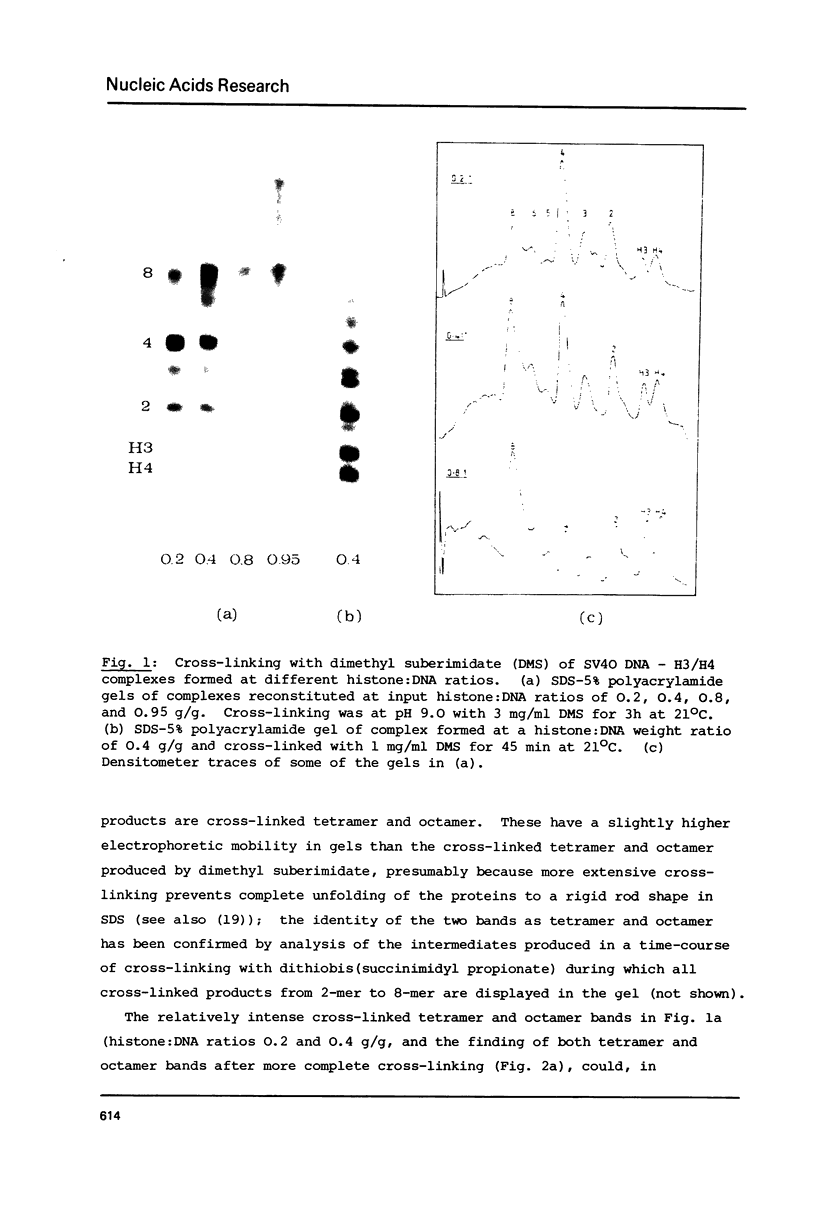
Abstract
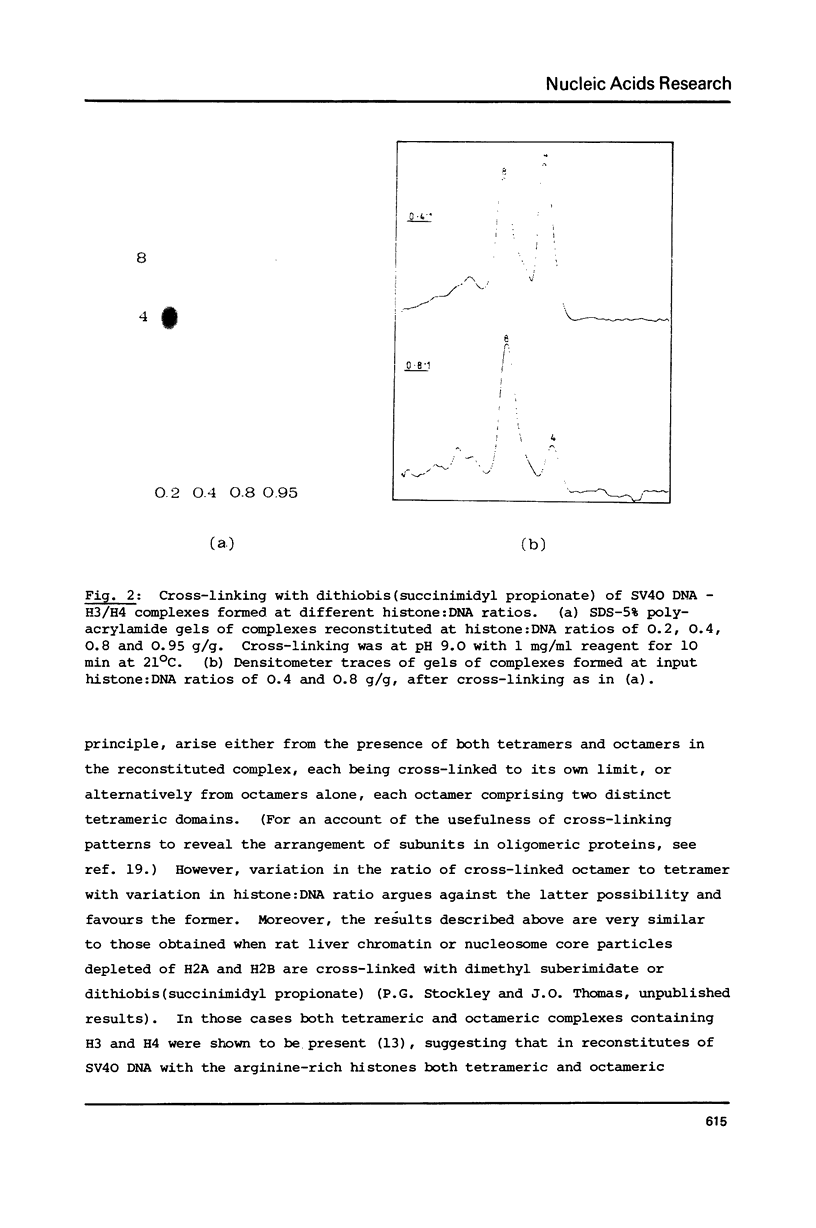
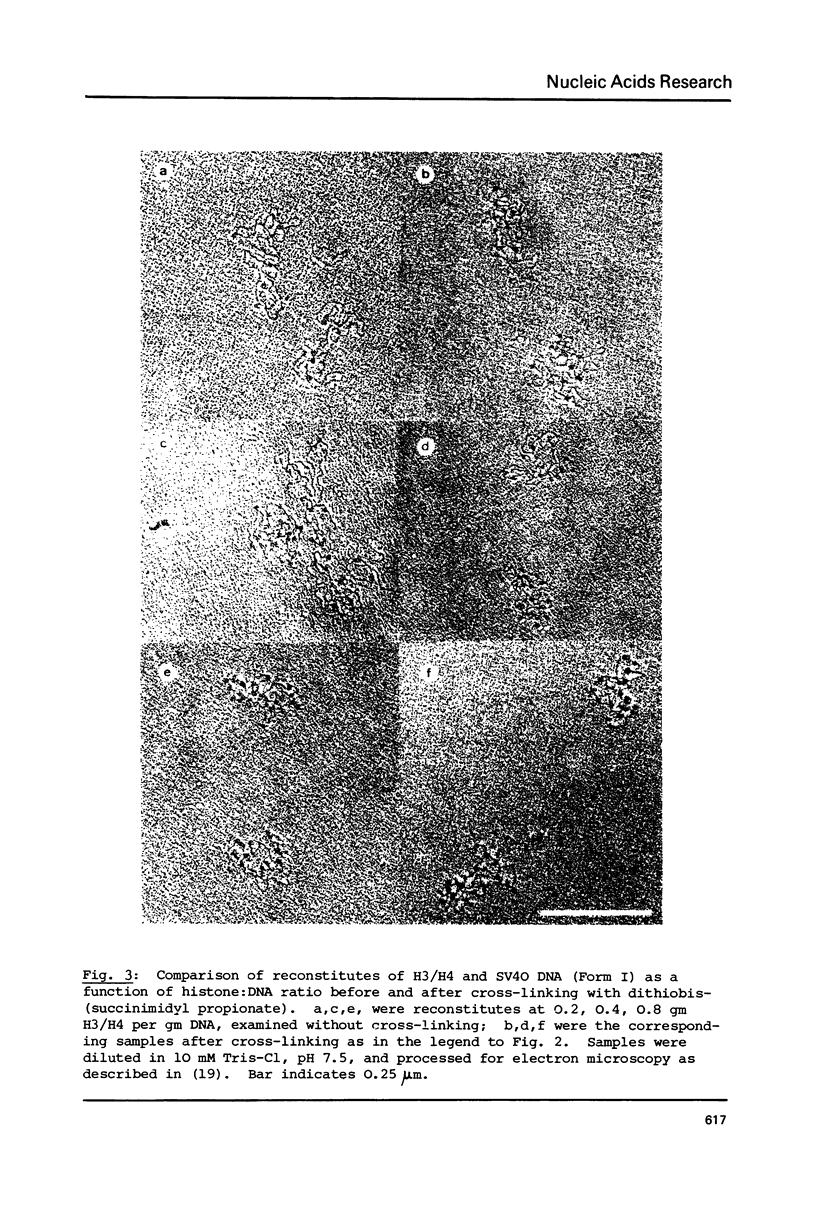
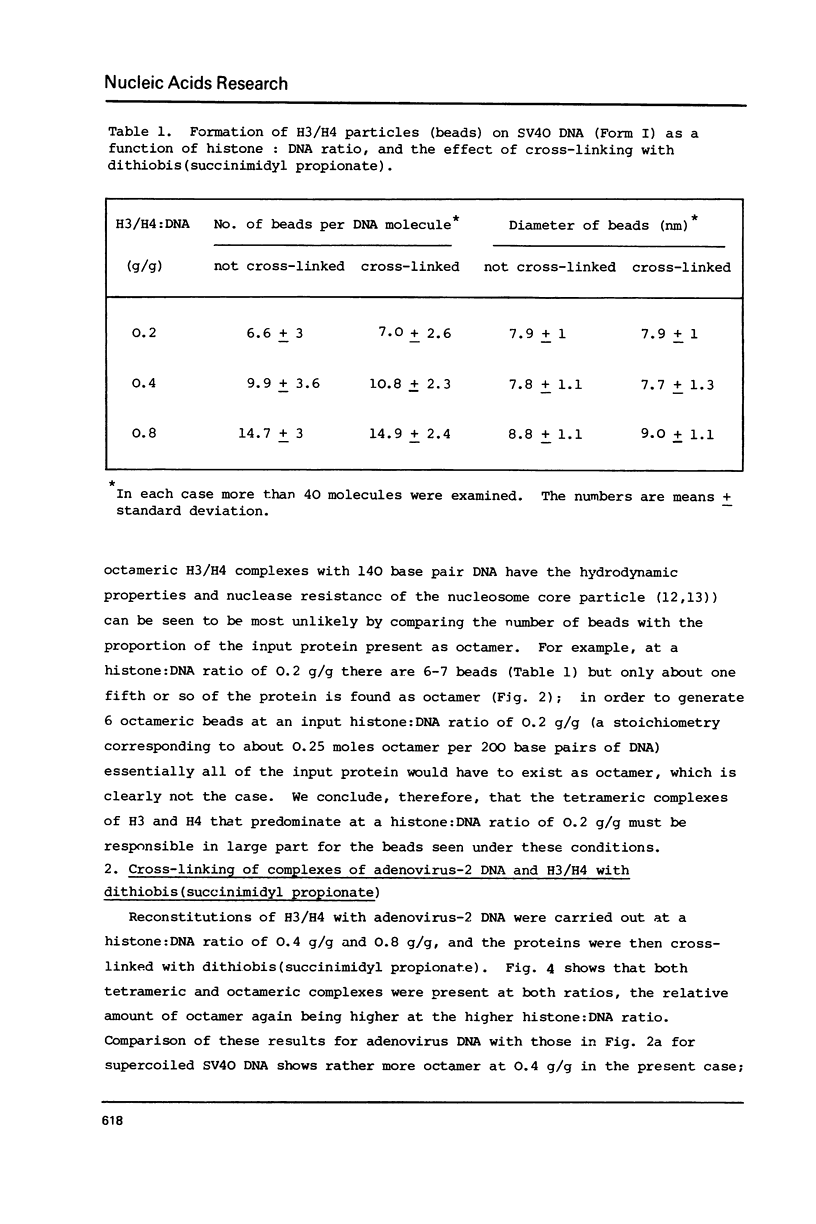
Tetramers of the arginine-rich histones H3 and H4 associate with supercoiled SV40 DNA either singly, giving tetrameric nucleoprotein complexes or in pairs giving octameric complexes, both of which are visualized as beads in the electron microscope. The relative amounts of the two complexes may be revealed by complete cross-linking of the proteins, followed by analysis in SDS-polyacrylamide gels. By electron microscopy of unmodified and of cross-linked complexes, both the tetrameric and the octameric complexes are shown to have a diameter of 8-9 nm and to contain about 145 base pairs (a nucleosome core length) of DNA. The compaction of the DNA in both cases is thus similar to that in the nucleosome, which has a diameter of about 12.5 nm and contains 200 base pairs of DNA.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellard M., Oudet P., Germond J. E., Chambon P. Subunit structure of simian-virus-40 minichromosome. Eur J Biochem. 1976 Nov 15;70(2):543–553. doi: 10.1111/j.1432-1033.1976.tb11046.x. [DOI] [PubMed] [Google Scholar]
- Bina-Stein M. Folding of 140-base pair length DNA by a core of arginine-rich histones. J Biol Chem. 1978 Jul 25;253(14):5213–5219. [PubMed] [Google Scholar]
- Bina-Stein M., Simpson R. T. Specific folding and contraction of DNA by histones H3 and H4. Cell. 1977 Jul;11(3):609–618. doi: 10.1016/0092-8674(77)90078-2. [DOI] [PubMed] [Google Scholar]
- Boseley P. G., Bradbury E. M., Butler-Browne G. S., Carpenter B. G., Stephens R. M. Physical studies of chromatin. The recombination of histones with DNA. Eur J Biochem. 1976 Feb 2;62(1):21–31. doi: 10.1111/j.1432-1033.1976.tb10093.x. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Felsenfeld G. Supercoiling energy and nucleosome formation: the role of the arginine-rich histone kernel. Nucleic Acids Res. 1977;4(5):1159–1181. doi: 10.1093/nar/4.5.1159-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Sollner-Webb B., Felsenfeld G. The organization of histones and DNA in chromatin: evidence for an arginine-rich histone kernel. Cell. 1976 Jul;8(3):333–347. doi: 10.1016/0092-8674(76)90145-8. [DOI] [PubMed] [Google Scholar]
- Finch J. T., Lutter L. C., Rhodes D., Brown R. S., Rushton B., Levitt M., Klug A. Structure of nucleosome core particles of chromatin. Nature. 1977 Sep 1;269(5623):29–36. doi: 10.1038/269029a0. [DOI] [PubMed] [Google Scholar]
- Germond J. E., Hirt B., Oudet P., Gross-Bellark M., Chambon P. Folding of the DNA double helix in chromatin-like structures from simian virus 40. Proc Natl Acad Sci U S A. 1975 May;72(5):1843–1847. doi: 10.1073/pnas.72.5.1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klevan L., Dattagupta N., Hogan M., Crothers D. M. Physical studies of nucleosome assemble. Biochemistry. 1978 Oct 17;17(21):4533–4540. doi: 10.1021/bi00614a027. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D. Structure of chromatin. Annu Rev Biochem. 1977;46:931–954. doi: 10.1146/annurev.bi.46.070177.004435. [DOI] [PubMed] [Google Scholar]
- Kornberg R. D., Thomas J. O. Chromatin structure; oligomers of the histones. Science. 1974 May 24;184(4139):865–868. doi: 10.1126/science.184.4139.865. [DOI] [PubMed] [Google Scholar]
- Mirzabekov A. D., Shick V. V., Belyavsky A. V., Bavykin S. G. Primary organization of nucleosome core particle of chromatin: sequence of histone arrangement along DNA. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4184–4188. doi: 10.1073/pnas.75.9.4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss T., Stephens R. M., Crane-Robinson C., Bradbury E. M. A nucleosome-like structure containing DNA and the arginine-rich histones H3 and H4. Nucleic Acids Res. 1977 Jul;4(7):2477–2485. doi: 10.1093/nar/4.7.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudet P., Germond J. E., Sures M., Gallwitz D., Bellard M., Chambon P. Nucleosome structure I: all four histones, H2A, H2B, H3, and H4, are required to form a nucleosome, but an H3-H4 subnucleosomal particle is formed with H3-H4 alone. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):287–300. doi: 10.1101/sqb.1978.042.01.031. [DOI] [PubMed] [Google Scholar]
- Oudet P., Gross-Bellard M., Chambon P. Electron microscopic and biochemical evidence that chromatin structure is a repeating unit. Cell. 1975 Apr;4(4):281–300. doi: 10.1016/0092-8674(75)90149-x. [DOI] [PubMed] [Google Scholar]
- Roark D. E., Geoghegan T. E., Keller G. H. A two-subunit histone complex from calf thymus. Biochem Biophys Res Commun. 1974 Jul 24;59(2):542–547. doi: 10.1016/s0006-291x(74)80014-8. [DOI] [PubMed] [Google Scholar]
- Simon R. H., Camerini-Otero R. D., Felsenfeld G. An octamer of histones H3 and H4 forms a compact complex with DNA of nucleosome size. Nucleic Acids Res. 1978 Dec;5(12):4805–4818. doi: 10.1093/nar/5.12.4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spadafora C., Oudet P., Chambon P. The same amount of DNA is organized in in vitro-assembled nucleosomes irrespective of the origin of the histones. Nucleic Acids Res. 1978 Oct;5(10):3479–3489. doi: 10.1093/nar/5.10.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockley P. G., Thomas J. O. A nucleosome-like particle containing an octamer of the arginine-rich histones H3 and H4. FEBS Lett. 1979 Mar 1;99(1):129–135. doi: 10.1016/0014-5793(79)80264-1. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Butler P. J. Characterization of the octamer of histones free in solution. J Mol Biol. 1977 Nov;116(4):769–781. doi: 10.1016/0022-2836(77)90270-4. [DOI] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. An octamer of histones in chromatin and free in solution. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2626–2630. doi: 10.1073/pnas.72.7.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. O., Kornberg R. D. The study of histone--histone associations by chemical cross-linking. Methods Cell Biol. 1978;18:429–440. [PubMed] [Google Scholar]
- van der Westhuyzen D. R., von Holt C. A new procedure for the isolation and fractionation of histones. FEBS Lett. 1971 May 20;14(5):333–337. doi: 10.1016/0014-5793(71)80294-6. [DOI] [PubMed] [Google Scholar]