Abstract
Background
Species of the genus Aeromonas are native inhabitants of aquatic environments and have recently been considered emerging human pathogens. Although the gastrointestinal tract is by far the most common anatomic site from which aeromonads are recovered, their role as etiologic agents of bacterial diarrhea is still disputed. Aeromonas-associated diarrhea is a phenomenon occurring worldwide; however, the exact prevalence of Aeromonas infections on a global scale is unknown.
Methodology/Principal Findings
The prevalence and virulence potential of Aeromonas in patients suffering from diarrhea in Israel was studied using molecular methods. 1,033 diarrheal stools were sampled between April and September 2010 and Aeromonas species were identified in 17 (∼2%) patients by sequencing the rpoD gene. Aeromonas species identity and abundance was: A. caviae (65%), A. veronii (29%) and Aeromonas taiwanensis (6%). This is the first clinical record of A. taiwanensis as a diarrheal causative since its recent discovery from a wound infection in a patient in Taiwan. Most of the patients (77%) from which Aeromonas species were isolated were negative for any other pathogens. The patients ranged from 1 to 92 years in age. Aeromonas isolates were found to possess different virulence-associated genes: ahpB (88%), pla/lip/lipH3/apl-1 (71%), act/hlyA/aerA (35%), alt (18%), ast (6%), fla (65%), lafA (41%), TTSS ascV (12%), TTSS ascF-ascG (12%), TTSS-dependent ADP-ribosylating toxins aexU (41%) and aexT (6%) in various combinations. Most of the identified strains were resistant to beta-lactam antibiotics but susceptible to third-generation cephalosporin antibiotics.
Conclusions
Aeromonas may be a causative agent of diarrhea in patients in Israel and therefore should be included in routine bacteriological screenings.
Introduction
Aeromonas species are waterborne, Gram-negative, oxidase-positive, rod-shaped bacteria that are ubiquitous in water. This includes chlorinated drinking water, as the bacteria can grow and survive in biofilms in the water distribution systems [1]. The prevalence of Aeromonas species in the aquatic environment has been recognized as a potential health risk, and some countries have adopted aeromonad counts as an additional indicator of water quality [2].
The most common clinical manifestations of Aeromonas infections are diarrhea, bacteremia and localized soft-tissue infections [3]. Patients may acquire Aeromonas infections both in community and hospital settings [3]–[5]. Both immunocompetent and immunocompromised patients are susceptible to Aeromonas infections [3], [6]. The gastrointestinal tract is by far the most common anatomic site from which aeromonads are recovered [3], [6]. Aeromonas have been isolated from children with acute diarrhea and from adults with traveler's diarrhea [3], [6]–[8]. The following species are frequently associated with diarrhea in humans: A. hydrophila, A. veronii bv. sobria and A. cavia [3], [6], [9], [10], [11].
The mechanism of Aeromonas pathogenesis is complex and not well understood. Aeromonas virulence is considered to be multifactorial. The virulence factors that were associated with Aeromonas pathogenicity are: cytotoxic enterotoxin, haemolysins, proteases [serine protease (aspA), elastase (ahpB)], lipases (pla and plc, sat), DNAses and adhesins [type IV pili and polar flagella (flaA and flaB)] [1], [6], [9], [10], [12], [13]. Several of these virulence factors have been identified in strains isolated from water [1]. In addition, genes for a type III secretion system (TTSS) were identified in this genus [14], [15]. TTSS has a role in delivering toxins directly into the host cell and in inducing apoptosis [14], [15].
In Israel, diarrhea patients are tested routinely by clinical laboratories for the presence of several bacterial pathogens, such as: Campylobacter spp., Shigella spp. and Salmonella spp., but not for Aeromonas. The aim of this research was to study the prevalence and virulence potential of Aeromonas spp. in diarrheal stools in Israel.
Materials and Methods
Ethics Statement
N/A. The data was analyzed anonymously.
We have applied to the ethics committee at Carmel Hospital, Clalit Health Services, Haifa, Israel, and the committee stated that such a research does not fall under the scope of the Helsinki Committee.
Aeromonas Prevalence in Diarrheal Stools
The presence of Aeromonas was monitored in fecal specimens from diarrheal patients submitted to the Microbiology Laboratory of Clalit Health Services in Haifa. This Laboratory provides services to a wide range of population, from the district of Haifa and West Galilee in Israel (this is a community health service, not a hospital). The surveillance was conducted between April 13 and September 15, 2010 (five months). All specimens were checked routinely for the following enteropathogens: Shigella, Salmonella and Campylobacter spp. were isolated and identified by conventional methods [16]; Rotavirus was detected by an antigen detection method (Novamed, Israel); parasites were studied according to methods described in Garcia and Isenberg [17]. For the isolation of Aeromonas spp. the fecal specimens were either enriched in alkaline peptone water (APW) containing peptone (1%, wt/vol) and NaCl (1%, wt/vol) pH 8.5, or directly streaked on a selective m-Aeromonas agar base (Havelaar Biolife, Milano, Italy). In the case of enrichment, the tubes were incubated at 37°C without shaking for 6–18 h, and then streaked on m-Aeromonas selective agar. The agar plates were incubated overnight at 37°C. Colonies that were morphologically suspected as Aeromonas (yellow, smooth and rounded) were subcultured onto LB agar (Himedia, India), and then tested for oxidase (1% tetramethyl-phenylenediamine, Sigma). The identity of the isolates with positive results was further verified by Aeromonas genus specific PCR assay in accordance with Kong et al. (1999) [18]. Reddy Mix PCR master mixture (ABgene, Epsom, UK) was used for the DNA amplification. All the isolates that were found to belong to the Aeromonas genus were maintained in LB with 30% glycerol (−80°C).
Aeromonas isolates were further identified by amplifying and sequencing the housekeeping gene rpoD, encoding σ 70 factor, which is one of the sigma factors that confer promoter-specific transcription initiation on RNA polymerase [19]. The PCR products were sequenced by MCLAB (San Francisco, CA). Newly determined sequences were compared to those available in the GenBank database, using the standard nucleotide–nucleotide BLAST program (BLASTN; http://www.ncbi.nlm.nih.gov), to ascertain their closest relatives. The sequences were submitted to the GenBank database under accession numbers JF738005–JF738021. A phylogenetic tree was generated using the neighbor-joining method with NJPlot (MEGA 4.1) based on alignments from CLUSTAL W.
Virulence Factors and Antimicrobial Susceptibility
The presence of the following genes encoding virulence factors was determined in all Aeromonas isolates: cytotoxic enterotoxin (act)/aerolysin (aerA)/haemolysin (hlyA) by using one set of primers AHCF1/AHCR1 [12]; alt and ast genes for cytotonic enterotoxins; ahyB gene for elastase; pla/lipH3/apl-1/lip genes for phospholipase; and fla gene for flagellin [1]. The presence of the genes act/aerA/hlyA and ast; fla and alt; ahyB and pla/lipH3/apl-1/lip was tested simultaneously in the same reaction mixture, in accordance with Sen and Rodgers (2004) [1]. The presence of genes encoding the components of the type III secretion system, ascV, ascF-ascG [15], type III secretion dependent ADP-ribosylating toxins, aexT and aexU [20], and of lafA gene encoding a lateral flagella [13] was determined as well.
The disk diffusion antimicrobial susceptibility tests were performed by a standardized method [21], [22]. All disks were purchased from OXOID (UK).
Results
Aeromonas Prevalence in Diarrheal Stools
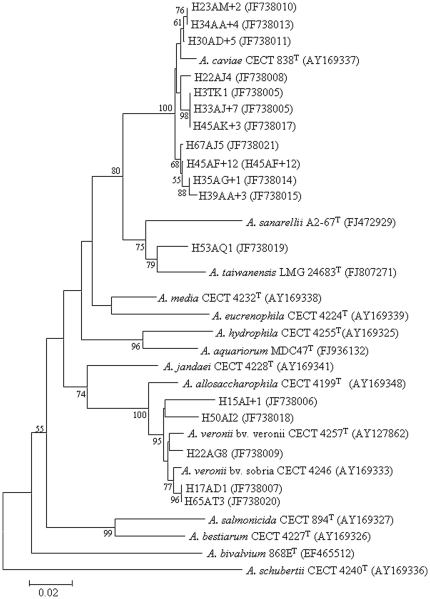
A total of 1,033 stool specimens from patients suffering from diarrhea were monitored for the presence of Aeromonas during a five month period between April 13 and September 15, 2010. Seventeen patients (∼2%) tested positive for Aeromonas species which included 11 (65%) A. caviae, five (29%) A. veronii, and one strain, (H53AQ1). This strain showed the highest rpoD gene similarity (96%) to the deposited sequence of the type strain of A. taiwanensis, and clustered with this species in the phylogenetic tree (Figure 1).
Figure 1. Phylogenetic tree of Aeromonas isolates recovered from diarrhea patients.
The tree shows the relationships based on partial sequences of rpoD gene of type strains of Aeromonas species and the isolates from the current study. The sequence alignments were performed using the CLUSTAL W program, and the tree was generated using the neighbor–joining method with Kimura 2 parameter distances in MEGA 4.1 software. Bootstrap values (from 1,000 replicates) greater than 50% are shown at the branch points. The bar indicates 2% sequence divergence.
The specimens of the diarrhea patients were also checked for other enteropathogenes. The results revealed that pathogenic bacteria, Rotavirus and parasites were recovered from about 15% (155 of 1033) of the diarrhea patients. The prevalence of the detected enterophathogenes was; Campylobacter sp. 5.2%, Shigella 3.3%, Salmonella enterica 2%, Aeromonas spp. 2%, Rotavirus 0.4%, Giardia lamblia 2.3%, and Cryptosporidium parvum 0.15%. Mixed infections were found in four patients that were positive for Aeromonas as well as for other known enteropathogens (Table S1).
Virulence Factors and Antimicrobial Susceptibility
All Aeromonas isolates were screened for the presence of virulence genes (Table 1). The most prevalent genes were ahyB (88%) and pla/lipH3/apl-1/lip (71%). The two types of flagella that were screened (polar and lateral) were quite prevalent as well (65% and 41%, respectively). In every strain that was positive for the genes encoding the TTSS, a gene for the effector aexU was present as well. The ast gene was found only in one isolate (H65AT3), which was identified as A. veronii. The virulence genotypes were found in different combinations: three isolates (18%) possessed five different genes, four (24%) possessed three or four different genes and two (12%) possessed two different genes (Table 1).
Table 1. Prevalence of virulence genes in Aeromonas isolates from diarrheal patients.
| Isolatename | Virulence genes | ||||||||||
| ahpB | pla/lip/lipH3/apl-1 | act/aerA/hlyA | ast | alt | fla | lafA | TTSSascV | TTSSascF-ascG | aexT | aexU | |
| H3TK1 | + | + | − | − | − | + | − | − | − | − | − |
| H15AI+1 | + | + | + | − | + | − | − | − | − | − | − |
| H17AD1 | − | − | + | − | + | + | − | − | − | + | − |
| H22AJ4 | + | + | − | − | − | − | − | − | − | − | − |
| H22AG8 | − | − | + | − | − | + | − | + | + | − | + |
| H23AM+2 | + | + | + | − | − | + | + | − | − | − | − |
| H30AD+5 | + | + | − | − | − | − | + | − | − | − | − |
| H33AJ+7 | + | − | − | − | − | + | − | − | − | − | + |
| H34AA+4 | + | + | + | − | − | + | + | − | − | − | − |
| H35AG+1 | + | + | − | − | − | + | + | − | − | − | − |
| H39AA+3 | + | + | − | − | + | − | − | − | − | − | + |
| H45AF+12 | + | + | − | − | − | + | + | − | − | − | + |
| H45AK+3 | + | + | − | − | − | + | − | − | − | − | − |
| H50AI2 | + | + | + | − | − | + | − | + | − | − | + |
| H53AQ1 | + | − | − | − | − | + | − | − | + | − | + |
| H65AT3 | − | + | − | + | − | − | + | − | − | − | − |
| H67AJ5 | + | + | − | − | − | − | + | − | − | − | + |
For more details on the isolates and on the patients see Table S1.
The susceptibility of Aeromonas isolates was evaluated against 15 antimicrobial agents. All isolates were susceptible to amikacin, cefotaxime, ceftazidime, ceftriaxone, ciprofloxacin and chloramphenicol; however, they varied in their susceptibility to other antimicrobial agents (Table 2). The three Aeromonas species displayed the same antibiotic sensitivity patterns.
Table 2. Susceptibility of Aeromonas isolates to antimicrobial agents.
| Antimicrobial agent | Number (%) of strains | ||
| (number of strains tested) | susceptible | intermediate resistance | resistant |
| amikacin (11) | 11 (100) | - | - |
| cefotaxime (11) | 11 (100) | - | - |
| ceftazidime (11) | 11 (100) | - | - |
| ceftriaxone (E test) (11) | 11 (100) | - | - |
| ciprofloxacin (11) | 11 (100) | - | - |
| chloramphenicol (11) | 11 (100) | - | - |
| gentamicin (11) | 10 (91) | - | 1 (9) |
| piperacillin–tazobactam (11) | 10 (91) | - | 1 (9) |
| trimethoprim–sulfamethoxazole (11) | 10 (91) | - | 1 (9) |
| imipenem (also meropenem) (11) | 8 (73) | - | 3 (27) |
| cefoxitin (10) | 7 (70) | 2 (20) | 1 (10) |
| nalidixic acid (11) | 7 (64) | - | 4 (36) |
| tetracycline (11) | 6 (55) | - | 5 (45) |
| amoxicillin+clavulanic acid (11) | 3 (27) | 3 (27) | 5 (46) |
| cephalotin (10) | 2 (20) | - | 8 (80) |
Most of the identified strains were resistant to beta-lactam antibiotics but susceptible to third-generation cephalosporin antibiotics.
Discussion
Despite the existence of detailed case reports and epidemiological case control investigations, the role of Aeromonas as the etiological agent of bacterial diarrhea has been questioned and debated several times [3], [6], [11], . Figueras et al. [26] rebutted arguments provided by several authors against considering Aeromonas a true enteropathogenic bacterium one by one. Today it is well accepted that if Aeromonas can cause different infections like cellulitis, meningitis, pneumonia, wound infections and more in healthy humans, it can also have the capacity to produce diarrhea [3], [6], [26].
In several reported studies throughout the world, Aeromonas species have been isolated at a rate of 0.6 to 7.2% in patients with diarrhea, predominantly in infants and children [2], [27]. In the current study, Aeromonas positive patients ranged in age and only two out of 17 isolates were taken from children (Table S1). The current study relied on a limited amount of strains as it was performed only during a period of five months; however, the Aeromonas isolation rate amounted to about 2%, which is similar to the rate obtained in other studies performed in other countries [3,6 and references therein], as well as in Israel in 1990 [28]. In a recent study performed by Pablos et al. [29] in León (Spain) they found a frequency of Aeromonas of 4% (32 positive patients of the 800 investigated), mainly associated with infant or pediatric patients (68.8%). Furthermore they found mixed infections with other pathogens in 12 patients [29]. In our study, only two of the patients were infants (12%), and all four patients that had mixed infections were adults (Table S1).
In mixed infections, Aeromonas may be transient colonizers lacking a causal relationship with a disease, but in some cases, multiple pathogens may act synergistically to produce diarrhea [30]. Aeromonas species are carried asymptomatically by some individuals [3], [6] as occurs with other recognized enteropathogens like Salmonella. However, a study that was performed in 1990 in Israel compared the prevalence of Aeromonas in the stools obtained from 932 adult patients with acute diarrhea (recovered between 1986 and 1987) to 500 stools from asymptomatic controls. They found an Aeromonas prevalence of about 2% in the diarrhea cases, which conforms to our study. But no Aeromonas were detected in the controls [28]. This seems to indicate a clear association of Aeromonas with diarrhea cases in Israel, as we found in our study.
Among the recognized Aeromonas species, A. veronii bv. sobria, A. caviae and A. hydrophila are more frequently associated with diarrhea in humans, representing 85% of clinical isolates [11]. Interestingly, none of the identified strains in the current study belonged to A. hydrophila. This is in agreement with the false importance attributed to this species on the basis of phenotypic identifications [3], [26]. In the current study, A. caviae was the predominating species (65%, 11/17), followed by A. veronii that was isolated from five patients (29%). One patient carried a strain that was identified as A. taiwanensis (Figure 1). All the strains were identified using the rpoD gene sequencing method. Aeromonas identification on the basis of rpoD gene sequencing is considered to be much more accurate than 16S rRNA gene sequencing or biochemical identification methods. The fact that many studies found A. hydrophila a major species to cause diarrhea (among Aeromonas species) may be due to limitations in the identification methods that were used in those studies [3], [26].
The current study provides the first clinical record of A. taiwanensis as a diarrheal causative since this species was identified [31]. So far, the only available strain (the type strain) was recovered from an infected burn wound of a 40 years old male [31] and in the current study the strain was isolated from feces of a 35 years old diarrheal female patient.
The clinical manifestations of Aeromonas associated gastroenteritis can range from mild self-limiting watery diarrhea to a more severe and invasive dysenteric form. Chronic diarrhea episodes and isolated cases of a cholera-like illness have also been described [11]. The bacterial flagella are thought to play an important role in pathogenicity. Aeromonas produces two types of flagella: a constitutively expressed polar flagellum (fla) and multiple inducible lateral flagella (laf). Both types play a role in the attachment of the bacteria to the gastrointestinal epithelium, biofilm formation and long-term colonization [6]. Both types of flagella (fla and lafA) were common among the Aeromonas isolates from the patients in the current study (Table 1). The occurrence of genes encoding hemolytic, cytotonic, cytotoxic, and enterotoxic activities (aerA, hlyA, alt, ast, act) may contribute to diarrheal-related virulence [6], [31], [32]. In the present study, 35% of the Aeromonas isolates possessed the act/aerA/hlyA gene. The most prevalent virulence-associated genes in the isolates from our study were ahpB for elastase (88%) and pla/lip/lipH3/apl-1 for lipase (71%) (Table 1). These genes may be essential for the ability of the bacterium to adhere and invade the intestinal mucosa [1].
Type III secretion system (TTSS) plays crucial roles in host-pathogen interactions [14], [15]. One of the best-described toxins that are translocated via a TTSS is the ADP-ribosylating toxin, AexT. This toxin was found to be more common among the environmental, rather than the clinical Aeromonas strains [13]. In our study, the gene for this toxin was detected only in one strain. Recently, a novel type-three-secretion-dependent effector, AexU, was discovered in Aeromonas. AexU is an ADP-ribosylating toxin and is required for virulence of Aeromonas hydrophila in mice [20]. The gene for this toxin was quite prevalent among the strains in our study (41%). The prevalence of the genes encoding TTSS apparatus (12%) was lower than the aexU gene prevalence (41%). The TTSS is probably underrepresented, as may happen in PCR based studies. Nevertheless, the presence of aexU gene strengthens the case of Aeromonas being recognized as a stronger pathogen.
In another study that surveyed the distribution of virulence associated genes among Aeromonas species from human stool specimens in Spain, it was found that alt, ast, laf, aerA, and hlyA genes were present in 72, 19, 3, 25, and 28% of the strains, respectively. None of the strains harbored ascF – G [29]. In clinical diarrheic isolates of A. hydrophila in Spain the distribution of associated virulence genes was different: alt – 82%, ast – 96%, laf – 77%, aexT – 5%, ascV – 5% [13].
Aeromonas species are known to be intrinsically susceptible to all antibiotics active against non-fastidious Gram-negative bacilli, except for many beta–lactams, due to the production of multiple inducible, chromosomally encoded β–lactamases [33]. In our study, most strains (80%) were resistant to cephalotin and partially resistant to amoxicillin combined with clavulanic acid (46%) (Table 2). All strains were susceptible to third-generation cephalosporin antibiotics (cefotaxime, ceftazidime, ceftriaxone), second-generation fluoroquinolone antibiotics – ciprofloxacin, aminoglycoside antibiotic – amikacin, and to chloramphenicol.
Recently, it was found that the egg masses of chironomids, non-biting midges, (Diptera; Chironomidae) serve as a natural reservoir for Aeromonas pathogenic species [34], [35] as well as for Vibrio cholerae [36]. Chironomid infestations in drinking water supply systems are an existing problem in Israel [37] and worldwide [38]. Chironomids may disseminate pathogenic species of Aeromonas between drinking water reservoirs, as was suggested for V. cholerae [39].
The source of Aeromonas in diarrheal patients was not investigated in the current study. In order to investigate the route of transmission of Aeromonas pathogenic strains an extensive study on strains from various origins should be performed. Chironomid egg masses in drinking water ponds and tap waters should be screened for Aeromonas isolates and compared with isolates from diarrheal patients.
Aeromonas infections are self–limiting, but their diagnosis may be crucial in young children, old and immunocompromised patients. We conclude that Aeromonas may be a causative agent of diarrhea in patients in Israel and therefore should be included in routine bacteriological screenings.
Supporting Information
Characterization of Aeromonas isolates from diarrheal patients. Aeromonas rpoD sequences were deposited in the GenBank database under the accession numbers JF738005–JF738021 (see also Figure 1).
(DOC)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This study was supported by the Research Authority, University of Haifa, Israel. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Sen K, Rodgers M. Distribution of six virulence factors in Aeromonas species isolated from US drinking water utilities: a PCR identification. J Appl Microbiol. 2004;97:1077–1086. doi: 10.1111/j.1365-2672.2004.02398.x. [DOI] [PubMed] [Google Scholar]
- 2.Borchardt MA, Stemper ME, Standridge JH. Aeromonas isolates from human diarrheic stool and groundwater compared by pulsed-field gel electrophoresis. Emerg Infect Dis. 2003;9:224–228. doi: 10.3201/eid0902.020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Figueras MJ. Clinical relevance of Aeromonas sM503. Rev Med Microbiol. 2005;16:145–153. [Google Scholar]
- 4.Essers B, Burnens AP, Lanfranchini FM, Somaruga SGE, von Vigier RO, et al. Acute community-acquired diarrhea requiring hospital admission in Swiss children. Clin Infect Dis. 2000;30:192–196. doi: 10.1086/313901. [DOI] [PubMed] [Google Scholar]
- 5.Anaissie EJ, Penzak SR, Dignani MC. The hospital water supply as a source of nosocomial infections: a plea for action. Arch Intern Med. 2002;162:1483–1492. doi: 10.1001/archinte.162.13.1483. [DOI] [PubMed] [Google Scholar]
- 6.Janda JM, Abbott SL. The Genus Aeromonas: taxonomy, pathogenicity, and infection. Clin Microbiol Rev. 2010;23:35–73. doi: 10.1128/CMR.00039-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Echeverria P, Blocklow NR, Sanford IB, Cakor GG. Traveler's diarrhea among American Peace Corps volunteers in rural Thailand. J Infect Dis. 1981;143:767–771. doi: 10.1093/infdis/143.6.767. [DOI] [PubMed] [Google Scholar]
- 8.Burke V, Gracey M, Robinson J, Peck D, Beaman J, et al. The microbiology of childhood gastroenteritis: Aeromonas species and other infective agents. J Infect Dis. 1983;148:68–74. doi: 10.1093/infdis/148.1.68. [DOI] [PubMed] [Google Scholar]
- 9.Ormen O, Regue MQ, Tomas JM, Granum PE. Studies of aerolysin promoters from different Aeromonas spp. Microb Pathog. 2003;35:189–196. doi: 10.1016/s0882-4010(03)00124-4. [DOI] [PubMed] [Google Scholar]
- 10.Agarwal RK, Kapoor KN, Kumar A. Virulence factors of aeromonads – an emerging food borne pathogen problem. J Commun Dis. 1998;30:71–78. [PubMed] [Google Scholar]
- 11.Janda JM, Abbott SL. Evolving concepts regarding the genus Aeromonas: an expanding panorama of species, disease presentations, and unanswered questions. Clin Infect Dis. 1998;27:332–344. doi: 10.1086/514652. [DOI] [PubMed] [Google Scholar]
- 12.Kingombe CI, Huys G, Tonolla M, Albert MJ, Swings J, et al. PCR detection, characterization, and distribution of virulence genes in Aeromonas spp. Appl Environ Microbiol. 1999;65:5293–5302. doi: 10.1128/aem.65.12.5293-5302.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aguilera-Arreola G, Hernandez-Rodrıguez C, Zuniga G, Figueras MJ, Castro-Escarpulli G. Aeromonas hydrophila clinical and environmental ecotypes as revealed by genetic diversity and virulence genes. FEMS Microbiol Lett. 2005;242:231–240. doi: 10.1016/j.femsle.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Yu HB, Srinivasa Rao PS, Lee HC, Vilches S, Merino S, et al. A type III secretion system is required for Aeromonas hydrophila AH-1 pathogenesis. Infect and Immun. 2004;72:1248–1256. doi: 10.1128/IAI.72.3.1248-1256.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chacón M, Soler L, Groisman E, Guarro J, Figueras MJ. Type III secretion system genes in clinical Aeromonas isolates. J Clin Microbiol. 2004;42:1285–1287. doi: 10.1128/JCM.42.3.1285-1287.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Murray PR, Baron EJ, Jorgensen JH, Pfaller MA, Yolken RH. Manual of Clinical Microbiology, 8th ed. Washington, D. C.: ASM press; 2003. pp. 654–671 and 902–914. [Google Scholar]
- 17.Garcia LS, Isenberg HD. Clinical Microbiology Procedures Handbook, 3rd ed. Washington, D. C.: ASM press. Section. 2010;9 [Google Scholar]
- 18.Kong R, Pelling A, So C, Wu R. Identification of oligonucleotide primers targeted at the 16S–23S rDNA intergenic spacers for genus-and species-specific detection of aeromonads. Mar Pollut Bull. 1999;38:802–808. [Google Scholar]
- 19.Soler L, Yanez MA, Chacon MR, Aguilera-Arreola MG, Catalan VM, et al. Phylogenetic analysis of the genus Aeromonas based on two housekeeping genes. Int J Syst Evol Microbiol. 2004;54:1511–1519. doi: 10.1099/ijs.0.03048-0. [DOI] [PubMed] [Google Scholar]
- 20.Silver AC, Graf G. Prevalence of genes encoding the Type Three Secretion System and the effectors AexT and AexU in the Aeromonas veronii group. DNA Cell Bio. 2009;28:383–388. doi: 10.1089/dna.2009.0867. [DOI] [PubMed] [Google Scholar]
- 21.Miller R, Walker R, Baya A, Clemens K, Coles M, et al. Antimicrobial susceptibility testing of aquatic Bacteria: quality control disk diffusion ranges for Escherichia coli ATCC 25922 and Aeromonas salmonicida subsp. salmonicida ATCC 33658 at 22 and 28°C. J Clin Microbiol. 2003;41:4318. doi: 10.1128/JCM.41.9.4318-4323.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jorgensen JH. Methods for antimicrobial dilution and disk susceptibility testing of infrequently isolated or fastidious Bacteria. 2010. Approved Guideline. Clinical and Laboratory Standards Institute. CLSI/NCCLS M45-A2. [DOI] [PubMed]
- 23.Ogunsanya T, Rotimi V, Adenuga A. A study of the aetiological agents of childhood diarrhoea in Lagos, Nigeria. J Med Microbiol. 1994;40:10–14. doi: 10.1099/00222615-40-1-10. [DOI] [PubMed] [Google Scholar]
- 24.Janda JM, Abbott SL. New gram-negative enteropathogens: fact or fancy? Rev Med Microbiol. 2006;17:27–37. [Google Scholar]
- 25.Morgan D, Johnson P, DuPont H, Satterwhite T, Wood L. Lack of correlation between known virulence properties of Aeromonas hydrophila and enteropathogenicity for humans. Infect Immun. 1985;50:62–65. doi: 10.1128/iai.50.1.62-65.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueras MJ, Horneman AJ, Martinez-Murcia A, Guarro J. Controversial data on the association of Aeromonas with diarrhea in a recent Hong Kong study. J Med Microbiol. 2007;56:996–998. doi: 10.1099/jmm.0.47062-0. [DOI] [PubMed] [Google Scholar]
- 27.Moyer NP. Clinical significance of Aeromonas species isolated from patients with diarrhea. J Clin Microbiol. 1987;25:2044–2048. doi: 10.1128/jcm.25.11.2044-2048.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Golik A, Modai D, Gluskin I, Schechter I, Cohen N, et al. Aeromonas in adult diarrhea: an enteropathogen or an innocent bystander? J Clin Gastroenterol. 1990;12:148–152. doi: 10.1097/00004836-199004000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Pablos M, Remacha MA, Rodríguez-Calleja JM, Santos JA, Otero A, et al. Identity, virulence genes, and clonal relatedness of Aeromonas isolates from patients with diarrhea and drinking water. Eur J Clin Microbiol Infect Dis. 2010;29:1163–1172. doi: 10.1007/s10096-010-0982-3. [DOI] [PubMed] [Google Scholar]
- 30.Albert MJ, Ansaruzzaman M, Talukder KA, Chopra AK, Kuhn I, et al. Prevalence of enterotoxin genes in Aeromonas spp. isolated from children with diarrhea, healthy controls, and the environment. J Clin Microbiol. 2000;38:3785–3790. doi: 10.1128/jcm.38.10.3785-3790.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alperi A, Martínez-Murcia AJ, Ko WC, Monera A, Saavedra M, et al. Aeromonas taiwanensis sp. nov. and Aeromonas sanarellii sp. nov., clinical species from Taiwan. Int J Syst Evol Microbiol. 2010;60:2048–2055. doi: 10.1099/ijs.0.014621-0. [DOI] [PubMed] [Google Scholar]
- 32.Heuzenroeder MW, Wong CYF, Flower RLP. Distribution of two hemolytic toxin genes in clinical and environmental isolates of Aeromonas spp.: correlation with virulence in a suckling mouse model. FEMS Microbiol Lett. 1999;174:131–136. doi: 10.1111/j.1574-6968.1999.tb13559.x. [DOI] [PubMed] [Google Scholar]
- 33.Jones BL, Wilcox MH. Aeromonas infections and treatment. J antimicrob chemother. 1995;35:453–461. doi: 10.1093/jac/35.4.453. [DOI] [PubMed] [Google Scholar]
- 34.Senderovich Y, Gershtein Y, Halewa E, Halpern M. Vibrio cholerae and Aeromonas: do they share a mutual host? ISME J. 2008;2:276–83. doi: 10.1038/ismej.2007.114. [DOI] [PubMed] [Google Scholar]
- 35.Figueras MJ, Beaz-Hidalgo R, Senderovich Y, Laviad S, Halpern M. Re-identification of Aeromonas isolates from chironomid egg masses as the potential pathogenic bacteria Aeromonas aquariorum. Environ Microbiol Rep. 2011;3:239–244. doi: 10.1111/j.1758-2229.2010.00216.x. [DOI] [PubMed] [Google Scholar]
- 36.Broza M, Halpern M. Chironomid egg masses and Vibrio cholerae. Nature. 2001;412:40. doi: 10.1038/35083691. [DOI] [PubMed] [Google Scholar]
- 37.Halpern M, Gasith A, Broza M. Does the tube of a benthic chironomid larva play a role in protecting its dweller against chemical toxicants? Hydrobiologia. 2002;470:49–55. [Google Scholar]
- 38.Sun XB, Cui FY, Zhang JS, Xu F, Liu LJ. Inactivation of chironomid larvae with chlorine dioxide. J Hazard Mater. 2007;142:348–353. doi: 10.1016/j.jhazmat.2006.08.030. [DOI] [PubMed] [Google Scholar]
- 39.Broza M, Gancz H, Halpern M, Kashi Y. Adult non-biting midges: possible windborne carriers of Vibrio cholerae non-O1 non-O139. Environ Microbiol. 2005;7:576–585. doi: 10.1111/j.1462-2920.2005.00745.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Characterization of Aeromonas isolates from diarrheal patients. Aeromonas rpoD sequences were deposited in the GenBank database under the accession numbers JF738005–JF738021 (see also Figure 1).
(DOC)