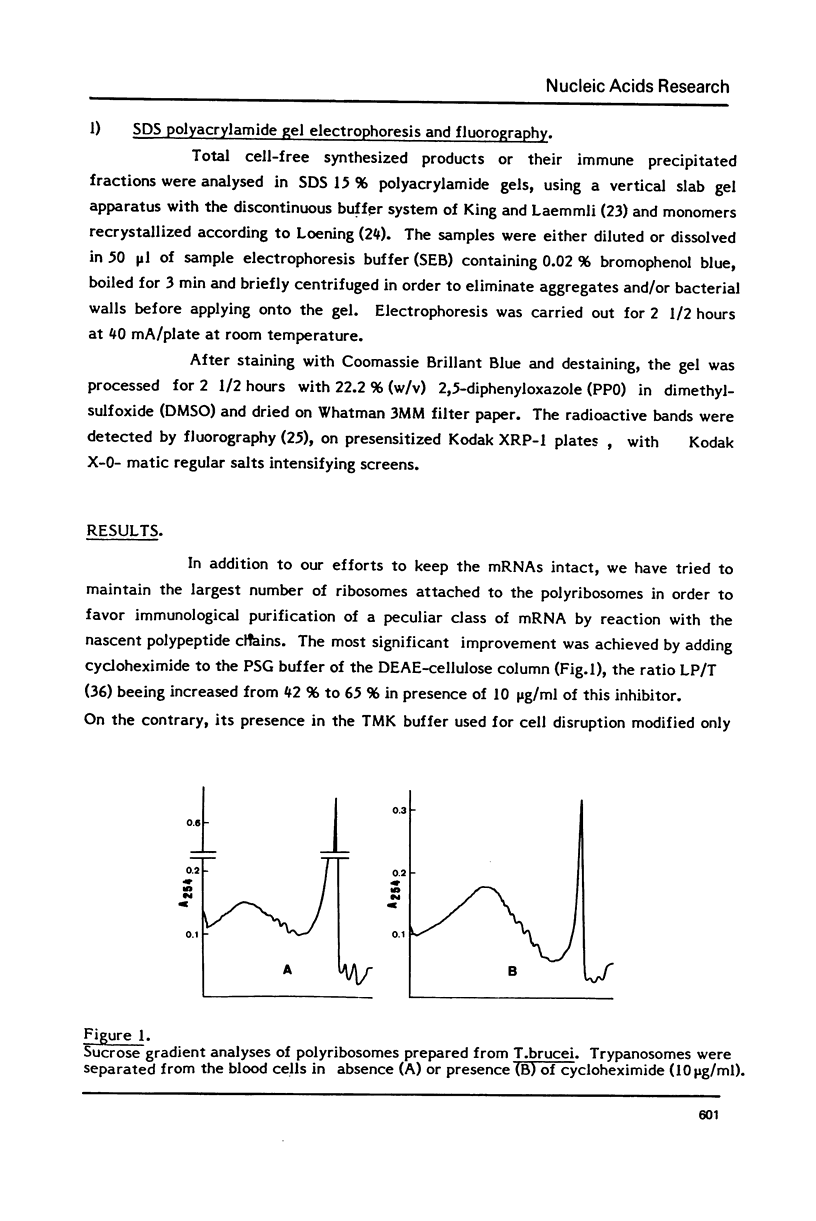
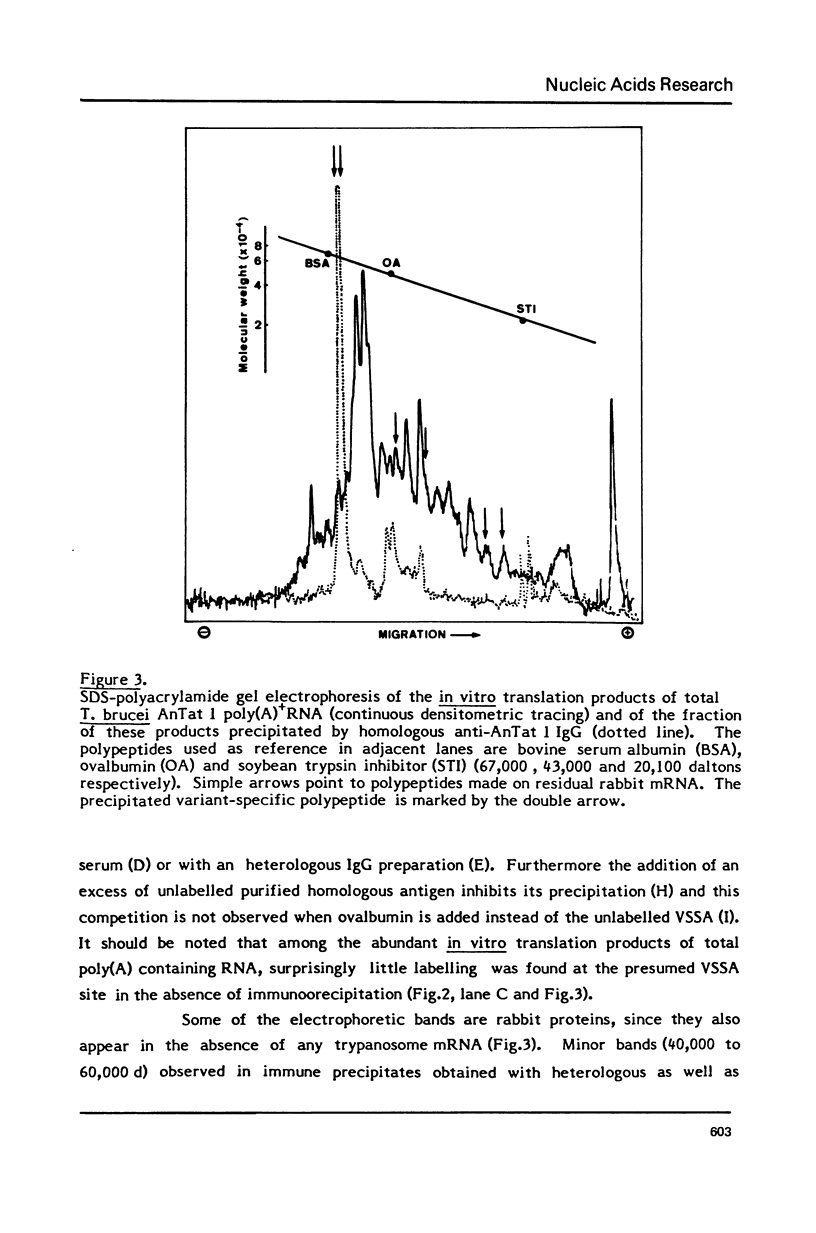
Abstract
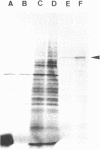
Polyadenylated RNA isolated from total polyribosomes of two variable antigen types (VATs) of T. brucei brucei were shown to program the synthesis, in mRNA-dependant reticulocyte lysates, of a wide variety of polypeptides. After immunoprecipitation of these cell-free products with an homologous antiserum raised against purified variant-specific surface antigen (VSSA), a major electrophoretic band was apparent on fluorography. It was confirmed that this band corresponds to the variable antigen since only an excess of purified homologous antigen will provoke competition. The apparent molecular weight of the in vitro synthesized antigen is about 63,000 daltons. The VSSA mRNA has been found in membrane-bound polyribosomes and a 15 fold immunological purification of this mRNA has been obtained, using partially purified anti-VSSA IgG in conjunction with inactivated Staphylococcus aureus.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnemann J., Heins B., Beato M. Purification and properties of rabbit uterus preuteroglobin mRNA. Nucleic Acids Res. 1977 Nov;4(11):4023–4036. doi: 10.1093/nar/4.11.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benicourt C., Haenni A. L. Differential translation of turnip yellow mosaic virus mRNAs in vitro. Biochem Biophys Res Commun. 1978 Oct 30;84(4):831–839. doi: 10.1016/0006-291x(78)91659-5. [DOI] [PubMed] [Google Scholar]
- COWIE D. B., SPIEGELMAN S., ROBERTS R. B., DUERKSEN J. D. Ribosome-bound beta-galactosidase. Proc Natl Acad Sci U S A. 1961 Jan 15;47:114–122. doi: 10.1073/pnas.47.1.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capbern A., Giroud C., Baltz T., Mattern P. Trypanosoma equiperdum: etude des variations antigéniques au cours de la trypanosomose experimentale du lapin. Exp Parasitol. 1977 Jun;42(1):6–13. doi: 10.1016/0014-4894(77)90055-8. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Antigenic variation in trypanosomes. Proc R Soc Lond B Biol Sci. 1978 Jun 5;202(1146):55–72. doi: 10.1098/rspb.1978.0057. [DOI] [PubMed] [Google Scholar]
- Cross G. A. Identification, purification and properties of clone-specific glycoprotein antigens constituting the surface coat of Trypanosoma brucei. Parasitology. 1975 Dec;71(3):393–417. doi: 10.1017/s003118200004717x. [DOI] [PubMed] [Google Scholar]
- Davies E., Larkins B. A., Knight R. H. Polyribosomes from peas: an improved method for their isolation in the absence of ribonuclease inhibitors. Plant Physiol. 1972 Nov;50(5):581–584. doi: 10.1104/pp.50.5.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggitt M. J., Tappenden L., Brown K. N. Translation in a reticulocyte cell-free system of RNA isolated from blood and culture forms of Trypanosoma brucei. Parasitology. 1977 Oct;75(2):133–141. doi: 10.1017/s0031182000062272. [DOI] [PubMed] [Google Scholar]
- Ghysdael J., Hubert E., Trávnícek M., Bolognesi D. P., Burny A., Cleuter Y., Huez G., Kettmann R., Marbaix G., Portetelle D. Frog oocytes synthesize and completely process the precursor polypeptide to virion structural proteins after microinjection of avian myeloblastosis virus RNA. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3230–3234. doi: 10.1073/pnas.74.8.3230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglioni B., Gianni A. M., Comi P., Ottolenghi S., Rungger D. Translational control of globin synthesis by haemin in Xenopus oocytes. Nat New Biol. 1973 Nov 28;246(152):99–102. doi: 10.1038/newbio246099a0. [DOI] [PubMed] [Google Scholar]
- Gurdon J. B., Lingrel J. B., Marbaix G. Message stability in injected frog oocytes: long life of mammalian alpha and beta globin messages. J Mol Biol. 1973 Nov 5;80(3):539–551. doi: 10.1016/0022-2836(73)90421-x. [DOI] [PubMed] [Google Scholar]
- Housman D., Pemberton R., Taber R. Synthesis of and chains of rabbit hemoglobin in a cell-free extract from Krebs II ascites cells. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2716–2719. doi: 10.1073/pnas.68.11.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kettmann R., Portetelle D., Mammerickx M., Cleuter Y., Dekegel D., Galoux M., Ghysdael J., Burny A., Chantrenne H. Bovine leukemia virus: an exogenous RNA oncogenic virus. Proc Natl Acad Sci U S A. 1976 Apr;73(4):1014–1018. doi: 10.1073/pnas.73.4.1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King J., Laemmli U. K. Polypeptides of the tail fibres of bacteriophage T4. J Mol Biol. 1971 Dec 28;62(3):465–477. doi: 10.1016/0022-2836(71)90148-3. [DOI] [PubMed] [Google Scholar]
- Lanham S. M., Godfrey D. G. Isolation of salivarian trypanosomes from man and other mammals using DEAE-cellulose. Exp Parasitol. 1970 Dec;28(3):521–534. doi: 10.1016/0014-4894(70)90120-7. [DOI] [PubMed] [Google Scholar]
- Lodish H. F. Alpha and beta globin messenger ribonucleic acid. Different amounts and rates of initiation of translation. J Biol Chem. 1971 Dec 10;246(23):7131–7138. [PubMed] [Google Scholar]
- Loening U. E. The fractionation of high-molecular-weight ribonucleic acid by polyacrylamide-gel electrophoresis. Biochem J. 1967 Jan;102(1):251–257. doi: 10.1042/bj1020251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews M. B. Further studies on the translation of globin mRNA and encephalomyocarditis virus RNA in a cell-free system from Krebs II ascites cells. Biochim Biophys Acta. 1972 Jun 22;272(1):108–118. doi: 10.1016/0005-2787(72)90038-x. [DOI] [PubMed] [Google Scholar]
- Mueller-Lantzsch N., Fan H. Monospecific immunoprecipitation of murine leukemia virus polyribosomes: identification of p30 protein-specific messenger RNA. Cell. 1976 Dec;9(4 Pt 1):579–588. doi: 10.1016/0092-8674(76)90040-4. [DOI] [PubMed] [Google Scholar]
- Palacios R., Palmiter R. D., Schimke R. T. Identification and isolation of ovalbumin-synthesizing polysomes. I. Specific binding of 125 I-anti-ovalbumin to polysomes. J Biol Chem. 1972 Apr 25;247(8):2316–2321. [PubMed] [Google Scholar]
- Palmiter R. D., Oka T., Schimke R. T. Modulation of ovalbumin synthesis by estradiol-17 beta and actinomycin D as studied in explants of chick oviduct in culture. J Biol Chem. 1971 Feb 10;246(3):724–737. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Shih D. S., Kaesberg P. Translation of brome mosaic viral ribonucleic acid in a cell-free system derived from wheat embryo. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1799–1803. doi: 10.1073/pnas.70.6.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiger R. F. On the ultrastructure of Trypanosoma (Trypanozoon) brucei in the course of its life cycle and some related aspects. Acta Trop. 1973;30(1):64–168. [PubMed] [Google Scholar]
- Van Meirvenne N., Janssens P. G., Magnus E. Antigenic variation in syringe passaged populations of Trypanosoma (Trypanozoon) brucei. 1. Rationalization of the experimental approach. Ann Soc Belg Med Trop. 1975;55(1):1–23. [PubMed] [Google Scholar]
- Vickerman K. Antigenic variation in trypanosomes. Nature. 1978 Jun 22;273(5664):613–617. doi: 10.1038/273613a0. [DOI] [PubMed] [Google Scholar]
- Vickerman K. On the surface coat and flagellar adhesion in trypanosomes. J Cell Sci. 1969 Jul;5(1):163–193. doi: 10.1242/jcs.5.1.163. [DOI] [PubMed] [Google Scholar]
- Williams R. O., Marcu K. B., Young J. R., Rovis L., Williams S. C. A characterization of mRNA activites and their sequence complexities in Trypanosoma brucei: partial purification and properties of the VSSA mRNA. Nucleic Acids Res. 1978 Sep;5(9):3171–3182. doi: 10.1093/nar/5.9.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]