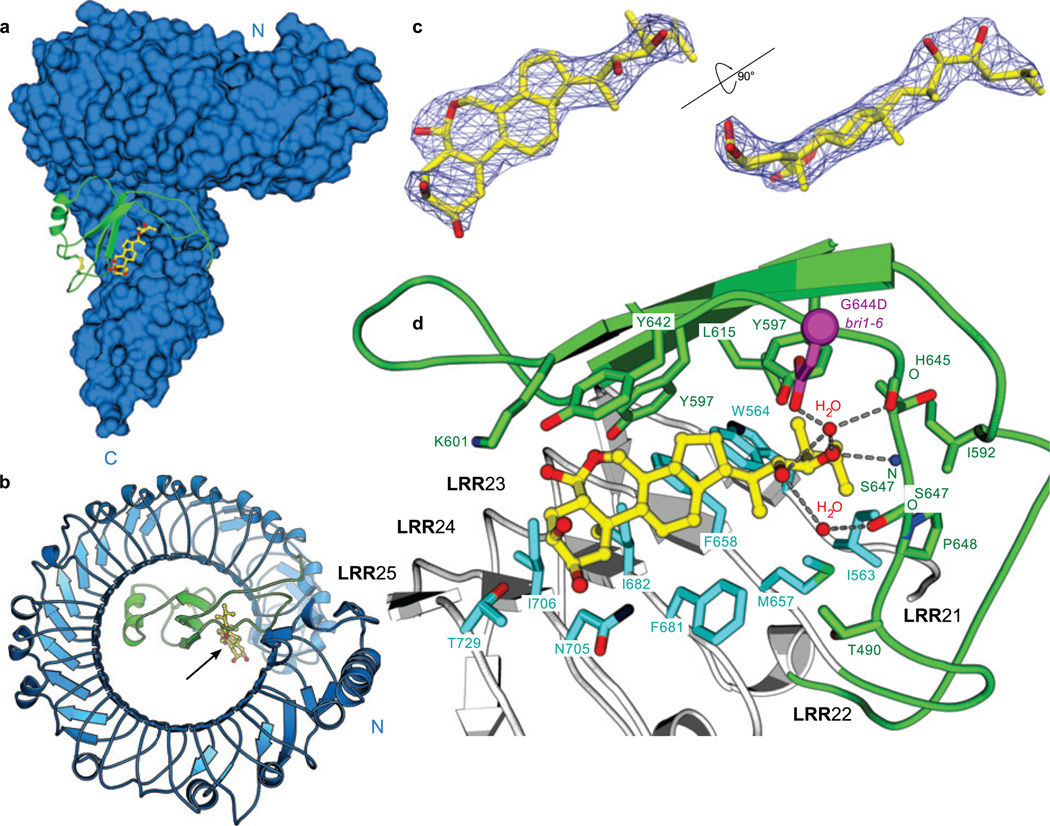
The island domain in BRI1 corresponds to a large insertion in the regular repeat-structure between LRRs 21 and 22 (residues 584–654) (Fig. 1a). The resulting ~70 residue segment forms a small domain that folds back into the interior of the superhelix, where it makes extensive polar and hydrophobic interactions with LRRs 13–25 (Fig 1a, Supplementary Fig. 6 and Table 2). The domain fold is characterised by an anti-parallel β-sheet, which is sandwiched between the LRR core and a 310 helix and stabilised by a disulfide bridge (Fig. 3a, Supplementary Fig. 4). The loss-of-function alleles bri1-9 (Ser662Phe, weak)32 and bri1-113 (Gly611Glu, strong)2 map to this island domain – LRR interface (Supplementary Fig. 6), and likely interfere with folding of the island domain33. Two long loops that connect the island domain to the LRR core appear partially disordered in the unliganded receptor (Supplementary Fig. 7). The insertion of a folded domain into the LRR repeat has not been observed in other LRR receptor structures and is likely an adaptation to the challenge of sensing a small steroid ligand, compared, for example, to recognising larger ligands, such as proteins, nucleic acids, or lipids25,26.
Figure 3. The steroid hormone binding site maps to C-terminal inner surface of the superhelix.
a, Brassinolide (in yellow sticks) binds to a surface provided by the LRR domain (in blue) and by parts of the island domain (green ribbon), b, Location of the steroid in centre of the BRI1 superhelix. c, Close-up view of the brassinolide in two orientations, including an omit 2Fo-Fc electron density map contoured at 1.5 σ. d, Protein-hormone interactions in the BRI1 steroid binding site. Ribbon diagram of LRRs 21–25 (in grey) are shown together with parts of the island domain (in green). Contacting residues are shown in full side-chain representation, polar interactions as dotted lines, and water molecules as red balls, bril-6 (Gly644Asp) is depicted in magenta.
We next solved a 2.5 Å co-crystal structure with brassinolide, a potent Arabidopsis steroid that binds BRI1 with nanomolar affinity7,34. One molecule of brassinolide per BRI1 monomer binds in close proximity to the island domain (Fig. 3 a–c), which was previously implicated in steroid binding7,16. Our structure reveals that the LRR superhelix and the island domain both extensively contribute to formation of the hormone binding site. The A–D rings of the steroid bind to a hydrophobic surface which is provided by LRRs 23–25 and that maps to the inner side of the BRI1 superhelix (Fig. 3b,d, Supplementary Fig. 8). The alkyl chain of the hormone fits into a small pocket formed by residues originating from LRRs 21 and 22 (Ile563, Trp564, Met657, Phe658) and from two loops connecting the island domain with the LRR core (Fig. 3d). The hydrophobic nature and restricted size of this pocket now explain why steroid ligands with bulkier or charged alkyl side chains, such as the arthropod steroid ecdysone (Supplementary Fig. 8), cannot be recognised by BRI17. A few polar interactions with brassinolide's second diol moiety (Fig. 3d) are established with Tyr597 and main chain atoms from His645 and Ser647 in the island domain, and are mediated by water molecules (Fig. 3d). Mutation of the neighbouring Gly644 to Asp may interfere with this hydrogen bonding network, and explain why this mutation greatly reduces the binding activity of the receptor7 and causes the loss-of-function phenotype bri1-632 (Fig. 3d). No polar contacts are observed with the seven-membered B-ring lactone (Fig. 3d), consistent with B-ring modifications as found in e.g. castasterone (Supplementary Fig. 8) being tolerated by BRI17,35.
The steroid-complex reveals a hormone-binding site that involves a much larger portion of the LRR domain than previously anticipated16. Major interactions between the steroid and the BRI1 ectodomain originate from the very C-terminal LRRs 23–25, which brings the hormone in close proximity to the membrane (Fig. 3a,d). Importantly, while there is a significant hormone-receptor interface (550 Å2) for such a small molecule ligand, large parts of the steroid are exposed to the solvent, including the 2α,3α-diol moiety in brassinolide that is important for biological activity35. Thus, protein-protein interactions may be involved in the recognition of the steroid ligand, with the hormone itself providing a docking platform. Importantly, steroid binding induces a conformational rearrangement and fixing of the island domain, which becomes fully ordered and competent to participate in protein-protein interactions that could be critical for receptor activation (Supplementary Fig. 7).
A protein interaction platform
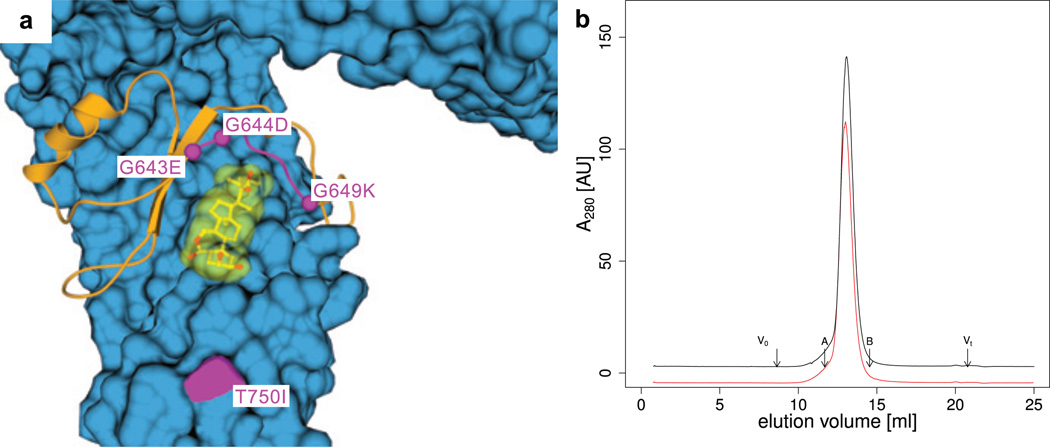
Four known BRI1 missense alleles map to the inner surface of last 5 LRRs (Fig. 5a). This surface is not masked by carbohydrate (Supplementary Fig. 9) and contains both the hormone-binding site and the island domain (Fig. 3a,d and 4a,c). Three mutations cluster in a loop connecting the island domain with LRR 22 (Fig. 5a). This loop is partially disordered in the unliganded structure but is well-defined in the brassinolide complex (Supplementary Fig. 7). We speculate that this loop, when ordered, is engaged in protein-protein interactions that are critical for receptor activation, and that missense alleles in BRI1 modulate these interactions: The gain-of-function allele sud136 (Gly643-Glu) may establish contact with Ser623 in the island domain, and lead to an ordered loop even in the absence of steroid ligand (Supplementary Fig. 10). Mutation of the neighbouring Gly644 to Asp causes the loss-of-function phenotype bri1-632 (see above, Fig. 3d, 4a), and mutation of conserved Thr649 to Lys inactivates barley BRI137. These mutations, when modelled in silico, induce steric clashes with residues in the island domain and in the underlying LRR domain (Supplementary Fig. 10), and thus may distort the position of the loop. Interestingly, bri1-102, a strong loss-of-function mutation (Thr750-Ile)38 that does not affect steroid binding7, maps to a distinct surface area in LRR 25 (Fig. 4a). Thus protein-protein interactions critical for receptor activation may not be restricted to the island domain, but also involve residues from the LRR core.
Figure 5. An accessible membrane-proximal region of BRI1 may provide a protein-protein interaction platform.
a, Overview of the C-terminal surface area (in blue) that is not masked by carbohydrate. Brassinolide is shown in yellow, the island domain in orange, and genetic alleles connected in magenta, b, Analytical gel-filtration 280 nm absorbance trace. The free ectodomain eluates as a monomer (black line), as does a putative complex with brassinolide (red line). Void (V0) and total volume (Vt) are shown together with elution volumes for molecular weight standards (A, aldolase, MW 158,000 Da; B, conalbumin, MW 75,000 Da). The estimated molecular weight for the monomer peak is ~125 kDa. The approximate molecular weight of the purified BRI1 is 110 kDa.
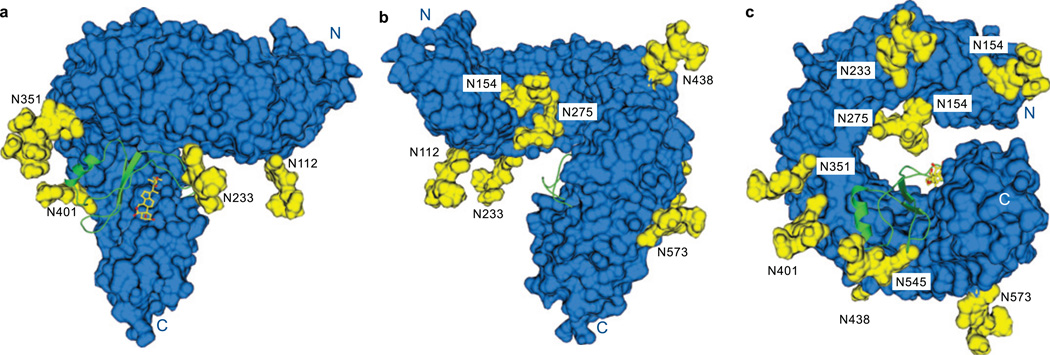
Figure 4. N-linked glycans mask large surface areas of the BRI1 superhelix.
Oligomannose core stmctures (containing two N-acetylglucosamines and three mannose units) as found in insect cells and plants were modelled onto the nine glycosylation sites in the structure to visualise the BRI1 surface that is potentially masked by carbohydrate. The LRR domain in surface representation is shown in blue, and the glycan structures are highlighted in yellow. A ribbon diagram of the island domain (in green) and the steroid ligand (in yellow) is included. The views are a, front b, back and c, perspective along the superhelix from the C-terminus.
Receptor activation
BRI1 has been shown to exist at least partially as a omooligomer in planta13,39,40. Thus steroid binding to the island domain and the concomitant rearrangements of the island domain loop could induce a conformational change in a preformed BRI1 homodimer13, or allow for ligand-dependent dimerisation of the BRI1 ectodomain. However, models of BRI1 dimers that bring the C-termini of their ectodomains into close proximity (n.b. it is known that the cytoplasmic kinase domains of BRI1 can interact13,18) and that make use of the interaction surface outlined above, encounter steric clashes with the N-terminal LRRs (Supplementary Fig. 11). Furthermore, in contrast to TLR ectodomains, which in crystals tend to form homodimers even in the absence of ligand24,25, dimers cannot be seen in BRI1 crystals, which grew under the same acidic pH conditions that are typically associated with the plant cell wall. The largest interface area between two neighbouring BRI1 molecules amounts to only ~1.5% of the total accessible surface area, consistent with the high solvent content of our crystals (see Supplementary Methods). The main crystal contact involves a head-to-head arrangement of two BRI1 monomers, a configuration that would place the cytoplasmic kinase domains far apart (Supplementary Fig. 12). The recombinant BRI1 ectodomain eluates as a monomer in the absence of steroid ligand, and shows no tendency to dimer- or oligomerise in the presence of a ~4x molar excess of brassinolide in size-exclusion chromatography experiments (Fig. 4b).
Our analyses suggest that the superhelical BRI1 LRR domain alone has no tendency to oligomerise, indicating that BRI1 receptor activation may not be mediated by ligand-induced homodimerisation of the ectodomain (as described for TLRs25,26) or by conformational changes in preformed homodimers13. We do not want to dismiss the fact that the cytoplasmic kinase domain of BRI1 can dimerise18, or that BRI1 homooligomers are present in vivo13,39,40. However, our structures reinforce the notion that homooligomerisation of BRI1 may be constitutive and independent of ligand stimulus39. The presence of an interaction platform that undergoes conformational changes upon steroid binding and that harbours several loss- and gain-of-function alleles suggests that interaction with another protein factor may control BRI1 activation.
Discussion
The structure of the BRI1 ectodomain offers several new insights, and its twisted shape will likely characterise the architecture of many plant LRR-RKs1. The presence of a folded domain-insert appears to be an adaptation to recognition of a small molecule ligand, a challenge that smaller LRR proteins have met by generating loop insertions into their capping motifs41. This fascinating mode of ligand recognition reveals how steroids can be sensed at membranes and rationalises a large set of genetic and biochemical findings2,7,32,38.
Different BRI1 receptor activation mechanisms have been proposed including ligand-dependent dimerisation as seen for TLRs25,26 and ligand-induced conformational changes in preformed homodimers13. Our analyses suggest that the superhelical shape of the BRI1 ectodomain is incompatible with homodimerisation, and that the isolated ectodomain behaves as a monomer even in the presence of steroid. These findings leave us with the alternative hypothesis that another protein factor could bind to the interaction platform in BRI1 that would minimally encompass the steroid ligand, LRRs 21–25 and parts of the island domain (Fig. 4a). While it is possible that an unknown protein fulfils this role and provides a dimerisation interface for two BRI1 molecules (as seen for example for TLR426), genetic and biochemical screens have not uncovered this protein. It is thus possible that the small receptor kinase BAK1 acts as a direct brassinosteroid co-receptor, as suggested previously10,20. It has been demonstrated that BAK1 is a genetic component of the brassinosteroid pathway20,21, that BRI1 and BAK1 interact in a steroid-dependent manner22 and that both receptors trans-phosphorylate each other upon ligand stimulus22. Notably, a homology model of the BAK1 ectodomain (Supplementary Fig. 13) is compatible in size and shape with the interaction platform in BRI1, and the BAK1 elg allele, which maps to the BAK1 ectodomain (Supplementary Fig. 14), renders plants hypersensitive to brassinosteroid treatment42. We speculate that the sud1, bri1-6, bri1-102 and elg mutations modulate the interaction between the BRI1 and BAK1 ectodomains in a brassinosteroid-dependent manner (Supplementary Fig. 14). The demonstration that BAK1 is essential for brassinosteroid sensing may have been obscured owing to genetic redundancy20, with at least two BAK1-like proteins interacting with BRI1 in vivo43,44. We recently overcame this limitation by showing that the BRI1 inhibitor protein BKI1 blocks the interaction between the BAK1 and BRI1 kinase domains18. Importantly, transgenic lines that constitutively deliver BKI1 to the site of BRI1 signalling, resemble strong BRI1 loss-of-function mutants, suggesting an important role for receptor - co-receptor association in brassinosteroid signal initiation18.
Future studies will undoubtedly test this heteromerisation model and dissect the relative contributions of the BRI1 and BAK1 ectodomains, their transmembrane segments and their cytoplasmic kinase domains to receptor activation. It will become important then to understand how BAK1 could serve as co-receptor for other LRR-RK signalling pathways5,19.
Methods Summary
The StrepII-9xHis fused BRI1 ectodomain (residues 29–788) was produced by secreted expression in baculovirus-infected insect cells, harvested 4 d post-infection by ultrafiltration and purified by tandem affinity chromatography, and by gel filtration. BRI1 was concentrated to 15 mg/ml and crystallised by vapour diffusion using a reservoir solution containing 14% PEG 4,000, 0.2 M (NH4)2SO4, 0.1 M citric acid (pH 4.0). The brassinolide complex was obtained by co-crystallisation. Diffraction data to 2.5 Å resolution were collected on a rotating anode X-ray generator and at beam-line 8.2.1 of the Advanced Light Source (ALS), Berkeley. The structure was solved using the SIRAS method. Data and refinement statistics are summarised in Supplementary Table 1.
Supplementary Material
Acknowledgements
We thank J. Vanhnasy and W. Yu for maintaining insect cell stocks, M. Jinek and B.W. Han for advice, W. Kwiatowski for excellent maintenance of the Salk X-ray equipment, Y. Jaillais for discussion, and F. V. Chisari for encouragement and support. This work was supported by the Howard Hughes Medical Institute and a grant from the National Science Foundation (IOS-0649389) to J.C. M.H. was supported by long-term fellowships from the European Molecular Biology Organisation and the International Human Frontier Science Program Organisation. Y.B. was a Howard Hughes Medical Institute fellow of the Life Sciences Research Foundation and was also a recipient of the Philippe Foundation. I.A.W. was supported by NIH grant AI042266 and by the Skaggs Institute for Chemical Biology.
Footnotes
Author Information Atomic coordinates and structure factors for the reported crystal structures have been deposited in the Protein Data Bank under accession numbers 3RIZ for the unliganded BRI1 ectodomain and 3RJ0 for the BRI1-brassinolide complex. Reprints and permissions information is available at www.nature.com/reprints. The authors declare no competing financial interests.
Author Contributions M.H., Y.B., J.P.N. and J.C. designed the project. M.H. expressed the BRI1 ectodomain in I.A.W.s laboratory with initial help from Y.B. M.H. purified and crystallised the protein, and phased and refined the structures. M.D. determined viral titers and optimised production of viruses. T.D. cloned the modified transfer vector. M.H., I.A.W. and J.C. analysed the data. J.C. supervised the project. M.H. wrote the paper with input from the other authors.
References
- 1.Shiu SH, Bleecker AB. Receptor-like kinases from Arabidopsis form a monophyletic gene family related to animal receptor kinases. Proc. Natl. Acad. Sci. U.S.A. 2001;98:10763–10768. doi: 10.1073/pnas.181141598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Chory J. A putative leucine-rich repeat receptor kinase involved in brassinosteroid signal transduction. Cell. 1997;90:929–938. doi: 10.1016/s0092-8674(00)80357-8. [DOI] [PubMed] [Google Scholar]
- 3.Clark SE, Williams RW, Meyerowitz EM. The CLAVATA1 gene encodes a putative receptor kinase that controls shoot and floral meristem size in Arabidopsis. Cell. 1997;89:575–585. doi: 10.1016/s0092-8674(00)80239-1. [DOI] [PubMed] [Google Scholar]
- 4.Nadeau JA, Sack FD. Control of stomatal distribution on the Arabidopsis leaf surface. Science. 2002;296:1697–1700. doi: 10.1126/science.1069596. [DOI] [PubMed] [Google Scholar]
- 5.Gómez-Gómez L, Boller T. FLS2: an LRR receptor-like kinase involved in the perception of the bacterial elicitor flagellin in Arabidopsis. Mol. Cell. 2000;5:1003–1011. doi: 10.1016/s1097-2765(00)80265-8. [DOI] [PubMed] [Google Scholar]
- 6.Nishimura R, et al. HAR1 mediates systemic regulation of symbiotic organ development. Nature. 2002;420:426–429. doi: 10.1038/nature01231. [DOI] [PubMed] [Google Scholar]
- 7.Wang ZY, Seto H, Fujioka S, Yoshida S, Chory J. BRI1 is a critical component of a plasma-membrane receptor for plant steroids. Nature. 2001;410:380–383. doi: 10.1038/35066597. [DOI] [PubMed] [Google Scholar]
- 8.Ogawa M, Shinohara H, Sakagami Y, Matsubayashi Y. Arabidopsis CLV3 peptide directly binds CLV1 ectodomain. Science. 2008;319:294. doi: 10.1126/science.1150083. [DOI] [PubMed] [Google Scholar]
- 9.Belkhadir Y, Chory J. Brassinosteroid signaling: a paradigm for steroid hormone signaling from the cell surface. Science. 2006;314:1410–1411. doi: 10.1126/science.1134040. [DOI] [PubMed] [Google Scholar]
- 10.Vert G, Nemhauser JL, Geldner N, Hong F, Chory J. Molecular mechanisms of steroid hormone signaling in plants. Annu. Rev. Cell Dev. Biol. 2005;21:177–201. doi: 10.1146/annurev.cellbio.21.090704.151241. [DOI] [PubMed] [Google Scholar]
- 11.Mangelsdorf DJ, et al. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geldner N, Hyman DL, Wang X, Schumacher K, Chory J. Endosomal signaling of plant steroid receptor kinase BRI1. Genes Dev. 2007;21:1598–1602. doi: 10.1101/gad.1561307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X, et al. Autoregulation and homodimerization are involved in the activation of the plant steroid receptor BRI1. Dev. Cell. 2005;8:855–865. doi: 10.1016/j.devcel.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Wang X, Chory J. Brassinosteroids regulate dissociation of BKI1, a negative regulator of BRI1 signaling, from the plasma membrane. Science. 2006;313:1118–1122. doi: 10.1126/science.1127593. [DOI] [PubMed] [Google Scholar]
- 15.He Z, et al. Perception of brassinosteroids by the extracellular domain of the receptor kinase BRI1. Science. 2000;288:2360–2363. doi: 10.1126/science.288.5475.2360. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita T, et al. Binding of brassinosteroids to the extracellular domain of plant receptor kinase BRI1. Nature. 2005;433:167–171. doi: 10.1038/nature03227. [DOI] [PubMed] [Google Scholar]
- 17.Wang X, et al. Identification and functional analysis of in vivo phosphorylation sites of the Arabidopsis BRASSINOSTEROID-INSENSITIVE1 receptor kinase. Plant Cell. 2005;17:1685–1703. doi: 10.1105/tpc.105.031393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jaillais Y, et al. Tyrosine phosphorylation controls brassinosteroid receptor activation by triggering membrane release of its kinase inhibitor. Genes Dev. 2011;25:232–237. doi: 10.1101/gad.2001911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chinchilla D, Shan L, He P, de Vries S, Kemmerling B. One for all: the receptor-associated kinase BAK1. Trends Plant Sci. 2009;14:535–541. doi: 10.1016/j.tplants.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nam KH, Li J. BRI1/BAK1, a receptor kinase pair mediating brassinosteroid signaling. Cell. 2002;110:203–212. doi: 10.1016/s0092-8674(02)00814-0. [DOI] [PubMed] [Google Scholar]
- 21.Li J, et al. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002;110:213–222. doi: 10.1016/s0092-8674(02)00812-7. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, et al. Sequential transphosphorylation of the BRI1/BAK1 receptor kinase complex impacts early events in brassinosteroid signaling. Dev. Cell. 2008;15:220–235. doi: 10.1016/j.devcel.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Kim T-W, Wang Z-Y. Brassinosteroid signal transduction from receptor kinases to transcription factors. Annu. Rev. Plant Biol. 2010;61:681–704. doi: 10.1146/annurev.arplant.043008.092057. [DOI] [PubMed] [Google Scholar]
- 24.Choe J, Kelker MS, Wilson IA. Crystal structure of human toll-like receptor 3 (TLR3) ectodomain. Science. 2005;309:581–585. doi: 10.1126/science.1115253. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, et al. Structural basis of toll-like receptor 3 signaling with double-stranded RNA. Science. 2008;320:379–381. doi: 10.1126/science.1155406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park BS, et al. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature. 2009;458:1191–1195. doi: 10.1038/nature07830. [DOI] [PubMed] [Google Scholar]
- 27.Bella J, Hindle KL, McEwan PA, Lovell SC. The leucine-rich repeat structure. Cell. Mol. Life Sci. 2008;65:2307–2333. doi: 10.1007/s00018-008-8019-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Evdokimov AG, Anderson DE, Routzahn KM, Waugh DS. Unusual molecular architecture of the Yersinia pestis cytotoxin YopM: a leucine-rich repeat protein with the shortest repeating unit. J. Mol. Biol. 2001;312:807–821. doi: 10.1006/jmbi.2001.4973. [DOI] [PubMed] [Google Scholar]
- 29.Schubert WD, et al. Structure of internalin, a major invasion protein of Listeria monocytogenes, in complex with its human receptor E-cadherin. Cell. 2002;111:825–836. doi: 10.1016/s0092-8674(02)01136-4. [DOI] [PubMed] [Google Scholar]
- 30.Di Matteo A, et al. The crystal structure of polygalacturonase-inhibiting protein (PGIP), a leucinerich repeat protein involved in plant defense. Proc. Natl. Acad. Sci. U.S.A. 2003;100:10124–10128. doi: 10.1073/pnas.1733690100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kajava AV. Structural diversity of leucine-rich repeat proteins. J. Mol. Biol. 1998;277:519–527. doi: 10.1006/jmbi.1998.1643. [DOI] [PubMed] [Google Scholar]
- 32.Noguchi T, et al. Brassinosteroid-insensitive dwarf mutants of Arabidopsis accumulate brassinosteroids. Plant Physiol. 1999;121:743–752. doi: 10.1104/pp.121.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jin H, Yan Z, Nam KH, Li J. Allele-specific suppression of a defective brassinosteroid receptor reveals a physiological role of UGGT in ER quality control. Mol. Cell. 2007;26:821–830. doi: 10.1016/j.molcel.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujioka S, et al. The Arabidopsis deetiolated2 mutant is blocked early in brassinosteroid biosynthesis. Plant Cell. 1997;9:1951–1962. doi: 10.1105/tpc.9.11.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Back TG, Pharis RP. Structure-Activity Studies of Brassinosteroids and the Search for Novel Analogues and Mimetics with Improved Bioactivity. J. Plant Growth Regul. 2003;22:350–361. doi: 10.1007/s00344-003-0057-0. [DOI] [PubMed] [Google Scholar]
- 36.Diévart A, Hymes MJ, Li J, Clark SE. Brassinosteroid-independent function of BRI1 / CLV1 chimeric receptors. Funct. Plant Biol. 2006;33:723–730. doi: 10.1071/FP06080. [DOI] [PubMed] [Google Scholar]
- 37.Gruszka D, Szarejko I, Maluszynski M. New allele of HvBRI1 gene encoding brassinosteroid receptor in barley. J. Appl. Genet. 2011 doi: 10.1007/s13353-011-0031-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Friedrichsen DM, Joazeiro CA, Li J, Hunter T, Chory J. Brassinosteroid-insensitive-1 is a ubiquitously expressed leucine-rich repeat receptor serine/threonine kinase. Plant Physiol. 2000;123:1247–1256. doi: 10.1104/pp.123.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hink MA, Shah K, Russinova E, de Vries SC, Visser AJWG. Fluorescence fluctuation analysis of Arabidopsis thaliana somatic embryogenesis receptor-like kinase and brassinosteroid insensitive 1 receptor oligomerization. Biophys. J. 2008;94:1052–1062. doi: 10.1529/biophysj.107.112003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Russinova E, et al. Heterodimerization and endocytosis of Arabidopsis brassinosteroid receptors BRI1 and AtSERK3 (BAK1) Plant Cell. 2004;16:3216–3229. doi: 10.1105/tpc.104.025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han BW, Herrin BR, Cooper MD, Wilson IA. Antigen recognition by variable lymphocyte receptors. Science. 2008;321:1834–1837. doi: 10.1126/science.1162484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whippo CW, Hangarter RP. A brassinosteroid-hypersensitive mutant of BAK1 indicates that a convergence of photomorphogenic and hormonal signaling modulates phototropism. Plant Physiol. 2005;139:448–457. doi: 10.1104/pp.105.064444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He K, et al. BAK1 and BKK1 regulate brassinosteroid-dependent growth and brassinosteroidindependent cell-death pathways. Curr. Biol. 2007;17:1109–1115. doi: 10.1016/j.cub.2007.05.036. [DOI] [PubMed] [Google Scholar]
- 44.Karlova R, et al. The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR-LIKE KINASE1 protein complex includes BRASSINOSTEROID-INSENSITIVE1. Plant Cell. 2006;18:626–638. doi: 10.1105/tpc.105.039412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.