Abstract
The white shark (Carcharodon carcharias) is a wide-ranging apex predator in the northeastern Pacific (NEP). Electronic tagging has demonstrated that white sharks exhibit a regular migratory pattern, occurring at coastal sites during the late summer, autumn and early winter and moving offshore to oceanic habitats during the remainder of the year, although the purpose of these migrations remains unclear. The purpose of this study was to use stable isotope analysis (SIA) to provide insight into the trophic ecology and migratory behaviors of white sharks in the NEP. Between 2006 and 2009, 53 white sharks were biopsied in central California to obtain dermal and muscle tissues, which were analyzed for stable isotope values of carbon (δ13C) and nitrogen (δ15N). We developed a mixing model that directly incorporates movement data and tissue incorporation (turnover) rates to better estimate the relative importance of different focal areas to white shark diet and elucidate their migratory behavior. Mixing model results for muscle showed a relatively equal dietary contribution from coastal and offshore regions, indicating that white sharks forage in both areas. However, model results indicated that sharks foraged at a higher relative rate in coastal habitats. There was a negative relationship between shark length and muscle δ13C and δ15N values, which may indicate ontogenetic changes in habitat use related to onset of maturity. The isotopic composition of dermal tissue was consistent with a more rapid incorporation rate than muscle and may represent more recent foraging. Low offshore consumption rates suggest that it is unlikely that foraging is the primary purpose of the offshore migrations. These results demonstrate how SIA can provide insight into the trophic ecology and migratory behavior of marine predators, especially when coupled with electronic tagging data.
Introduction
The white shark (Carcharodon carcharias) is a large apex predator that ranges across coastal and pelagic habitats in the northeastern Pacific (NEP) [1], [2], [3], [4]. White sharks are primarily concentrated along the west coast of North America during the late summer, autumn and early winter months and exhibit a regular offshore migration to oceanic habitats during the winter, spring and summer. Although some white sharks move as far as the Hawaiian island archipelago (traveling at least as far west as Midway Atoll), many sharks generally move to an area that has been referred to as the “offshore focal area” [2], “shared offshore foraging area” [3], or “white shark café” (hereafter referred to as the Café) [4]. This region is located approximately midway between Baja California, Mexico, and the Hawaiian Islands.
It has been theorized that the migratory behaviors elucidated by electronic tagging studies are related to foraging or reproduction [2], [3]; however the purpose of these offshore movements remains unclear. Recent electronic tagging studies have demonstrated that white sharks spend a considerable amount of their time in offshore habitats. Over the course of a year, sharks utilize pelagic habitats for 7 to 8 months or longer (mean 234 d±45 SD from pop-up archival transmitting (PAT) tags) [2], [4], indicating that these habitats play an important role in the life history of white sharks in the NEP. White sharks are listed as vulnerable by the World Conservation Union (IUCN) and protected under the Convention on the International Trade in Endangered Wild Flora and Fauna (CITES) [5]. Therefore it is important to understand how and why white sharks use these regions in order to have a more complete understanding of their life history and effectively manage and conserve the species.
To date, there has been no direct evidence of white sharks foraging during these offshore periods. It has been hypothesized that white sharks may be able to fast for extended periods of time following consumption of large quantities of high caloric marine mammal tissue [6]. It seems highly unlikely that an active, endothermic shark could fast for the extensive periods of time they spend offshore away from productive neritic habitats, suggesting that foraging in offshore habitats must occur. However, it has been noted that upon returning to aggregation sites off the central California coast (e.g., the Farallon Islands) after being offshore, white sharks often appear lean [7]. When in the Hawaii focal area, and to a lesser extent the Café, white sharks exhibit diel vertical migrations [4], suggesting that sharks may forage within the deep scattering layer, possibly upon mid-upper trophic level prey that track the deep scattering layer [8], [9], [10], [11]. White sharks in the Café, especially males, exhibit rapid oscillatory diving, a behavior which appears to be unique to this region and the purpose of which is unknown, but it has been theorized that it may represent searching or reproductive behavior [2].
The Café is an oligotrophic open ocean habitat in the North Pacific subtropical gyre where prey availability is presumably lower than in more productive neritic habitats [12]. Upon first examination, extensive use of this low-productivity region by a large apex predator is puzzling. However, there may be a more abundant forage base than apparent, as pelagic fishes (tuna, billfish) [13], [14], sharks [13], [14], and squid [15], [16], [17] occur in this region when white sharks are present. In addition, it is possible that shoaling of the oxygen minimum layer in the vicinity of the Café may aggregate prey by compressing vertical habitat [18], [19].
The Hawaiian Islands are a more productive region than the Café [20] with a diversity of pelagic, shelf and slope habitats that may offer white sharks more diverse and abundant prey. When in the coastal waters of Hawaii, white sharks may consume neritic teleosts, elasmobranchs, and cephalopods [21], [22], [23], [24]. In addition, Weng et al. [25] noted that white sharks have been observed near spinner dolphin (Stenella longirostris) aggregations and Hawaiian monk seal (Monachus schauinslandi) colonies, and that the timing of their occurrence in Hawaiian waters coincided with calving season of humpback whales (Megaptera novaeangliae) [26], suggesting that they may forage upon these species.
Although advances in electronic tag technology have provided great insight into the movements and habitat use of white sharks in the NEP, additional analytical techniques are required to help resolve some of the unanswered questions regarding the ecology of white sharks during the offshore component of their migratory cycle. SIA is a biogeochemical technique that uses the ratio of heavy to light isotopes of elements present in tissues as an intrinsic marker to understand the foraging and migration of an organism [27]. The stable isotope composition of an animal is directly related to that of its prey and the isotopic value generally shifts in a predictable manner through successive trophic levels [28], [29]. The stable isotope composition of an animal reflects that of local food webs [28] and because isotopic composition of different food webs vary spatially due to differences in biogeochemical processes, every animal is “tagged” [27], [30] and isotopes can be used as tracers to learn about trophic ecology and migration of organisms [30], [31], [32], [33].
Spatial differences in biogeochemical processes related to primary production create isotopic gradients, or isoscapes [27], [29], [30], [34], which can be used to understand the general patterns of movements and trophic ecology of white sharks. There is a gradient in 13C/12C ratios between nearshore or benthic derived food webs versus offshore or pelagic food webs, with 13C/12C ratios increasing from oligotrophic offshore to productive nearshore ecosystems [35], [36], [37], [38]. The nitrogen isotope composition at the base of the food web varies based on the 15N/14N ratios of nutrient sources (e.g., N2, nitrate, ammonium) and differential importance of biological processes such as denitrification, N2-fixation, and isotopic fractionation associated with nitrogen assimilation dynamics [39], [40]. These patterns generally result in offshore oligotrophic regions of the NEP, (i.e., subtropical gyre) being depleted in 15N due to the predominance of N2-fixation and nearshore regions of the California Current being enriched from the upwelling of 15N-enriched nitrate.
Because it directly reflects diet integrated over time and space, SIA is a useful complement to electronic tagging data, and an integrative approach combining both methods has the potential to be a useful tool that can be used to address a variety of ecological questions. White sharks move between isotopically distinct oceanographic regions during the course of their migrations. More productive coastal habitats are generally enriched in 13C and 15N relative to oligotrophic offshore habitats of the Café and Hawaiian Islands [29], [35], [39], [41], [42], [43], [44]. Because white sharks move between isotopically distinct oceanographic regions in a well-defined manner identified through electronic tagging studies, SIA has the potential to elucidate if sharks are foraging during offshore periods and improve our overall understanding of white shark trophic ecology. Indeed, because of the difficulty in studying white sharks due to their low population size, highly mobile nature, and protected status, SIA or other biochemical analyses of tissues collected non-destructively from sharks may be one of the few ways to understand trophic ecology and general patterns of habitat use of this species throughout its ontogeny and when they are away from visible, well-studied coastal regions.
The purpose of this study was to couple SIA and electronic tagging results from white sharks to provide insight into trophic ecology and migratory behavior of white sharks, a protected species in the NEP. By interpreting SIA results within the spatial and temporal context provided by electronic tag data, we demonstrate the efficacy of using this integrative approach to study the movements and trophic ecology of a large, highly migratory marine species. In particular, our goals were to directly incorporate electronic tagging data and isotopic incorporation rates into mixing models to 1) provide evidence of offshore foraging, 2) estimate relative importance of nearshore and offshore focal areas to white shark diet and 3) estimate relative consumption rates in the different focal areas.
Materials and Methods
Ethics statement
The project was conducted with permits from the California Department of Fish and Game, Monterey Bay National Marine Sanctuary, Gulf of the Farallones National Marine Sanctuary, U. S. National Park Service, Naval Postgraduate School, and under Stanford University animal care protocol 10765.
Sample collection
A biopsy probe attached to a tagging pole was used to collect tissue samples from free-swimming white sharks during the tagging operations reported by Jorgensen et al. [4] at shark aggregation sites at South Farallon Island, Point Reyes, and Tomales Point in central California. Biopsies were from the epaxial musculature adjacent to the dorsal fin in the same region as tag implantation. Tissue was kept on ice until it was frozen several hours after collection, and stored at −80°C. Biopsies provided two tissue types, dermal and/or white muscle tissue. When both tissues were present, biopsies were subsampled for analysis of each tissue.
Stable isotope analysis
Tissue samples were loaded in Accelerated Solvent Extractor (ASE, Dionex) cells between GF/F (Whatman), and sand was used to fill the remaining headspace. Each cell was rinsed 2× with Petroleum Ether (PE) and 3× with deionized water (DIW) to remove lipids and urea, respectively. Each rinse consisted of 9 ml of solution at 1500 psi and 50°C that was driven through samples for 5 min [45]. After PE and DIW treatment, ASE cells (containing filters and samples) were dried overnight in an oven set to 50°C. Following processing, 0.5–0.7 mg of dried tissue was weighed into tin boats (3×5 mm, Costech). Samples were analyzed at the Stable Isotope Laboratory at the University of California, Santa Cruz, using an Elemental Analyzer coupled to an isotope ratio monitoring mass spectrometer (Delta XP-EA, Thermo-Finnagen IRMS). Isotopic composition is expressed in δ values (parts per thousand differences from a standard or per mil (‰)) and is calculated using the equation: δX = [(R sample/R standard)−1)] * 1000; where X = 13C or 15N, R = ratio of 13C/12C, 15N/14N, and the standards are Vienna Pee Dee Belemnite limestone (V-PDB) for carbon and AIR for nitrogen. Replicates of a gelatin standard within each analysis allowed for mass and drift corrections. Comparisons of this standard within and between runs yielded standard deviations of <0.1‰ and <0.2‰ for δ13C and δ 15N values, respectively.
Isotopic characterization of focal areas
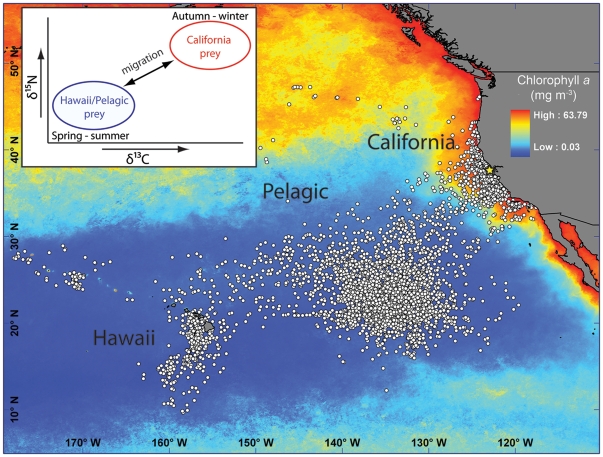
Based on electronic tagging studies of central California white sharks [2], [4], we defined three general regions for white sharks: 1) the California Current, 2) offshore pelagic habitats of the subtropical gyre (i.e., the Café), and 3) neritic habitats of the Hawaiian Island Archipelago and pelagic habitats to the south of Hawaii. We refer to these regions as the California, Pelagic, and Hawaii regions (Figure 1).
Figure 1. White shark focal areas from satellite tag data from Jorgensen et al. [4].
Regions used in the mixing model are indicated (California, Pelagic, Hawaii), see text for details. White shark aggregation sites in central California where tissue collection occurred are designated with the star. Mean chlorophyll-a concentration for 2006 (Aqua MODIS, http://oceanwatch.pfeg.noaa.gov) showing productivity gradients which isotopic gradients generally follow. Inset shows conceptual diagram showing how the seasonal migration of white sharks take them between regions with isotopically distinct prey.
The stable isotope values for all known and potential white shark prey in the NEP were collected from the literature, and prey were grouped according to the three defined regions in order to isotopically characterize the different focal areas (Table 1). Because marine mammals are believed to be the primary prey of large white sharks in central California [46], [47], [48], [49], we used marine mammal isotope values to estimate the California Current region value. Nothing is known about white shark diet in the Pelagic and Hawaiian focal areas. Therefore, we included all potential prey species from offshore habitats that have published stable isotope values. Potential prey items were defined as species that a white shark could reasonably be expected to encounter and consume either through active predation or scavenging and were from taxonomic groups known to be consumed by white sharks [50]. It is important to note that there are no published values of potential prey collected directly in the Café. However, the Café is part of the eastern subtropical gyre; thus species sampled from nearby regions of this large oceanographic region that have similar oceanographic conditions and biogeochemical processes [29], [30], [39], [42], [51] should be isotopically similar to those species in the Café. In addition, potential prey of white sharks in offshore habitats includes species that are not residents of the Café or Hawaii but occur seasonally in these regions and therefore might be encountered by white sharks in offshore habitats. As a result, stable isotope values from organisms sampled in different regions of the NEP were used to define the Pelagic region, which generally covers offshore, pelagic habitats between Hawaii and California, and serves as our proxy for the Café and similar habitats.
Table 1. Isotopic composition of potential white shark prey from different regions of the NEP.
| Carbon | Nitrogen | |||||||||
| ID | Species | Mean | SD | Mean | SD | Lipids | Region | Tissue | N | Reference |
| 1 | Grey whale (Eschrichtius robustus) | −13.1/−15.3 | 0.8 | 14.2 | 0.7 | E | California | Bone collagen | 13 | [120] |
| 2 | Humpback whale (Megaptera novaeangliae) | −16.3 | 1.1 | 14.7 | 1.1 | E | California | Skin | 128 | [121] |
| 3 | porpoise (Phoecena phoecena) | −16.2 | 0.5 | 15.2 | 0.8 | E | California | Muscle | 29 | [122] |
| 4 | California sea lion (Zalophus californianus) | −13.8/−16.0 | 0.9 | 18.6 | 1.0 | E | California | Bone collagen | 15 | [123] |
| 5 | elephant seal (Mirounga angustirostris) | −14.0/−16.2 | 1.0 | 18.2 | 1.0 | E | California | Bone collagen | 24 | [123] |
| 6 | harbor seal (Phoca vitulina) | −12.4/−14.6 | 0.8 | 18.6 | 0.9 | E | California | Bone collagen | 18 | [123] |
| 7 | northern fur seal (Callorhinus ursinus) | −14.5/−16.7 | 0.4 | 18.4 | 0.4 | E | California | Bone collagen | 6 | [124] |
| 8 | Sperm whale (Physeter macrocephalus) | −13.3/−15.5 | 0.5 | 17.5 | 0.5 | E | California | Tooth dentin | 24 | [120] |
| 9 | steller sea lion (Eumetopias jubatus) | −15.2 | 0.5 | 19.8 | 0.6 | E | California | Muscle | 5 | [125] |
| 10 | humpback whale (Megaptera novaeangliae) | −18.0 | 0.9 | 13.0 | 1.4 | E | Hawaii | Skin | 310 | [126] |
| 11 | yellowfin tuna (Thunnus albacares) | −16.5 | 0.4 | 10.2 | 1.8 | E | Hawaii | Muscle | 84 | [127] |
| 12 | purpleback flying squid (Sthenoteuthis oualaniensis) | −17.8 | 0.3 | 12.8 | 1.0 | C | Hawaii | Muscle | 11 | McCauley unpub. data |
| 13 | bigeye tuna (Thunnus obesus) | −16.3 | 0.4 | 11.3 | 0.9 | E | Hawaii | Muscle | 37 | [128] |
| 14 | purpleback flying squid (Sthenoteuthis oualaniensis) | −18.7 | 0.5 | 8.1 | 1.1 | N | Pelagic | Muscle | 156,14* | [129], [130] |
| 15 | blue shark (Prionace glauca) | −17.6 | 0.6 | 16.8 | 0.8 | E | Pelagic | Muscle | 20 | Leaf unpub. data |
| 16 | neon flying squid (Ommastrephes bartrami) | −18.4 | 0.7 | 11.7 | 1.3 | E | Pelagic | Whole animal | 44 | [131] |
| 17 | Pacific pomfret (Brama japonica) | −19.2 | 0.8 | 10.9 | 2.1 | E | Pelagic | Whole animal | 10 | [131] |
| 18 | northern right whale dolphin (Lissodelphis borealis) | −18.7 | 0.2 | 11.8 | 1.9 | E | Pelagic | Unknown | 23 | [131] |
| 19 | bluefin tuna (Thunnus orientalis) | −18.2 | 0.3 | 12.6 | 0.8 | C | Pelagic | Muscle | 32 | Madigan unpub. data |
| 20 | albacore tuna (Thunnus alalunga) | −19.2 | 0.4 | 13.4 | 0.6 | C | Pelagic | Muscle | 14 | Madigan unpub. data |
‘ID’ is the number used to identify this species on Figure 2. For species with values based on collagen, both the reported values and values corrected to reflect muscle are reported respectively. ‘Lipids’ indicates if and how lipids were accounted for in the study (E = extracted, C = mathematically corrected, N: not accounted for), ‘Region’ denotes which focal area the prey are from and ‘N’ is sample size.
*156 squid used in estimating nitrogen, 14 in carbon.
All but one of the studies used lipid extraction or mathematical corrections to account for lipids in the tissues. For studies that measured collagen isotope values (bone and tooth dentin) in marine mammals, we adjusted collagen δ13C values to resemble muscle values by subtracting 2.2‰ [52]. Nitrogen was not adjusted because there is little variation in nitrogen fractionation between muscle and collagen in mammals [52], [53].
We calculated average δ13C and δ15N values for each of the three regions using a random effects meta-analysis in MetaWin version 2.0 [54], which has been used previously to synthesize stable isotope data [55]. Random effects meta-analysis weights species means by the inverse of sample variance and the between-sample variance [56]. These mean values were used as an estimate of generalized prey isotopic composition for the different regions. By including an array of potential prey items for each region, variability of mean estimates increased, which provided a more conservative estimate of regional values. See supporting information for more details about selection of prey used in the characterization of focal areas (Text S1).
Stable isotope dynamics in white sharks
Two important biological parameters necessary to interpret stable isotope data are discrimination (or trophic enrichment) factors and tissue incorporation (turnover) rates. When an organism consumes a prey item, there is enrichment in 15N and 13C in the consumer's tissues relative to the prey due to preferential assimilation of the heavy isotope and preferential excretion of the light isotope [29], [31], [57], [58], [59]. This discrimination factor (Δ13C or Δ15N) is calculated as δXconsumer – δXprey = Δ13C or Δ15N [58], [60], [61]. Studies often use a discrimination factor of 3.4‰ for nitrogen to estimate trophic level in elasmobranchs [62], [], [63,64], which is the average of terrestrial and aquatic organisms from Post [65]. However, elasmobranchs have unique physiological adaptations, such as urea retention for osmoregulation and lipid storage in the liver [62], [66], which may alter the fractionation processes during metabolism. We used discrimination factors from leopard sharks (Triakis semifasciata; 3.7‰±0.4 SD for nitrogen, 1.7‰±0.5 SD for carbon) reported by Kim [67] because they represent the first rigorous, lab-defined discrimination factors for elasmobranchs.
Isotopic incorporation, also known as turnover, is the period of time for a consumer's tissue to isotopically reflect a new dietary source following a shift between isotopically distinct diets. A variety of factors, including body size, growth rate (synthesis of new tissue), and tissue replacement due to catabolic turnover [68], [69], [70], [71] can affect an organism's tissue incorporation rate. Rates vary among tissues, with more metabolically active tissues (e.g., liver, blood, etc.) having more rapid incorporation rates than less active tissues (e.g., muscle, bone, etc.) [58], [60], [61]. The change in isotope composition is most commonly modeled by an exponential decay function based on exponential growth [58], [60], [71], [72], [73]. White shark tissue incorporation rates (λ) are unknown; therefore in our analyses we used three estimates of muscle incorporation rate that should bound white shark incorporation rate (Table 2). We used the tissue incorporation rate estimated for juvenile sandbar sharks [74] (Table 2), which were small, rapidly growing individuals [68]. Because incorporation rates are known to scale allometrically with body mass and the mass of white sharks is much greater than the mass of the juvenile sandbar sharks, the sandbar shark incorporation rate is likely faster than a large white shark's incorporation rate; thus, the sandbar shark rate was used as the upper limit of potential white shark incorporation rates. We also used two different relationships that scale incorporation rates allometrically with body mass (Table 2). White shark weights were estimated using the length-weight relationship from Compagno [50]. The mean weight of biopsied sharks was 781 kg±351 (SD). The allometrically scaled incorporation rate based on teleosts from Weidel et al. [75] was the slowest, lower limit of possible white shark incorporation rates. Although this relationship was based on small ectothermic fish (2.4×10−8 to 0.4 kg), muscle incorporation rates for leopard sharks (∼4 kg) were estimated within 90% confidence using this relationship [76]. We also used allometrically scaled incorporation rates from the equation in Carleton and Martinez del Rio [77] as a the intermediate rate as it was calculated for small endothermic birds (0.01 to 1.2 kg), which have relatively high incorporation rates, but being scaled for mass, this incorporation rate was slower than the sandbar shark rate. Both allometric equations describe carbon incorporation rate.
Table 2. Range of tissue incorporation rates used to approximate white shark incorporation rate.
| Species | Incorporation rate (d−1) | Half life (d) | Reference |
| Sandbar shark | 0.0060 (0.003) | 129 (66) | [74] |
| Allometrically scaled bird | 0.0028 (0.0001) | 258 (46) | [77] |
| Allometrically scaled fish | 0.0018 (0.0001) | 394 (42) | [75] |
All rates are for muscle tissue. Incorporation rate estimates are in days (d) and are reported as mean ± SE, whereas half life estimates are mean ± SD.
Tissue incorporation rate and metabolic activity are positively related in other taxa, including elasmobranchs, teleosts, crustaceans, birds and mammals [58], [60], [61], [78], [79]. White sharks are large active, endothermic sharks with increased metabolic rates [6], [80], [81], [82], hence they likely have increased tissue incorporation rates relative to other ectothermic elasmobranchs. Given their increased metabolic rate and active life style, white sharks likely have a incorporation rate between the allometrically scaled bird incorporation rate, which may be too rapid given the high metabolic rate of small endothermic birds, and the allometrically scaled fish incorporation rate, which is likely too slow due to the slow metabolic rate of small ectothermic fish. Although our extrapolation is beyond the size range of the studied species in these allometric relationships, we used three incorporation rates that should encompass the actual white shark incorporation rate for our analyses.
Mixing model
Regions with different oceanographic and biogeochemical processes (i.e. coastal areas, upwelling currents, oligotrophic gyres) have different isotopic dynamics at the primary producer level. These differences propagate up the regional food webs and thus an oceanographic region can be represented by the isotopic values of prey species that occur in that region. If aggregate prey estimates differ sufficiently between regions, relative inputs of prey from different regions to a predator's diet can be estimated using Bayesian mixing models (i.e. MixSIR [83]). This approach is especially relevant to animals that show predictable migratory movements and in high trophic level organisms where regional baseline isotopic values may be difficult to identify and interpret after multiple trophic transfers (i.e., NEP white sharks). In addition, there is evidence that the stable isotope composition of apex predators better reflects large scale gradients in isotopes than primary producers [84], as apex predators integrate temporal and spatial variability in the isotopic composition of primary producers and lower trophic level organisms. Therefore, as the white shark is a highly migratory apex predator, with potentially migratory prey species, we chose to use mean prey values for the different focal regions based on known and potential prey as sources in our mixing model rather than using baseline (i.e., particulate organic matter or plankton) derived isoscapes for which data are lacking and which may be obscured by trophic transfer, seasonality, and migration patterns of prey at multiple trophic levels.
Because sharks use the focal areas at different times, the known movement patterns and dynamics of isotopic incorporation into new tissues (turnover rate) should be considered when using mixing models to elucidate use of the different focal areas. Existing Bayesian mixing models assume that tissues are in steady-state with their dietary components, which is unlikely in large, migratory organisms with slow incorporation rates. Due to their large size, white sharks likely have a slow incorporation rate that results in non steady-state conditions with diet. Thus information regarding spatio-temporal distribution of white sharks should be directly included into mixing models to provide a more robust estimate of focal area use. Fortunately, recent studies have demonstrated the extensibility of Bayesian mixing models by accounting for individual consumer variability [85] and the influence of spatial habitat patterns on consumer diets [86].
In this paper we extend the Moore and Semmens [83] model to account for both the temporal patterns in the distribution of sharks across the three focal regions (California, Pelagic, Hawaii) and the isotopic incorporation rate of tissues. Our modeling approach integrates satellite tagging data, tissue isotope values, and assumptions about isotopic incorporation in white shark muscle to characterize the relative contribution of different focal areas to white shark tissues and the relative feeding rate of sharks in the different ocean regions. While the modeling approach is described in detail below, we have also provided the JAGS/WinBUGS code as a digital appendix (Text S2).
In the original Bayesian mixing model formulation of Moore and Semmens [83], the n isotope signatures of i white shark tissue samples (Ison,i) are described by:
where k represents the number of sources considered and f represents the proportional contribution of each source to the mixture. In the above equations, m and s2 represent the discrimination factor adjusted source isotope means and variances (i.e., source means and variances have been additively adjusted for discrimination factor means and variances).
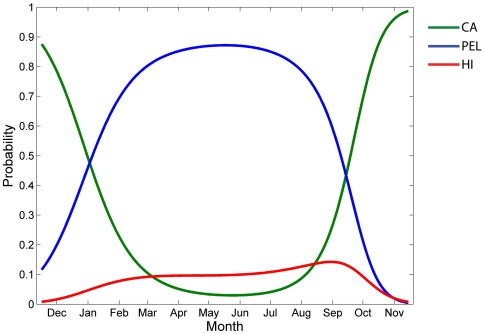
We used satellite tagging data from 65 white sharks tagged as part of Jorgensen et al. [4] to estimate the proportion of the population residing in each of the focal areas as a function of Julian day of the year. The probability that a tagged shark i is in region j (1–3, for California, Pelagic, and Hawaii) on a given day t (1–365) is estimated by:
Where yi,t is the observed location of tagged sharks, and P is a matrix of region and time specific probabilities estimated from the 4th order multinomial Logit function:
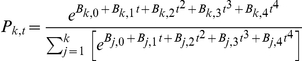 |
where all elements of B1 are set to 0. As was noted in Jorgensen et al. [4], the probability of finding a white shark from the study population in coastal habitats was high during a relatively short period of time during the autumn and winter (October through December), while the probability of finding sharks in offshore habitats was greater between January and September (Figure 2). In our modeling framework, the P matrix is simultaneously treated as a daily estimate of the proportion of the population in each ocean region where, under a constant consumption rate across regions, we would expect the f vector (proportional contribution of each region to the shark isotope signatures) to be equivalent to the relative amount of time the population spends in each region (defined by the P matrix across all Julian dates). However, we are interested in determining whether there appears to be a differential contribution of each region to the diet of white sharks, beyond that expected from differences in the proportional occupancy of the shark population in each region though time. Therefore, we introduce region specific daily isotope uptake adjustment terms, E, to the elements of the P matrix:
Here, we set the first element of the E vector (that applied to California) to 1 in order to make the isotope uptake adjustment of the other regions relative to the daily uptake rate in California.
Figure 2. Probability of white sharks occurring in different focal areas by time of year based on satellite tag data from Jorgensen et al. [4].
Finally, because isotope values in white shark tissue “decay” at a rate defined by the isotopic incorporation rate, we expect that the contribution of a given day's foraging to white shark tissue isotopes will lessen the further into the future the sample is collected. Specifically, for a particular future day t, the fraction of original material remaining Ck is:
where h represents the tissue turnover rate (in half-lives) of stable isotopes (assumed to be the same for all stable isotopes). The t/h term thus represents the number of half-lives into the future that the sample was taken. Using this formula, we adjust the expected proportional contribution of each source:
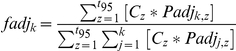 |
where t95 is the number of days until 5% of less of isotope signature remains in the tissue. The modified mixing model was run for the three different estimates of incorporation rate.
Results
A total of 53 white sharks (35 ♂, 7 ♀, 11 unknown) were biopsied from 2006–2009 (Table 3) from August to January. Most samples were collected from September to November (87%), with the highest number being collected during October (64%). Samples were primarily from male sharks. Sharks were sampled at Pt. Reyes (n = 15), South Farallon Island (n = 33), Tomales Point (n = 4), and Oregon (n = 1). All samples were from free-swimming sharks with the exception of the shark from Oregon, which was killed after becoming entangled in a crab pot. Sharks ranged in total length (TL) from 2.6 m to 5.3 m (mean ± standard deviation [SD] 4.3±0.6; hereafter all errors similarly expressed as SD). Based on an estimated TL at first maturity of 3.8 m for males [87] and 4.5 m for females [88], 31 sharks were mature, 11 were immature, and 11 were of unknown maturity (due to inability to identify gender of the shark).
Table 3. δ13C and δ 15N of white shark muscle and dermal tissue.
| Tissue | N | Mean | SD | Min | Max | |
| White muscle | δ13C | 21 | −15.5 | 0.5 | −16.9 | −14.4 |
| δ15N | 21 | 18.4 | 1.0 | 17.4 | 21.1 | |
| C∶N | 21 | 3.3 | 0.1 | 3.2 | 3.5 | |
| Dermis | δ13C | 48 | −12.8 | 0.5 | −14.1 | −11.9 |
| δ15N | 48 | 19.2 | 0.9 | 17.2 | 21.1 | |
| C∶N | 48 | 2.8 | 0.1 | 2.7 | 3.1 |
We analyzed 48 dermal (32 ♂, 6 ♀, 10 U) and 21 muscle (14 ♂, 2 ♀, 5 U) samples. Muscle and dermal tissues had distinct δ15N and δ13C values (Table 3). Sixteen biopsies contained both dermal and muscle tissue. For these paired samples, dermal tissue was significantly enriched in 13C and 15N relative to muscle (paired t-test, p<0.001) (Table 3). Because shark dermis is primarily collagen [89], mean dermal isotope values were corrected to resemble muscle according to tissue-specific differences reported by Hussey et al. [90] (corrected dermal values: −14.9‰±0.5 for δ13C and 21.5‰±0.9 for δ15N).
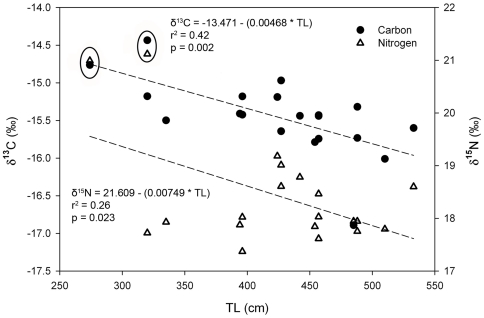
There was a significant negative relationship between shark length and muscle δ13C values (linear regression, n = 20, p = 0.002, r2 = 0.42) and δ15N values (linear regression, n = 20, δ15N = 21.609−(0.00749 * TL), p = 0.023, r2 = 0.26) (Figure 3). Two of the smallest sharks (2.7 and 3.2 m TL) had the highest muscle δ13C and δ15N values of all sharks (−14.8‰, 21.0‰ and −14.4‰, 21.1‰ respectively). There was no significant relationship between dermal δ13C values and length (linear regression, n = 47, p = 0.368, r2 = 0.02). There was a significant negative relationship between dermal δ15N values and length (linear regression, n = 47, p = 0.048, r2 = 0.08), but the low r2 indicates that this relationship is very weak. As with muscle, some of the smallest sharks (2.6, 3.2, 3.3, and 3.4 m) had the highest δ15N values (20.4‰ to 21.1‰) of all the sharks. Of the muscle samples, 14 were from males, 2 from females, and 5 from sharks of undetermined sex.
Figure 3. Relationship between white shark length (TL) and δ13C and δ15N of white shark muscle.
The two ellipses contain the δ13C and δ15N values of two of the smallest sharks that had the highest δ13C and δ15N values.
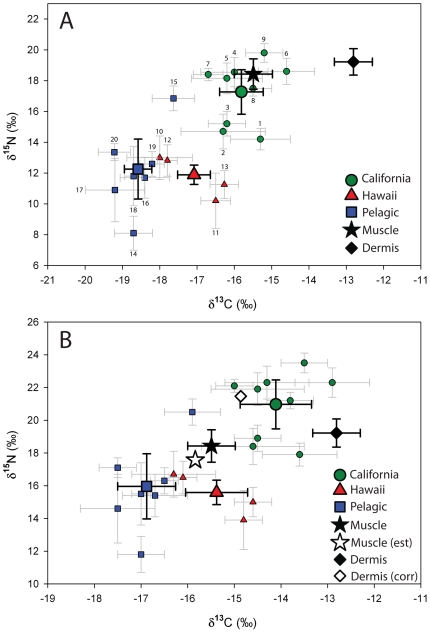
Isotopic values of potential prey clustered by region (Figure 4, Table 4). The mean isotopic composition of prey from the California region was highly enriched in 15N and 13C relative to Pelagic and Hawaii prey. Hawaii prey were more enriched in 13C than Pelagic prey, but mean δ15N of Hawaii and Pelagic prey were similar, although the latter were more variable. The mean δ13C and δ15N values of all potential offshore prey items grouped together were −18.0‰±0.8 and 12.2‰±2.0, respectively. Muscle δ13C and δ15N values, adjusted to account for discrimination factor, were intermediate between California prey and offshore (Pelagic and Hawaii) prey (Figure 4b), demonstrating consumption of prey from offshore habitats. Dermis δ13C and δ15N values, adjusted to account for discrimination factor and corrected to resemble muscle tissue, was similar to California prey (Dermis (corr), Figure 4b), indicating coastal foraging.
Figure 4. δ13C and δ15N values of white shark tissues and potential prey items from different focal areas.
Regions and shark tissues are designated with different symbols and colors. For regions, smaller symbols show mean (SD) isotopic values of individual species and larger symbols indicating mean regional average (SD). 4a: δ13C and δ15N values for different prey, regions and white sharks tissues. Individual species are labeled according to Table 1. 4b: Mixing polygon used to estimate contribution of different regions to white shark tissue. Regional (and prey) values are adjusted to account for discrimination factor (Kim et al. [67]). Error in discrimination factors was propagated into error of mean regional values. Muscle (est) is the predicted mean stable isotope composition of white shark muscle if sharks foraged at the same rate in the different regions. Dermis (corr) is the mean dermal stable isotope composition adjusted to resemble muscle by accounting for differences in discrimination factors between the tissues; the adjustment is based on Hussey et al. [90]. Note that the closer the tissue values are to source (region) values, the higher the contribution of that source to the tissue.
Table 4. Estimated δ13C and δ 15N values of white shark focal areas.
| δ13C | δ15N | |||
| Focal area | Mean | SD | Mean | SD |
| California | −15.8 | 0.6 | 17.3 | 1.4 |
| Pelagic | −18.6 | 0.4 | 12.3 | 1.9 |
| Hawaii | −17.1 | 0.4 | 11.9 | 0.6 |
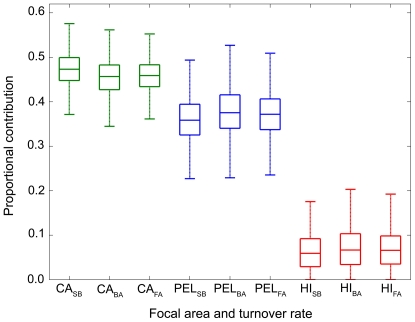
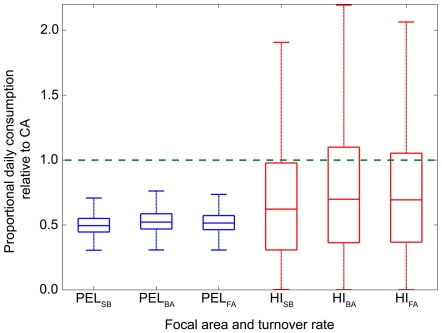
To ensure convergence, the modified Bayesian stable isotope mixing model was run on white muscle data to produce 100,000 posterior draws and the chain was thinned for every 10th draw. Mixing model results indicate that regardless of incorporation rate, white shark muscle primarily reflects California and Pelagic sources with a lower contribution from Hawaii prey (Table 5, Figure 5). Depending on the incorporation rate, the median estimated contribution of California prey to muscle tissue ranged from 45.7 to 47.3%, Pelagic prey 35.9 to 37.5%, and Hawaii prey 5.9 to 6.7% (Table 5, Figure 5). Estimated daily consumption rates were approximately half in the Pelagic region relative to the California region (median 49.5 to 52.2%), with consumption rates in Hawaii slightly higher than the Pelagic region (62.2 to 69.3% relative to California) (Table 6, Fig. 6). The estimated daily consumption rate when in Hawaii had much higher variability relative to the Pelagic region, probably due to the relative lack of tagging data from sharks going to Hawaii and the model's inability to precisely discern the location of sharks in the distant past relative to tissue incorporation rates based on isotope data. The expected stable isotope values of white shark muscle, δ13C −15.8‰±0.08 and δ15N 17.6‰±0.2 (Muscle (est), Figure 4b), reflects expectations based upon observed migratory pattern (Figure 2) and assumed equal consumption rates in different focal areas. This assumes that the different focal areas serve the same function and are used in the same manner by white sharks, an assumption that is almost certainly invalid for this species and many migratory species which move between foraging and reproductive habitats [91]. That the observed values were different from expected values indicates that this assumption is likely incorrect and demonstrates the importance of incorporating consumption rate into the mixing model.
Table 5. Overall contribution of different focal areas to white shark muscle tissue using different tissue incorporation rates.
| California | Pelagic | Hawaii | ||||
| Incorporation rate | Median | 95% | Median | 95% | Median | 95% |
| Sandbar shark | 47.3 | 40.1–54.4 | 35.9 | 26.2–46.7 | 5.9 | 0.3–15.3 |
| Allometric bird | 45.7 | 38.1–52.8 | 37.5 | 27.8–48.9 | 6.7 | 0.4–17.7 |
| Allometric fish | 45.9 | 38.7–53.2 | 37.2 | 27.8–49.4 | 6.6 | 0.6–16.6 |
Estimates incorporate tissue incorporation rate, satellite tag data, and consumption rate. Median and 95% credible intervals are presented.
Figure 5. Overall contribution of different focal areas to white shark tissues estimated using the spatially explicit Bayesian mixing model.
Results show posterior model estimates (median, interquartile range and max/min values) of source (CA: California, PEL: Pelagic, HI: Hawaii) contribution to muscle. The estimated contribution of the different focal areas incorporates electronic tag data from Jorgensen et al. [4] and is estimated for three different tissue incorporation rates (SB: juvenile sandbar shark, BA: allometrically scaled bird, FA: allometrically scaled fish, see text for details).
Table 6. Estimated rate of consumption in offshore focal areas relative to consumption rate in California using different tissue incorporation rates.
| Pelagic | Hawaii | |||
| Incorporation rate | Median | 95% | Median | 95% |
| Sandbar shark | 49.5 | 35.7–66.7 | 62.2 | 3.0–162.9 |
| Allometric bird | 52.5 | 37.3–72.3 | 69.8 | 3.9–191.9 |
| Allometric fish | 51.5 | 37.6–71.8 | 69.4 | 6.0–178.2 |
Median and 95% credible intervals are presented.
Figure 6. Rate of consumption in offshore focal areas (PEL: Pelagic, HI: Hawaii) relative to California (CA) estimated using the spatially explicit Bayesian mixing model.
Results show posterior model estimates (median, interquartile range and max/min values). The green dashed line designates the relative consumption rate in the California focal area. Consumption rate is estimated using three different tissue incorporation rates for both offshore focal areas (SB: juvenile sandbar shark, BA: allometrically scaled bird, FA: allometrically scaled fish, see text for details).
Discussion
Over the past decade, electronic tagging data have shown that a variety of large marine predators, including fish, sharks, pinnipeds, whales, marine reptiles, and marine birds, exhibit seasonally recurring migrations in the northeastern Pacific, often between coastal and oceanic habitats [92]. Seasonal migrations often take animals between areas used for foraging and ones used for reproduction [91], so understanding how a migratory species uses different parts of its range is fundamental to understanding its ecology and life history. Because productive coastal habitats and oligotrophic offshore habitats of the NEP have very different stable isotope baseline values [30], the opportunity exists to use stable isotope analysis to investigate the relative importance of different regions as foraging habitats for white sharks, and thereby shed light on the underlying function of white shark migrations.
In this study we developed a novel approach to directly integrate movement data and tissue incorporation rates into stable isotope mixing models to investigate the trophic ecology of a highly migratory marine predator, the white shark, in order to better understand how the species uses different focal areas and elucidate the life history function of its migratory behavior. Mixing model results show considerable dietary inputs from offshore pelagic habitats, in particular oceanic regions like the Café, and provide direct evidence of offshore foraging in NEP white sharks. Estimated rates of daily consumption indicate that although white sharks forage while offshore, they do so at a lower rate in offshore habitats relative to coastal ones. This is the first use of biogeochemical methods to estimate relative foraging rates in different food webs. These results suggest that white sharks may seasonally play an important role in offshore ecosystems of the NEP and provide insight of the underlying function of white shark migratory behavior.
Evidence of offshore foraging
Although the resolution of stable isotope data generally precludes identification of specific prey species, the results of this study indicate that white sharks are foraging in offshore food webs that are depleted in 13C and 15N relative to marine mammals in California. Mixing model results indicate that while offshore, white sharks forage more on prey that are isotopically similar to the Pelagic prey rather than Hawaiian prey, indicating increased foraging in the Pelagic region relative to the Hawaiian Region. These results are consistent with the results from previous studies, which show white sharks have higher occupancy of the Café waters relative to Hawaiian waters [2], [3], [4]. Furthermore, the different incorporation rates did not greatly change mixing model results and suggests that our model was not sensitive to differences in incorporation rates in the range that we used.
The reduced consumption rate in offshore focal areas relative to California is logical given the abundance and diversity of calorically-rich prey in productive coastal habitats such as the California Current. Marine mammals, pinnipeds in particular, are especially abundant in California waters and are known to be important prey for white sharks in the region [46], [47], [48]. The oligotrophic Subtropical Gyre supports a less abundant and diverse prey field that is more patchily distributed, so there are fewer opportunities to forage, especially upon calorically-rich marine mammals. The increased consumption rate in Hawaii relative to the Pelagic region suggests that the Hawaiian region, and neritic habitats of the Hawaiian Islands in particular, may provide white sharks with increased foraging opportunities relative to the Pelagic region. This is logical given the increased abundance of prey available for white sharks to exploit in the Hawaiian Islands, including marine mammals such as monk seals and humpback whales.
A reduced rate of consumption in offshore habitats is consistent with visual observations that white sharks are often lean when they return from offshore regions to aggregation sites in central California [7]. Once sharks return to coastal aggregation sites, they rapidly gain mass by feeding on pinnipeds [7]. Hence, despite the fact that white sharks generally spend less time in coastal habitats [4], these coastal regions may play a critical role in the life history of large white sharks as foraging areas. White sharks also demonstrate a high degree of inter- and intra-annual fidelity to coastal aggregation sites, which indicates the important role of these habitats in the life history of the NEP white shark population [3], [4]. Marine mammal consumption in coastal habitats may play an important role in generating the caloric reserves that are used by sharks over the course of their offshore migrations.
Despite the likely reduction in foraging opportunities in the Subtropical Gyre, white sharks are offshore during a period of time when prey availability in this region may be relatively high. It is logical that white sharks would time their offshore migrations to coincide with periods of increased prey availability. The reported spawning area of the neon flying squid (Ommastrephes bartrami) generally overlaps temporally and spatially with white sharks in offshore waters [16] as does that of the purpleback flying squid (Sthenoteuthis oualaniensis) [17], suggesting that spawning aggregations of Ommastrephid squid may be an important prey resource in offshore waters. Similar to the neon flying squid, Pacific pomfret (Brama japonica) also make seasonal migrations, foraging in the transition zone or subarctic waters during the summer then moving south to subtropical waters to spawn during winter and spring [93], [94]. Catches of pelagic fishes and sharks by the Japanese long line fishery can be substantial, though not necessarily at their peak, in this region during the time of year that white sharks are present [13], [14]. It has also been noted that the occurrence of white sharks in Hawaii coincides with birthing of humpback whales [26]. Therefore, it appears that white sharks may be using these offshore habitats when prey availability, though likely still lower than in coastal habitats, is relatively greater than at other times of the year.
Although these offshore habitats may not provide optimal foraging conditions at present, it is important to remember that historical conditions may have been very different. It is possible that with the decline of large pelagic fishes, sharks and whales [5], [95], [96] the historical forage base of white sharks in these habitats has diminished. In addition, the decline of the Hawaiian monk seals [97], may have also removed an important prey item for white sharks. It seems likely that historically there was a more abundant prey field available for white sharks to exploit in pelagic habitats. Therefore, to some extent white shark migrations may be vestigial and reflect historical conditions.
There are no empirical data on white shark diet in NEP offshore waters; however, the list of potential white shark prey represents a reasonable first estimate of white shark prey in the offshore focal areas. Despite possible biases of the offshore regional estimates and potential limitations in our ability to differentiate between use of the Pelagic and Hawaiian regions, our estimates of the relative contribution of the California region vs. offshore regions (Pelagic and Hawaii) are useful because they clearly demonstrate considerable offshore foraging. It is also important to note that our data were primarily from male sharks, so our inferences largely pertain to males.
Shift in muscle isotopic composition with size
The decrease in muscle δ13C and δ15N values with shark length is the opposite of what would be expected with ontogenetic increases in trophic level, which would lead to increased δ13C and δ15N values, and may instead be indicative of ontogenetic changes in habitat use. There is an ontogenetic shift in white shark diet with smaller sharks (<300 cm) primarily being piscivorous and larger sharks primarily consuming marine mammals [21], [98]. Therefore, we would predict that δ13C and δ15N values would increase with size as sharks consume larger, higher trophic level prey, especially marine mammals, as has been noted in Atlantic white sharks [64]. However, the opposite trend was found. The highest muscle δ13C and δ15N values in this study population were the two smallest sharks (2.74 and 3.20 m TL) and larger sharks being more depleted in 13C and 15N.
This result suggests that the smallest sharks were residential within the coastal waters of the California Current and foraged primarily in the productive coastal food webs that are enriched in 13C and 15N. Smaller juveniles (<2.5 m) are known to be coastal residents that utilize the neritic habitats of the Southern California Bight and Baja California, Mexico [25], [99]. Interestingly, two sharks (320 and 335 cm TL), which were slightly larger than the smallest two sharks, had lower isotopic values and more resembled larger sharks. In particular, the δ15N values of these sharks were dramatically lower. One explanation is that these two sharks specialized on lower trophic level prey (hence lower δ13C and δ15N values) than the two smallest sharks. However there is currently no evidence of such dietary specialization in white sharks. Alternatively, the decline in δ13C and δ15N in these sharks may be attributed to a threshold age at which white sharks begin offshore migrations and consumption of offshore prey. Patterns of movement and habitat use in these intermediate size white sharks (∼2.5 to 3.5+ m) are poorly understood as relatively few sharks in this range have been tagged and tracked [2], [3], [4]. The size at which white sharks begin to migrate to offshore regions is unknown and may differ with gender. Based on the similarity of the isotopic values between these two small sharks and larger sharks, for which movement patterns are well understood, the most parsimonious explanation for the δ13C and δ15N shift in smaller size classes of sharks is due to a shift in foraging habitat (onshore vs. offshore) rather than dietary differences in coastal waters.
If this shift in δ13C and δ15N values with size is due to a change in habitat use, it suggests that white sharks may start using offshore waters at lengths of 3 to 4 m (∼5 to 9 years) [87], [88], [100], a size range that encompasses the estimated size of first maturity in males (3.8 m [87]). Our results are supported by Kerr et al. [101] who reported the same decrease in δ13C values with white shark size based on SIA of NEP white shark vertebrae. The decrease Kerr et al. [101] reported in δ13C values appears to have started at similar size/age as we observed here. Kerr et al. [101] also determined that radiocarbon (14C) values were depleted in larger sharks. They attributed this decrease in δ13C and radiocarbon to ontogenetic shifts in foraging location, including increased foraging in offshore, deep, radiocarbon depleted waters [35], [102], [103]. Our sample size in this smaller size range is limited, so additional sampling of smaller sharks (<4 m) is needed and would provide insight to the timing of ontogenetic shifts in white shark habitat use.
Our observation of decreasing muscle δ13C and δ15N values with shark size may also reflect an allometric shift in incorporation rate [77], [104], with larger sharks having a slower incorporation rate. If this is the case, shark tissue would be expected to increasingly reflect offshore prey as sharks get larger since they spend more time in offshore (mean 234 d±45 SD) rather than coastal habitats. Alternatively, it is possible that this pattern is due to larger white sharks spending increasingly more time in offshore habitats relative to smaller sharks. However, currently there are not enough data to test this.
Purpose of white shark migrations
One of the fundamental questions regarding white sharks in the NEP is why they undertake these extensive and regular offshore migrations, leaving the highly productive California Current for oligotrophic waters of the central Pacific. The two primary hypotheses are that these movements are related to foraging or reproduction. While our data are not able to directly address the reproductive hypothesis, they are useful for evaluating the foraging hypothesis. Our results indicate that 1) white sharks do forage in offshore habitats, though at a lower rate, and 2) white sharks may initiate these offshore migrations around the size of first maturity. These results, in combination with the observation that white sharks returning to central California aggregation sites often are lean [7], suggest that although white sharks feed offshore, it appears foraging may not be the primary motivation for offshore migration.
If white sharks are not moving offshore to feed, an alternative explanation is that the offshore migrations have a reproductive purpose, possibly playing a role in gestation, parturition or mating [2], [3], [4]. The particular reproductive function that offshore habitats may play in white shark life history is unclear, but it is possible that use of these habitats is related to gestation and/or mating as parturition is believed to occur in the southern California Bight [24]. It is possible that females use the warm waters of the Subtropical Gyre to aid in gestation [105], [106], and indeed there is some evidence that females segregate from males in offshore habitats, especially when in the Hawaiian focal area [107], [108]. If female white sharks have an extended gestation period (12 to 18 months [109]), extended use of warm offshore waters may explain the return of some large females to coastal aggregation sites every other year [110], [111].
Weng et al. [2] and Jorgensen et al. [4] noted that when males were in the Café they exhibited a very unusual and distinct rapid oscillatory diving behavior. Weng et al. [2] noted that this unique behavior was similar to the oscillatory diving behavior recorded by electronic tags during the spawning of Atlantic bluefin tuna in the Gulf of Mexico [112], and suggested that these behaviors may represent some sort of courtship or other reproductive behavior. Furthermore, Jorgensen et al. [4] suggested that it was possible that mating occurred offshore based on the pattern of distribution, estimated gestation time and timing of parturition, and behavior of sharks when offshore. It is unclear why sharks would not mate when both sexes are gathered at coastal aggregation sites, but several decades of visual observations at these coastal sites have yielded no evidence of mating behavior [3], [4], [110].
Because our samples are primarily from males, it is possible that the reduced offshore consumption rate that we observed is a reflection of the males engaging in periods of courtship or reproductive behavior, possibly including rapid oscillatory diving behavior, at the expense of time spent foraging. As Jacoby et al. [108] noted, it is possible that sex-specific differences in life-history traits may result in female sharks that allocate more time and energy to finding environmental conditions that aid in gestation whereas male sharks primarily focus on the pursuit of mates. Although we only had two muscle samples from females, they were indistinguishable from males, suggesting that females may also have a reduced rate of consumption. However, our ability to draw inferences for females is limited by low sample size, and possible differences in time spent offshore relative to males would need to be taken into account [110], [111].
In addition to a reduced foraging rate in offshore habitats, initiation of offshore migration around the age of first maturity is also consistent with a reproductive function to the migration. Our results suggest that sharks appeared to exhibit a shift to offshore prey between 3–4 m, the size range that encompasses the estimated size of first maturity for males. It is possible that there is no impetus for smaller, immature sharks to leave the productive nearshore habitats until there is a reproductive imperative. Our sample size is low in these smaller size ranges, so these inferences are speculative and require more research. It is possible that given the dietary shift that occurs around 3 m, when sharks start to consume marine mammals, that some shift in trophic ecology plays a role as well.
Although based on our data and modeling framework foraging does not appear to be the primary driver of offshore migration, it is likely an important component given the amount of time white sharks spend offshore. Importantly, the foraging and reproduction hypotheses are not mutually exclusive and these migrations are likely driven by a suite of factors, including 1) the physiological and reproductive condition of the sharks and 2) environmental and prey dynamics in offshore and coastal habitats. Environmental conditions or prey availability in the coastal areas may play a role as well. Jorgensen et al. [4] noted that the offshore phase coincides with the period of peak upwelling of cold water in the California Current, so it is possible that white sharks, or their prey, may have some physiological thermal limitations, leading to a reduction in use of the California Current during cold periods. Additionally, recent electronic tagging work has shown that timing of offshore migration in white sharks is coincident with offshore movements of a number of upper trophic level species from California Current, including pinnipeds, sharks, and tuna [92], suggesting a possible decrease in prey base on coastal habitats.
Regardless of the function of offshore migration, our results show that sharks clearly feed when offshore, although at a lower rate than in coastal habitats. These results do not provide support for foraging being the primary purpose of offshore migrations, and therefore suggest an alternative purpose such as reproduction. However, whether it is indeed reproduction or some other factor or suite of factors remains to be demonstrated.
Dermal tissue
The dermis was highly enriched in 13C and 15N relative to muscle tissue and more closely resembled values of marine mammals in California. The mean dermis δ13C and δ15N value, corrected to resemble muscle (Dermis (corr), Figure 4b, mean δ13C and δ15N −14.9‰ and 21.5‰, respectively), is very similar to what would be expected for a diet composed of elephant seals, sea lions, and northern fur seals (prey items 4, 5 and 7, Figure 4). Elephant seals and sea lions are believed to be the primary prey of white sharks at coastal aggregation sites in central California [46], [48]. These results are consistent with a more rapid incorporation rate in dermis than muscle, thus dermis may represent more recent foraging while muscle represents a longer integration of foraging and movement. However, it is possible that isotopic routing [68], [113] or differences in amino acid composition may account for this difference.
The stable isotope dynamics of dermis have not been described for elasmobranchs or teleosts. Studies featuring human subjects show that dermal collagen, the primary component of dermis in mammals as well as elasmobranchs, is a dynamic tissue that responds rapidly to physical activity [114] and has a incorporation rate similar to or potentially faster than skeletal muscle [115], [116]. In addition to serving as a protective sheath, shark dermal collagen is believed to serve a biomechanical function where it serves as an anchorage for the body musculature [89]. Thus, dermal collagen could be subject to relatively high rates of mechanical stress and could have an increased incorporation rate. This is speculative, and experimental work is needed to assess the dynamics of isotopic incorporation of dermal collagen in elasmobranchs. If dermal collagen does incorporate dietary carbon and nitrogen more rapidly than muscle, it would be a valuable tissue for SIA because other tissues with increased incorporation rates can only be sampled destructively (e.g., liver) or require extensive handling (e.g., blood). If dermal tissue has an increased tissue incorporation rate relative to muscle, researchers could easily and non-destructively sample two tissues that provide an estimate of recent and longer term patterns of foraging and movement.
Future directions
Given the low cost of SIA and existence of electronic tag data sets for a variety of species, the approach of integrating movement data and SIA described in this paper has the potential to be used on a variety of species and may prove useful as a monitoring tool. This approach can be used to monitor long-term patterns in trophic ecology and movements of white sharks or other marine organisms relative to fluctuations in prey availability or oceanographic conditions. NEP white sharks are ideal organisms for this approach given their small population size and relatively well-defined migratory patterns. A long-term sampling regime using these and improved techniques to monitor NEP white sharks may provide valuable information on the influence of environmental variability in offshore and nearshore habitats on white shark behavior. These techniques could also be applied to the white shark population at Guadalupe Island, Mexico, which exhibit a similar migratory pattern as central California white sharks [3]. White sharks in New Zealand [117] and South Africa [118] have also been noted to migrate to subtropical or tropical regions and comparative analyses with eastern Pacific sharks would be valuable. Additionally, in order to begin to validate this approach, efforts should be made to biopsy individual sharks with a known migratory history. If the previous year's migratory pattern of an individual shark, recorded using an electronic tag, matches the inferred pattern of habitat use based on the stable isotope composition of its muscle, it would provide some validation that this approach is accurately recording migratory pattern.
In addition to increasing the sample size of females and juvenile sharks, future work should focus on improving the parameters used in this study. Much work needs to be done to characterize the isoscapes over which these sharks move [34], generating robust isotopic data sets of potential prey in different focal regions (especially the Café and Hawaii) and refining estimates of white shark incorporation rates and discrimination factors. Such information will lead to a better understanding of the trophic ecology of this species and a more robust estimate of the dietary contribution of the different focal areas than is currently possible. Determining a better estimate of tissue incorporation rates that include body size, growth rate, and metabolic turnover of tissue for white sharks would be ideal; however the size and handling difficulty of adult white sharks makes it unlikely that we will be able to determine species-specific isotopic incorporation parameters and we may remain limited to using estimates from proxy species. In addition, obtaining a robust time series of samples from sharks throughout the coastal period, ranging from newly returned sharks to ones that have been foraging coastally for several months, may provide insight into tissue incorporation rates as sharks consume coastal prey in addition to providing information on use of coastal prey resources. Concurrent with the refinement of these parameters, use of compound-specific isotopic analysis would provide great insight into the ecology of white sharks, and elucidate how metabolic processes, trophic ecology, and movements across isotopic gradients influence the isotopic composition of a shark's tissues [119].
Conclusions
Our results demonstrate how stable isotope analysis, when used in concert with electronic tagging data, can provide valuable insight into the ecology of white sharks in the NEP. The stable isotope composition of white shark tissues serves as a natural tracer that provides a record of their use of different regions of the NEP. The isotopic composition of white shark muscle provides strong evidence of offshore foraging, especially in pelagic habitats like the Café, by NEP white sharks, whereas dermal tissue appears to reflect recent foraging on prey in the California Current. Our results indicate that when in oligotrophic offshore waters, white sharks feed at approximately half the rate they do when in productive nearshore habitats. The decrease in muscle δ13C and δ15N values with shark length is likely indicative of ontogenetic changes in habitat use, possibly related to the onset of maturity. Overall our results suggest that although sharks feed when offshore, foraging is unlikely to be the primary purpose of the offshore migrations. If this is the case, it is possible that the extensive migrations serve a reproductive function, though this remains to be proven.
The results presented here are based on models with data-driven parameter estimates, and this study has produced results that are concordant with and add to previous research while generating new hypotheses that can be addressed in future research. Stable isotopes provide an inexpensive intrinsic tracer that can be used to infer movements and foraging in large numbers of animals. Consequently, SIA may be a cheap and cost effective way to further study and monitor general patterns of white shark migration and foraging ecology and be a useful tool for white shark conservation and management efforts. In addition, the techniques used in this study could easily be applied to other species, especially ones for which spatio-temporal patterns of distribution are relatively well understood.
Supporting Information
Expanded description of selection of prey species used to estimate mean δ13C and δ15N values for different focal areas (doc).
(DOC)
JAGS/WinBUGS code for spatially explicit Bayesian mixing model. This file includes descriptions of the types and formatting of data needed to run this code (R).
(R)
Acknowledgments
We would like to thank S. Litvin, K. Kroeker, W. Smith, D. Ebert, A. Brown, J. Barlow, C. Farwell, C. Harrold, J. O'Sullivan, M. Castleton, A. P. Klimley, L. Rodriguez, and C. Logan for their invaluable assistance with this project. We would also like to acknowledge SeaLife Conservation, R. Repass, E. Homer and crew of the R.S.V. Derek M. Bayliss and the Bodega Marine Lab boating program for vessel support.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Funding was provided by the Alfred P. Sloan Foundation, the David and Lucile Packard Foundation, the Gordon and Betty Moore Foundation, Monterey Bay Aquarium Foundation, the Office of Naval Research, and Stanford University's School of Humanities and Sciences. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Boustany AM, Davis SF, Pyle P, Anderson SD, Le Boeuf BJ, et al. Satellite tagging - Expanded niche for white sharks. Nature. 2002;415:35–36. doi: 10.1038/415035b. [DOI] [PubMed] [Google Scholar]
- 2.Weng KC, Boustany AM, Pyle P, Anderson SD, Brown A, et al. Migration and habitat of white sharks (Carcharodon carcharias) in the eastern Pacific Ocean. Mar Biol. 2007;152:877–894. [Google Scholar]
- 3.Domeier ML, Nasby-Lucas N. Migration patterns of white sharks Carcharodon carcharias tagged at Guadalupe Island, Mexico, and identification of an eastern Pacific shared offshore foraging area. Mar Ecol Prog Ser. 2008;370:221–237. [Google Scholar]
- 4.Jorgensen SJ, Reeb CA, Chapple TK, Anderson S, Perle C, et al. Philopatry and migration of Pacific white sharks. Proc R Soc Biol Sci Ser B. 2010;277:679–688. doi: 10.1098/rspb.2009.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dulvy NK, Baum JK, Clarke S, Compagno LJV, Cortes E, et al. You can swim but you can't hide: the global status and conservation of oceanic pelagic sharks and rays. Aquatic Conserv Mar Freshw Ecosyst. 2008;18:459–482. [Google Scholar]
- 6.Carey FG, Kanwisher JW, Brazier O, Gabrielson G, Casey JG, et al. Temperature and activities of a white shark, Carcharadon carcharias. Copeia. 1982;1982:254–260. [Google Scholar]
- 7.Chapple TK, Jorgensen SJ, Anderson SD, Kanive PE, Klimley AP, et al. A first estimate of white shark, Carcharodon carcharias, abundance off Central California. Biol Lett. 2011 doi: 10.1098/rsbl.2011.0124. doi: 10.1098/rsbl.2011.0124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dagorn L, Bach P, Josse E. Movement patterns of large bigeye tuna (Thunnus obesus) in the open ocean, determined using ultrasonic telemetry. Mar Biol. 2000;136:361–371. [Google Scholar]
- 9.Musyl MK, Brill RW, Boggs CH, Curran DS, Kazama TK, et al. Vertical movements of bigeye tuna (Thunnus obesus) associated with islands, buoys, and seamounts near the main Hawaiian Islands from archival tagging data. Fish Oceanogr. 2003;12:152–169. [Google Scholar]
- 10.Roper CFE, Young RE. Vertical distribution of pelagic cephalopods. Smithson Contrib Zool. 1975;209:1–51. [Google Scholar]
- 11.Watanabe H, Kubodera T, Moku M, Kawaguchi K. Diel vertical migration of squid in the warm core ring and cold water masses in the transition region of the western North Pacific. Mar Ecol Prog Ser. 2006;315:187–197. [Google Scholar]
- 12.Berger WH. Global maps of ocean productivity. Berger WH, Smetacek VS, Wefer G, editors. Productivity of the ocean: present and past: Wiley, New York. 1989:429–455. [Google Scholar]
- 13.Matsumoto T, Bayliff WH. A review of the Japanese longline fishery for tunas and billfishes in the eastern Pacfic Ocean, 1998–2003. Inter-Am Trop Tuna Comm Bull. 2008;24:1–187. [Google Scholar]
- 14.Okamoto H, Bayliff WH. A review of the Japanese longline fishery for tunas and billfishes in the eastern Pacific Ocean, 1993–1997. Inter-Am Trop Tuna Comm Bull. 2003;22:221–431. [Google Scholar]
- 15.Bower JR, Ichii T. The red flying squid (Ommastrephes bartramii): a review of recent research and the fishery in Japan. Fish Res. 2005;76:39–55. [Google Scholar]
- 16.Ichii T, Mahapatra K, Sakai M, Okada Y. Life history of the neon flying squid: effect of the oceanographic regime in the North Pacific Ocean. Mar Ecol Prog Ser. 2009;378:1–11. [Google Scholar]
- 17.Zuyev G, Nigmatullin C, Chesalin M, Nesis K. Main results of long-term worldwide studies on tropical nektonic oceanic squid genus Sthenoteuthis: an overview of Soviet investigations. Bull Mar Sci. 2002;71:1019–1060. [Google Scholar]
- 18.Hongyan Z, Ludsin SA, Mason DM, Adamack AT, Brandt SB, et al. Hypoxia-driven changes in the behavior and spatial distribution of pelagic fish and mesozooplankton in the northern Gulf of Mexico. J Exp Mar Biol Ecol. 2009;381:580–591. [Google Scholar]
- 19.Prince E, Goodyear C. Hypoxia-based habitat compression of tropical pelagic fishes. Fish Oceanogr. 2006:451–464. [Google Scholar]
- 20.Gilmartin M, Revelante N. The ‘island mass effect’ on the phytoplankton and primary production of the Hawaiian Islands. J Exp Mar Biol Ecol. 1974;16:181–204. [Google Scholar]
- 21.Tricas TC, McCosker JE. Predatory behavior of the white shark (Carcharodon carcharias), with notes on its biology. Proc Calif Acad Sci. 1984;43:221–238. [Google Scholar]
- 22.Malcolm H, Bruce BD, Stevens JD. A review of the biology and status of white sharks in Australian waters. 2001. 81 Report to Environment Australia, Marine Species Protection Program, CSIRO Research, Hobart.
- 23.Casey JG, Pratt HL. Distribution of the white shark, Carcharodon carcharias, in the western North Atlantic. Mem South Calif Acad Sci. 1985;9:2–14. [Google Scholar]
- 24.Klimley AP. The aerial distribution and autecology of the white shark (Carcharodon carcharias), off the west coast of North America. Mem South Calif Acad Sci. 1985;9:15–40. [Google Scholar]
- 25.Weng KC, O'Sullivan JB, Lowe CG, Winkler CE, Dewar H, et al. Movements, behavior and habitat preferences of juvenile white sharks Carcharodon carcharias in the eastern Pacific. Mar Ecol Prog Ser. 2007;338:211–224. [Google Scholar]
- 26.Taylor L. White sharks in Hawaii: historical and contemporary records. Mem South Calif Acad Sci. 1985;9:41–48. [Google Scholar]
- 27.Hobson KA. Tracing origins and migration of wildlife using stable isotopes: a review. Oecologia. 1999;120:314–326. doi: 10.1007/s004420050865. [DOI] [PubMed] [Google Scholar]
- 28.Peterson BJ, Fry B. Stable isotopes in ecosystem studies. Annu Rev Ecol Syst. 1987;18:293–320. [Google Scholar]
- 29.Michener RH, Kaufman L. Stable isotope ratios as tracers in marine food webs: an update. In: Michener R, Lajtha K, editors. Stable isotopes in ecology and environmental science. 2nd ed. Boston: Blackwell; 2007. pp. 238–282. [Google Scholar]
- 30.Graham BS, Koch PL, Newsome SD, McMahon KW, Aurioles D. Using isoscapes to trace the movements and foraging behavior of top predators in oceanic ecosystems. In: West JB, Bowen GJ, Dawson TE, Tu KP, editors. Isoscapes: understanding movement, pattern, and process on earth through isotope mapping. New York: Springer; 2010. pp. 299–318. [Google Scholar]
- 31.DeNiro MJ, Epstein S. Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta. 1978;42:495–506. [Google Scholar]
- 32.DeNiro MJ. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta. 1981;45:341–345. [Google Scholar]
- 33.Hobson KA, Barnett-Johnson R, Cerling TE. Using isoscapes to track animal migration. In: West JB, Bowen GJ, Dawson TE, Tu KP, editors. Isoscapes: understanding movement, pattern, and process on earth through isotope mapping. New York: Springer; 2010. pp. 273–298. [Google Scholar]
- 34.West JB, Bowen GJ, Dawson TE, Tu KP, editors. Isoscapes: understanding movement, pattern, and process on Earth through isotope mapping. New York: Springer; 2010. 487 [Google Scholar]
- 35.France RL. Carbon-13 enrichment in benthic compared to planktonic algae - foodweb implications. Mar Ecol Prog Ser. 1995;124:307–312. [Google Scholar]
- 36.Hobson KA, Piatt JF, Pitocchelli J. Using stable isotopes to determine seabird trophic relationships. J Anim Ecol. 1994;63 [Google Scholar]
- 37.Dunton KH, Saupe SM, Golikov N, Schell DM, Schonberg SV. Trophic relationships and isotopic gradients among arctic and subarctic marine fauna. Mar Ecol Prog Ser. 1989;56 [Google Scholar]
- 38.Thomas CJ, Cahoon LB. Stable isotope analyses differentiate between different trophic pathways supporting rocky-reef fishes. Mar Ecol Prog Ser. 1993;95:19–24. [Google Scholar]
- 39.Montoya JP. Natural abundance of 15N in marine planktonic ecosystems. In: Michener R, Lajtha K, editors. Stable isotopes in ecology and environmental science. 2nd ed. Boston: Blackwell; 2007. pp. 176–201. [Google Scholar]
- 40.Mahaffey C, Michaels AF, Capone DG. The conundrum of marine N2 fixation. Am J Sci. 2005;305:546–595. [Google Scholar]
- 41.Saino T, Hattori A. Geographical variation of the water column distribution of suspended organic nitrogen and its 15N natural abundance in the Pacific and its marginal seas. Deep Sea Research. 1987;34:807–827. [Google Scholar]
- 42.Clementz MT, Koch PL. Differentiating aquatic mammal habitat and foraging ecology with stable isotopes in tooth enamel. Oecologia. 2001;129:461–472. doi: 10.1007/s004420100745. [DOI] [PubMed] [Google Scholar]
- 43.Miller TW, Brodeur RD, Rau GH. Carbon stable isotopes reveal relative contribution of shelf-slope production to the northern California Current pelagic community. Limnol Oceanogr. 2008;53:1493–1503. [Google Scholar]
- 44.Waite AM, Muhling BA, Holl CM, Beckley LE, Montoya JP, et al. Food web structure in two counter-rotating eddies based on δ15N and δ13C isotopic analyses. Deep-Sea Research II. 2007;54:1055–1075. [Google Scholar]
- 45.Kim SL, Koch PL. Methods to collect, preserve, and prepare elasmobranch tissues for stable isotope analysis. Environ Biol Fishes. 2011 DOI 10.1007/s10641-10011-19860-10649. [Google Scholar]
- 46.Ainsley DG, Henderson RP, Huber HR, Boekelheide RJ, Allen SG, et al. Dynamics of white shark/pinniped interactions in the Gulf of the Farallons. Mem South Calif Acad Sci. 1985;9:109–122. [Google Scholar]
- 47.Le Boeuf BJ, Riedman M, Keyes RS. White shark predation on pinnipeds in California coastal waters. Fish Bull. 1982;80:891–895. [Google Scholar]
- 48.Long DJ, Hanni KD, Pyle P, Roletto J, Jones RE, et al. White shark predation on four pinniped species in central California waters: geographic and temporal patterns inferred from wounded carcasses. In: Klimley PA, Ainsley DG, editors. Great white sharks: the biology of Carcharadon carcharias. San Diego: Academic Press; 1996. pp. 263–274. [Google Scholar]
- 49.Long DJ, Jones RE. White shark predation and scavenging on cetaceans in the eastern North Pacific Ocean. In: Klimley PA, Ainsley DG, editors. Great white sharks: the biology of Carcharadon carcharias. San Diego: Academic Press; 1996. pp. 293–307. [Google Scholar]
- 50.Compagno LJV. Sharks of the world. An annotated and illustrated catalogue of shark species known to date. Volume 2. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes) Rome: FAO Species Catalogue for Fishery Purposes No. 1 Vol. 2; 2001. 269 [Google Scholar]
- 51.Goericke R, Fry B. Variations of marine plankton δ13C with latitude, temperature, and dissolved CO2 in the world ocean. Global Biogeochemical Cycles. 1994;8:85–90. [Google Scholar]
- 52.Nardoto GB, Godoy PB, Ferraz ESB, Ometto JPHB, Martinelli LA. Stable carbon and nitrogen isotopic fractionation between diet and swine tissues. Sci Agric. 2006;63:579–582. [Google Scholar]
- 53.Roth JD, Hobson KA. Stable carbon and nitrogen isotopic fractionation between diet and tissue of captive red fox: implications for dietary reconstruction. Can J Zool. 2000;78:848–852. [Google Scholar]
- 54.Rosenberg MS, Adams DC, Gurevitch J. Statistical software for meta-analysis. 2.0 ed: Sinauer Associates, Inc, Sunderland, MA; 2000. [Google Scholar]
- 55.Johnson SP, Schindler DE. Trophic ecology of Pacific salmon (Oncorhynchus spp.) in the ocean: a synthesis of stable isotope research. Ecol Res. 2009;24:855–863. [Google Scholar]
- 56.Hedges LV, Olkin I. Statistical methods for meta-analysis. San Diego, California, USA: Academic Press; 1985. 369 [Google Scholar]
- 57.Rau GH, Mearns AJ, Young DR, Olson RJ, Schafer HA, et al. Animal 13C/12C correlates with trophic level in pelagic food webs. Ecology. 1983;64:1314–1318. [Google Scholar]
- 58.Tieszen LL, Boutton TW, Tesdahl KG, Slade NA. Fractionation and turnover of stable carbon isotopes in animal tissues: implications for δ13C analysis of diet. Oecologia. 1983;57:32–37. doi: 10.1007/BF00379558. [DOI] [PubMed] [Google Scholar]
- 59.Fry B. Stable isotope ecology. New York: Springer; 2006. 308 [Google Scholar]
- 60.Hobson KA, Clark RW. Assessing avian diets using stable isotopes I: turnover of δ13C. Condor. 1992;94:181–188. [Google Scholar]
- 61.Fry B, Arnold C. Rapid 13C/12C turnover during growth of brine shrimp (Penaeus aztecus). Oecologia. 1982;54:200–204. doi: 10.1007/BF00378393. [DOI] [PubMed] [Google Scholar]
- 62.Fisk AT, Tittlemier SA, Pranschke JL, Norstrom RJ. Using anthropogenic contaminants and stable isotopes to assess the feeding ecology of Greenland sharks. Ecology. 2002;83:2162–2172. [Google Scholar]
- 63.Estrada JA, Rice AN, Lutcavage ME, Skomall GB. Predicting trophic position in sharks of the north-west Atlantic Ocean using stable isotope analysis. J Mar Biol Assoc U K. 2003;83:1347–1350. [Google Scholar]
- 64.Estrada JA, Rice AN, Natanson LJ, Skomal GB. Use of isotopic analysis of vertebrae in reconstructing ontogenetic feeding ecology in white sharks. Ecology. 2006;87:829–834. doi: 10.1890/0012-9658(2006)87[829:uoiaov]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 65.Post DM. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology. 2002;83:703–718. [Google Scholar]
- 66.Evans DH, Piermarini PM, Choe KP. Homeostasis: osmoregulation, pH regulation and Nitrogen excretion. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. Boca Raton: CRC Press; 2004. pp. 247–268. [Google Scholar]
- 67.Kim SL, Casper DR, Galván-Magaña F, Ochoa-Díaz R, Hernández-Aguilar SB, et al. Carbon and nitrogen discrimination factors for elasmobranch soft tissues based on a long-term controlled feeding study. Environ Biol Fishes: . 2011 DOI 10.1007/s10641-10011-19919-10647. [Google Scholar]
- 68.Martinez del Rio C, Wolf N, Carleton SA, Gannes LZ. Isotopic ecology ten years after a call for more laboratory experiments. Biol Rev. 2009;84:91–111. doi: 10.1111/j.1469-185X.2008.00064.x. [DOI] [PubMed] [Google Scholar]
- 69.Newsome SD, Clementz MT, Koch PL. Using stable isotope biogeochemistry to study marine mammal ecology. Mar Mamm Sci. 2010;26:509–572. [Google Scholar]
- 70.Martinez del Rio C, Wolf BO. Mass balance models for animal isotopic ecology. In: Starck MA, Wang T, editors. Physiological and ecological adaptations to feeding in vertebrates. Enfield, , NH: Science Publishers; 2005. pp. 141–174. [Google Scholar]
- 71.Hesslein RH, Hallard KA, Ramlal P. Replacement of sulfur, carbon, and nitrogen in tissue of growing broad whitefish (Coregonus nasus) in response to a change in diet traced by δ34S, δ13C, and δ15N. Can J Fish Aquat Sci. 1993;50:2071–2076. [Google Scholar]
- 72.MacAvoy SE, Macko SA, Garman GC. Isotopic turnover in aquatic predators: quantifying the exploitation of migratory prey. Can J Fish Aquat Sci. 2001;58:923–932. [Google Scholar]
- 73.Phillips DL, Eldridge PM. Estimating the timing of diet shifts using stable isotopes. Oecologia. 2006;147:195–203. doi: 10.1007/s00442-005-0292-0. [DOI] [PubMed] [Google Scholar]
- 74.Logan JM, Lutcavage M. Stable isotope dynamics in elasmobranch fishes. Hydrobiologia. 2010;644:231–244. [Google Scholar]
- 75.Weidel BC, Carpenter SR, Kitchell JF, Vander Zanden MJ. Rates and components of carbon turnover in fish muscle: insights from bioenergetics models and a whole-lake 13C addition. Can J Fish Aquat Sci. 2011;68:387–399. [Google Scholar]
- 76.Kim SL. Insights into shark ecology and physiology with stable isotope analysis [Ph.D. dissertation] Santa Cruz: University of California; 2010. 227 [Google Scholar]
- 77.Carleton SA, Martinez Del Rio C. The effect of cold-induced increased metabolic rate on the rate of 13C and 15N incorporation in house sparrows (Passer domesticus). Oecologia. 2005;144:226–232. doi: 10.1007/s00442-005-0066-8. [DOI] [PubMed] [Google Scholar]
- 78'.MacNeil MA, Drouillard KG, Fisk AT. Variable uptake and elimination of stable nitrogen isotopes between tissues in fish. Can J Fish Aquat Sci. 2006;63:345–353. [Google Scholar]
- 79.Logan J, Haas H, Deegan L, Gaines E. Turnover rates of nitrogen stable isotopes in the salt marsh mummichog, Fundulus heteroclitus, following a laboratory diet switch. Oecologia. 2006;147:391–395. doi: 10.1007/s00442-005-0277-z. [DOI] [PubMed] [Google Scholar]
- 80.Goldman KJ. Regulation of body temperature in the white shark, Carcharodon carcharias. J Comp Physiol B Biochem Syst Environ Physiol. 1997;167:423–429. [Google Scholar]
- 81.Graham JB, Dewar H, Lai NC, Lowell WR, Arce SM. Aspects of shark swimming performance determined using a large water tunnel. J Exp Biol. 1990;151:175–192. [Google Scholar]
- 82.Carlson JK, Goldman KJ, Lowe CG. Metabolism, energetic demand, and endothermy. In: Carrier JC, Musick JA, Heithaus MR, editors. Biology of sharks and their relatives. Boca Raton: CRC Press; 2004. pp. 203–224. [Google Scholar]
- 83.Moore JW, Semmens BX. Incorporating uncertainty and prior information into stable isotope mixing models. Ecol Lett. 2008;11:470–480. doi: 10.1111/j.1461-0248.2008.01163.x. [DOI] [PubMed] [Google Scholar]
- 84.Bump JK, Fox-Dobbs K, Bada JL, Koch PL, Peterson RO, et al. Stable isotopes, ecological integration and environmental change: wolves record atmospheric carbon isotope trend better than tree rings. Proc R Soc Biol Sci Ser B. 2007;274:2471–2480. doi: 10.1098/rspb.2007.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Semmens BX, Ward EJ, Moore JW, Darimont CT. Quantifying inter- and intra-population niche variability using hierarchical Bayesian stable isotope mixing models. PLoS ONE. 2009;4:e6187. doi: 10.1371/journal.pone.0006187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Francis TB, Schindler DE, Holtgrieve GW, Larson ER, Scheuerell MD, et al. Habitat structure determines resource use by zooplankton in temperate lakes. Ecol Lett. 2011;14:364–372. doi: 10.1111/j.1461-0248.2011.01597.x. [DOI] [PubMed] [Google Scholar]
- 87.Pratt HLJ. Reproduction in the male white shark. In: Klimley PA, Ainsley DG, editors. Great white sharks: the biology of Carcharadon carcharias. San Diego: Academic Press; 1996. pp. 131–138. [Google Scholar]
- 88.Francis MP. Observations on a pregnant white shark with a review of reproductive biology. In: Klimley PA, Ainsley DG, editors. Great white sharks: the biology of Carcharadon carcharias. San Diego: Academic Press; 1996. pp. 157–172. [Google Scholar]
- 89.Motta PJ. Anatomy and functional morphology of dermal collagen fibers in sharks. Copeia. 1977:454–464. [Google Scholar]
- 90.Hussey NE, Brush J, McCarthy ID, Fisk AT. δ15N and δ13C diet-tissue discrimination factors for large sharks under semi-controlled conditions. Comp Biochem Physiol A-Mol Integr Physiol. 2010;155:445–453. doi: 10.1016/j.cbpa.2009.09.023. [DOI] [PubMed] [Google Scholar]
- 91.Dingle H. Migration: the biology of life on the move. New York, , NY: Oxford University Press; 1996. [Google Scholar]
- 92.Block BA, Jonsen ID, Jorgensen SJ, Winship AJ, Shaffer SA, et al. Tracking apex marine predator movements in a dynamic ocean. Nature. 2011;475:86–90. doi: 10.1038/nature10082. [DOI] [PubMed] [Google Scholar]
- 93.Pearcy WC, Fisher JP, Yoklavich MM. Biology of the Pacific pomfret (Brama japonica) in the North Pacific Ocean. Can J Fish Aquat Sci. 1993;50 [Google Scholar]
- 94.Watanabe H, Kubodera T, Kawahara S. Summer feeding habits of the Pacific pomfret Brama japonica in the transitional and subarctic waters of the central North Pacific. J Fish Biol. 2006;68:1436–1450. [Google Scholar]
- 95.Springer AM, Estes JA, van Vliet GB, WIlliams TM, Doak DF, et al. Sequential megafaunal collapse in the North Pacific Ocean: an ongoing legacy of industrial whaling? Proceedings of the National Academy of Sciences. 2003;100:12223–12228. doi: 10.1073/pnas.1635156100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sibert J, Hampton J, Kleiber P, Maunder M. Biomass, size, and trophic status of top predators in the Pacific Ocean. Science. 2006;314:1773–1776. doi: 10.1126/science.1135347. [DOI] [PubMed] [Google Scholar]
- 97.Ragen TJ, Lavigne DM. The Hawaiian monk seal: biology of an endangered species. In: Twiss JR, Reeves RR, editors. Conservation and management of marine mammals. Washington: Smithsonian Institution Press; 1999. pp. 223–245. [Google Scholar]
- 98.McCosker JE. White shark attack behavior: observations of and speculations about predator and prey strategies. Mem South Calif Acad Sci. 1985;9:123–135. [Google Scholar]
- 99.Dewar H, Domeier M, Nasby-Lucas N. Insights into young of the year white shark, Carcharodon carcharias, behavior in the Southern California Bight. Environ Biol Fishes. 2004;70:133–143. [Google Scholar]
- 100.Cailliet GM, Natanson LJ, Welden BA, Ebert DA. Preliminary studies on the age and growth of the white shark, Carcharadon carcharias, using vertebral bands. Mem South Calif Acad Sci. 1985;9:49–60. [Google Scholar]
- 101.Kerr LA, Andrews AH, Cailliet GM, Brown TA, Coale KH. Investigations of Δ14C, δ13C, and δ15N in vertebrae of white shark (Carcharodon carcharias) from the eastern North Pacific Ocean. Environ Biol Fishes. 2006;77:337–353. [Google Scholar]
- 102.Perry RI, Thompson PA, Mackas DL, Harrison PJ, Yelland DR. Stable carbon isotopes as pelagic food web tracers in adjacent shelf and slope regions off British Columbia, Canada. Can J Fish Aquat Sci. 1999;56:2477–2486. [Google Scholar]
- 103.Pearcy WG, Stuiver M. Vertical transport of carbon-14 into deep-sea food webs. Deep-Sea Res Part A Oceanogr Res Pap. 1983;30:427–440. [Google Scholar]
- 104.McIntyre PB, Flecker AS. Rapid turnover of tissue nitrogen of primary consumers in tropical freshwaters. Oecologia. 2006;148:12–21. doi: 10.1007/s00442-005-0354-3. [DOI] [PubMed] [Google Scholar]
- 105.Hight BV, Lowe CG. Elevated body temperatures of adult female leopard sharks, Triakis semifasciata, while aggregating in shallow nearshore embayments: Evidence for behavioral thermoregulation? J Exp Mar Biol Ecol. 2007;352:114–128. [Google Scholar]
- 106.Wallman HL, Bennett WA. Effects of parturition and feeding on thermal preference of Atlantic stingray, Dasyatis sabina (Lesueur). Environ Biol Fishes. 2006;75:259–267. [Google Scholar]
- 107.Wearmouth VJ, Sims DW. Sexual segregation in marine fish, reptiles, birds, and mammals: behaviour patterns, mechanisms and conservation implications. Adv Mar Biol. 2008;54:107–170. doi: 10.1016/S0065-2881(08)00002-3. [DOI] [PubMed] [Google Scholar]
- 108.Jacoby DMP, Croft DP, Sims DW. Social behaviour in sharks and rays: analysis, patterns and implications for conservation. Fish Fish: . 2011 DOI: 10.1111/j.1467-2979.2011.00436.x. [Google Scholar]
- 109.Mollet HF, Cliff G, Pratt HL, Stevens JD. Reproductive biology of the female shortfin mako, Isurus oxyrinchus Rafinesque, 1810, with comments on the embryonic development of lamnoids. Fish Bull. 2000;98:299–318. [Google Scholar]
- 110.Anderson S, Pyle P. A temporal, sex-specific occurence pattern among white sharks at the South Farallon Islands, California. California Department of Fish and Game. 2003;89:96–101. [Google Scholar]
- 111.Domeier ML, Nasby-Lucas N. Annual re-sightings of photographically identified white sharks (Carcharodon carcharias) at an eastern Pacific aggregation site (Guadalupe Island, Mexico). Mar Biol. 2007;150:977–984. [Google Scholar]
- 112.Teo SLH, Boustany A, Dewar H, Stokesbury MJW, Weng KC, et al. Annual migrations, diving behavior, and thermal biology of Atlantic bluefin tuna, Thunnus thynnus, on their Gulf of Mexico breeding grounds. Mar Biol. 2007;151:1–18. [Google Scholar]
- 113.Gannes LZ, O'Brien DM, Martinez Del Rio C. Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology. 1997;78:1271–1276. [Google Scholar]
- 114.Smith K, Rennie MJ. New approaches and recent results concerning human-tissue collagen synthesis. Curr Opin Clin Nutr Metab Care. 2007;10 doi: 10.1097/MCO.0b013e328285d858. [DOI] [PubMed] [Google Scholar]
- 115.Babraj JA, Cuthbertson DJR, Smith K, Langberg H, Miller B, et al. Collagen synthesis in human musculoskeletal tissues and skin. Am J Physiol-Endocrinol Metab. 2005;289:E864–E869. doi: 10.1152/ajpendo.00243.2005. [DOI] [PubMed] [Google Scholar]
- 116.El-Harake WA, Furman MA, Cook B, Nair KS, Kukowski J, et al. Measurement of dermal collagen synthesis rate in vivo in humans. American Journal of Physiology, Endocrinology, and Metabolism. 1998;274:E586–E591. doi: 10.1152/ajpendo.1998.274.4.E586. [DOI] [PubMed] [Google Scholar]
- 117.Bonfil R, Francis MP, Duff C, Manning MJ, O'Brien S. Large-scale tropical movements and diving behavior of white sharks Carcharadon carcharias tagged off New Zealand. Aquat Biol. 2010;8:115–123. [Google Scholar]
- 118.Bonfil R, Meyer M, Scholl MC, Johnson R, O'Brien S, et al. Transoceanic migration, spatial dynamics, and population linkages of white sharks. Science. 2005;310:100–103. doi: 10.1126/science.1114898. [DOI] [PubMed] [Google Scholar]
- 119.Popp BN, Graham BS, Olson RJ, Hannides CC, Lott MJ, et al. Insight into the trophic ecology of yellowfin tuna, Thunnus albacares, from compound-specific nitrogen isotope analysis of proteinaceous amino acids. In: Dawson TE, Siegwolf RTW, editors. Stable isotopes as indicators of ecological change. London: Academic Press; 2007. pp. 173–190. [Google Scholar]
- 120.Newsome SD, Etnier MA, Monson DH, Fogel ML. Retrospective characterization of ontogenetic shifts in killer whale diets via δ13C and δ15N analysis of teeth. Mar Ecol Prog Ser. 2009;374:229–242. [Google Scholar]
- 121.Witteveen BH, Worthy GAJ, Wynne KM, Roth JD. Population structure of North Pacific humpback whales on their feeding grounds revealed by stable carbon and nitrogen isotope ratios. Mar Ecol Prog Ser. 2009;379:299–310. [Google Scholar]
- 122.Topperoff AK. Diet of harbor porpoise (P. phocoena) using stomach contents and stable isotope analyses [MS] San Jose: San Jose State University; 1997. 103 [Google Scholar]
- 123.Burton RK, Koch PL. Isotopic tracking of foraging and long-distance migration in northeastern Pacific pinnipeds. Oecologia. 1999;119:578–585. doi: 10.1007/s004420050822. [DOI] [PubMed] [Google Scholar]
- 124.Burton RK, Snodgrass JJ, Gifford-Gonzalez D, Guilderson T, Brown T, et al. Holocene changes in the ecology of northern fur seals: insights from stable isotopes and archaeofauna. Oecologia. 2001;128:107–115. doi: 10.1007/s004420100631. [DOI] [PubMed] [Google Scholar]
- 125.Jarman WM, Hobson KA, Sydeman WJ, Bacon CE, McLaren EB. Influence of trophic position and feeding location on contaminant levels in the Gulf of the Farallones food web revealed by stable isotope analysis. Environ Sci Technol. 1996;30:654–660. [Google Scholar]
- 126.Witteveen BH, Worthy GAJ, Roth JD. Tracing migratory movements of breeding North Pacific humpback whales using stable isotope analysis. Mar Ecol Prog Ser. 2009;393:173–183. [Google Scholar]
- 127.Graham BS, Grubbs D, Holland K, Popp BN. A rapid ontogenetic shift in the diet of juvenile yellowfin tuna from Hawaii. Mar Biol. 2007;150:647–658. [Google Scholar]
- 128.Graham BS. Trophic dynamics and movements of tuna in tropical Pacific Ocean inferred from stable isotope analyses [Ph.D.] Manoa: University of Hawaii; 2007. 237 [Google Scholar]
- 129.Parry MP. The trophic ecology of two ommastrephid squid species, Ommastrephes bartamii and Sthenoteuthis oualaniensis, in the North Pacific sub-tropical gyre [Ph.D.] Manoa: University of Hawaii; 2003. 287 [Google Scholar]
- 130.Parry MP. Trophic variation with length in two ommastrephid squids, Ommastrephes bartramiii and Sthenoteuthis oualaniensis. Mar Biol. 2008;153 [Google Scholar]
- 131.Gould P, Ostrom P, Walker W. Trophic relationship of albatrosses associated with squid and large-mesh drift-net fisheries in the North Pacific Ocean. Can J Zool. 1997;75:549–562. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded description of selection of prey species used to estimate mean δ13C and δ15N values for different focal areas (doc).
(DOC)
JAGS/WinBUGS code for spatially explicit Bayesian mixing model. This file includes descriptions of the types and formatting of data needed to run this code (R).
(R)