Abstract
Background
Spontaneously hypertensive rats (SHR)-derived vascular smooth muscle cells (VSMCs) show exaggerated growth with a synthetic phenotype and angiotensin II (Ang II)-production. To evaluate the contribution of complement 3 (C3) or C3a toward these abnormalities in SHR, we examined effects of a C3a receptor inhibitor on proliferation, phenotype, and Ang II-production in VSMCs from SHR and Wistar–Kyoto (WKY) rats.
Methods
Expression of pre-pro-C3 messenger RNA (mRNA) and C3 protein was evaluated by reverse transcription-PCR and western blot analyses, and C3a receptor mRNA was evaluated by reverse transcription-PCR analysis in quiescent VSMCs from SHR and WKY rats. We examined the effects of the C3a inhibitor, SB290157, on proliferation and the expression of phenotype-marker and Krueppel-like factor 5 (KLF-5) mRNAs in VSMCs from SHR and WKY rats. We examined effects of C3a receptor inhibitor, SB290157, on Ang II-production in conditioned medium of VSMCs from SHR and WKY rats by a radioimmunoassay.
Results
Expression of pre-pro-C3 mRNA and C3 protein was significantly higher in SHR VSMCs than WKY VSMCs. SB290157 significantly inhibited proliferation of VSMCs from SHR, but not in cells from WKY rats. Relative to WKY VSMCs, SB290157 significantly increased the low expression of SM22α mRNA and decreased the high expression of osteopontin mRNA in SHR VSMCs. SB290157 significantly decreased the high expression of KLF-5 and Ang II-production in VSMCs from SHR, but not in cells from WKY rats.
Conclusions
C3a induces exaggerated growth, a synthetic phenotype and Ang II-production in SHR-derived VSMCs. C3a may be primarily involved in cardiovascular remodeling in hypertension.
Keywords: angiotensin II, blood pressure, complement 3, hypertension, Kruppel-like factor 5, phenotype, proliferation, spontaneously hypertensive rat, vascular smooth muscle cell
Patients with essential hypertension, a hereditary polygenic disease, are eventually complicated with stroke, cardiovascular remodeling, and nephrosclerosis. These complications are practical targets for therapy of essential hypertension with antihypertensive medicines. Spontaneously hypertensive rats (SHR), a genetic animal model for essential hypertension, show exaggerated growth of cardiovascular organs in comparison with normotensive Wistar–Kyoto (WKY) rats.1,2 Enhanced DNA synthesis and organ hypertrophy have been described in SHR even as early as the day of birth.3,4,5 SHR-derived vascular smooth muscle cells (VSMCs) in culture show a higher specific growth rate, abnormal contact inhibition, accelerated entry into S phase of the cell cycle, and nonspecific hyperproliferation in response to various growth factors in comparison to cells from WKY rats.6,7 These behaviors may reflect intrinsic abnormalities in SHR that are not caused by pressure overload because there is no blood pressure in culture. Therefore, these characteristics of VSMCs from SHR appear to be associated with genetic abnormalities. We found that SHR-derived VSMCs generate angiotensin II (Ang II) in homogenous cultures.8 We have reported that the mechanism underlying this enhanced generation of Ang II in SHR-derived VSMCs appears to be a change from the contractile to the synthetic phenotype in comparison to cells from WKY rats.9,10
It is possible that genetic abnormalities are involved in the exaggerated growth and synthetic phenotype of VSMCs from SHR. We investigated the genes that are responsible and found by microarray analysis that the messenger RNA (mRNA) encoding complement 3 (C3) is expressed only in VSMCs from SHR and is associated with both the synthetic phenotype and exaggerated growth.11 At the same time, we demonstrated that C3 also changes renal mesangial cells to the synthetic phenotype.12 We investigated mechanisms underlying the C3-induced phenotypic changes and found that C3 stimulates Kruppel-like factor 5 (KLF-5) promoter activity through extracellular signal-regulated kinase (ERK) signaling.13
C3 is a 190 kDa glycoprotein that consists of two polypeptide chains, which are produced from liver, monocytes, and macrophages, and it is essential for eliciting the complement response.14 C3 from pre-pro-C3 mRNA is secreted after cleavage of the heterodimer, and then C3 is proteolytically cleaved into C3a (molecular weight 9,000) and C3b (molecular weight 185,000). C3a is an anaphylotoxin, and C3b serves as an opsonizing agent.15 We have demonstrated that the increases in Ang II-production in VSMCs in homogeneous culture are associated with changes to the synthetic phenotype in VSMCs.8,16
In the current study, in order to evaluate whether C3 or C3a is associated with the exaggerated growth of the synthetic phenotype and increased Ang II-production in VSMCs from SHR, we examined effects of the C3a receptor inhibitor on proliferation, phenotype, and Ang II-production in VSMCs from SHR and WKY rats.
Methods
Ethics and animals. Our investigation conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, 1996). The ethics committee of the Nihon University School of Medicine examined every research protocol involving the use of living animals. SHR/Izm and WKY/Izm rats were obtained from Japan SLC (Hamamatsu, Japan).
Cell culture and establishment of quiescence. VSMCs were obtained from aortic explants from 3-week-old prehypertensive male SHR/Izm and WKY/Izm rats. VSMCs were maintained in Dulbecco's modified Eagle's medium (DMEM) with 10% calf serum (Gibco Life Technologies, Gaithersburg, MD), 100 U/ml penicillin, and 100 mg/ml streptomycin. Experiments were performed on cells between the 5th and 10th passages. Trypsinized cells were plated into 24-well culture dishes at a density of 105 cells/cm2. They were allowed to grow in DMEM containing 10% calf serum for 24 h, and the culture medium was then changed to DMEM with 0.2% calf serum. The cells were incubated in this medium for 48–72 h to establish quiescence.
Proliferation of VSMCs. VSMCs from SHR and WKY rats were inoculated and grown into DMEM containing 5% calf serum in the absence or presence of 0.1 µmol/l SB290157 in 24-well culture dishes at a density of 105 cells/cm2. Cells were trypsinized with 0.05% trypsin at 24, 48, and 72 h after inoculation, and cell numbers were counted in a Coulter counter (Coulter Electronics, Luton, UK).
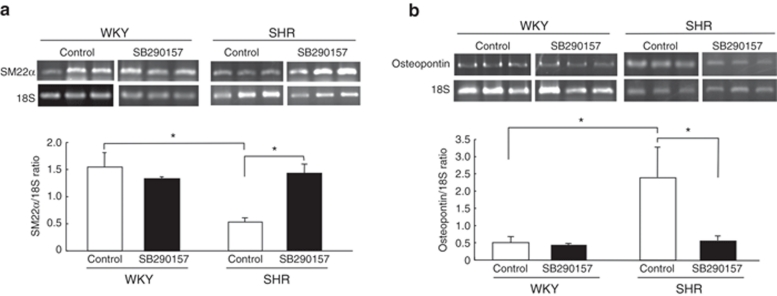
Semiquantitative reverse transcription-PCR analysis. Total RNAs from samples were extracted from quiescent VSMCs from SHR and WKY rats with ISOGEN (Nippon Gene, Tokyo, Japan). Primer sequences are listed in Table 1. 18S ribosomal RNA was used as an internal control. To confirm that no genomic DNA was co-amplified by PCR, control reverse transcription-PCR experiments were performed with each set of primers but without reverse transcriptase; no product was amplified. For semiquantative analysis of mRNA, the kinetics of the PCR reaction were monitored; the number of cycles at which the PCR products were detectable on the gel was compared between samples.17 Serial tenfold dilutions of complementary DNA (100, 10, and 1 ng) were amplified; the PCR products were detectable at earlier cycles with increasing amounts of complementary DNA. PCR was performed for 30 cycles in a DNA thermal cycler (Perkin-Elmer Cetus, Waltham, MA), and products were separated by electrophoresis on 1.5% agarose gels, stained with ethidium bromide, and visualized by ultraviolet illumination.
Table 1. PCR primers and product sizes.
Western blot analysis for C3 protein in VSMCs. VSMCs (5 × 104 cells/cm2) were disrupted with lysis buffer (50 mmol/l Tris-HCl (pH 8.0), 150 mmol/l NaCl, 0.02% sodium azide, 100 µg/ml phenylmethylsulfonyl fluoride, 1 µg/ml aprotinin, 1% Triton X-100). Total proteins were extracted and purified with 100 µl of chloroform and 400 µl of methanol. Protein samples were boiled for 3 min and subjected to electrophoresis on 8% polyacrylamide gels and then transblotted to nitrocellulose membranes (Bio-Rad Laboratories, Hercules, CA). Blots were incubated with rabbit polyclonal antibodies specific for C3 (Santa Cruz Biotechnology, Santa Cruz, CA) or a mouse monoclonal antibody specific for α-tubulin (Sigma, St Louis, MO) as an internal control, and were then incubated with goat anti-rabbit immunoglobulin G or goat anti-mouse immunoglobulin G (Bio-Rad Laboratories). Immunocomplexes were detected by enhanced chemiluminescence (ECL, Amersham, UK).
Measurement of Ang II in conditioned medium. VSMCs (106) from SHR and WKY rats were inoculated in 10 cm2 wells with DMEM containing 10% calf serum and incubated for 24 h. The cells were then washed twice with phosphate-buffered saline, and incubated first for 24 h with serum-free DMEM and then for 48 h with 2 ml of fresh serum-free DMEM in the absence or presence of 0.1 µmol/l SB290157. The culture medium was collected and centrifuged at 600g for 10 min, and the resulting supernatant (conditioned medium) was collected and treated with 1 mmol/l each of aprotinin, leupeptin, and pepstatin A as well as 0.1 mmol/l phenylmethylsulfonyl fluoride. Samples were applied to a Sep-Pak C18 cartridge (Waters Associates, Milford, MA), and peptides were eluted with 3 ml of methanol-water-trifluoroacetic acid (80:19.9:0.1, vol/vol). The eluate was dried in a vacuum centrifuge and subjected to a radioimmunoassay for Ang II. The antiserum to Ang II (GE Healthcare, Buckinghamshire, UK) showed <1% cross-reactivity with Ang I, and 100% cross-reactivity with Ang III (heptapeptide), Ang II (3–8) (hexapeptide), and Ang II (4–8) (pentapeptide).
Statistical analysis. Values are reported as the mean ± s.e.m. Statistical analysis was done with Student's t-test for unpaired data, two-way analysis of variance, or Duncan's multiple range test.
Results
Expression of C3 and the C3a receptor in VSMCs
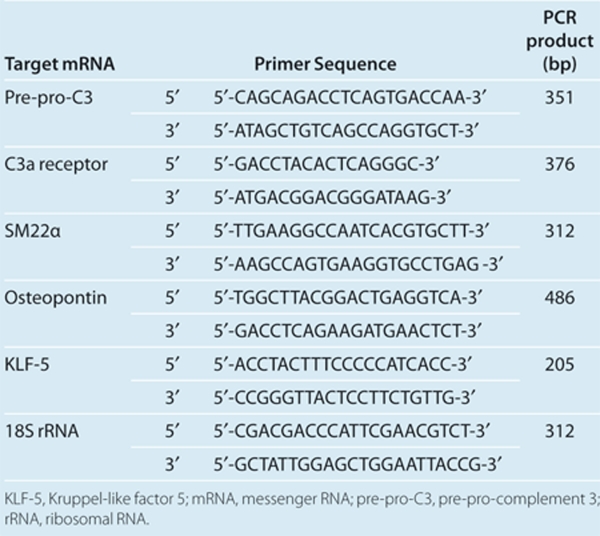
To assess C3 and receptor systems, we evaluated expression of pre-pro-C3 mRNA, C3 protein and C3a receptor mRNA in VSMCs from SHR and WKY rats. The abundance of pre-pro-C3 mRNA was significantly (P < 0.01) higher in the VSMCs from SHR than in cells from WKY rats (Figure 1a). C3 protein was not expressed in cells from WKY rats, although it was increased with 10 ng/ml interferon-γ, whereas the abundance of C3 protein was signicantly (P < 0.05) higher in VSMCs from SHR rats than WKY rats, and C3 was also increased significantly (P < 0.05) with interferon-γ (Figure 1b). VSMCs from SHR and WKY rats expressed C3a receptor mRNA. There were no differences in the abundances of C3a receptors between either strain (Figure 1c).
Figure 1.
Expression of complement 3 (C3) and the C3a receptor in vascular smooth muscle cells (VSMCs) from spontaneously hypertensive rats (SHR) and Wistar–Kyoto (WKY) rats. Total RNA was extracted from quiescent VSMCs of 3-week-old SHR and WKY rats. Semiquantitative determination of the abundance of (a) C3 and (c) C3a receptor messenger RNA (mRNA) was performed by reverse transcription and PCR analysis. The ratio of each mRNA to 18S ribosomal RNA (rRNA) was evaluated by analysis of amplified PCR products. Data were expressed as mean ± s.e.m. (n = 6 from two analyses). (b) Western blot analysis of expression of C3 protein in cultured VSMCs from SHR and WKY rats without or with interferon-γ (IFN-γ). Quiescent VSMCs from 3-week-old SHR and WKY rats were incubated without (control) or with 10 ng/ml IFN-γ for 20 h. The ratio of abundance of IFN-γ protein to α-tubulin was evaluated by densitometric analysis. Data are means ± s.e.m. (n = 3). *P < 0.05, **P < 0.01 between indicated column.
Effects of the C3a receptor inhibitor on proliferation of VSMCs
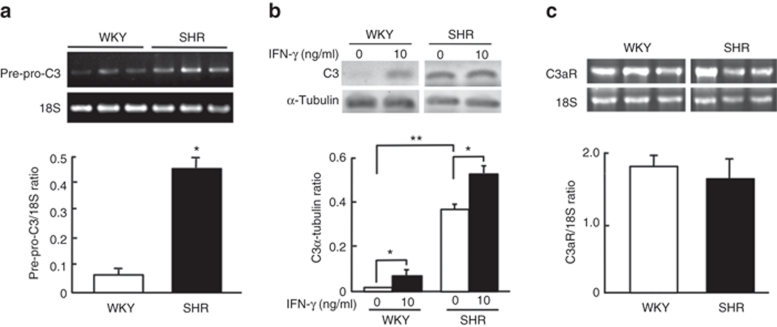
Figure 2 shows the effects of C3a receptor inhibitor, SB290157, on proliferation of VSMCs from SHR and WKY rats. The increase in cell numbers in cultures grown in DMEM containing 5% calf serum was higher in VSMCs from SHR than in cells from WKY rats. SB290157 significantly (P < 0.05) inhibited the increase in the number of VSMCs in SHR rats, whereas SB290157 did not affect the increase in the number of cells in WKY rats.
Figure 2.
Effects of the complement 3a receptor inhibitor, SB290157, on proliferation of vascular smooth muscle cells (VSMCs) from spontaneously hypertensive rats (SHR) and Wistar–Kyoto (WKY) rats. VSMCs were inoculated at 5 × 104 cells/cm2 in DMEM containing 5% calf serum without (control) or with 0.1 µmol/l SB290157. Cells were replenished with DMEM containing 5% calf serum and 0.1 µmol/l SB290157 every 24 h. They were trypsinized at 24, 48, and 72 h after inoculation, and cell number was determined in a Coulter counter. Data are means ± s.e.m. (n = 8). *P < 0.01 vs. control. DMEM, Dulbecco's modified Eagle's medium.
Effects of the C3a receptor inhibitor on the phenotype of VSMCs
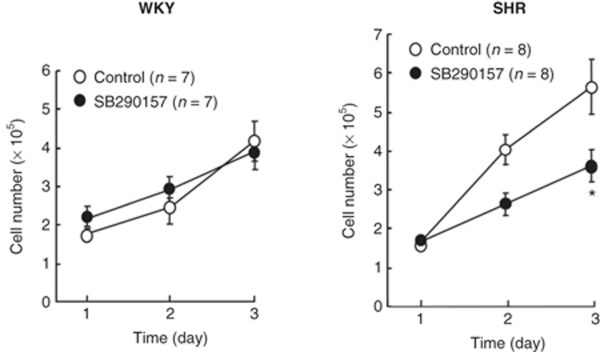
Basal abundances of SM22α mRNA as a marker for the contractile phenotype of VSMCs were significantly (P < 0.05) lower in VSMCs from SHR rats than in cells from WKY rats (Figure 3a). Basal abundances of osteopontin mRNA as a marker for the synthetic phenotype of VSMCs were significantly (P < 0.05) higher in VSMCs from SHR rats than in cells from WKY rats (Figure 3b). These findings indicate that the phenotype of VSMCs from SHR is more synthetic than in cells from WKY rats in serum deprivation. The C3a receptor inhibitor SB290157 significantly (P < 0.05) increased the abundance of SM22α mRNA (Figure 3a) and decreased the abundance of osteopontin mRNA in VSMCs from SHR rats (Figure 3b), but these did not occur in cells from WKY rats, indicating that endogenous C3a induces the synthetic phenotype of SHR-derived VSMCs.
Figure 3.
Effects of the complement 3a receptor inhibitor, SB290157, on expression of the mRNAs of the phenotypic markers, (a) SM22α and (b) osteopontin, in vascular smooth muscle cells (VSMCs) from spontaneously hypertensive rats (SHR) and Wistar–Kyoto (WKY) rats. Quiescent VSMCs from 3-week-old SHR and WKY rats were incubated without (control) or without 0.1 µmol/l SB290157 for 24 h. Total RNA was extracted and the abundance of SM22α and osteopontin mRNAs was evaluated by reverse transcription and PCR analysis. The ratio of abundance of each mRNA to 18S rRNA was evaluated by densitometric analysis. Data are means ± s.e.m. (n = 8 from two analyses). *P < 0.05 between indicated columns. mRNA, messenger RNA; rRNA, ribosomal RNA.
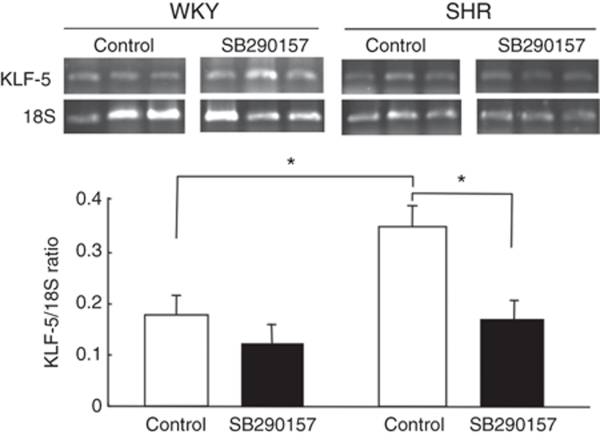
Effects of the C3a receptor inhibitor on expression of KLF-5 mRNA in VSMCs
Figure 4 shows the effects of the SB290157 C3a receptor inhibitor on expression of KLF-5 mRNA in VSMCs from SHR and WKY rats. Basal abundances of KLF-5 mRNA were significantly higher (P < 0.05) in VSMCs from SHR than in cells from WKY rats. SB290157 significantly (P < 0.05) decreased the abundance of KLF-5 mRNA in VSMCs from SHR rats, but SB290157 did not affect the abundance of KLF-5 mRNA in cells from WKY rats.
Figure 4.
Effects of the complement 3a receptor inhibitor, SB290157, on expression of Kruppel-like factor 5 (KLF-5) mRNAs in vascular smooth muscle cells (VSMCs) from spontaneously hypertensive rats (SHR) and Wistar–Kyoto (WKY) rats. Quiescent VSMCs from 3-week-old SHR and WKY rats were incubated without (control) or without 0.1 µmol/l SB290157 for 24 h. Total RNA was extracted and the abundance of KLF-5 mRNAs was evaluated by reverse transcription and PCR analysis. The ratio of abundance of each mRNA to 18S rRNA was evaluated by densitometric analysis. Data are means ± s.e.m. (n = 8 from two analyses). *P < 0.05 between the indicated columns. mRNA, messenger RNA.
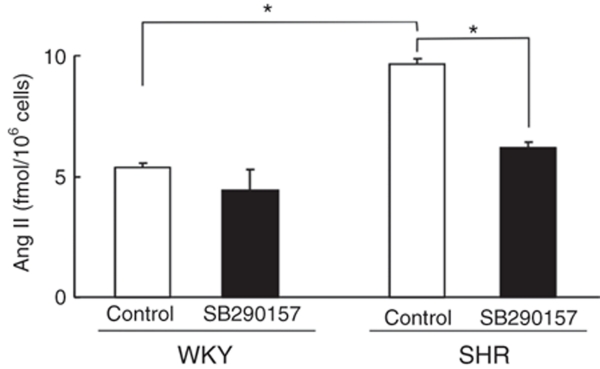
Effects of the C3a receptor inhibitor on Ang II-production in VSMCs
Figure 5 shows the effects of the SB290157 C3a receptor inhibitor on Ang II-production in VSMCs from SHR and WKY rats. Basal production of Ang II was significantly higher (P < 0.05) in VSMCs from SHR than in cells from WKY rats. Incubation with SB290157 significantly (P < 0.05) decreased production of Ang II in VSMCs from SHR, but it did not affect production of Ang II in cells from WKY rats.
Figure 5.
Effects of the complement 3a receptor inhibitor, SB290157, on production of angiotensin II (Ang II) in conditioned medium of vascular smooth muscle cells (VSMCs) from spontaneously hypertensive rats (SHR) and Wistar–Kyoto (WKY) rats. VSMCs (106) were incubated first for 24 h with serum-free DMEM and then for 48 h with 2 ml of fresh serum-free DMEM in the absence or presence of 0.1 µmol/l SB290157. The culture medium was collected and subjected to radioimmunoassay for Ang II. Data are means ± s.e.m. (n = 6). *P < 0.05 between indicated columns. DMEM, Dulbecco's modified Eagle's medium.
Discussion
In the present study, we confirmed that SHR-derived VSMCs abundantly express pre-pro-C3 mRNA and produce C3 protein when compared to cells from WKY rats. We previously evaluated the expression of 1,300 transcripts by microarray analysis with total mRNA extracted from the mid-layer of smooth aortic muscle from 3-week-old prehypertensive SHR and WKY rats in vivo. mRNAs encoding six genes including pre-pro-C3 were expressed only in aortic smooth muscle from SHR, and not in smooth muscle from WKY rats. Moreover, pre-pro-C3 mRNA was detected only in cultured VSMCs from SHR, not in cells from WKY rats in vitro.11 In the microarray analysis, there were no differences in the levels of mRNA for C4, C8, and C9 in aortic smooth muscle from SHR and WKY rats. These results indicated that the abundant expression of C3 in SHR-derived VSMCs is independent of the complement generating pathways.
Ueda et al.18 showed that C3 is produced by human aortic VSMCs but not by a human smooth muscle cell line or VSMCs from human umbilical cord vein. Thus C3-production may differ between smooth muscle cell types. Thus since the enhanced expression of pre-pro-C3 in VSMCs from SHR might be associated with genetic alterations, we therefore performed the direct sequencing of PCR products for promoter lesions of pre-pro-C3 in genomic DNA extracted from liver of SHR and WKY rats. However, there were no sequence differences in pre-pro-C3 promoter lesions between the two strains (data not shown). It is possible that the enhanced expression of pre-pro-C3 in VSMCs from SHR is associated with epigenetic alterations. Further investigation is required to clarify the different expression of pre-pro-C3 in VSMCs from SHR and WYK rats.
In the present study, we examined effects of a C3a receptor inhibitor on proliferation, phenotype, and Ang II-production of VSMCs from SHR and WKY rats, to evaluate whether C3 or C3a is associated with the exaggerated growth in the synthetic phenotype and Ang II-production in SHR-derived VSMCs. Expression of SM22α mRNA was relatively low, whereas expression of osteopontin mRNA was relatively high in quiescent VSMCs from SHR compared to cells from WKY rats, indicating that SHR-derived VSMCs are rather synthetic. SB290157 increased the low expression of SM22α and decreased the high expression of osteopontin mRNAs in VSMCs from SHR. These findings indicate that endogenous C3a acts to induce the synthetic phenotype of SHR-derived VSMCs as a final effector.
C3 has roles in immune defenses but has also been reported to exert several other biological functions. C3 produced by endothelial cells, monocytes/macrophages, and fibroblasts induces atherosclerosis via activation of several cytokines.19 C3 is synthesized by embryonic cells and participates in cell differentiation and proliferation.20 C3 enhances growth of cancer cells,21 contributes to contraction of VSMCs directly or indirectly through the activation of basophils,22 and activates MAP kinase in endothelial cells.23 Schraufstatter et al.24 recently demonstrated that C3a maintains dedifferentiation of mesenchymal stem cells by the phosphorylation of ERK1/2. Thus the C3 and C3a system may induce the synthetic phenotype of VSMCs by its dedifferentiating effects on the mesenchymal cells.
Concerning mechanisms underlying the synthetic phenotype of VSMCs from SHR compared to cells from WKY rats, the expression of KLF-5 mRNA was higher in SHR-derived VSMCs, and was inhibited by the C3a receptor antagonist in the present experiments. These results indicate that the increased expression of the C3–C3a system induces KLF-5, which promotes the synthetic phenotype of SHR-derived VSMCs. In the cardiovascular system, KLF-5 is expressed abundantly in embryonic VSMCs but is decreased in the adult vasculature.25 KLF-5 expression is markedly upregulated in activated VSMCs and myofibroblasts during atherosclerosis.25,26 KLF-5 is implicated in alterations in VSMCs during the change to the synthetic phenotype, which includes alterations in gene expression, at the site of vascular injury.27 We previously demonstrated that exogenous C3a increased the expression of KLF-5 mRNA and promoter activity via ERK signaling in VSMCs.13 These findings and the present study suggest that endogenous C3a induces the synthetic phenotype of SHR VSMCs from the stimulation of KLF-5.
Production of Ang II was increased in VSMCs from SHR, which was suppressed with the C3a receptor antagonist, indicating that increases in the endogenous C3–C3a system are associated with increased Ang II-production. We have previously demonstrated that the increased Ang II-production in SHR-derived VSMCs is associated with the Ang II-generating proteinases such as cathepsin D and angiotensin-converting enzyme.7 VSMCs that display the synthetic phenotype have a reduced fractional volume of myofilaments and many synthetic organelles such as Golgi, mitochondria, and endoplasmic reticulum, which generate proteolytic enzymes including cathepsin D and angiotensin-converting enzyme, which produce Ang II, as well as growth factors and cytokines.28,29 It is possible that endogenous C3a contributes to the increased Ang II-production through the induction of the synthetic phenotype of SHR-derived VSMCs.
Taken together, SHR-derived VSMCs primarily produce C3–C3a, but this does not occur in cells from WKY rats. Endogenous C3a promotes transcriptional activation of KLF-5 to induce the exaggerated growth with a synthetic phenotype and the production of Ang II in VSMCs from SHR.
Acknowledgments
This work was supported in part by a Grant-in-Aid for the Nihon University Multidisciplinary Research Grant for 2011.
The authors declared no conflict of interest.
References
- Sen S, Tarazi RC, Khairallah PA, Bumpus FM. Cardiac hypertrophy in spontaneously hypertensive rats. Circ Res. 1974;35:775–781. doi: 10.1161/01.res.35.5.775. [DOI] [PubMed] [Google Scholar]
- Folkow B. Structure and function of the arteries in hypertension. Am Heart J. 1987;114:938–948. doi: 10.1016/0002-8703(87)90591-6. [DOI] [PubMed] [Google Scholar]
- Folkow B. The fourth Volhard lecture: cardiovascular structural adaptation; its role in the initiation and maintenance of primary hypertension. Clin Sci Mol Med Suppl. 1978;4:3s–22s. doi: 10.1042/cs055003s. [DOI] [PubMed] [Google Scholar]
- Gray SD. Spontaneous hypertension in the neonatal rat. A review. Clin Exp Hypertens A. 1984;6:755–781. doi: 10.3109/10641968409044037. [DOI] [PubMed] [Google Scholar]
- Walter SV, Hamet P. Enhanced DNA synthesis in heart and kidney of newborn spontaneously hypertensive rats. Hypertension. 1986;8:520–525. doi: 10.1161/01.hyp.8.6.520. [DOI] [PubMed] [Google Scholar]
- Hadrava V, Tremblay J, Hamet P. Abnormalities in growth characteristics of aortic smooth muscle cells in spontaneously hypertensive rats. Hypertension. 1989;13:589–597. doi: 10.1161/01.hyp.13.6.589. [DOI] [PubMed] [Google Scholar]
- Hu WY, Fukuda N, Kanmatsuse K. Growth characteristics, angiotensin II generation, and microarray-determined gene expression in vascular smooth muscle cells from young spontaneously hypertensive rats. J Hypertens. 2002;20:1323–1333. doi: 10.1097/00004872-200207000-00019. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Satoh C, Hu WY, Soma M, Kubo A, Kishioka H, Watanabe Y, Izumi Y, Kanmatsuse K. Production of angiotensin II by homogeneous cultures of vascular smooth muscle cells from spontaneously hypertensive rats. Arterioscler Thromb Vasc Biol. 1999;19:1210–1217. doi: 10.1161/01.atv.19.5.1210. [DOI] [PubMed] [Google Scholar]
- Fukuda N, Hu WY, Satoh C, Nakayama M, Kishioka H, Kubo A, Kanmatsuse K. Contribution of synthetic phenotype on the enhanced angiotensin II-generating system in vascular smooth muscle cells from spontaneously hypertensive rats. J Hypertens. 1999;17:1099–1107. doi: 10.1097/00004872-199917080-00009. [DOI] [PubMed] [Google Scholar]
- Hu WY, Fukuda N, Satoh C, Jian T, Kubo A, Nakayama M, Kishioka H, Kanmatsuse K. Phenotypic modulation by fibronectin enhances the angiotensin II-generating system in cultured vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2000;20:1500–1505. doi: 10.1161/01.atv.20.6.1500. [DOI] [PubMed] [Google Scholar]
- Lin ZH, Fukuda N, Jin XQ, Yao EH, Ueno T, Endo M, Saito S, Matsumoto K, Mugishima H. Complement 3 is involved in the synthetic phenotype and exaggerated growth of vascular smooth muscle cells from spontaneously hypertensive rats. Hypertension. 2004;44:42–47. doi: 10.1161/01.HYP.0000129540.83284.ca. [DOI] [PubMed] [Google Scholar]
- Wan JX, Fukuda N, Endo M, Tahira Y, Yao EH, Matsuda H, Ueno T, Matsumoto K. Complement 3 is involved in changing the phenotype of human glomerular mesangial cells. J Cell Physiol. 2007;213:495–501. doi: 10.1002/jcp.21129. [DOI] [PubMed] [Google Scholar]
- Yao EH, Fukuda N, Ueno T, Tsunemi A, Endo M, Matsumoto K. Complement 3 activates the KLF5 gene in rat vascular smooth muscle cells. Biochem Biophys Res Commun. 2008;367:468–473. doi: 10.1016/j.bbrc.2007.12.160. [DOI] [PubMed] [Google Scholar]
- de Bruijn MH, Fey GH. Human complement component C3: cDNA coding sequence and derived primary structure. Proc Natl Acad Sci USA. 1985;82:708–712. doi: 10.1073/pnas.82.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu A, Lambris JD. Structure and biology of complement protein C3, a connecting link between innate and acquired immunity. Immunol Rev. 2001;180:35–48. doi: 10.1034/j.1600-065x.2001.1800103.x. [DOI] [PubMed] [Google Scholar]
- Hu WY, Fukuda N, Ikeda Y, Suzuki R, Tahira Y, Takagi H, Matsumoto K, Kanmatsuse K, Mugishima H. Human-derived vascular smooth muscle cells produce angiotensin II by changing to the synthetic phenotype. J Cell Physiol. 2003;196:284–292. doi: 10.1002/jcp.10299. [DOI] [PubMed] [Google Scholar]
- Mocharla H, Mocharla R, Hodes ME. Coupled reverse transcription-polymerase chain reaction (RT-PCR) as a sensitive and rapid method for isozyme genotyping. Gene. 1990;93:271–275. doi: 10.1016/0378-1119(90)90235-j. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Nagasawa K, Tsukamoto H, Horiuchi T, Nishizaka H, Ikeda K, Niho Y. Production of the third and fourth component of complement (C3, C4) by smooth muscle cells. Immunology. 1996;89:183–188. doi: 10.1046/j.1365-2567.1996.d01-725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buono C, Come CE, Witztum JL, Maguire GF, Connelly PW, Carroll M, Lichtman AH. Influence of C3 deficiency on atherosclerosis. Circulation. 2002;105:3025–3031. doi: 10.1161/01.cir.0000019584.04929.83. [DOI] [PubMed] [Google Scholar]
- Hong MH, Jin CH, Sato T, Ishimi Y, Abe E, Suda T. Transcriptional regulation of the production of the third component of complement (C3) by 1 alpha, 25-dihydroxyvitamin D3 in mouse marrow-derived stromal cells (ST2) and primary osteoblastic cells. Endocrinology. 1991;129:2774–2779. doi: 10.1210/endo-129-5-2774. [DOI] [PubMed] [Google Scholar]
- di Renzo L, Longo A, Morgante E, Mardente S, Prodinger WM, Russo M, Pontieri GM, Lipari M. C3 molecules internalize and enhance the growth of Lewis lung carcinoma cells. Immunobiology. 1999;200:92–105. doi: 10.1016/s0171-2985(99)80035-7. [DOI] [PubMed] [Google Scholar]
- Hugli TE, Müller-Eberhard HJ. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- Monsinjon T, Gasque P, Chan P, Ischenko A, Brady JJ, Fontaine MC. Regulation by complement C3a and C5a anaphylatoxins of cytokine production in human umbilical vein endothelial cells. FASEB J. 2003;17:1003–1014. doi: 10.1096/fj.02-0737com. [DOI] [PubMed] [Google Scholar]
- Schraufstatter IU, Discipio RG, Zhao M, Khaldoyanidi SK. C3a and C5a are chemotactic factors for human mesenchymal stem cells, which cause prolonged ERK1/2 phosphorylation. J Immunol. 2009;182:3827–3836. doi: 10.4049/jimmunol.0803055. [DOI] [PubMed] [Google Scholar]
- Nagai R, Suzuki T, Aizawa K, Shindo T, Manabe I. Significance of the transcription factor KLF5 in cardiovascular remodeling. J Thromb Haemost. 2005;3:1569–1576. doi: 10.1111/j.1538-7836.2005.01366.x. [DOI] [PubMed] [Google Scholar]
- Shiojima I, Aikawa M, Suzuki J, Yazaki Y, Nagai R. Embryonic smooth muscle myosin heavy chain SMemb is expressed in pressure-overloaded cardiac fibroblasts. Jpn Heart J. 1999;40:803–818. doi: 10.1536/jhj.40.803. [DOI] [PubMed] [Google Scholar]
- Watanabe N, Kurabayashi M, Shimomura Y, Kawai-Kowase K, Hoshino Y, Manabe I, Watanabe M, Aikawa M, Kuro-o M, Suzuki T, Yazaki Y, Nagai R. BTEB2, a Krüppel-like transcription factor, regulates expression of the SMemb/Nonmuscle myosin heavy chain B (SMemb/NMHC-B) gene. Circ Res. 1999;85:182–191. doi: 10.1161/01.res.85.2.182. [DOI] [PubMed] [Google Scholar]
- Mosse PR, Campbell GR, Wang ZL, Campbell JH. Smooth muscle phenotypic expression in human carotid arteries. I. Comparison of cells from diffuse intimal thickenings adjacent to atheromatous plaques with those of the media. Lab Invest. 1985;53:556–562. [PubMed] [Google Scholar]
- Glukhova MA, Frid MG, Koteliansky VE. Developmental changes in expression of contractile and cytoskeletal proteins in human aortic smooth muscle. J Biol Chem. 1990;265:13042–13046. [PubMed] [Google Scholar]