Abstract
The wireless motility/pH capsule (WMC) is an orally ingested, nondigestible, data recording device that enables the simultaneous assessment of regional and whole gut transit. Approved by the US Food and Drug Administration for the evaluation of patients with suspected delayed gastric emptying and the evaluation of colonic transit time in patients with chronic idiopathic constipation, this capsule continuously measures the temperature, pH, and pressure of its surrounding environment while traveling through the gastrointestinal tract (via gut peristalsis) until exiting the body through the anus. Validated patterns in pH and temperature recordings allow for accurate measurement of gastric emptying, small bowel transit, colonic transit, and whole gut transit times. The WMC is a nonradioactive, office-based, gastrointestinal transit testing modality shown in several clinical trials to be a suitable alternative to scintigraphy and radiopaque marker studies in measuring gastric emptying, small bowel, colonic, and whole gut transit times. Unlike widely available transit tests, which provide only region-specific transit data, the WMC offers the benefit of measuring gastric, small bowel, and colonic transit times in a single examination. The WMC also provides intraluminal pressure readings throughout the digestive tract, offering a noninvasive means by which to assess gastrointestinal motility. The WMC should be considered the transit study of choice for individuals suspected of having altered transit in more than one region of the gastrointestinal tract. This review summarizes the features and performance characteristics of the WMC as well as provides a summary on how this diagnostic modality is most effectively used in the assessment of gastrointestinal symptom complexes due to suspected abnormalities in transit.
Keywords: Wireless motility capsule, gastrointestinal transit testing, gastrointestinal motility, gastroparesis, constipation
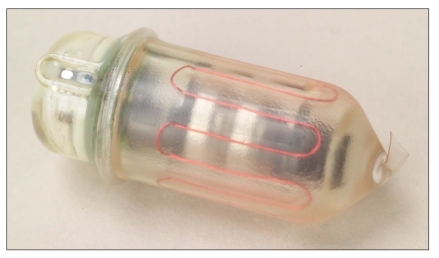
The wireless motility/pH capsule (WMC; SmartPill Wireless Motility Capsule, SmartPill Corporation) is a data recording device measuring 26.8 mm in length and 11.7 mm in diameter that provides real-time measurements of the temperature (range, 25–49°C), pH (range, 0.05–9.0), and pressure (range, 0–350 mmHg) of its immediate surroundings. The capsule consists of a rigid polyurethane shell containing a battery that lasts for a minimum of 120 hours; sensors for pH, temperature, and pressure; and a transmitter that operates at a wavelength of 434 MHz (Figure 1). The WMC is a single-use, orally ingested, nondigestible capsule that is capable of measuring gastric emptying time (GET), small bowel transit time (SBTT), colonic transit time (CTT), and whole gut transit time (WGTT). The capsule can also record intraluminal pressure and, therefore, provide pressure patterns in these regions of the gastrointestinal tract. The US Food and Drug Administration approved the WMC for the evaluation of patients with suspected delayed gastric emptying (gastroparesis) in 2006 and for the evaluation of colonic transit in patients with chronic idiopathic constipation in 2009 (Table 1). In addition, the American and European Neurogastroenterology and Motility Societies recently recognized the capsule as a validated and objective measure of gastrointestinal transit.1
Figure 1.
The wireless motility capsule.
Table 1.
US Food and Drug Administration-Approved Indications for the Wireless Motility Capsule
|
Testing Procedure
A WMC study can be performed in a physician's office after the patient undergoes an overnight fast and discontinues medications that could potentially alter gastric pH and gastrointestinal motility (Table 2). The WMC is ingested immediately following ingestion of a standardized 260-kcal nutrient bar (SmartBar, which consists of 17% protein, 66% carbohydrate, 2% fat, and 3% fiber) and 50 mL of water. (Transit times observed after ingestion of this nutrient bar are similar to those observed in healthy controls and patients with dysmotility after consumption of a low-fat egg-substitute meal, although these times have not been directly compared; egg substitute with jam and toast has been used as an alternative meal for WMC diagnostic testing.2) Prior to ingestion, the capsule has to be calibrated and activated (via an activation fixture provided by the manufacturer). A small (6″ x 4″ x 1.5″) lightweight external data recorder containing a rechargeable battery is attached to a specially designed belt worn around the patient's waist, and the patient is instructed to keep the data receiver within 5 feet of his or her body during the testing period (3—5 days). The patient is instructed regarding how to manually record activities such as meals, sleep, and bowel movements by pushing an event button on the data receiver. Following ingestion of the capsule, the patient is allowed to leave the physician's office. In order to obtain an accurate measurement of GET, the patient has to wait 6 hours after ingesting the capsule before eating another meal. For the remainder of the testing period, the patient can consume meals according to his or her usual schedule. In order to avoid the potential effects of exercise on transit measurement, the patient is instructed to avoid strenuous or vigorous exercise during the testing period. The patient returns to the physician's office at the end of the testing period (3—5 days later) to return the data receiver. A loss of the recording signal and/ or an abrupt temperature drop on the recording profile confirm passage of the capsule from the body. Upon completion of the study, the physician receives an automated report that includes measured regional transit times. If desired, the physician can perform a manual review and interpretation of the data (for which on-site training is required and provided by the manufacturer).
Table 2.
Medications That Should Be Discontinued Prior to and During the Wireless Motility Capsule (WMC) Test
| Medications that potentially alter gastric pH | Time period of medication discontinuation |
|---|---|
| Proton pump inhibitors | 7 days prior to WMC ingestion |
| Histamine receptor antagonists | 3 days prior to WMC ingestion |
| Antacids | 1 day prior to WMC ingestion |
| Medications that potentially alter gastrointestinal motility | Time period of medication discontinuation |
| Prokinetics (metoclopramide, domperidone, erythromycin, azithromycin) | 3 days prior to and during WMC testing |
| Antiemetic agents | 3 days prior to and during WMC testing |
| Anticholinergic agents (dicyclomine, hyoscyamine) | 3 days prior to and during WMC testing |
| Laxatives | 2 days prior to and during WMC testing |
| Antidiarrheal agents | 3 days prior to and during WMC testing |
| Narcotic analgesics | 3 days prior to and during WMC testing |
| Nonsteroidal anti-inflammatory drugs | 3 days prior to and during WMC testing |
Definitions of Transit Time Periods
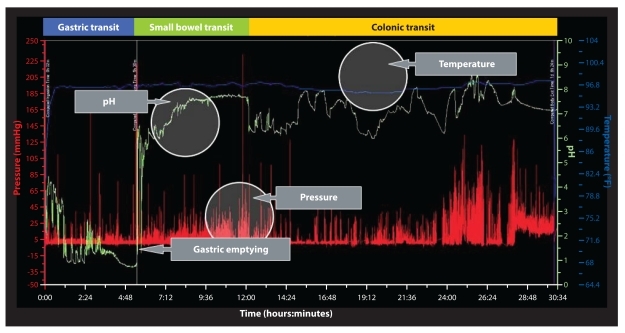
Combinations of pH and temperature profiles are used to calculate GET, SBTT, CTT, and WGTT (Figure 2). GET is defined as the time from the capsule's ingestion to its departure from the stomach. Immediately following ingestion of the WMC, there is an abrupt rise in recorded temperature from ambient temperature to normal body temperature (approximately 36°C). The WMC's exit from the stomach is defined by an abrupt rise in pH (≥2 pH units), which corresponds to the capsule's passage from the acidic environment of the stomach to the more alkaline environment of the proximal duodenum.2,3 Discontinuing acid-suppressive medications facilitates detection of the pH increase associated with the pyloric passage; however, smaller increases can still be observed in a patient who is taking a proton pump inhibitor.4 Furthermore, the WMC, representing a nondigestible solid, has been shown to empty with return of phase 3 of the migrating motor complex, which occurs upon complete emptying of solid food from the stomach.3
Figure 2.
A sample motility graph showing data from a wireless motility capsule. Temperature, pH, and pressure measurements can be used to calculate gastric emptying, small bowel transit, colonic transit, and whole gut transit times, as well as other motility information.
SBTT is defined as the time from the WMC's entrance into the duodenum to its passage through the ileocecal valve into the cecum. Passage into the cecum is defined by a sustained (>10 minutes in duration) pH drop of at least 1 pH unit, which occurs as the WMC leaves the alkaline environment of the terminal ileum and enters the more acidic environment of the cecum.5,6 If the pH drop in the ileocecal junction is not evident, abrupt changes in pressure wave frequency or amplitude may provide supportive evidence of the transition from the small bowel to the colon.
CTT is defined as the time from the WMC's entry into the cecum to its passage from the body. Once again, passage of the WMC outside the body is confirmed by a loss of recording signal and/or an abrupt temperature drop on the recording profile.
WGTT is defined as the time from the capsule's ingestion to its exit from the body.
Assessment of Gastrointestinal Transit with the Wireless Motility Capsule
Gastric Emptying Time
In an industry-sponsored, multicenter study, GET (as measured by the WMC) was compared to gastric emptying scintigraphy with simultaneous measurements in 87 healthy adults and 61 adults with gastroparesis. Healthy adults were 18—75 years of age, did not have any chronic diseases, and did not report any gastrointestinal symptoms on the Mayo Gastrointestinal Disease Screening Questionnaire, whereas adults with gastroparesis were 18—75 years of age, had nausea, vomiting, early satiety, and epigastric discomfort for a minimum of 6 months, and had documented abnormal gastric emptying scintigraphy within the past 2 years.2,7 In this study, GET demonstrated a correlation coefficient of 0.73 compared to a concurrently performed 4-hour scintigraphic gastric emptying study (GES-4) and a correlation coefficient of 0.63 compared to a 2-hour scintigraphic gastric emptying study (GES-2). Based on receiver operating characteristics, the diagnostic accuracy of GET was 0.83, compared to 0.82 for GES-4 and 0.79 for GES-2. Using a cutoff time of 300 minutes, GET identified delayed gastric emptying in 65% of adults with an established scintigraphic diagnosis of gastroparesis, compared to 44% with GES-4 and 34% with GES-2. Using the same cutoff time, 13% of healthy adults were found to have delayed gastric emptying with GET, compared to 7% with GES-4 or GES-2. Based on these data, a GET of 5 hours or less, as measured by the WMC, was defined as normal.
Rapid gastric emptying quantified by scintigraphy has recently been reported in select patients with functional dyspepsia, cyclic vomiting syndrome, and fat intolerance.8–10 Data from the previously discussed WMC gastroparesis trial were extracted to define the 95% cutoff for abnormally rapid gastric emptying at 2 hours; however, the sample size of rapid emptiers from this trial was too small to determine the clinical relevance of this finding.11 To date, no prospective trials have validated this WMC cutoff for rapid gastric emptying compared to scintigraphy.
Small Bowel Transit Time
The measurement of SBTT with the WMC has been assessed in several industry-sponsored, multicenter trials involving healthy adults, adults with gastroparesis, and adults with chronic constipation.2,12–14 A single-center study of 10 healthy adults who received simultaneous gastrointestinal transit measurements via the WMC and whole gut scintigraphy demonstrated comparable small bowel transit measurements between the 2 methods.15 However, it may not be possible to determine SBTT in all patients, as clinical trials have been unable to accurately identify the necessary pH landmarks in 5—10% of those studies.12,13 A normal range for SBTT, based on pooled results from healthy adults in WMC clinical trials, has been reported in the manufacturer's user manual; based on 95% cutoff values from control studies, a SBTT less than 2.5 hours has been defined as rapid transit, and a SBTT exceeding 6 hours has been defined as delayed small bowel transit. To date, no prospective trials have characterized abnormal SBTT in disease states such as intestinal pseudo-obstruction.
Colonic Transit Time
The WMC has been tested and subsequently validated for the measurement of CTT in patients with chronic constipation. An initial, industry-sponsored, multicenter clinical trial conducted in 78 adults who met Rome II criteria for functional constipation and 87 healthy adults compared the WMC to 2-day and 5-day radiopaque marker (ROM) studies for identifying delayed colonic transit.12 Healthy adults were 18—79 years of age, did not report any chronic diseases, and did not have any gastrointestinal symptoms on the Mayo Gastrointestinal Disease Screening Questionnaire, whereas constipated adults fulfilled a minimum of 2 of the 6 symptoms included in the Rome II criteria for chronic functional constipation.7,16 In this study, CTT (as measured by the WMC) demonstrated an overall correlation coefficient of 0.78 (95% confidence interval [CI], 0.70—0.84) with the number of retained ROMs at Day 2 and a correlation coefficient of 0.59 (95% CI, 0.46—0.69) with the number of retained ROMs at Day 5.12 Using a cutoff time of 59 hours for CTT, 46% of patients with constipation demonstrated delayed transit with the WMC, compared to 37% with the 5-day ROM study. Among healthy controls, 5% had delayed colonic transit with both the WMC and the 5-day ROM study.12
A subsequent industry-sponsored, multicenter validation study was conducted in 158 adults who met modified Rome III criteria for chronic functional constipation emphasizing abnormal stool consistency.13 This study demonstrated overall device agreement of 87% between the WMC and a widely used quantitative segmental ROM protocol in the determination of delayed versus normal colonic transit in this constipated cohort.13,17 Based on these clinical trials, the WMC's manufacturer defined rapid transit as a CTT less than 5 hours and delayed colonic transit as a CTT greater than 59 hours.
Utility of Pressure Measurements in the Assessment of Gastrointestinal Motility
Pressure measurements provided by the WMC may offer motility information beyond that of regional transit times and WGTT. Normal pressure patterns have been characterized in healthy controls, and abnormal contractile activities have been identified in adults with gastroparesis or chronic constipation. In a post-hoc analysis, individuals with gastroparesis exhibited a marked decrease in stomach contractions compared to healthy controls.18 Taken as a group, 33% of the 42 gastroparesis patients exhibited pressure profiles that fell below the fifth percentile of the 71 healthy controls in terms of the number of gastric contractions. Furthermore, when patients with gastroparesis were stratified by the degree of delayed gastric emptying, 75% of patients with a GET over 12 hours had a number of gastric contractions below the fifth percentile of normal, compared to 8% of patients with a GET less than 12 hours. This observation suggests that WMC pressure measurements can facilitate classification of gastroparesis patients into distinct severity categories.
Differences in contractility patterns within the colon have also been demonstrated in patients with constipation compared to healthy controls.19 Furthermore, contractility patterns appear to differ among the subtypes of chronic constipation. When adding together WMC contractile activity by measuring areas under pressure curves, constipated patients with normal colonic transit (<59 hours) or mildly to moderately delayed transit (59—100 hours) exhibited contractility that was 33—50% higher than in healthy controls. This increase was most prominent in patients with constipation-predominant irritable bowel syndrome. In contrast, a preliminary evaluation of a cohort enriched with severely constipated patients found that profound delays in colonic transit (>100 hours) were associated with significantly reduced contractile activity. These preliminary findings suggest that contractility patterns obtained from the WMC may help define subtypes of constipation and may provide valuable insight into underlying pathophysiologic mechanisms.
Future Applications of Pressure Measurements Quantifying WMC pressure parameters has been suggested as an alternative means of obtaining some of the data that are traditionally obtained from stationary antroduodenal and colonic motility studies, which use orally or anally placed manometric catheters, respectively. These invasive methods are particularly useful for distinguishing visceral neuropathy from myopathy as the cause of dysmotility.20 However, these methods are typically limited by lack of availability, patient discomfort, and the need for specialized personnel. Preliminary unpublished analyses of WMC recordings in healthy controls have identified increases in small intestinal pressure activity after the consumption of liquid nutrient meals. Similar preliminary WMC studies have quantified gastrocolonic responses to eating and suppression of colonic pressure activity during sleep in healthy subjects and patients with constipation. These findings indicate the potential for WMC analyses to detect neuropathic dysmotility. However, the WMC only has one pressure sensor that migrates within the gut lumen. Consequently, it cannot characterize organized small bowel patterns such as migrating motor complex or propagating contractions in the colon. Despite this limitation, WMC pressure measurements may have important complementary roles, in addition to transit determinations, because of the ease of test performance and superior patient tolerance compared to manometry.
Contraindications to Wireless Motility Capsule Testing
The WMC should not be administered to patients with a history of gastric bezoar, swallowing disorders, dysphagia to food or pills for any reason, suspected strictures or fistulae along the gastrointestinal tract, physiologic gastrointestinal obstruction, gastrointestinal surgery within the previous 3 months, Crohn's disease, diverticulitis, or an implanted or portal electromechanical medical device (eg, a cardiac pacemaker or infusion pump). The capsule is not approved for use in children.
Adverse Events of Wireless Motility Capsule Testing
Test failure has been reported in a small number of cases for reasons including inability of the patient to swallow the capsule, failure of the capsule to record or transmit data, failure of the receiver to record or download data, and software malfunction. Of the 495 patients who participated in WMC clinical trials, 3 patients were unable to swallow the capsule, yielding a failure-to-swallow incidence rate of 0.6%. In these clinical trials, 36 incidences of equipment or software malfunction prevented the acquisition of interpretable transit data from the WMC. Many of these incidences involved prototypic equipment that has since been upgraded. In a postmarketing analysis of approximately 5,000 WMC ingestions, the incidence of equipment failure was reported to be 0.8—0.9%.
The most serious adverse events associated with the WMC are inability to confirm passage of the capsule outside the body, capsule retention, and obstruction. In the WMC clinical trials, there were several incidences of prolonged capsule retention, with all but 2 cases demonstrating spontaneous passage of the capsule via radiograph imaging 21 days after ingestion. In one case, a gastroparesis patient who had ingested a fiber supplement had a retained capsule in the stomach, which passed into the small bowel following a single dose of intravenous erythromycin. In the second case, a patient with chronic constipation had a peptic stricture in the proximal duodenum that prevented passage of the capsule; the capsule was extracted from the stomach via endoscopy.
In a postmarketing analysis of nearly 6,000 shipped capsules, there have been 20 reports of prolonged capsule retention (5 in the stomach, 2 in the small intestine, and 13 in the colon), which corresponds to a retention rate of 0.33%. An upper endoscopy was required for extraction of the 5 capsules that were retained in the stomach. Of the remaining 15 cases, only 1 capsule required drug intervention; the other 14 capsules passed spontaneously. There were no obstructions requiring surgical intervention.
If the capsule's exit cannot be confirmed 5 days after ingestion, the physician should consult the manufacturer's follow-up recommendations, which are based on the capsule's location (determined via the pH profile obtained during the study). Due to the low risk of obstruction once the capsule is in the colon, no specific follow-up is necessary if the capsule is retained in this area. If the pH profile suggests that the capsule is retained in the stomach or small bowel, serial radiograph imaging at 3-week intervals is recommended until the capsule moves into the colon or exits the body. If clinical evidence of obstruction (nausea, vomiting, abdominal pain, or abdominal distention) develops at any time, abdominal imaging should be immediately pursued, and the capsule should be extracted.
Other side effects in the WMC clinical trials included abdominal pain, dysphagia, nausea, and diarrhea. However, it is difficult to determine whether these side effects were caused by the capsule, as many patients had these symptoms at baseline. Therefore, these side effects have not been followed in postmarketing studies.
Use of the Wireless Motility Capsule in Clinical Practice
The WMC is a nonradioactive alternative to the transit testing modalities that are currently available for assessing gastric emptying in patients with diabetic, idiopathic, postinfectious, or postsurgical gastroparesis or for evaluating patients with functional dyspepsia who failed medical therapy and are suspected of having delayed gastric transit. The WMC is particularly useful in gastroparesis cases in which an individual is suspected of having both delayed gastric emptying and delayed transit in other regions of the gastrointestinal tract, including the small bowel and/or colon.
The WMC is also a nonradioactive alternative to the colonic transit testing modalities that are currently available for evaluating chronic constipation. The capsule is particularly useful in idiopathic constipation cases in which a quantitative assessment of CTT is needed to more accurately define the severity of delayed colonic transit. The capsule is also useful in cases of refractory slow transit constipation when obtaining transit times of the stomach and small bowel is necessary in order to exclude a more global gastrointestinal transit disorder.
Finally, the WMC is the only nonradioactive transit testing modality available for individuals who present with overlapping symptoms suggestive of delayed upper and lower gastrointestinal transit (eg, upper gastrointestinal symptoms such as nausea, vomiting, bloating, or postprandial fullness with concomitant lower gastrointestinal symptoms such as constipation). The following 3 vignettes illustrate the use of the WMC for evaluating syndromes of suspected altered gastrointestinal transit.
A Gastroparesis Patient with Associated Intestinal Pseudo-Obstruction
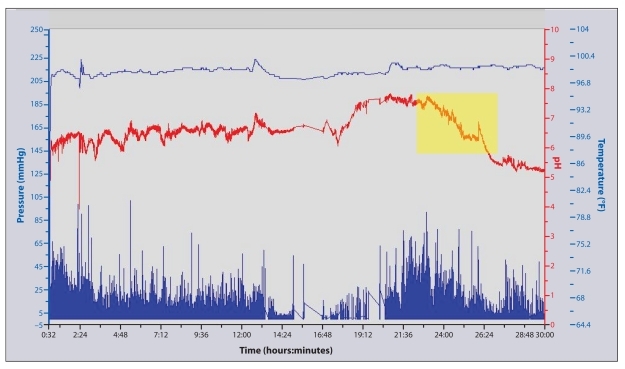
A 35-year-old woman presented to the gastroenterology clinic with a 1-year history of postprandial nausea, vomiting, and abdominal pain with associated weight loss. An upper endoscopy and computed axial tomography scan of the abdomen had unremarkable findings. A scintigraphic GES-4 revealed delayed gastric emptying. Medication trials with metoclopramide, domperidone, erythromycin, nortriptyline, and mirtazapine were either poorly tolerated or ineffective for controlling symptoms. Various peripheral and central-acting antiemetic agents were minimally effective as well. Due to persistent symptoms and further weight loss, the patient underwent a total gastrectomy. Following surgery, her nausea, vomiting, poor oral intake, and abdominal pain progressed in severity. The patient was then referred to another gastroenterologist for a second opinion. This physician suspected that there was a problem in the patient's small bowel and/or colonic transit and performed a WMC study. The capsule revealed a markedly delayed SBTT of 23 hours and 15 minutes and a normal CTT of 47 hours (Figure 3). Furthermore, the pressure-tracing pattern was abnormal, demonstrating numerous high-amplitude contractions throughout the small bowel and colon, with loss of the normal sleep-wake cycle in pressure activity. This finding was interpreted as demonstrating intestinal pseudo-obstruction with a contractile profile that was most consistent with visceral neuropathy. The capsule findings altered the patient's therapy (which included intermittent use of antibiotics for small bowel decontamination and treatment trials of octreotide and pyridostigmine). Moreover, the gastrectomy could have been avoided if the WMC had been used earlier.
Figure 3.
A portion of the wireless motility capsule tracing spanning the time of ingestion to the time of capsule passage into the cecum in a gastroparesis patient with associated intestinal pseudo-obstruction. The sustained drop in pH (shaded box) at 23 hours and 15 minutes indicates the capsule's exit from the small bowel and its entrance into the cecum. The drop and subsequent rise in pH characteristically seen with passage of the capsule through the stomach is not found in this tracing, as the patient had previously undergone a gastrectomy.
In a patient with suspected or confirmed gastro-paresis that is refractory to medical therapy, the WMC provides physicians with a single transit study to assess gastric emptying and determine the presence of altered transit in other portions of the digestive tract. This information may help direct therapy and, more importantly, prevent the pursuit of therapeutic interventions that may worsen symptoms.
A Patient with Refractory Idiopathic Chronic Constipation
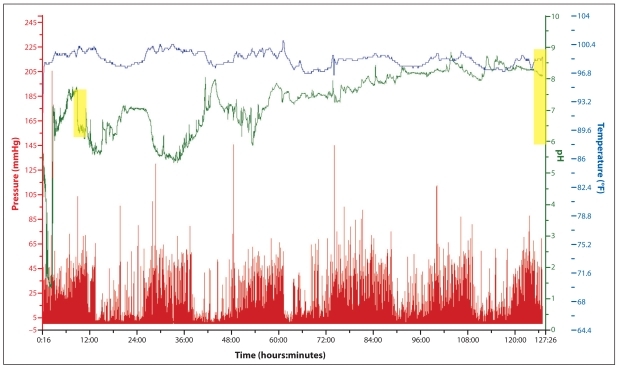
A 21-year-old woman presented with progressive symptoms of constipation and abdominal pain since childhood. She reported having once-monthly bowel movements and generalized abdominal pain every other day. The abdominal pain varied in location, was severe in intensity, and was associated with episodic nausea and vomiting at least once every week. Trials of fiber supplementation, magnesium, polyethylene glycol, bisacodyl, and lubiprostone (Amitiza, Sucampo) were either poorly tolerated or ineffective for controlling her symptoms. A colonoscopy was remarkable for a poor bowel preparation, and a magnetic resonance image of the patient's abdomen and pelvis was remarkable for moderate fecal loading. At the time of her presentation, the patient was taking polyethylene glycol (3 times daily) and bisacodyl (5 mg every evening) and was still going up to 5 days without having a bowel movement as well as experiencing occasional episodes of generalized abdominal pain. The physician administered a WMC study to assess colonic transit, as slow transit constipation was strongly suspected. The capsule confirmed this suspicion, finding a CTT of 116 hours (Figure 4). Equally important, the WMC confirmed a normal GET and a normal SBTT, at 4 hours and 5 hours, respectively. Due to the failure of medical therapy and confirmation of an isolated colonic inertia problem, the patient was referred for elective colectomy without the need for further testing.
Figure 4.
A tracing of a wireless motility capsule in a patient with refractory idiopathic chronic constipation. The capsule entered the cecum at 9 hours (shown by a sustained drop in pH; shaded box on the left side) and exited the body at 125 hours (shown by a drop in temperature; shaded box on the right side), yielding a colonic transit time of 116 hours.
In this case, the additional transit information regarding GET and SBTT helped to determine the suitability of an elective colectomy. Excluding small bowel and gastric motility disorders is important prior to pursuing an elective colectomy for colonic inertia. In order to obtain the necessary transit information for the colon, stomach, and small bowel via conventional modalities, evaluation of this patient would have included a 7-day ROM study (to provide a measurable CTT) followed by a scintigraphic gastric emptying study and a small bowel follow-through.
A Patient with Overlapping Upper and Lower Gastrointestinal Symptom Complex
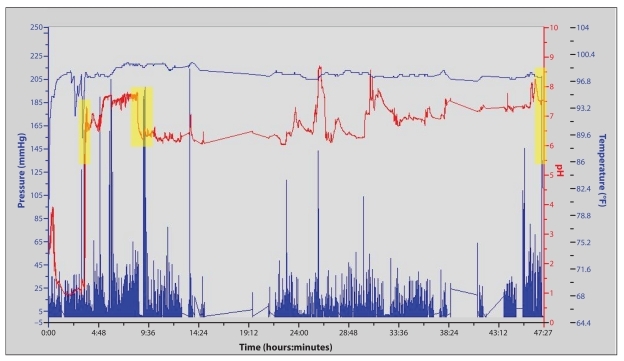
A 19-year-old woman presented with a 2-month history of persistent nausea and vomiting associated with worsening heartburn, recurrent episodes of dehydration, and 15-lb weight loss. Her upper gastrointestinal symptoms developed shortly after recovering from an upper respiratory tract infection. A review of her symptoms revealed a several-month history of constipation manifested by infrequent bowel movements, with the patient going up to 5 days without a bowel movement. Trials of various antisecretories, antiemetic agents, and laxatives did not improve symptoms. The patient had been admitted to the hospital twice since the onset of her upper gastrointestinal symptoms. An upper endoscopy had unremarkable findings, and an abdominal radiograph was remarkable for fecal loading. The patient was unable to complete a scintigraphic gastric emptying study due to emesis after ingestion of the test meal. The patient was frustrated with her persistent symptoms, which were affecting her social life, studies, and participation on her college soccer team. The WMC was administered, revealing a normal GET, SBTT, and CTT (Figure 5). Due to these results, the patient was placed on low-dose mirtazapine and was advised to use an antiemetic as needed. She reported considerable symptom improvement and did not require further testing.
Figure 5.
A tracing of a wireless motility capsule in a patient with overlapping upper and lower gastrointestinal symptom complex. The capsule exited the stomach at 3 hours and 16 minutes (shown by an abrupt rise in pH; first shaded box), demonstrating a normal gastric emptying time. The capsule exited the small bowel at 7 hours and 32 minutes (shown by a sustained drop in pH; second shaded box), yielding a normal small bowel transit time of 4 hours and 16 minutes. The capsule exited the body at 46 hours and 52 minutes (shown by an abrupt drop in temperature; third shaded box), yielding a normal colonic transit time of 39 hours and 20 minutes.
In this case, the patient's presentation was suggestive of a motility disorder involving both the upper and lower digestive tracts. The WMC provided the physician with a single study that could assess gastric, small bowel, and colonic transit. This study provided the physician and patient with valuable objective information that helped direct therapy and provided a better understanding of the cause of the patient's symptoms. This appro ach also reduced the cost, complexity, and risk of further radiation exposure, as conventional testing modalities would have necessitated a second attempt at a scintigraphic gastric emptying study, a ROM study to assess colonic transit, and a small bowel follow-through to assess small bowel transit.
Diagnostic Utility of the Wireless Motility Capsule
Rao and colleagues recently reported on the diagnostic utility of the WMC for assessing patients with suspected gastrointestinal dysmotility.11 The researchers conducted a retrospective review at their tertiary care center in which they examined 86 consecutive patients with various symptoms of dysmotility, including abdominal pain, nausea, vomiting, bloating, postprandial fullness, constipation, straining, a feeling of incomplete evacuation, hard stool, use of digital maneuvers during defecation, gas, or flatulence. All of the patients had documented normal endoscopic and radiographic evaluations prior to presentation and were assessed with both the WMC and conventional motility tests (a scintigraphic gastric emptying study or a 5-day ROM study). The patients were separated by their predominant symptoms into an upper gastrointestinal (UGI) symptom complex group or a lower gastrointestinal (LGI) symptom complex group. Compared to conventional motility testing, the WMC provided new diagnostic information in 53% of the LGI group (P=.006) and 47% of the UGI group (P=.001). Furthermore, the WMC identified a generalized motility disorder in 51% of all patients and influenced management in 30% of the LGI group and 88% of the UGI group.
A recent retrospective study by Kuo and colleagues also assessed the diagnostic utility of the WMC for evaluating patients with suspected gastrointestinal transit delay.21 This analysis involved 83 patients who presented to 2 tertiary care centers with suspected gastroparesis, intestinal dysmotility, or slow transit constipation. In 93% of the cases, the capsule provided complete recordings of gastric, small bowel, and colonic transit. Compared to conventional motility tests, the WMC demonstrated discordant results in 38% of cases and, more importantly, provided a new diagnosis in 53% of cases. Furthermore, WMC testing influenced management in two thirds of the cases and eliminated the need for additional motility testing in more than two thirds of patients. As with conventional motility testing, patients' presenting symptoms were found to be a poor predictor of gastrointestinal transit delay.
There are several possible reasons for the discordant results between the WMC and conventional motility testing modalities. In the 2 previously mentioned retrospective studies, WMC results were compared to conventional test results that had often been obtained months earlier in the disease course when motor impairments may have been more or less severe. Indeed, inconsistencies in gastric and colonic physiology in patients with dysmotility can lead to major day-to-day variability on repeat transit testing. In addition, standardized methods of gastric scintigraphy and colonic transit testing recommended by professional societies may not be widely applied. For example, many institutions use gastric scintigraphy techniques that quantify 90—120-minute values or half-times of emptying, which correlate poorly with both WMC emptying times and 4-hour scintigraphic retention values. Likewise, colonic transit is usually assessed with a radiograph obtained 5 days after ROM ingestion, which correlates poorly with more rigorous conventional and WMC methods.
These retrospective reports show that, despite observed discordances with conventional test findings, WMC results impact decisions made by clinicians who have expertise in the management of dysmotility syndromes. As there is no true gold standard for assessing transit, discordant results between the WMC and conventional testing will require interpretation on a case-by-case basis.
Summary
The WMC offers physicians and patients an office-based, nonradioactive motility test that is capable of providing accurate GET, SBTT, CTT, and WGTT in a single study, thus enabling objective assessment of a constellation of symptoms suggestive of gastrointestinal motility disorders. The capsule should be considered a suitable alternative to the conventional motility testing modalities currently being used for assessing gastric emptying or colonic transit. In addition, the WMC should be used when there is suspicion of a generalized motility disorder or a motility disorder affecting more than one region of the gastrointestinal tract. In this setting, this single diagnostic test is an alternative to administering 3 separate regional transit tests.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Rao SS, Camilleri M, Hasler WL, et al. Evaluation of gastrointestinal transit in clinical practice: position paper of the American and European Neurogastroenterology and Motility Societies. Neurogastroenterol Motil. 2011;23:8–23. doi: 10.1111/j.1365-2982.2010.01612.x. [DOI] [PubMed] [Google Scholar]
- 2.Kuo B, McCallum RW, Koch KL, et al. Comparison of gastric emptying of a nondigestible capsule to a radio-labelled meal in healthy and gastroparetic subjects. Aliment Pharmacol Ther. 2008;27:186–196. doi: 10.1111/j.1365-2036.2007.03564.x. [DOI] [PubMed] [Google Scholar]
- 3.Cassilly D, Kantor S, Knight LC, et al. Gastric emptying of a non-digestible solid: assessment with simultaneous SmartPill pH and pressure capsule, antroduodenal manometry, gastric emptying scintigraphy. Neurogastroenterol Motil. 2008;20:311–319. doi: 10.1111/j.1365-2982.2007.01061.x. [DOI] [PubMed] [Google Scholar]
- 4.Michalek W, Semler JR, Kuo B. Impact of acid suppression on upper gastrointestinal pH and motility. Dig Dis Sci. 2011;56:1735–1742. doi: 10.1007/s10620-010-1479-8. [DOI] [PubMed] [Google Scholar]
- 5.Evans DF, Pye G, Bramley R, Clark AG, Dyson TJ, Hardcastle JD. Measurement of gastrointestinal pH profiles in normal ambulant human subjects. Gut. 1988;29:1035–1041. doi: 10.1136/gut.29.8.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zarate N, Mohammed SD, O'Shaughnessy E, et al. Accurate localization of a fall in pH within the ileocecal region: validation using a dual-scintigraphic technique. Am J Physiol Gastrointest Liver Physiol. 2010;299:G1276–G1286. doi: 10.1152/ajpgi.00127.2010. [DOI] [PubMed] [Google Scholar]
- 7.Locke GR, Talley NJ, Weaver AL, Zinsmeister AR. A new questionnaire for gastroesophageal reflux disease. Mayo Clin Proc. 1994;69:539–547. doi: 10.1016/s0025-6196(12)62245-9. [DOI] [PubMed] [Google Scholar]
- 8.Hejazi RA, Patil H, McCallum RW. Dumping syndrome: establishing criteria for diagnosis and identifying new etiologies. Dig Dis Sci. 2010;55:117–123. doi: 10.1007/s10620-009-0939-5. [DOI] [PubMed] [Google Scholar]
- 9.Hejazi RA, Lavenbarg TH, McCallum RW. Spectrum of gastric emptying patterns in adult patients with cyclic vomiting syndrome. Neurogastroenterol Motil. 2010;22:1298–1302, e1338. doi: 10.1111/j.1365-2982.2010.01584.x. [DOI] [PubMed] [Google Scholar]
- 10.Lin HC, Van Citters GW, Zhao XT, Waxman A. Fat intolerance depends on rapid gastric emptying. Dig Dis Sci. 1999;44:330–335. doi: 10.1023/a:1026606601767. [DOI] [PubMed] [Google Scholar]
- 11.Rao SS, Mysore K, Attaluri A, Valestin J. Diagnostic utility of wireless motility capsule in gastrointestinal dysmotility. J Clin Gastroenterol. 2011;45:684–690. doi: 10.1097/MCG.0b013e3181ff0122. [DOI] [PubMed] [Google Scholar]
- 12.Rao SS, Kuo B, McCallum RW, et al. Investigation of colonic and whole gut transit with wireless motility capsule and radiopaque markers in constipation. Clin Gastroenterol Hepatol. 2009;7:537–544. doi: 10.1016/j.cgh.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Camilleri M, Thorne NK, Ringel Y, et al. Wireless pH-motility capsule for colonic transit: prospective comparison with radiopaque markers in chronic constipation. Neurogastroenterol Motil. 2010;22:874–882, e233. doi: 10.1111/j.1365-2982.2010.01517.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sarosiek I, Selover KH, Katz LA, et al. The assessment of regional gut transit times in healthy controls and patients with gastroparesis using wireless motility technology. Aliment Pharmacol Ther. 2010;31:313–322. doi: 10.1111/j.1365-2036.2009.04162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maqbool S, Parkman HP, Friedenberg FK. Wireless capsule motility: comparison of the SmartPill GI monitoring system with scintigraphy for measuring whole gut transit. Dig Dis Sci. 2009;54:2167–2174. doi: 10.1007/s10620-009-0899-9. [DOI] [PubMed] [Google Scholar]
- 16.Thompson WG, Longstreth GF, Drossman DA, Heaton KW, Irvine EJ, Muller-Lissner SA. Functional bowel disorders and functional abdominal pain. Gut. 1999;45(suppl 2):II43–II47. doi: 10.1136/gut.45.2008.ii43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Metcalf AM, Phillips SF, Zinsmeister AR, MacCarty RL, Beart RW, Wolff BG. Simplified assessment of segmental colonic transit. Gastroenterology. 1987;92:40–47. doi: 10.1016/0016-5085(87)90837-7. [DOI] [PubMed] [Google Scholar]
- 18.Kloetzer L, Chey WD, McCallum RW, et al. Motility of the antroduodenum in healthy and gastroparetics characterized by wireless motility capsule. Neurogas-troenterol Motil. 2010;22:527–533 e117. doi: 10.1111/j.1365-2982.2010.01468.x. [DOI] [PubMed] [Google Scholar]
- 19.Hasler WL, Saad RJ, Rao SS, et al. Heightened colon motor activity measured by a wireless capsule in patients with constipation: relation to colon transit and IBS. Am J Physiol Gastrointest Liver Physiol. 2009;297:G1107–G1114. doi: 10.1152/ajpgi.00136.2009. [DOI] [PubMed] [Google Scholar]
- 20.Camilleri M, Bharucha AE, di Lorenzo C. American Neurogastroenterology and Motility Society consensus statement on intraluminal measurement of gastrointestinal and colonic motility in clinical practice. Neurogastroenterol Motil. 2008;20:1269–1282. doi: 10.1111/j.1365-2982.2008.01230.x. [DOI] [PubMed] [Google Scholar]
- 21.Kuo B, Maneerattanaporn M, Lee AA, et al. Generalized transit delay on wireless motility capsule testing in patients with clinical suspicion of gastroparesis, small intestinal dysmotility, or slow transit constipation. Dig Dis Sci. 2011;56:2928–2938. doi: 10.1007/s10620-011-1751-6. [DOI] [PubMed] [Google Scholar]