Abstract
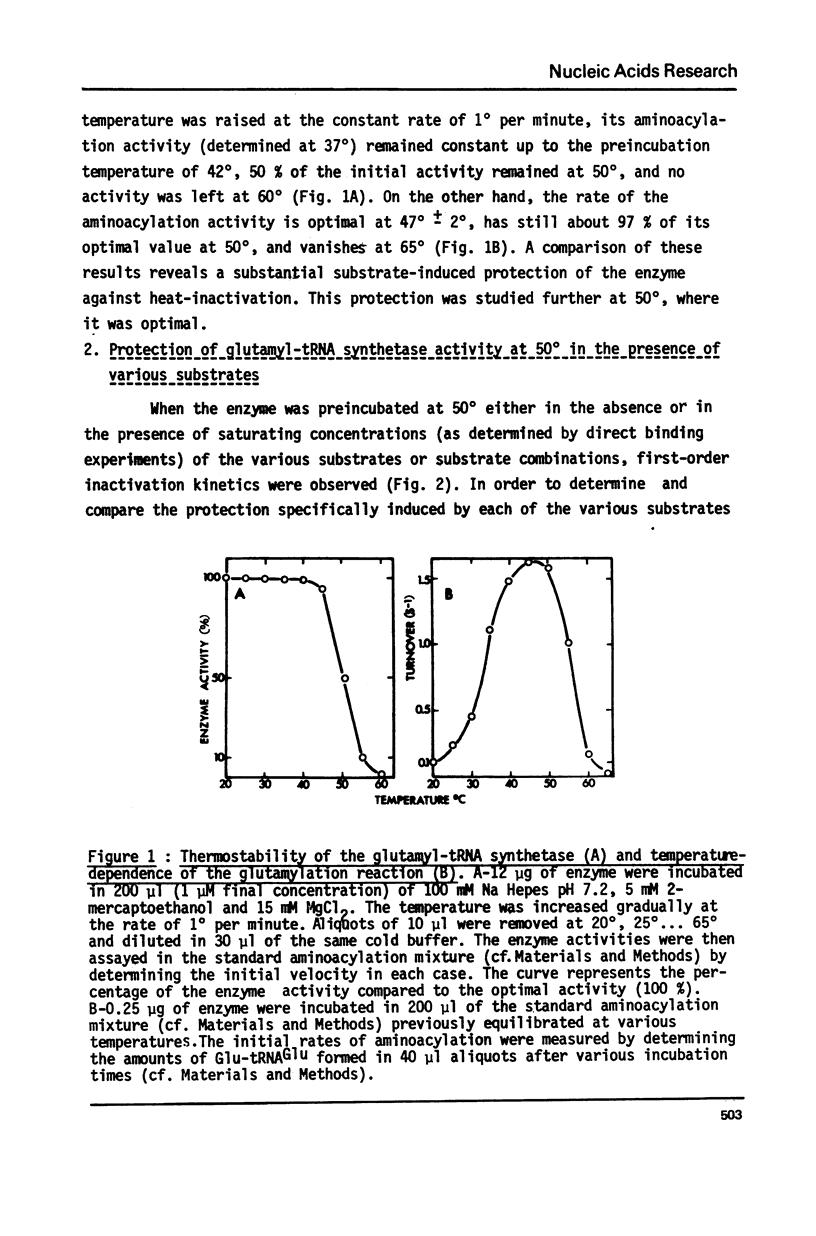
The substrates-induced protection against the heat-inactivation of the glutamyl-tRNA synthetase has been investigated. tRNAGlu and ATP protect efficiently the enzyme, whereas glutamate does not. In the presence of tRNAGlu, glutamate induces an additional protection to that given by the tRNAGlu alone. A weak synergism was observed between ATP and tRNAGlu, whereas no synergism was detected between ATP and glutamate. These results suggest that tRNAGlu and ATP, but not glutamate are able to bind to the free enzyme form; glutamate binds only to the Enzyme.tRNAGlu and to the Enzyme.tRNAGlu.ATP complexes. The presence of the three substrates induces a higher stabilization of the enzyme than that expected from the protection observed for the various other substrates combinations, suggesting the existence of a marked synergism between the three substrates against the heat-inactivation of the enzyme. The protection constants determined from this study are similar to the dissociation constants determined by direct binding experiments and to the Km values determined kinetically.
Full text
PDF












Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende C. C., Chaimovich H., Gatica M., Allende J. E. The aminoacyl transfer ribonucleic acid synthetases. II. Properties of an adenosine triphosphate-threonyl transfer ribonucleic acid synthetase complex. J Biol Chem. 1970 Jan 10;245(1):93–101. [PubMed] [Google Scholar]
- BURTON K. The stabilization of D-amino acid oxidase by flavin-adenine dinucleotide, substrates and competitive inhibitors. Biochem J. 1951 Apr;48(4):458–467. doi: 10.1042/bj0480458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang H. Y., Bell F. E. Use of a thermal inactivation technique to obtain binding constants for the Escherichia coli valyl-tRNA synthetase. Arch Biochem Biophys. 1972 Oct;152(2):502–514. doi: 10.1016/0003-9861(72)90245-7. [DOI] [PubMed] [Google Scholar]
- Fersht A. R., Gangloff J., Dirheimer G. Reaction pathway and rate-determining step in the aminoacylation of tRNAArg catalyzed by the arginyl-tRNA synthetase from yeast. Biochemistry. 1978 Sep 5;17(18):3740–3746. doi: 10.1021/bi00611a011. [DOI] [PubMed] [Google Scholar]
- Folk W. R. Molecular weighr of Escherichia coli glutaminyl transfer ribonucleic acid synthetase, and isolation of its complex with glutamine transfer ribonucleic acid. Biochemistry. 1971 Apr 27;10(9):1728–1732. doi: 10.1021/bi00785a034. [DOI] [PubMed] [Google Scholar]
- Gangloff J., Schutz A., Dirheimer G. Arginyl-tRNA synthetase from baker's yeast. Purification and some properties. Eur J Biochem. 1976 May 17;65(1):177–182. doi: 10.1111/j.1432-1033.1976.tb10403.x. [DOI] [PubMed] [Google Scholar]
- Kern D., Potier S., Boulanger Y., Lapointe J. The monomeric glutamyl-tRNA synthetase of Escherichia coli. Purification and relation between its structural and catalytic properties. J Biol Chem. 1979 Jan 25;254(2):518–524. [PubMed] [Google Scholar]
- Kim J. J., Chakraburtty K., Mehler A. H. Evidence for single mechanism for aminoacyl-tRNA synthetases including aminoacyl adenylates as intermediates. J Biol Chem. 1977 Apr 25;252(8):2698–2701. [PubMed] [Google Scholar]
- Kisselev L. L., Favorova O. O. Aminoacyl-tRNA synthetases: sone recent results and achievements. Adv Enzymol Relat Areas Mol Biol. 1974;40(0):141–238. doi: 10.1002/9780470122853.ch5. [DOI] [PubMed] [Google Scholar]
- Lapointe J., Söll D. Glutamyl transfer ribonucleic acid synthetase of Escherichia coli. I. Purification and properties. J Biol Chem. 1972 Aug 25;247(16):4966–4974. [PubMed] [Google Scholar]
- Lin C. S., Irwin R., Chirikjian J. G. Kinetic studies of leucyl transfer RNA synthetase from bakers' yeast. Order of addition of substrates and release of products. J Biol Chem. 1975 Dec 25;250(24):9299–9303. [PubMed] [Google Scholar]
- Loftfield R. B., Eigner E. A. Mechanism of action of amino acid transfer ribonucleic acid ligases. J Biol Chem. 1969 Apr 10;244(7):1746–1754. [PubMed] [Google Scholar]
- Loftfield R. B. The mechanism of aminoacylation of transfer RNA. Prog Nucleic Acid Res Mol Biol. 1972;12:87–128. doi: 10.1016/s0079-6603(08)60660-1. [DOI] [PubMed] [Google Scholar]
- Mehler A. H., Mitra S. K. The activation of arginyl transfer ribonucleic acid synthetase by transfer ribonucleic acid. J Biol Chem. 1967 Dec 10;242(23):5495–5499. [PubMed] [Google Scholar]
- Midelfort C. F., Chakraburtty K., Steinschneider A., Mehler A. H. Kinetic demonstration of the intermediate role of aminoacyl-adenylate-enzyme in the formation of valyl transfer ribonucleic acid. J Biol Chem. 1975 May 25;250(10):3866–3873. [PubMed] [Google Scholar]
- Mitra K., Mehler A. H. The role of transfer ribonucleic acid in the pyrophsphate exchange reaction of arginine-transfer ribonucleic acid synthetase. J Biol Chem. 1966 Nov 10;241(21):5161–5162. [PubMed] [Google Scholar]
- Mitra S. K., Chakraburtty K., Mehler A. H. Binding of transfer RNA and arginine to the arginine transfer RNA synthetase of Escherichia coli. J Mol Biol. 1970 Apr 14;49(1):139–156. doi: 10.1016/0022-2836(70)90382-7. [DOI] [PubMed] [Google Scholar]
- Myers G., Blank H. U., Söll D. A comparative study of the interactions of Escherichia coli leucyl-, seryl-, and valyl-transfer ribonucleic acid synthetases with their cognate transfer ribonucleic acids. J Biol Chem. 1971 Aug 25;246(16):4955–4964. [PubMed] [Google Scholar]
- Mérault G., Graves P. V., Labouesse B., Labouesse J. Influence of magnesium on the steady-state-derived order of substrate addition and product release in tRNATrp aminoacylation by beef pancreas tryptophan: tRNA ligase: significance of the deduced mechanism. Eur J Biochem. 1978 Jul 3;87(3):541–550. doi: 10.1111/j.1432-1033.1978.tb12405.x. [DOI] [PubMed] [Google Scholar]
- Nazario M., Evans J. A. Physical and kinetic studies of arginyl transfer ribonucleic acid ligase of Neurospora. A sequential ordered mechanism. J Biol Chem. 1974 Aug 10;249(15):4934–4936. [PubMed] [Google Scholar]
- Papas T. S., Mehler A. H. Kinetic studies of the prolyl transfer ribonucleic acid synthetase of Escherichia coli. Order of addition of substrates and release of products. J Biol Chem. 1971 Oct 10;246(19):5924–5928. [PubMed] [Google Scholar]
- Parfait R. Arginyl-tRNA synthetase from Bacillus stearothermophilus: Heat inactivation and substrate induced protection. FEBS Lett. 1973 Feb 1;29(3):323–325. doi: 10.1016/0014-5793(73)80049-3. [DOI] [PubMed] [Google Scholar]
- Parfait R., Grosjean H. Arginyl-transfer ribonucleic-acid synthetase from Bacillus stearothermophilus. Purification, properties and mechanism of action. Eur J Biochem. 1972 Oct;30(2):242–249. doi: 10.1111/j.1432-1033.1972.tb02092.x. [DOI] [PubMed] [Google Scholar]



