Abstract
AIM: To assess the role of hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels in regulating the excitability of vagal and spinal gut afferents.
METHODS: The mechanosensory response of mesenteric afferent activity was measured in an ex vivo murine jejunum preparation. HCN channel activity was recorded through voltage and current clamp in acutely dissociated dorsal root ganglia (DRG) and nodose ganglia (NG) neurons retrogradely labeled from the small intestine through injection of a fluorescent marker (DiI). The isoforms of HCN channels expressed in DRG and NG neurons were examined by immunohistochemistry.
RESULTS: Ramp distension of the small intestine evoked biphasic increases in the afferent nerve activity, reflecting the activation of low- and high-threshold fibers. HCN blocker CsCl (5 mmol/L) preferentially inhibited the responses of low-threshold fibers to distension and showed no significant effects on the high-threshold responses. The effect of CsCl was mimicked by the more selective HCN blocker ZD7288 (10 μmol/L). In 71.4% of DiI labeled DRG neurons (n = 20) and 90.9% of DiI labeled NG neurons (n = 10), an inward current (Ih current) was evoked by hyperpolarization pulses which was fully eliminated by extracellular CsCl. In neurons expressing Ih current, a typical “sag” was observed upon injection of hyperpolarizing current pulses in current-clamp recordings. CsCl abolished the sag entirely. In some DiI labeled DRG neurons, the Ih current was potentiated by 8-Br-cAMP, which had no effect on the Ih current of DiI labeled NG neurons. Immunohistochemistry revealed differential expression of HCN isoforms in vagal and spinal afferents, and HCN2 and HCN3 seemed to be the dominant isoform in DRG and NG, respectively.
CONCLUSION: HCNs differentially regulate the excitability of vagal and spinal afferent of murine small intestine.
Keywords: Hyperpolarization-activated cyclic nucleotide-gated cation, Vagal afferent, Spinal afferent, Gastrointestinal tract, CsCl
INTRODUCTION
The gastrointestinal tract is innervated by vagal and spinal afferent nerves, whose cell bodies lie in the dorsal root ganglion (DRG) and nodose ganglion (NG), respectively[1]. Previous studies have shown that vagal and spinal afferents differ in their responsiveness to a variety of stimuli and hence may play different roles in gastrointestinal (GI) physiology and pathophysiology. The mesenteric nerves (consisting of both vagal and spinal afferents) of the small intestine exhibited biphasic increases in afferent activity in response to ramp distension in the rat in vivo and in the ex vivo mouse or rat jejunum preparations, suggesting the presence of low- and high-threshold mechanoreceptors[2,3]. Booth et al[3] demonstrated that the low-threshold response in rats was markedly reduced following chronic vagotomy whereas the high-threshold response remained unaltered. These data were consistent with the notion that vagal mechanoreceptors are primarily low-threshold fibers that convey innocuous signals in the GI tract and contribute to the control of satiety and food intake as well as reflex regulation of motility, secretion and absorption[1]. The spinal afferents, on the other hand, are mainly composed of high-threshold and wide dynamic range fibers and may therefore encode nociceptive signals in the GI tract. There has been extensive evidence suggesting that altered sensitivity of vagal and spinal afferents may underlie some of the debilitating symptoms such as bloating and pain seen in functional GI diseases[4]. However, the molecular mechanisms of sensory dysfunction remain poorly understood.
Previous studies have identified a number of ion channels and G-protein coupled receptors that are involved in sensory transduction and modulation of the excitability of primary afferents including those innervating the GI tract. In mammals, the hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channel family consists of 4 cation channels, named HCN1-4. In cells expressing these channels, hyperpolarization of the membrane potential would activate HCN channels, resulting in a slow inward current. The kinetics of these channel activity are regulated by cyclic adenosine 3’,5’-monophosphate (cAMP). Because these channels are activated near the resting membrane potential, they can modulate the membrane excitability. Several groups have explored the possible role of HCN channels in sensory processing. It has been shown that HCN channels are expressed in DRG and nodose neurons[5,6]. Importantly, it has been reported that HCN channels were up-regulated in DRG neurons following nerve injury and neuropathic pain was reversed by HCN blockers, suggesting that HCN channels have an excitatory influence on sensory neurons and may represent a potential therapeutic target in pain management. However, another study demonstrated that in nodose neurons and aortic baroreceptors, HCN blockers reduced the threshold for activation,indicating that HCN channels have an inhibitory influence on the excitability of nodose neurons and baroreceptors[5,7].
Matsuyoshi et al[8] examined the expression of HCN channels in bladder afferent neurons. Among HCN-1, HCN-2 and HCN-4, positive staining with HCN-2 antibodies was found in approximately 60% of small- and medium-sized bladder afferent neurons. However, the amplitude and current density of hyperpolarization-activated current (Ih) was significantly larger in medium-sized bladder afferent neurons than in small-sized bladder neurons, suggesting that Ih currents could control the excitability of mechanoceptive Aδ-fiber bladder afferent neurons[9]. HCN channels are also localized in enteric nervous system[10]. Linden and colleagues[11] found that the enhancement of afterhyperpolarization neuronal excitability in inflamed guinea pig colon involves an increase in Ih current. However, there has been little information regarding the potential role of HCN channels in the function of extrinsic afferents of the GI tract.
In the present study, we investigated the effect of HCN channel blockers on the mechanosensory responses of mesenteric afferent nerves of the murine jejunum in vitro and compared the Ih current in vagal and spinal primary afferent neurons retrogradely labeled from the small intestine.
MATERIALS AND METHODS
Animals
Male Kunming mice weighing 20-25 g were purchased from Shanghai Jiaotong University School of Medicine. The mice were allowed free access to normal laboratory food and tap water and were kept under conditions of constant temperature and humidity with a 12-h light/dark cycle. Mesenteric afferent nerve recording experiments were performed on 6 mice for CsCl and 3 mice for ZD-7288. Immunohistochemistry was performed on 3 DiI labeled mice and 3 as control. All procedures involving use of animals were approved by the Institutional Animal Care and Use Committee at Shanghai Jiaotong University.
Tissue preparation and afferent nerve recording
Mice were deeply anaesthetized with pentobarbital (80 mg/kg, ip) and then killed by cervical dislocation. A midline laparotomy was performed and sections of jejunum were rapidly removed[2]. One of the jejunum segments was placed in a recording chamber (10 mL) and superfused with oxygenated Krebs solution (composition in mmol/L: NaCl 113, KCl 5.9, CaCl2 1.25, MgSO4 1.2, NaH2PO4 1.2, NaHCO3 25, Glucose 11.5). Chamber temperature was kept at 34 °C. The jejunum was cannulated at each end to allow intraluminal infusion (0.1 mL/min) and ramp distension of the gut. A branch of the mesenteric nerve was dissected and the electrical activity was recorded using a suction electrode connected to a Neurolog headstage (NL100, Digitimer Ltd., United Kingdom). The signal was amplified (NL104), filtered (NL 215, bandpass 300-3000Hz), digitized (Micro1401, CED, United Kingdom) and then captured by a computer with the Spike2 software (version 5.14, Cambridge Electronic Design, United Kingdom). The jejunum segment was distended gradually to an intraluminal pressure of 60 mmHg by closing the outflow port to observe the mechanosensitivity of mesenteric nerves and this was repeated at an interval of 15 min. The effect of HCN channel blockers (5 mmol/L CsCl or 10 μmol/L ZD7288) on the mechanosensory responses was tested by switching the bath and intraluminal solution to one that contains one of the antagonists.
Retrograde labeling of DRG and NG neurons innervating the jejunum
DRG and NG neurons were retrogradely labeled by injecting the fluorescent dye, DiI (Molecular Probes, Eugene, OR)[12] into the gut wall. Briefly, mice were anaesthetized with 1% pentobarbital (40 mg/kg, ip). Under sterile conditions, a midline laparotomy was performed to expose the jejunum and 25 μL DiI (0.1% in DMSO) was injected at 15-20 sites into the smooth muscle layer. Incisions were sutured in layers and mice were allowed to recover for 1-2 wk to permit DiI to be transported to the cell soma.
Dissociation of neurons and patch-clamp recording
Mice were killed by an overdose of pentobarbital (100 mg/kg, ip). T7-L2 DRG and NG were quickly isolated under a dissecting microscope and transferred into ice cold Hanks’ solution. The ganglia were minced with fine spring scissors after removing the connective tissue. The minced ganglia were placed in Hank’s solution containing type II collagenase (3 mg/mL) and trypsin (2.5 mg/mL) and incubated at 37 °C for 50 min, then washed 2-3 times in DMEM with 10% fetal calf serum, 100 U penicillin and 100 μg streptomycin. Cells were then dispersed by gentle titration with fire-polished Pasteur pipettes. The cell suspension was plated onto 35-mm culture dish coverslips pre-coated with 0.01% poly-lysine and incubated in 5% CO2 at 37 °C overnight.
For patch-clamp recording, one of the coverslips was placed in the recording chamber (1 mL) and the cells were continuously perfused with extracellular solution (in mmol/L: NaCl 130, KCl 6, MgCl2 1.2, CaCl2 2.5, HEPES 10, Glucose 10, BaCl2 1) at room temperature (21-25 °C). We added 1 mmol/L Ba2+ in the extracellular solution in order to block the inward-rectifier potassium current upon applying the hyperpolarizing voltage steps[13,14]. Whole cell recordings of the DiI-labelled cells were conducted using electrodes with a resistance of about 3-5 MΩ when filled with the internal solution (composition in mmol/L: K-gluconate 140, MgCl2 2, EGTA 1.1, HEPES 10).
Immunohistochemistry
Mice were killed by an overdose of pentobarbital and then perfused with saline followed by 4% paraformaldehyde in 0.1 mol/L sodium phosphate buffer with a pH of 7.4. T7-L2 DRG and NG were isolated and post-fixed in the same solution at 4 °C overnight and then transferred into 30% sucrose for 24 h at 4 °C. The ganglia were then embedded in an Optimal Cutting Temperature compound and serially sectioned (10 μm) using a cryostat for immunofluorescent staining. Briefly, sections were treated with phosphate-buffered saline (PBS) containing 1% normal goat serum and 1% TritonX-100 at room temperature for 30 min twice followed by incubation with one of the primary antibodies at 4 °C overnight. The sections were rinsed in PBS for four times and then incubated with the secondary antibody at room temperature for 1 h. The primary antibodies used were rabbit polyclonal antibodies: 1:200 anti-HCN1 (ab65706, Abcam), 1:200 anti-HCN2 (ab65704, Abcam), 1:200 anti-HCN3 (ab65705, Abcam) and 1:1000 anti-HCN4 (ab65703, Abcam ). The secondary antibody was a goat anti-rabbit Alexa Fluor 488 (Molecular Probes, Invitrogen) diluted in 10% NGS /PBS/Triton solution (1:1000).
Statistical analysis
Numerical data were expressed as mean ± SE. Statistical analysis of the data was performed using SPSS version 13.0 or Graphpad Prism version 5 (Graphpad software, San Diego, United States). The effect of CsCl on low- and high- threshold afferent responses were analyzed by paired Student’s t test. The pressure-afferent nerve response curves were analyzed using linear regression and then compared using an analysis of covariance (ANCOVA). Amplitude, density and time constants of Ih current were compared between DRG and NG neurons by Wilcoxon rank sum test. Mean voltages of half activation (V1/2) and slope factors (k) of Ih were acquired from the curves fitted by Boltzmann equation in Origin 6.0 (OriginLab, Northampton, Massachusetts, United States). The percentages of HCN-positive cells in DRG and NG were compared by the χ2 test. P < 0.05 was considered statistically significant.
RESULTS
Effect of HCN channel blockers on the mechanosensory responses of small intestinal afferent nerves
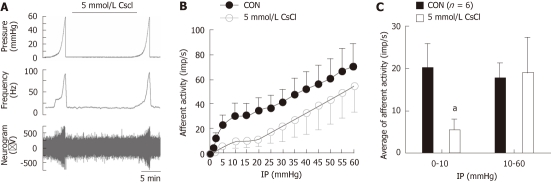
Ramp distension of the jejunum segments typically evoked biphasic increases in mesenteric afferent nerve discharge (Figure 1), which is consistent with previous reports[2,15]. The first phase was a rapid increase in afferent activity when the intraluminal pressure started to rise and the nerve activity reached the first peak at an intraluminal pressure of approximately 5 mmHg and then plateaued or increased slowly as the intraluminal pressure continued to rise. The second phase was an accelerated increase in nerve discharge starting at an intraluminal pressure of 20 mmHg. This pattern of ramp distension-evoked afferent responses was due to the activation of three functional populations of afferent fibers, namely low-threshold fibers, wide dynamic range fibers and high-threshold fibers[2]. As is exemplified in Figure 1A, simultaneous bath and intraluminal application of the HCN channel blocker, CsCl (5 mmol/L), attenuated the initial phase of the nerve responses to ramp distension but seemed to render the second phase of the mechanosensory response unaffected. Figure 1B shows the average pressure-afferent response curves of the mesenteric nerves (n = 6) under the control condition and in the presence of CsCl (5 mmol/L) (Figure 1B). For simplicity, each curve is divided into two parts (intraluminal pressure range, 0-10 mmHg and 10-60 mmHg) and analyzed using linear regression, reflecting the mechanosensitivity of low- and high-threshold fibers, respectively. Under the control condition, the initial part of the pressure-response curve had a slope of (2.95 ± 0.73) imp/s per mmHg and in the presence of 5 mmol/L CsCl, the slope was (0.90 ± 0.37) imp/s per mmHg, which was significantly smaller compared with the control curve (P < 0.05, ANCOVA, Figure 1B). The slope of the second part of the pressure-response curve (10-60 mmHg) in the presence of 5 mmol/L CsCl was not significantly different from that of the control curve [(0.86 ± 0.24) imp/s per mmHg vs (0.95 ± 0.27) imp/s per mmHg, P > 0.05, ANCOVA, Figure 1B]. Another way of addressing the responses of low- and high-threshold fibers is to calculate the changes in afferent discharge rate when the intraluminal pressure increased from the baseline to 10 mmHg (low-threshold response) and from 10 to 60 mmHg (high-threshold response). As shown in Figure 1C, 5 mmol/L CsCl attenuated the low-threshold response but rendered the high threshold response unaltered [0-10 mmHg: (19.91 ± 5.92) imp/s vs (5.65 ± 2.34) imp/s , paired t test, t = 3.29, P < 0.05; 10-60 mmHg: (17.48 ± 3.70) imp/s vs (18.83 ± 8.55) imp/s, paired t test, P > 0.05, Figure1C].
Figure 1.
Effects of CsCl on the mechenosensory activity of small intestinal afferents. A: Original recording of the mesenteric afferent nerve activity in response to distension in an ex vivo jejunum preparation; B: The pressure-afferent nerve response curves in control and in the presence of hyperpolarization-activated cyclic nucleotide-gated cation blocker, 5 mmol/L CsCl; C: Bar graph showing the low- and high- threshold mechanosensory responses with or without the presence of 5 mmol/L CsCl. aP < 0.05.
To confirm that CsCl-induced inhibition of the low-threshold mechanosensory response is due to the blockade of HCN channels, we observed the effects of ZD-7288, a more selective HCN channel blocker, on the responses of mesenteric nerves to ramp distension in 3 jejunum preparations. Indeed, 10 μmol/L ZD7288 mimicked the effects of CsCl in that it attenuated the low-threshold responses, but not significantly affected the high-threshold responses (Figure 2). These data clearly demonstrate that under the normal conditions, HCN channels exert a tonic facilitatory effect on the low-threshold mechanoreceptors but not on high-threshold mechanoreceptors.
Figure 2.
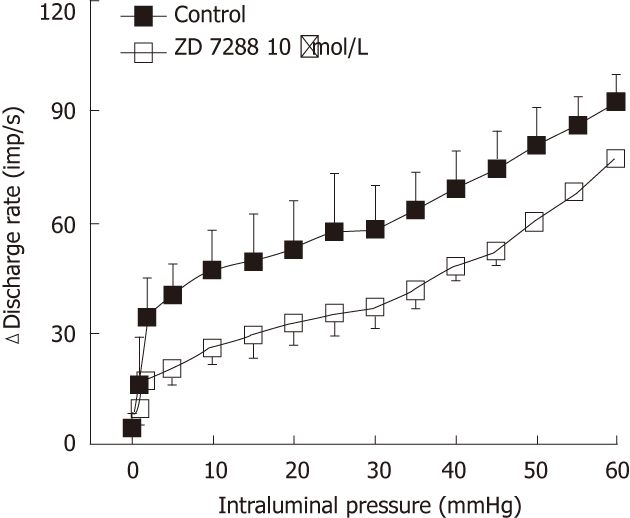
Effects of ZD-7288 on the mechanisensory responses of mesenteric afferent nerves. Note that the slopes of the average pressure-response curves in the pressure range of 10 mmHg - 60 mmHg were apparently similar with or without the hyperpolarization-activated cyclic nucleotide-gated cation channel blocker ZD-7288 (10 μmol/L). Data were pooled from 3 preparations.
Hyperpolarization-activated current in vagal and spinal primary afferent neurons projecting to the small intestine
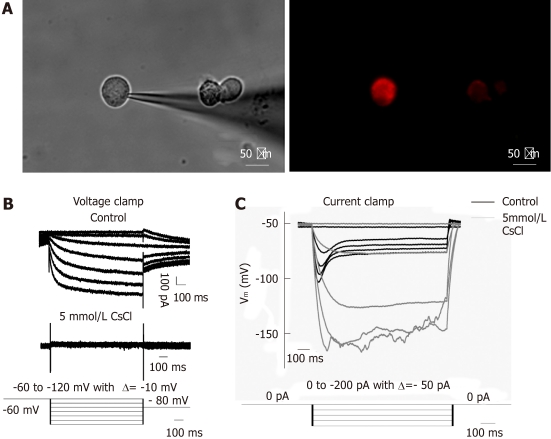
We focused on DRG and NG neurons retrogradely labeled from the small intestine by patch-clamp recording of Ih current. Ih current was present in 71.4% (20/28) of DiI labeled DRG neurons (mean diameter, 35.67 ± 0.65 μm; mean capacitance, 45.83 ± 5.13 pF) and 90.9% (10/11) of NG neurons (mean diameter, 29.63 ± 1.20 μm; mean capacitance, 25.84 ± 2.60 pF). Figure 3 shows an example of DiI labeled DRG neurons viewed under the inverted fluorescence microscope, as well as the current and voltage traces obtained by whole cell recording. Under voltage-clamp recording, hyperpolarization pulses activated inward currents which were virtually abolished by 5 mmol/L CsCl. Under current-clamp recording, hyperpolarizing currents elicited hyperpolarization of the membrane potential which exhibited a “sag” (depolarization) that was also prevented by 5 mmol/L CsCl. These results show that both vagal and spinal primary afferent neurons projecting to the small intestine express functional HCN channels, which seemed to exert a facilitatory effect on the afferent excitability.
Figure 3.
Ih recorded under whole-cell voltage and current clamp from DiI labeled dissociated dorsal root ganglia and nodose ganglia neurons innervating the jejunum. A: DiI labeled dissociated dorsal root ganglia neurons; B: Representative current traces of Ih current (top trace); Current recorded in the presence of 5 mmol/L CsCl (middle trace); voltage clamp protocal: Ih was induced from a holding potential of -60 mV in 1 s pulses from -60 mV to -120 mV in steps of 10 mV, followed by a final step to -80 mV to record the tail current (bottom trace); C: Voltage response to test current pulse before (black) and after (grey) application of 5 mmol/L CsCl (top trace); bottom trace shows the current clamp protocol, i.e., hyperpolarizing current pulses ranging from 0 pA to -200 pA in steps of 50 pA. Note that the current elicited an instantaneous hyperpolarization that was followed by depolarization (named “sag”) of membrane potential. DRG: Dorsal root ganglia; NG: Nodose ganglia.
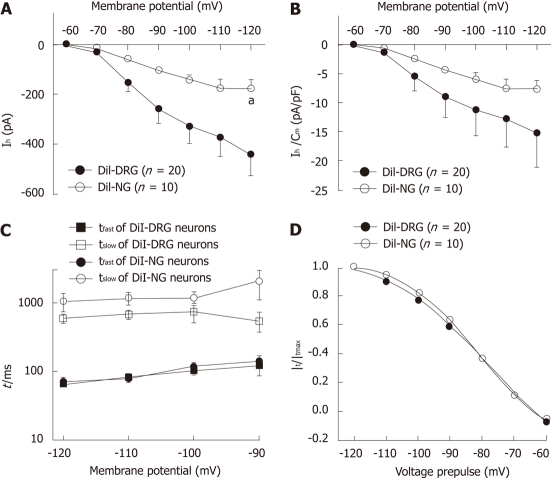
Hyperpolarization-activated currents in DiI-labeled DRG neurons were generally of larger amplitude than in NG neurons [-120 mV: (-439.08 ± 89.56) pA for DRG neurons and (-176.37 ± 36.96) pA for NG neurons, Wilcoxon rank sum test, P < 0.05, Figure 4A], although when normalized to the membrane capacitance, the difference in current density was not statistically significant between spinal and vagal afferents [-120 mV: (-15.22 ± 5.93) pA/pF for DRG neurons and (-7.64 ± 1.67) pA/pF for NG neurons, Wilcoxon rank sum test, P > 0.05, Figure 4B]. We compared the kinetics of Ih currents in these two populations. The time course of Ih activation was fitted by a two-exponential function (Figure 4C). There was no significant difference in the fast and slow time constant between DiI-labeled DRG and NG neurons [-120mV: (65.81 ± 6.89) ms and (590.57 ± 88.19) ms for DRG neurons, (70.12 ± 5.10) ms and (1044.54 ± 307.80) ms for NG neurons, respectively]. The activation parameters of Ih was V1/2 (-77.86 ± 3.38) mV, slope factor 16.06 ± 2.96 for DiI-labeled DRG neurons and V1/2 -81.31 ± 0.41 mV, slope factor 11.84 ± 0.40 for DiI-labeled NG neurons (Figure 4D).
Figure 4.
Ih steady-state parameters and activation kinetics in DiI-labeled DRG and NG neurons. A: Ih current-voltage relationship between DiI-labeled dissociated dorsal root ganglia (DRG) and nodose ganglia neurons (NG); B: Ih current density-voltage relationship between DiI-labeled DRG and NG neurons; C: The time constant of Ih current. Ih current traces were fitted with two exponentials according to the following equation: Ih(t) = Af exp(-t/τf) + As exp(-t/τs), where Ih(t) is the amplitude of the current at time t and Af and As are the initial amplitudes of the fast (τf) and slow (τs) activation time constant components, respectively. Time constants were obtained by fitting currents using pCLAMP; D: Ih current activation curves. Normalized activation curves were obtained from tail currents at -80 mV and fitted by Boltzmann function: Ih/Ih(max) =1/{1+ exp[(Vm – V1/2)/k]}, where Ih is the peak amplitude of the tail current recorded immediately after the pre-pulse, Ih(max) is the maximal current recorded after the maximal prepulse of -120 mV, Vm is the membrane potential, V1/2 is the membrane potential at which Ih conductance is half-activated, and k is a slope factor of the curve. Data were expressed as mean ± SE. aP < 0.05 vs DiI-labeled DRG neurons.
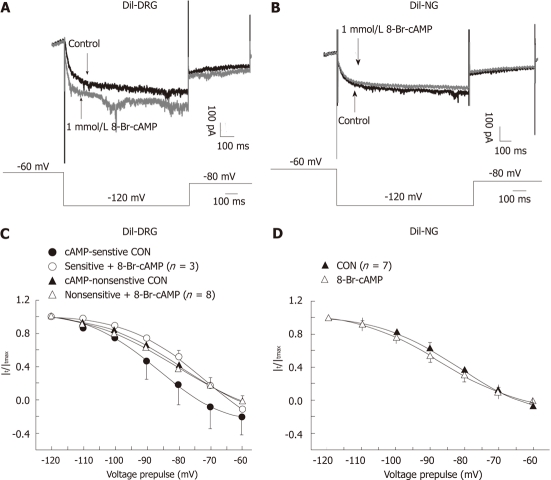
We applied 8-Br-cAMP (a membrane permeable cAMP) to test the sensitivity of Ih currents in spinal and vagal afferent neurons to cAMP. In 3 of 11 DiI-labeled DRG neurons, Ih currents were augmented by 8-Br-cAMP, as is shown in Figure 5A. In these neurons, 8-Br-cAMP (1 mmol/L) caused a 14 mV depolarizing shift in the midpoint of voltage activation without marked effect on the slope factor [V1/2 from (-86.49 ± 1.14) mV to (-72.16 ± 3.44) mV and slope factor from 10.59 ± 1.31 to 12.07 ± 2.04, Figure 5A and C]. Ih currents in the other 8 DRG neurons tested were not sensitive to 8-Br-cAMP [V1/2 from (-79.45 ± 1.70) mV to (-80.43 ± 2.07) mV and slope factor from 12.17 ± 1.52 to 14.00 ± 2.03, Figure 5C]. Seven NG neurons were tested, but the Ih currents in these neurons were not altered in the presence of 8-Br-cAMP [V1/2 from (-81.95 ± 0.78) mV to (-86.84 ± 0.29) mV and slope factor from 11.57 ± 0.78 to 11.98 ± 0.36, Figure 5B and D]. These data suggest that different isoforms of HCN channels may exist in subpopulations of spinal and vagal primary afferent neurons innervating the small intestine.
Figure 5.
Effects of 8-Br-cAMP on the activation curves of Ih current in DiI-labeled dissociated dorsal root ganglia and nodose ganglia neurons. A and B: Overdraw of the Ih current traces elicited by a hyperpolarizing pulse (-120 mV) in control and in the presence of 8-Br-cAMP in DiI-labeled dissociated dorsal root ganglia (DRG) (A) and nodose neurons (B); C: Activation curves of cAMP-sensitive and cAMP-insensitive DiI-labeled DRG neurons before (black block symbols) and after treatment with 8-Br-cAMP (empty symbols); D: Activation curves of DiI-labeled nodose ganglia neurons before (black block triangle) and after treatment with 8-Br-cAMP (empty triangle). Data were expressed as mean ± SE.
Immunohistochemical staining of HCN isoforms in DRG and NG neurons
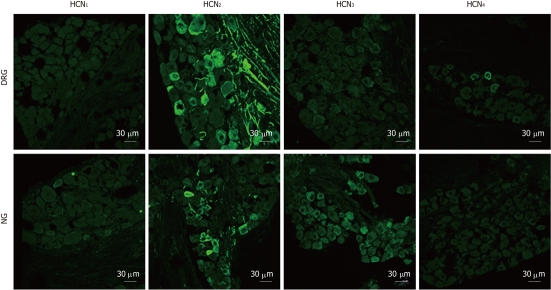
There was no apparent immunofluorescent staining for HCN1 in DRG or NG sections. HCN2 immunostaining was detected in both ganglia. In DRG sections, 12.3% of DRG neurons and numerous afferent fibers were stained for HCN2. In NG sections, HCN2 immunostaining was less extensive in that only 2.3% of NG neurons (P < 0.05, χ2 test, compared with DRG) and fewer fibers were stained for HCN2. Weak HCN3 immunoreactivity was detected in some DRG neurons. In contrast, moderate staining for HCN3 appeared to be present in the majority of NG neurons. HCN4 immunoreactivity was not detected in NG sections but was present in a minority of DRG neurons (Figure 6).
Figure 6.
Immunostaining for hyperpolarization-activated cyclic nucleotide-gated cation isoforms in dorsal root ganglia and nodose ganglia sections. Hyperpolarization-activated cyclic nucleotide-gated cation channel 1 (HCN1) immunoreactivity was not detectable in dorsal root ganglia (DRG) and nodose ganglia (NG) neurons. HCN2 immunoreactivity was prominent in DRG (cell bodies and fibers) and was also present in some NG neurons and fibers. HCN3 immunoreactivity was profuse in NG sections but was weaker in DRG sections. HCN4 immunoreactivity was present in a minority of DRG neurons and was absent in NG.
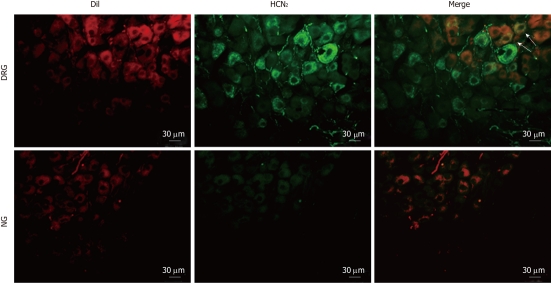
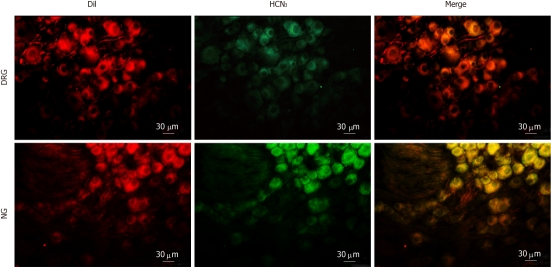
To determine the subtypes of HCN channels expressed in intestinal primary afferent neurons, we further observed the immunoreactivity of HCN1-4 in DRG and NG neurons retrogradely labeled from the small intestine via injection of DiI into the gut wall. As expected, HCN1 immunoreactivity was not detected in any of the DiI-labeled DRG or NG neurons. HCN2 immunoreactivity was present in some DiI-labeled DRG neurons but was not detected in DiI-labeled NG neurons (Figure 7). On the other hand, moderate HCN3 immunoreactivity was apparently present in most DiI-labeled NG neurons. DiI-labeled DRG neurons, however, were only weakly immunoreactive to HCN3 (Figure 8). HCN4 immunoreactivity was not detected in DiI-labeled DRG or NG neurons.
Figure 7.
Immunostaining for hyperpolarization-activated cyclic nucleotide-gated cation channel 2 in intestinal primary afferent neurons. Spinal and vagal afferent neurons innervating the small intestine were labeled via injection of DiI (red) into the gut wall. Arrows indicate positive hyperpolarization-activated cyclic nucleotide-gated cation channel 2 (HCN2) staining in DiI-labeled dorsal root ganglia neurons. DRG: Dissociated dorsal root ganglia; NG: Nodose ganglia.
Figure 8.
Immunostaining for hyperpolarization-activated cyclic nucleotide-gated cation channel 3 in intestinal primary afferent neurons. Intestinal afferents were labeled via injecting DiI into the gut wall. Note that weak hyperpolarization-activated cyclic nucleotide-gated cation channel 3 (HCN3) immunoreactivity (green) was present in some DiI-labeled (red) dorsal root ganglia neurons. In contrast, most DiI-labeled nodose ganglia neurons were moderately stained for HCN3.
DISCUSSION
Extrinsic afferent nerves play a major role in the control of GI function. Altered vagal and spinal afferent nerve sensitivity has been implicated in the pathophysiology of GI symptoms such as bloating, discomfort and pain. Previous studies have shown that there exist major differences in vagal and spinal primary afferent neurons in their sensitivity to mechanical and chemical stimulations. Delineating the molecular mechanisms that control the excitability of spinal and vagal afferents of the GI tract may potentially offer novel therapeutic targets. The present study provided evidence that HCN channels differentially regulate the excitability of spinal and vagal afferents innervating the small intestine.
In the first series of the study, we tested the effects of HCN channel blocker, CsCl, on the mechanosensory responses of mesenteric nerves of the jejunum. Consistent with previous reports, ramp distension of the jejunum evoked biphasic increases in mesenteric nerve discharge, which was due to the activation of three major types of afferent fibers: low-threshold fibers, wide dynamic range fibers and high-threshold fibers[2]. CsCl at 5 mmol/L was found to attenuate the first phase of the increase in afferent discharge (pressure rose by 10 mmHg from the baseline) and showed no significant effect on the second phase of the response (pressure rose from 10 to 60 mmHg). CsCl is a relatively weak HCN channel blocker and was non-selective among different HCN isoforms. We choose to test 5 mmol/L CsCl since this was the concentration used in many previous studies[7,16,17] and we noted in pilot experiments that the amplitude of action potential spikes was not altered, suggesting that conduction of nerve signals was not impaired in the presence of 5 mmol/L CsCl. To further exclude the possible off-target effects of 5 mmol/L CsCl, we tested a more potent HCN channel blocker ZD7288 (10 μmol/L) and found that it had a similar effect on the mechanosensory responses of mesenteric nerves. Thus, we could be relatively confident that the inhibitory effects of 5 mmol/L CsCl on the mechanosensory responses were due to blockade of HCN channels and that this set of data show certain HCN isoforms that exert a tonic facilitatory action on the excitability of low-threshold afferent fibers.
Booth et al[3] showed in rats that the low-threshold responses of mesenteric nerves to ramp distension of the jejunum diminished whilst the high- threshold responses remained intact following chronic vagotomy. Therefore, in the second series of the experiments, we tested the hypothesis that there were differences in the expression of functional HCN channels in vagal vs spinal primary afferent neurons. To this end, we conducted patch clamp recording of Ih in acutely dissociated DRG and NG neurons. Ih currents of varying amplitudes could be elicited by hyperpolarizing voltage pulses in approximately half of DRG and NG neurons. We then focused on DiI-labeled (intestinal) spinal and vagal sensory neurons. Ih current was present in 71.4% of DRG neurons and 90.9% of NG neurons. The Ih currents of DRG neurons were generally of greater amplitude than those of NG neurons, but the kinetics (fast and slow time constants and activation curves) of Ih currents in these two populations were not significantly different. At the present, subtype selective HCN blockers are not available, but different subtypes of HCN channels are known to show distinct cAMP sensitivity. HCN2 and HCN4 are strongly modulated by cAMP, whilst HCN1 and HCN3 show minimal or no sensitivity to cAMP[18]. We therefore tested the sensitivity of Ih currents to cAMP in DiI-labeled DRG and NG neurons. In 3 of 11 DRG neurons tested, the Ih currents showed a significant sensitivity to 8-Br-cAMP with a significant depolarizing shift of the haft activation voltage. In the other 8 DRG neurons and all (7/7) DiI-labeled NG neurons, Ih currents were not altered by 8-Br-cAMP. Admittedly, the number of cells included in this study was small. Nevertheless, this set of data does suggest that subpopulations of spinal afferents of the small intestine might express cAMP-sensitive (HCN2 or HCN4) and cAMP-insensitive HCN isoforms (HCN1 or HCN3), whilst vagal afferents of the small intestine appeared to express the cAMP-insensitive HCN channels only.
In the final series of experiments, we detected the expression of HCN isoforms in spinal and vagal primary afferent neurons by means of immunohistochemistry. HCN1 was not detected in DRG or NG sections. Intense HCN2 immunoreactivity was present in 12.3% of unidentified DRG and 2.3% unidentified NG neurons, respectively. Conversely, HCN3 was more abundant in NG than in DRG. HCN4 was seen in a minority of DRG neurons but not in NG neurons. Furthermore, we found that DRG neurons retrogradely labeled from the small intestine were partially immunoreactive to HCN2 and weakly stained for HCN3, whereas NG neurons retrogradely labeled from the small intestine were virtually all immunoreactive to HCN3 but not to HCN2. Combined with the patch-clamp and the afferent nerve recording data, these results would suggest that the vagal primary afferent nerves of the small intestine express cAMP-insensitive HCN3 channels. Since HCN blockers, CsCl and ZD7288, inhibited the low-threshold (i.e., vagal) mechanosensory responses of mesenteric nerves, it appears likely that HCN3 channels exert a tonic facilitatory action on the excitability of vagal afferents.
The expression of HCN isoforms in sensory neurons has been investigated in several previous studies. For spinal primary afferent neurons, Tu et al[19] reported presence of HCN1 immunoreactivity in large- and medium-sized neurons, HCN2 in proportions of neurons of all sizes and absence of HCN4 immunoreactivity in rat DRG (L4-L5). The distribution of immunoreactivity for HCN isoforms within the DRG found in the present study was consistent with the findings of Matsuyoshi et al[8], who demonstrated that HCN2 was the dominant isoform in L6-S1 rat DRG. In that study, HCN2 immunoreactivity was detected in 46.9%, 21.1% and 4.5% of small-, medium- and large-sized neurons, respectively; and there was no apparent staining for HCN1 and scarce staining for HCN4. For vagal afferent neurons, Tu et al[5] detected HCN1, HCN3, and HCN4 in A-fiber neurons and HCN2, HCN3 and HCN4 in C-fiber neurons in NG of rats using a double-staining technique. They further demonstrated that over-expression of HCN1-3 channels in nodose neurons contributed to the decreased excitability of vagal afferent neurons in diabetic rats. Li et al[7] also reported that over-expression of HCN1 and HCN2 was associated with the blunted excitability of A fiber aortic baroreceptor neurons in type-1 diabete rats. In the current study, most NG neurons were moderately stained for HCN3 and minorities of NG neurons were stained for HCN2, whilst HCN1 and HCN4 immunoreactivity was hardly detectable. It is likely that there exist subtle species differences in the distribution of HCN isoforms in vagal afferent neurons and that expression of HCN isoforms in vagal afferent neurons may be altered under certain pathological conditions.
Momin and colleagues demonstrated that Ih current had an important influence on action potential generation in sensory neurons[20]. We noted that CsCl and ZD7288 inhibited the low-threshold (vagal) mechanosenosry responses without significant effect on the high-threshold (presumably spinal) responses, although the patch-clamp and immunohistochemical data both showed that functional HCN channels were present in some spinal afferent neurons. It appears likely that under normal conditions, HCN channels expressed on spinal afferent neurons only have minimal effect on the excitability of afferent terminals. However, this does not preclude the possible contribution of HCN channels to the hyper-excitability of spinal afferents seen in pathological conditions such as inflammatory and non-inflammatory bowel diseases. In this regard, it is particularly interesting to note that a proportion of spinal afferents appeared to express HCN2, which is sensitive to modulation by cAMP, a key intracellular second messenger for a variety of inflammatory mediators[18,21]. Conceivably, spinal afferents expressing HCN2 may become hypersensitive due to the increased availability of inflammatory mediators such as bradykinin, ATP, 5-HT and prostaglandin E2[22].
In summary, the data obtained in this study suggest that HCN2 and HCN3 are differentially expressed in vagal vs spinal primary afferents innervating the small intestine. HCN2 is the major HCN isoforms in spinal afferents and HCN3 is more abundant in vagal afferent neurons and seems to exert a tonic facilitatory modulation on the excitability of afferent terminals.
COMMENTS
Background
The gastrointestinal (GI) tract is innervated by vagal and spinal afferent nerves, which play important roles in the control of GI function. Altered vagal and spinal afferent nerve sensitivity has been implicated in the pathophysiology of GI symptoms such as bloating, discomfort and pain, but the molecular mechanisms are still not clear. Hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channels underlie the pacemaker current in the heart and are also known to regulate CNS neuronal excitability. The current study investigated the possible role of HCN channels in the control of small intestinal afferent nerve sensitivity.
Research frontiers
Previous studies implicated HCN channels in neuropathic pain and in control of arterial baroreceptor and bladder afferent sensitivity. Linden and colleagues reported that enhanced Ih current contributes to the increased excitability of a subpopulation of enteric neurons in inflamed guinea pig colon. Until recently, little information is available regarding the potential role of HCN channels in the function of extrinsic afferents of the GI tract.
Innovations and breakthroughs
As reported in this study, HCN2 and HCN3 are differentially expressed in vagal vs spinal primary afferents innervating the small intestine. HCN2 is the major HCN isoform in spinal afferents whereas HCN3 is more abundant in vagal afferent neurons. HCN3 seems to exert a tonic facilitatory action on the excitability of vagal afferent terminals.
Applications
The current findings suggest that drugs targeting HCN2 or HCN3 may potentially reverse dysfunction of vagal and spinal afferents seen in functional and inflammatory GI diseases.
Terminology
HCN is a hyperpolarization-activated cyclic nucleotide-gated cation channel which mediates Ih current.
Peer review
The design of the study is correct and the findings are important, as these channels may redirect further research against bowel symptoms to other targeted drugs.
Footnotes
Supported by Science and Technology Commission of Shanghai Municipality, No. 10ZR1417300; Educational Commission of Shanghai Municipality, No. 10ZZ69
Peer reviewer: Rubén Ciria, MD, PhD, Hepatobiliary Surgery and Liver Transplantation Unit, Hospital Universitario Reina Sofía, Cordoba 14004, Spain
S- Editor Wu X L- Editor Ma JY E- Editor Xiong L
References
- 1.Blackshaw LA, Brookes SJ, Grundy D, Schemann M. Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil. 2007;19:1–19. doi: 10.1111/j.1365-2982.2006.00871.x. [DOI] [PubMed] [Google Scholar]
- 2.Rong W, Hillsley K, Davis JB, Hicks G, Winchester WJ, Grundy D. Jejunal afferent nerve sensitivity in wild-type and TRPV1 knockout mice. J Physiol. 2004;560:867–881. doi: 10.1113/jphysiol.2004.071746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Booth CE, Shaw J, Hicks GA, Kirkup AJ, Winchester W, Grundy D. Influence of the pattern of jejunal distension on mesenteric afferent sensitivity in the anaesthetized rat. Neurogastroenterol Motil. 2008;20:149–158. doi: 10.1111/j.1365-2982.2007.01003.x. [DOI] [PubMed] [Google Scholar]
- 4.Blackshaw LA, Gebhart GF. The pharmacology of gastrointestinal nociceptive pathways. Curr Opin Pharmacol. 2002;2:642–649. doi: 10.1016/s1471-4892(02)00211-4. [DOI] [PubMed] [Google Scholar]
- 5.Tu H, Zhang L, Tran TP, Muelleman RL, Li YL. Diabetes alters protein expression of hyperpolarization-activated cyclic nucleotide-gated channel subunits in rat nodose ganglion cells. Neuroscience. 2010;165:39–52. doi: 10.1016/j.neuroscience.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 6.Kouranova EV, Strassle BW, Ring RH, Bowlby MR, Vasilyev DV. Hyperpolarization-activated cyclic nucleotide-gated channel mRNA and protein expression in large versus small diameter dorsal root ganglion neurons: correlation with hyperpolarization-activated current gating. Neuroscience. 2008;153:1008–1019. doi: 10.1016/j.neuroscience.2008.03.032. [DOI] [PubMed] [Google Scholar]
- 7.Li YL, Tran TP, Muelleman R, Schultz HD. Blunted excitability of aortic baroreceptor neurons in diabetic rats: involvement of hyperpolarization-activated channel. Cardiovasc Res. 2008;79:715–721. doi: 10.1093/cvr/cvn141. [DOI] [PubMed] [Google Scholar]
- 8.Matsuyoshi H, Masuda N, Chancellor MB, Erickson VL, Hirao Y, de Groat WC, Wanaka A, Yoshimura N. Expression of hyperpolarization-activated cyclic nucleotide-gated cation channels in rat dorsal root ganglion neurons innervating urinary bladder. Brain Res. 2006;1119:115–123. doi: 10.1016/j.brainres.2006.08.052. [DOI] [PubMed] [Google Scholar]
- 9.Masuda N, Hayashi Y, Matsuyoshi H, Chancellor MB, de Groat WC, Yoshimura N. Characterization of hyperpolarization-activated current (Ih) in dorsal root ganglion neurons innervating rat urinary bladder. Brain Res. 2006;1096:40–52. doi: 10.1016/j.brainres.2006.04.085. [DOI] [PubMed] [Google Scholar]
- 10.Xiao J, Nguyen TV, Ngui K, Strijbos PJ, Selmer IS, Neylon CB, Furness JB. Molecular and functional analysis of hyperpolarisation-activated nucleotide-gated (HCN) channels in the enteric nervous system. Neuroscience. 2004;129:603–614. doi: 10.1016/j.neuroscience.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Linden DR, Sharkey KA, Mawe GM. Enhanced excitability of myenteric AH neurones in the inflamed guinea-pig distal colon. J Physiol. 2003;547:589–601. doi: 10.1113/jphysiol.2002.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Suckow SK, Caudle RM. Identification and immunohistochemical characterization of colospinal afferent neurons in the rat. Neuroscience. 2008;153:803–813. doi: 10.1016/j.neuroscience.2008.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vasilyev DV, Barish ME. Postnatal development of the hyperpolarization-activated excitatory current Ih in mouse hippocampal pyramidal neurons. J Neurosci. 2002;22:8992–9004. doi: 10.1523/JNEUROSCI.22-20-08992.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Welie I, Wadman WJ, van Hooft JA. Low affinity block of native and cloned hyperpolarization-activated Ih channels by Ba2+ ions. Eur J Pharmacol. 2005;507:15–20. doi: 10.1016/j.ejphar.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Rong W, Winchester WJ, Grundy D. Spontaneous hypersensitivity in mesenteric afferent nerves of mice deficient in the sst2 subtype of somatostatin receptor. J Physiol. 2007;581:779–786. doi: 10.1113/jphysiol.2006.125187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doan TN, Kunze DL. Contribution of the hyperpolarization-activated current to the resting membrane potential of rat nodose sensory neurons. J Physiol. 1999;514(Pt 1):125–138. doi: 10.1111/j.1469-7793.1999.125af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Doan TN, Stephans K, Ramirez AN, Glazebrook PA, Andresen MC, Kunze DL. Differential distribution and function of hyperpolarization-activated channels in sensory neurons and mechanosensitive fibers. J Neurosci. 2004;24:3335–3343. doi: 10.1523/JNEUROSCI.5156-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaupp UB, Seifert R. Molecular diversity of pacemaker ion channels. Annu Rev Physiol. 2001;63:235–257. doi: 10.1146/annurev.physiol.63.1.235. [DOI] [PubMed] [Google Scholar]
- 19.Tu H, Deng L, Sun Q, Yao L, Han JS, Wan Y. Hyperpolarization-activated, cyclic nucleotide-gated cation channels: roles in the differential electrophysiological properties of rat primary afferent neurons. J Neurosci Res. 2004;76:713–722. doi: 10.1002/jnr.20109. [DOI] [PubMed] [Google Scholar]
- 20.Momin A, Cadiou H, Mason A, McNaughton PA. Role of the hyperpolarization-activated current Ih in somatosensory neurons. J Physiol. 2008;586:5911–5929. doi: 10.1113/jphysiol.2008.163154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dray A. Inflammatory mediators of pain. Br J Anaesth. 1995;75:125–131. doi: 10.1093/bja/75.2.125. [DOI] [PubMed] [Google Scholar]
- 22.Bueno L, Fioramonti J. Visceral perception: inflammatory and non-inflammatory mediators. Gut. 2002;51 Suppl 1:i19–i23. doi: 10.1136/gut.51.suppl_1.i19. [DOI] [PMC free article] [PubMed] [Google Scholar]