Abstract
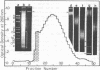
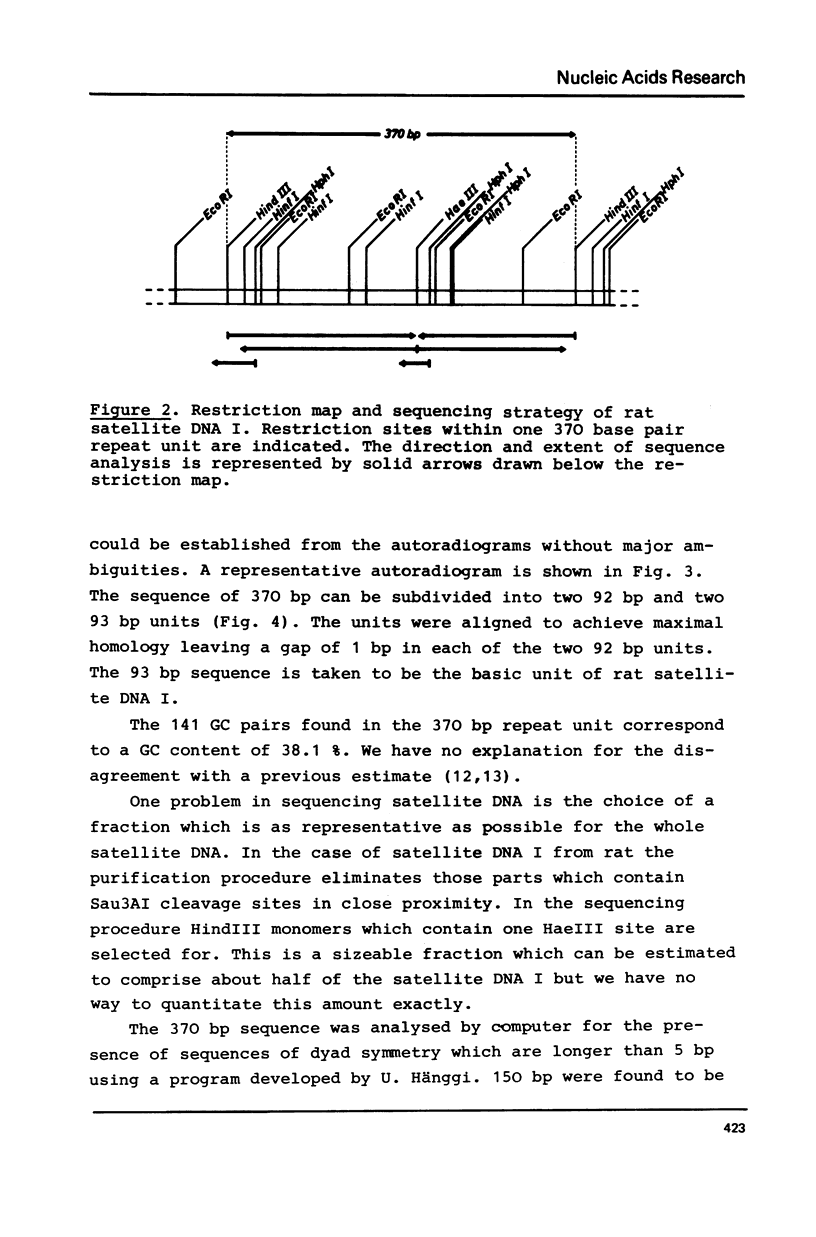
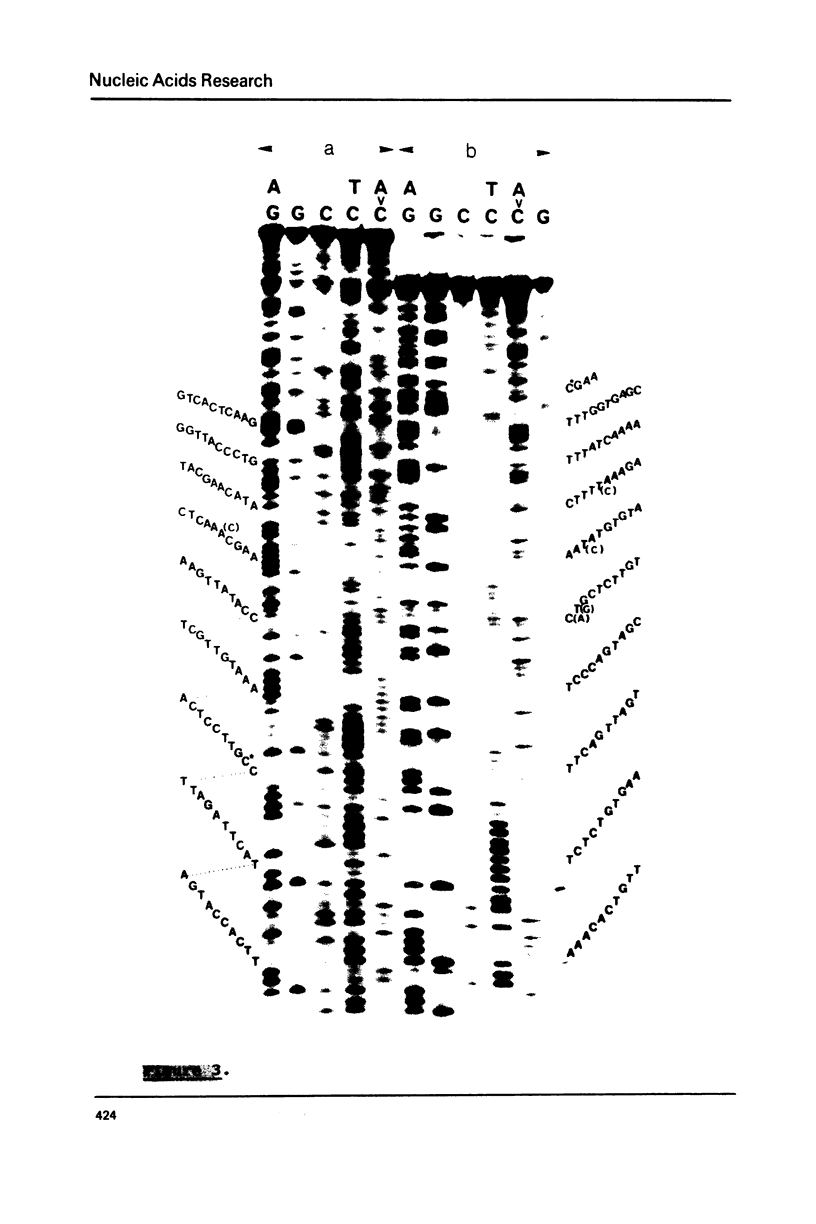
A highly repetitive component of rat DNA which could not yet be enriched by density gradient centrifugation was isolated with the help of the restriction nuclease Sau3AI. This nuclease converted the bulk of the DNA to small fragments and left a repetitive DNA component as large fragments which were subsequently purified by gel filtration and electrophoresis. This DNA component which was termed rat satellite DNA I is composed of tandemly repeated 370 bp blocks. According to sequence analysis the 370 bp repeats consist of alternating 92 and 93 bp units with homologous but not identical sequences. Methylation of CpG residues was correlated to the rate of cleavage by restriction nucleases. Significant homologies exist between the sequences of rat satellite DNA I and satellite DNAs of several other organisms. The divergence of the sequence of rat satellite DNA I was discussed with respect to evolutionary considerations.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appels R., Peacock W. J. The arrangement and evolution of highly repeated (satellite) DNA sequences with special reference to Drosophila. Int Rev Cytol Suppl. 1978;Suppl 8:69–126. doi: 10.1016/s0074-7696(08)60472-6. [DOI] [PubMed] [Google Scholar]
- Beauchamp R. S., Mitchell A. R., Buckland R. A., Bostock C. J. Specific arrangements of human satellite III DNA sequences in human chromosomes. Chromosoma. 1979 Feb 21;71(2):153–166. doi: 10.1007/BF00292820. [DOI] [PubMed] [Google Scholar]
- Bonner J., Garrard W. T., Gottesfeld J., Holmes D. S. Functional organization of the mammalian genome. Cold Spring Harb Symp Quant Biol. 1974;38:303–310. doi: 10.1101/sqb.1974.038.01.034. [DOI] [PubMed] [Google Scholar]
- Botchan M., McKenna G., Sharp P. A. Cleavage of mouse DNA by a restriction enzyme as a clue to the arrangement of genes. Cold Spring Harb Symp Quant Biol. 1974;38:383–395. doi: 10.1101/sqb.1974.038.01.041. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Brutlag D., Carlson M., Fry K., Hsieh T. S. DNA sequence organization in Drosophila heterochromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1137–1146. doi: 10.1101/sqb.1978.042.01.114. [DOI] [PubMed] [Google Scholar]
- DeFilippes F. M. A new method for isolation of a restriction enzyme from Hemophilus parainfluenzae. Biochem Biophys Res Commun. 1974 Jun 4;58(3):586–596. doi: 10.1016/s0006-291x(74)80460-2. [DOI] [PubMed] [Google Scholar]
- Fuke M., Busch H. HindIII-sensitive sites present once in every four repeats of EcoRI-sensitive sites in Novikoff rat hepatoma DNA. FEBS Lett. 1979 Mar 1;99(1):136–140. doi: 10.1016/0014-5793(79)80265-3. [DOI] [PubMed] [Google Scholar]
- Fuke M., Davis F. M., Busch H. Localization of highly repeated HindIII fragments of Novikoff rat DNA to the nucleolus. FEBS Lett. 1979 Jun 1;102(1):46–50. doi: 10.1016/0014-5793(79)80925-4. [DOI] [PubMed] [Google Scholar]
- Gray C. P., Sommer R., Polke C., Beck E., Schaller H. Structure of the orgin of DNA replication of bacteriophage fd. Proc Natl Acad Sci U S A. 1978 Jan;75(1):50–53. doi: 10.1073/pnas.75.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttann T., Votavová H., Pivec L. Base composition heterogeneity of mammalian DNAs in CsCl-netropsin density gradient. Nucleic Acids Res. 1976 Mar;3(3):835–845. doi: 10.1093/nar/3.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewish D. R., Burgoyne L. A. Chromatin sub-structure. The digestion of chromatin DNA at regularly spaced sites by a nuclear deoxyribonuclease. Biochem Biophys Res Commun. 1973 May 15;52(2):504–510. doi: 10.1016/0006-291x(73)90740-7. [DOI] [PubMed] [Google Scholar]
- Hörz W., Zachau H. G. Characterization of distinct segments in mouse satellite DNA by restriction nucleases. Eur J Biochem. 1977 Mar 1;73(2):383–392. doi: 10.1111/j.1432-1033.1977.tb11329.x. [DOI] [PubMed] [Google Scholar]
- Igo-Kemenes T., Greil W., Zachau H. G. Prepartation of soluble chromatin and specific chromatin fractions with restriction nucleases. Nucleic Acids Res. 1977 Oct;4(10):3387–3400. doi: 10.1093/nar/4.10.3387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igó-Kemenes T., Zachau H. G. Domains in chromatin structure. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):109–118. doi: 10.1101/sqb.1978.042.01.012. [DOI] [PubMed] [Google Scholar]
- Maio J. J., Brown F. L., Musich P. R. Subunit structure of chromatin and the organization of eukaryotic highly repetitive DNA: recurrent periodicities and models for the evolutionary origins of repetitive DNA. J Mol Biol. 1977 Dec 15;117(3):637–655. doi: 10.1016/0022-2836(77)90062-6. [DOI] [PubMed] [Google Scholar]
- Mann M. B., Smith H. O. Specificity of Hpa II and Hae III DNA methylases. Nucleic Acids Res. 1977 Dec;4(12):4211–4221. doi: 10.1093/nar/4.12.4211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manuelidis L., Wu J. C. Homology between human and simian repeated DNA. Nature. 1978 Nov 2;276(5683):92–94. doi: 10.1038/276092a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. R., Cartwright E. M., Brownlee G. G., Fedoroff N. V., Brown D. D. The nucleotide sequence of oocyte 5S DNA in Xenopus laevis. II. The GC-rich region. Cell. 1978 Apr;13(4):717–725. doi: 10.1016/0092-8674(78)90221-0. [DOI] [PubMed] [Google Scholar]
- Ohmori H., Tomizawa J. I., Maxam A. M. Detection of 5-methylcytosine in DNA sequences. Nucleic Acids Res. 1978 May;5(5):1479–1485. doi: 10.1093/nar/5.5.1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philippsen P., Streeck R. E., Zachau H. G. Defined fragments of calf, human, and rat DNA produced by restriction nucleases. Eur J Biochem. 1974 Jun 15;45(2):479–488. doi: 10.1111/j.1432-1033.1974.tb03573.x. [DOI] [PubMed] [Google Scholar]
- Pulleyblank D. E., Shure M., Vinograd J. The quantitation of fluorescence by photography. Nucleic Acids Res. 1977;4(5):1409–1418. doi: 10.1093/nar/4.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Roizés G. Analysis of eucaryotic DNAs with a restriction endonuclease from H. influenzae: isolation of "hidden" satellite DNAs. Nucleic Acids Res. 1974 Sep;1(9):1099–1120. doi: 10.1093/nar/1.9.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg H., Singer M., Rosenberg M. Highly reiterated sequences of SIMIANSIMIANSIMIANSIMIANSIMIAN. Science. 1978 Apr 28;200(4340):394–402. doi: 10.1126/science.205944. [DOI] [PubMed] [Google Scholar]
- Russell G. J., Walker P. M., Elton R. A., Subak-Sharpe J. H. Doublet frequency analysis of fractionated vertebrate nuclear DNA. J Mol Biol. 1976 Nov;108(1):1–23. doi: 10.1016/s0022-2836(76)80090-3. [DOI] [PubMed] [Google Scholar]
- Shmookler Reis R. J., Biro P. A. Sequence and evolution of mouse satellite DNA. J Mol Biol. 1978 May 25;121(3):357–374. doi: 10.1016/0022-2836(78)90369-8. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Long range periodicities in mouse satellite DNA. J Mol Biol. 1975 May 5;94(1):51–69. doi: 10.1016/0022-2836(75)90404-0. [DOI] [PubMed] [Google Scholar]
- Streeck R. E., Zachau H. G. A long-range and two short-range periodicities are superimposed in the 1.706-g/cm3 satellite DNA from calf thymus. Eur J Biochem. 1978 Aug 15;89(1):267–279. doi: 10.1111/j.1432-1033.1978.tb20923.x. [DOI] [PubMed] [Google Scholar]
- Sussenbach J. S., Monfoort C. H., Schiphof R., Stobberingh E. E. A restriction endonuclease from Staphylococcus aureus. Nucleic Acids Res. 1976 Nov;3(11):3193–3202. doi: 10.1093/nar/3.11.3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sussenbach J. S., Steenbergh P. H., Rost J. A., van Leeuwen W. J., van Embden J. D. A second site-specific restriction endonuclease from Staphylococcus aureus. Nucleic Acids Res. 1978 Apr;5(4):1153–1163. doi: 10.1093/nar/5.4.1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker P. M. How different are the DNAs from related animals? Nature. 1968 Jul 20;219(5151):228–232. doi: 10.1038/219228a0. [DOI] [PubMed] [Google Scholar]