Abstract
AIM: To evaluate whether contrast enhanced ultrasound (CEUS) might also be used for response prediction and early response evaluation in patients receiving bevacizumab based chemotherapy for metastasized colorectal cancer.
METHODS: Thirty consecutive patients with non primary resectable liver metastases from colorectal cancer underwent CEUS before treatment (CEUS date 1) and before the second (CEUS date 2) and fourth (CEUS date 3) cycle of bevacizumab based chemotherapy. Three parameters [PEAK, Time to peak (TTP) and RISE RATE]were correlated with radiological response.
RESULTS: For neoadjuvant purpose a reduction of tumour mass was required to assume clinical response. Based on these response criteria there was a significant (P < 0.001) correlation in TTP between metastases of responders (9.08 s) and non-responders (14.76 s) archived on CEUS date 1. By calculating a standardized quotient (metastases divided by normal liver tissue) we were able to define a cut off, predicting response with a sensitivity of 92.3 % and a specificity of 100 %. To reflect a palliative intention only those patients with progressive disease were classified as non-responders. In this stetting TTP was also significantly (P < 0.01) different between responders and non-responders. In contrast, Peak and Rise rate did not show any significant difference between responder and non-responder.
CONCLUSION: CEUS might serve as a surrogate marker to predict treatment response in patients with metastasized colorectal cancer who receive antiangiogenic therapy.
Keywords: Colorectal cancer, Liver metastases, Response prediction to chemotherapy, Contrast-enhanced ultrasound, Bevacizumab
INTRODUCTION
The liver is the most frequent site of colorectal cancer (CRC) metastases, with 15% to 25% of patients having liver metastases at diagnosis and further 30% developing liver metastases at a later point in the disease course[1-3]. While for decades prognosis for these patients was poor, introduction of multimodal management approaches such as highly active chemobiological therapy and innovative surgical techniques, have significantly improved both disease free and overall survival. Those patients who can have liver metastases resected even display 5-year survival rates of up to 40%, with 20% alive after 10 years[4]. Unfortunately, most patients initially present with unresectable disease. However, in some of these cases neoadjuvant treatment can reduce tumour load and allow secondary resections. Thus, the aim of systemic chemotherapy in patients with non primary respectable liver metastases has undergone a transition from a pure palliative to a neoadjuvant and thereby curative concept[5].
To improve anti-tumour activity, in clinical routine the vascular endothelial growth factor (VEGF)-antibody bevacizumab is often added to treatment[6-8]. While on the one hand such combination chemotherapies are associated with improved response rates, they are on the other hand related to high toxicity and costs, and it remains an unsolved problem to identify those patients that will benefit from therapy. Fluorodeoxyglucose positron emission tomography (FDG PET) demonstrated efficacy in early treatment monitoring of a variety of tumour diseases, however data supporting its use in patients with advanced colorectal cancer are conflicting[9,10]. A novel technique to improve response evaluation to anti-angiogenic agents represents contrast-enhanced ultrasound[11] with the opportunity to detect changes of tumour perfusion at early time-points after administration of chemotherapy.
In the present study we explore the use of CEUS for response prediction and early response evaluation in patients with liver metastases from CRC, which were treated with bevacizumab containing chemotherapy.
MATERIALS AND METHODS
Patients
Between October 2007 and October 2009, 30 consecutive patients with histological confirmed colorectal carcinoma and non primary resectable liver metastasis, according to the decision of our interdisciplinary tumour board, were enrolled. Five patients had metastatic rectal cancer and 25 patients had colonic cancer. The histological grading was in 13 cases G2 and in 17 cases G3. The number of individual liver metastases ranged from 4 to 25.
All eligible patients were > 18 years old and had no prior history of receiving chemo- or radiotherapy or major surgery within 28 d before initiation of study treatment. The protocol was approved by the institutional review board and carried out in accordance with the Declaration of Helsinki and local ethical and legal requirements.
Treatment
Chemotherapy consisted of Leucovorin (LV) 400 mg/m2 per day as a 2 h infusion followed by bolus 5-Fluorouracil (5-FU) 400 mg/m2 per day and a 46-h infusion of 5-FU 2400 mg/m2 per day (simplified FOLFIRI). Bevacizumab was administered every two weeks at a dose of 5 mg per kg.
Contrast-enhanced ultrasound
Within 1 d before the first (day 1), second (day 15) and forth (day 43) application of chemotherapy a contrast enhanced ultrasound (CEUS) of one liver metastasis was performed (CEUS - date 1, 2 and 3). In case of multiple liver metastases, the metastasis which could be best positioned in the CEUS was selected. Ultrasound examinations were performed using the Aplio system (Toshiba Medical Systems Europe, Neuss, Germany). All patients were examined with a convex 3.5 MHz transducer as baseline US. The CEUS was performed using the contrast enhancer SonoVue® and predefined settings using the 3.5 MHz transducer. All patients were examined in wideband harmonic mode (pulse inversion) at low energy (low mechanical index) optimizing real-time detection of harmonic contrast response. The agent was injected as bolus in units of 2.4 mL through a peripheral venous catheter over 2 s, followed by bolus injection of 10 mL of 0.9% NaCl solution. Video documentation of all examination steps was obtained from the beginning of injection for a period of at least 2 min, allowing recording all steps of contrast enhancement characteristics (Figure 1).
Figure 1.
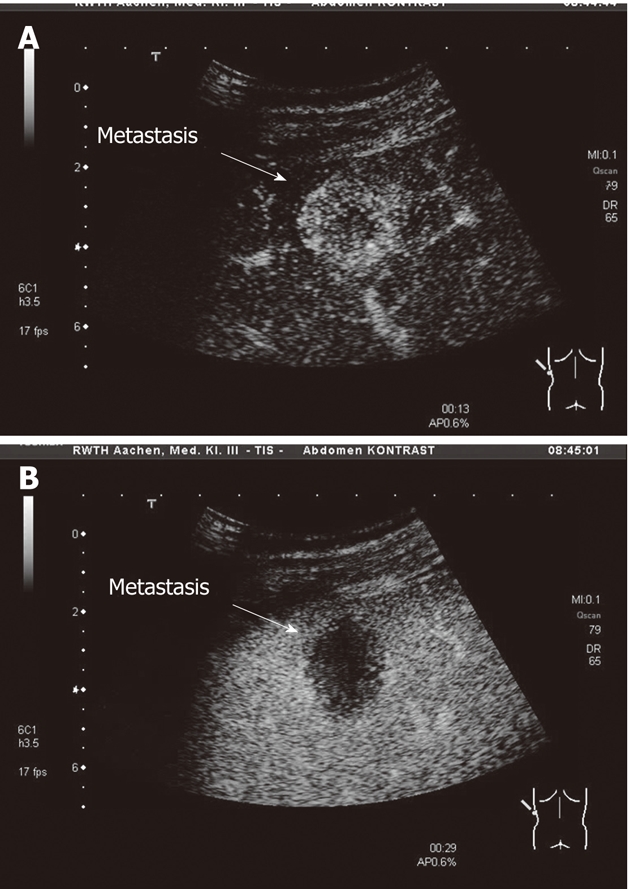
Contrast enhanced ultrasound. A: A representative liver metastasis after 13 s of contrast agent injection with early contrast enhancement; B: A representative liver metastasis after 29 s of contrast agent injection with lost of contrast enhancement.
For quantitatively measuring vascularity in the metastasis and normal liver tissue by contrast dye characteristics we used the Bracco QONTRAST software (Version 4.00). This software is a specific sonographic quantification software, based on pixel by pixel signal intensity over time to obtain contrast-enhanced sonographic perfusion maps for each metastasis. In all cases, regions of interest (ROIs) were chosen over the whole area of the metastasis. Additionally we selected ROIs at least two centimetres lateral (same deepness) of the metastasis in a region with normal liver tissue. The first contrast enhancement seen in ROI determines the beginning of the measurement (Figure 2).
Figure 2.
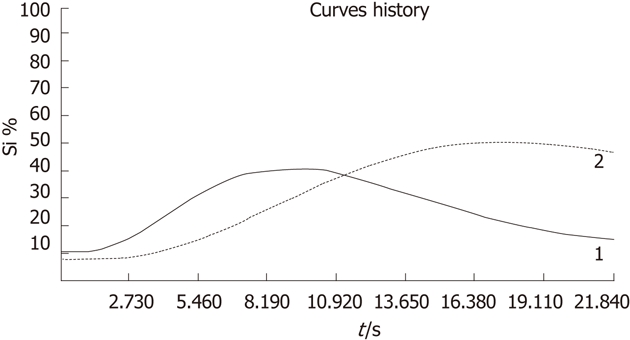
Curves of contrast behavior in a liver metastasis (solid line, 1) and normal liver tissue (dotted line, 2) over the time (s = seconds) and percent of contrast enhancement (Si %).
Three parameters were calculated including the PEAK (%; maximum peak of contrast intensity), the Time to peak (TTP) (s, time to reach the maximum peak of contrast intensity) and the RISE RATE (s-1), which is equal to contrast enhancement. The ultrasound exam was analyzed by two independent investigators blinded to the cases.
Response evaluation
All patients received before and after 3 mo of therapy a CT scan for response evaluation. Tumour response was defined by changes in diameter of target lesions on the basis of Multislice-CT results at baseline and after the first 3 mo of FOLFIRI-Avastin administration, according to published recommendations [Response Evaluation Criteria In Solid Tumors (RECIST) 1.1][12].
Statistical analysis
The multivariate analysis of variance model (MANOVA) was applied to test the within subject factors time (CEUS date 1, 2 and 3) and tissue (metastasis vs normal). Pair wise comparisons were performed with the paired t-test. The MANOVA was applied to test the between subject factor response (responder vs non-responder). For each separate date the unpaired t-test was used to compare the two groups. ROC curves were used to analyse the diagnostic power of the parameters. Cut points were chosen as to maximize the Youden index. Patients were classified as responder if the parameter is smaller or equal to the cut point.
RESULTS
Patients and response to chemotherapy
A total of 30 patients (8 women and 22 men) with a mean age of 62 (range: 50-78) were enrolled and received FOLFIRI supplemented by bevacizumab for metastasized colorectal cancer. All patients underwent radiological evaluation of tumour-load at baseline and after a period of 3 mo of treatment. According to RECIST criteria 1 patient showed a complete response (CR), 12 patients showed a partial response (PR), 8 patients had a stable disease (SD) and 9 patients had a progressive disease (PD). 4 of the 30 patients underwent resection of liver metastases within 4 mo after initiation of chemotherapy.
To reflect the clinical reality of patients with metastasized colorectal cancer, two distinct definitions of response were used: in a first scenario only those patients with PR and CR in radiological evaluation were classified as responders, while in a second scenario also patients with radiological SD were included in the group of responders.
Contrast-enhanced ultrasound
At baseline, all hepatic tumour sites were assessed by classical sonography. One metastasis was chosen as an index metastasis for further examination bei CEUS. The diameters of these selected lesions ranged from 1.9 cm to 15 cm (mean 2.7 cm). 26 lesions were classified as predominantly hypoechoic and 4 as hyperechoic according to B-mode ultrasound. In these lesions, all relevant quantitative parameters (TTP, PEAK and RISE RATE), were analyzed at baseline and during the course of treatment (CEUS-date 1, 2 and 3) by CEUS.
By applying the more strict criteria of the first scenario (neoadjuvant purpose), reflecting the need of tumour reduction in patients that could benefit from neoadjuvant therapy, we detected a significant (P < 0.001) difference of TTP in metastases between responders (9.08 s) and non-responders (14.76 s) already at baseline (CEUS date 1) shown in Table 1 and illustrated in Figure 3A. Furthermore in the group of the responders, a strong and continuous increase in TTP was observed during therapy and this reflects the effect of bevacizumab on tumour vascularisation. Strikingly no comparable therapy related increase in TTP was detectable within the group of non-responders. While on CEUS date 2 differences in TTP between responders and non-responders were still detectable, after 4 cycles (8 wk) of therapy identical TTP were detected in both groups. In contrast to these observations in metastatic tissue, no differences in TTP were found in normal liver tissue. To further standardize our data, a standard TTP-quotient was calculated by dividing the TTP measured in liver metastasis by TTP in the corresponding normal liver. Strikingly standardization of the described data did not change the described observations.
Table 1.
Time to peak and to peak quotient in liver metastasis
| Date 1 | Date 2 | Date 3 | |
| Time to peak | |||
| Responder (CR, PR) | |||
| mean ± SD | 9.1 ± 2.9 | 12.1 ± 2.3 | 14.6 ± 3.9 |
| 95% CI | 7.3-10.8 | 10.7-13.6 | 12.2-17.0 |
| Non-responder (SD1,PD) | |||
| mean ± SD | 14.8 ± 3.7 | 16.7 ± 2.8 | 16.4 ± 2.6 |
| 95% CI | 12.8-16.7 | 15.2-18.2 | 15.0-17.7 |
| P value compared responder with non-responder | < 0.001 | < 0.001 | NS |
| Time to peak quotient | |||
| Responder (CR, PR) | |||
| mean ± SD | 57.5 ± 11.8 | 80.5 ± 5.4 | 93.7 ± 9.2 |
| 95% CI | 50.3-64.6 | 77.2-83.7 | 88.2-99.2 |
| Non-responder (SD1, PD) | |||
| mean ± SD | 98.7 ± 12.6 | 100.6 ± 13.3 | 96.9 ± 9.7 |
| 95% CI | 92.2-105.2 | 93.8-107.5 | 91.9-101.9 |
| P value compared responder with non-responder | < 0.001 | < 0.001 | NS |
NS: Not significant; CR: Complete response; PR: Partial response; SD1: Stable disease; PD: Progressive disease; CI: Confidence interval.
Figure 3.
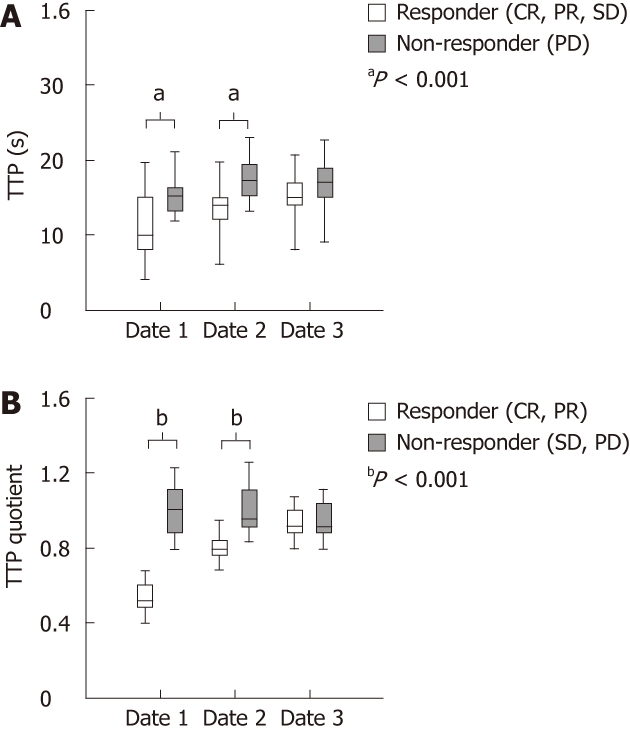
Time to peak parameters. A: Time to peak (TTP) values measured in the metastasis between responders and non-responders on contrast enhanced ultrasound (CEUS) date 1, date 2 and date 3 (responders with complete response (CR), partial response (PR) and stable disease (SD) (n = 21) vs non-responder with progressive disease (PD) (n = 9); B:The TTP quotient between responders and non-responders on CEUS date 1, date 2 and date 3 (CR and PR were classified as responders (n = 13) and patients with SD and PD as non-responders (n = 17).
Chemobiological therapies are related to high toxicity. Response prediction would therefore allow restricting treatment to patients that will benefit from therapy. We therefore attempted to calculate a cut of point which predicted response with high specificity and sensitivity. According to our data, a TTP-quotient < 0.7 predicted a decrease of tumour load according to RECIST with a sensitivity of 92.3% and a specificity of 100% (Table 1 and Figure 3B).
In a second scenario we used less strict criteria for response definition, as patients with stable disease were also included in the group of responders, reflecting the clinical reality of patients in a palliative setting. Interestingly, also based on this response definition TTP and TTP-quotient were significantly lower in the group of the responders compared to non responders (Figure 3A). Here, a TTP quotient of 0.8 predicted response with a sensitivity of 61.9% and a specificity of 100%.
In contrast, the PEAK and RISE RATE parameter did not show any significant difference between responder and non-responder independent of response definition, use of the quotient or date of CEUS. In addition, there was no significant correlation between tumour response and differentiation of the tumour or the number, the location or the size of liver metastases (data not shown).
DISCUSSION
In the last years intensive efforts were conducted to identify surrogate markers that predict response to antiangiogenic combination chemotherapies. Previously a correlation between early metabolic response according to PET and patients outcome was demonstrated in patients receiving bevacizumab. However comparable data regarding colorectal cancer are insufficient. Indeed just recently two studies investigating the role of FDG-PET for treatment monitoring in patients with metastasized colorectal cancer revealed conflicting results[9,10]. Furthermore, despite these advances in monitoring of treatment, to our knowledge no data describing surrogate markers for prediction of response before starting the treatment are available.
In the present study we demonstrate that CEUS might evolve as an innovative tool in prediction of response and response evaluation in patients with metastasized colorectal cancer receiving bevacizumab based therapy. We clearly demonstrate that baseline TTP and TTP quotient are significantly lower in the group of the responders compared to the non-responders, meaning that low baseline TTP significantly correlates with tumor response according to RECIST. Furthermore, correlating to the antiangiogenic effect of bevacizumab we observed a strong increase in TTP and TTP quotient during chemotherapy, which was restricted to the group of the responders. In line with these data Varallyay et al[13] recently demonstrated a decrease in tumor perfusion after bevacizumab based chemotherapy by using dynamic MRI.
Chemobiological combination chemotherapies are associated with considerable toxicity and prediction of response could help to prevent non-responders from undesiderable side-effects. Based on the described perfusion analysis we established a cut off for TTP which predicted response with a specificity and sensitivity of up to 90%. To our knowledge we here for the fist time describe a parameter which reliably predicts tumor response even before starting therapy.
Despite these encouraging results, the present study has some limitations. The number of patients included is limited and at present the follow up is only 3 mo, so that a conclusion on the use of CEUS for predicting survival is not possible. Additionally, we only included one index lesion that could be best positioned in the CEUS and thus it would be of interest to include different liver metastasis of one patient to further assess the power of this method.
In summary, our results indicate that CEUS might serve as a useful, noninvasive surrogate marker of early response in patients with liver metastases of a colorectal cancer receiving bevacizumab. However, larger trials with a longer follow-up are needed to clarify the clinical use of CEUS in this field.
COMMENTS
Background
In the last few years contrast enhanced ultrasound (CEUS) was established for differentiation between benign and malignant liver lesions. CEUS might also be used for response prediction and early response evaluation in patients receiving chemotherapy for metastasized colorectal cancer.
Research frontiers
In the present study we explore the use of CEUS for response prediction and early response evaluation in patients with liver metastases from CRC, which were treated with bevacizumab containing chemotherapy.
Innovations and breakthroughs
The results indicate that CEUS might serve as a useful, noninvasive surrogate marker of early response in patients with liver metastases of a colorectal cancer receiving bevacizumab.
Applications
CEUS for response evaluation may represent a future diagnostic pathway in the management of patients with liver metastases of a colorectal cancer.
Peer review
This is an interesting pilot study investigating the relevance of CEUS in metastatic colorectal cancer. For the first time a parameter, which reliably predicts tumor response even before starting therapy, was described.
Footnotes
Peer reviewer: Andrada Seicean, MD, PhD, Third Medical Clinic Cluj Napoca, University of Medicine and Pharmacy Cluj Napoca, Romania, 15, Closca Street, Cluj-Napoca 400039, Romania
S- Editor Tian L L- Editor Kerr C E- Editor Xiong L
References
- 1.Young H, Baum R, Cremerius U, Herholz K, Hoekstra O, Lammertsma AA, Pruim J, Price P. Measurement of clinical and subclinical tumour response using [18F]-fluorodeoxyglucose and positron emission tomography: review and 1999 EORTC recommendations. European Organization for Research and Treatment of Cancer (EORTC) PET Study Group. Eur J Cancer. 1999;35:1773–1782. doi: 10.1016/s0959-8049(99)00229-4. [DOI] [PubMed] [Google Scholar]
- 2.Adam R, Avisar E, Ariche A, Giachetti S, Azoulay D, Castaing D, Kunstlinger F, Levi F, Bismuth F. Five-year survival following hepatic resection after neoadjuvant therapy for nonresectable colorectal. Ann Surg Oncol. 2001;8:347–353. doi: 10.1007/s10434-001-0347-3. [DOI] [PubMed] [Google Scholar]
- 3.Scheele J, Stang R, Altendorf-Hofmann A, Paul M. Resection of colorectal liver metastases. World J Surg. 2011;19:59–71. doi: 10.1007/BF00316981. [DOI] [PubMed] [Google Scholar]
- 4.Power DG, Kemeny NE. Role of adjuvant therapy after resection of colorectal cancer liver metastases. J Clin Oncol. 2010;28:2300–2309. doi: 10.1200/JCO.2009.26.9340. [DOI] [PubMed] [Google Scholar]
- 5.Adam R, Pascal G, Castaing D, Azoulay D, Delvart V, Paule B, Levi F, Bismuth H. Tumor progression while on chemotherapy: a contraindication to liver resection for multiple colorectal metastases? Ann Surg. 2004;240:1052–1061; discussion 1052-1061. doi: 10.1097/01.sla.0000145964.08365.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 7.Kabbinavar F, Hurwitz HI, Fehrenbacher L, Meropol NJ, Novotny WF, Lieberman G, Griffing S, Bergsland E. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer. J Clin Oncol. 2003;21:60–65. doi: 10.1200/JCO.2003.10.066. [DOI] [PubMed] [Google Scholar]
- 8.Malavasi N, Ponti G, Depenni R, Bertolini F, Zironi S, Luppi G, Conte PF. Complete pathological response in a patient with multiple liver metastases from colon cancer treated with Folfox-6 chemotherapy plus bevacizumab: a case report. J Hematol Oncol. 2009;2:35. doi: 10.1186/1756-8722-2-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Geus-Oei LF, van Laarhoven HW, Visser EP, Hermsen R, van Hoorn BA, Kamm YJ, Krabbe PF, Corstens FH, Punt CJ, Oyen WJ. Chemotherapy response evaluation with FDG-PET in patients with colorectal cancer. Ann Oncol. 2008;19:348–352. doi: 10.1093/annonc/mdm470. [DOI] [PubMed] [Google Scholar]
- 10.Byström P, Berglund A, Garske U, Jacobsson H, Sundin A, Nygren P, Frödin JE, Glimelius B. Early prediction of response to first-line chemotherapy by sequential [18F]-2-fluoro-2-deoxy-D-glucose positron emission tomography in patients with advanced colorectal cancer. Ann Oncol. 2009;20:1057–1061. doi: 10.1093/annonc/mdn744. [DOI] [PubMed] [Google Scholar]
- 11.Strobel D, K Seitz, W Blank, A Schuler, C Dietrich, A von Herbay, M Friedrich-Rust, G Kunze, D Becker, U Will, et al. Contrast-enhanced ultrasound for the characterization of focal liver lesions--diagnostic accuracy in clinical practice (DEGUM multicenter trial) Ultraschall Med. 2008;29:499–505. doi: 10.1055/s-2008-1027806. [DOI] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 13.Varallyay CG, Muldoon LL, Gahramanov S, Wu YJ, Goodman JA, Li X, Pike MM, Neuwelt EA. Dynamic MRI using iron oxide nanoparticles to assess early vascular effects of antiangiogenic versus corticosteroid treatment in a glioma model. J Cereb Blood Flow Metab. 2009;29:853–860. doi: 10.1038/jcbfm.2008.162. [DOI] [PMC free article] [PubMed] [Google Scholar]


