Abstract
The risk of osteoporotic fracture can be viewed as a function of loading conditions and the ability of the bone to withstand the load. Skeletal loads are dominated by muscle action. Recently, it has become clear that bone and muscle share genetic determinants. Involution of the musculoskeletal system manifests as bone loss (osteoporosis) and muscle wasting (sarcopenia). Therefore, the consideration of pleiotropy is an important aspect in the study of the genetics of osteoporosis and sarcopenia. This Perspective will provide the evidence for a shared genetic influence on bone and muscle. We will start with an overview of accumulating evidence that physical exercise produces effects on the adult skeleton, seeking to unravel some of the contradictory findings published thus far. We will provide indications that there are pleiotropic relationships between bone structure/mass and muscle mass/function. Finally, we will offer some insights and practical recommendations as to the value of studying shared genetic factors and will explore possible directions for future research. We consider several related questions that together comprise the general paradigm of bone responses to mechanical loading and the relationship between muscle strength and bone parameters, including the genetic factors that modulate these responses. We believe that further progress in understanding the common genetic etiology of osteoporosis and sarcopenia will provide valuable insight into important biological underpinnings for both conditions and may translate into new approaches to reduce the burdens of both conditions through improved diagnosis, prevention, and early targeted treatment.
Keywords: genetics, pleiotropy, osteoporosis, sarcopenia, development, aging
INTRODUCTION
The risk of osteoporotic fracture can be viewed as a function of the ability of human bone to withstand loads. Skeletal loading conditions are largely dominated by muscle action, more so at younger ages. With advanced age, bones undergo a profound decline in their responsiveness to mechanical loads from exercise, which probably corresponds to the aging‐related changes in cellular and hormonal mechanisms and the decline of muscle input. Because bone and muscle cells share the same mesenchymal precursor, it is thus reasonable to hypothesize that adult bone strength and muscle mass share genetic determinants.
The aim of this perspective paper is therefore to provide evidence from experimental and epidemiological studies for a new conceptual framework for the biological, especially genetic, mechanisms underlying an interaction between bones and muscles in adults. We will start with an overview of accumulating evidence that muscle mass and physical exercise produce effects on the skeleton. We will then provide indications that the genetic influences are shared between the muscles and bone strength. Finally, we will offer some insights on the possible nature of shared genetic factors and conclude with some potential directions and practical recommendations for future research in this field. We thus consider several related questions that together comprise the general paradigm of bone responses to mechanical loading and the relationship between muscle strength and bone parameters. (1) What is the evidence that the age‐related decline in bone and muscle mass have a common etiology? Is it an artifact of general aging? (2) How do aging bones respond to the changes in muscle input/strength? (3) What genetic factors modulate these responses? (4) What is the best way to study the pleiotropic relationships between bone structure/mass and muscle mass/function?
In this perspective, we attempt to answer these important conceptual questions. Further progress in understanding common genetic etiologies of osteoporosis and sarcopenia may ultimately lead to approaches to reduce the burdens of both conditions through improved diagnosis and early‐targeted treatment.
AGING OF BONE AND OSTEOPOROSIS
The process of involution of the musculoskeletal system seems to be a general phenomenon and a “normal” manifestation of tissue atrophy with age. (1) Bones undergo a profound decline with age in their responsiveness to mechanical loads from exercise, (2) a decline that also probably corresponds to the decrease in muscle input that arises from the shortage in energy and fiber loss.
After the skeleton has reached maturity, bone remodeling is responsible for the complete replacement of old bone tissue with new tissue. (3) With advanced age, the placement and quality of new bone are altered. This alteration is caused by the change in areas of remodeling due to disease, accumulated microtrauma, and by variations in muscle strength and direction of applied force, caused by various kinds of work and leisure activity. Catabolic illness, such as surgery, injury, palsy, (4) and immobilization (bed rest or space flight (5) ), may contribute to rapid bone degeneration. The net result of the age‐related bone changes in older persons is chronic degenerative disease, which is characterized by the loss of bone mass and bone strength, development of fragility, and the alteration of bone morphology.
The most widely used and reliable clinical predictor of an osteoporotic fracture remains areal BMD, (6) as evaluated by DXA. The strength of bones is determined not only by the amount of bone but also by the spatial distribution of bone tissue. A growing body of evidence indicates that bone geometry contributes substantially to bone strength and fracture risk. 7 , 8 Femoral geometry may be assessed noninvasively also by DXA‐based hip structural analysis (HSA). The more sophisticated technique of QCT provides valid measures of volumetric BMD (especially trabecular) and precise estimates of the bone structural traits; however, it is not completely clear whether it is superior to DXA in predicting fracture risk. (9)
AGING OF MUSCLE AND SARCOPENIA
Similar to bone, muscle tissue deteriorates with the age. Age‐associated loss of muscle fibers, fatty degeneration, and decreased number of functioning motor units cause decline in muscle quality (i.e., force generated per unit of muscle mass). (10) The weight of skeletal muscle comprises ∼45% of body weight at age 21–30 yr and only ∼27% at age 70+ yr. (11) Sarcopenia (muscle wasting) is manifested by decreases in muscle strength and muscle mass with age, mainly as a result of the alterations in muscle morphology. This is caused mostly by a decreased proportion and cross‐sectional area of type II (fast‐twitch) fibers; however, single fiber analysis suggests that the contractile proteins also become less effective with age. (12) Hand grip strength is known to be associated with muscular functioning in other muscle groups and with activities of daily living. Reduction in muscle mass and hand grip strength is ∼25–30% between ages 30 and 70 yr. (11) Hand grip strength predicts disability 13 , 14 later in life and may also be a good predictor of future deficits in the lower extremities leading to locomotor and balance problems.
Although muscle biopsy is best able to quantify the magnitude of sarcopenia, obtaining such samples is not feasible for research in large human populations. Noninvasive imaging modalities such as QCT and MRI are particularly useful, providing quantitative estimates of muscle mass. (15) Muscle density measured by QCT is a reliable and valid measure of muscle degeneration that accompanies sarcopenia. 16 , 17 The area and radiographic density of the trunk musculature have been associated with both lower back pain and level of physical function in elderly individuals. 18 , 19 DXA‐measured lean mass also serves as an indirect indicator of mechanical loading on bone. (20) Higher lean mass is associated with greater muscle strength and better functioning 21 , 22 , 23 ; leg lean muscle mass measured by DXA has been shown to be associated with mobility disability. 21 , 24 , 25
RELATIONSHIP BETWEEN OSTEOPOROSIS AND SARCOPENIA
There are many indications that sarcopenia coexists with osteopenia. (26) The key to understanding the etiology of osteoporosis and sarcopenia lies in the recognition that bones and muscles act as two parts of the same functional unit of the musculoskeletal system, as proposed by Frost and colleagues 27 , 28 ; therefore, loading effects on muscle will also influence bone. Sievänen (29) postulated that “It is quite clear that the behavior of such a complex system is qualitatively and quantitatively different from what can be inferred from observations and phenomena in isolated units of the whole system.” There are both theoretical (evolutionary and allometric) and empirical (basic biologic and clinical epidemiological) underpinnings that support this view and provide evidence that the musculoskeletal system develops, functions, and ages as a whole.
EVOLUTIONARY VIEW: ALLOMETRY AND PLEIOTROPY
There are three major ontogenetic periods in the bone and muscle relationship: patterning in embryonic life, allometric growth in the postnatal period, and the homeostatic relationship in adult life. (30) For recent and comprehensive reviews of embryonic sources of muscle and bone development as well as progenitor cells, see Refs. 2, 31. In brief, muscle and bone cells share a common mesenchymal precursor. Furthermore, their production for the needs of organogenesis is regulated by a concerted action of genes, which is clearly evident during intrauterine development. The above concept of a universal biomechanical unit 27 , 28 was recently used by Matsuoka et al., (31) who proposed to identify genes involved in the cellular modularity of the muscle and bone.
The theory of allometry fully supports our assertion that genetic influences on bone and muscle are exerted in a pleiotropic manner. Allometry is usually defined as the form/size/shape covariance; the relationship between the growth and size of one body part to the growth and size of the whole organism. In regard to the musculoskeletal system, we expect that morphological traits, such as joint shapes and sizes, long bone diaphyseal morphology, muscle–bone interfaces (entheses), and bone–muscle lever orientations, would tend to covary significantly with gross morphological parameters because of the need to maintain an integrated, functioning system in light of variation in overall form and shape. (32) In theory, the pattern of (co)variation is expected to evolve to match fitness demands, (33) and alleles whose pleiotropic effects contribute to the attainment of appropriate proportions by interdependent parts that will generally be favored by natural selection. (34)
Models of allometric scaling of bone variables have been proposed. 35 , 36 In particular, Nevill et al. (37) supported the view that the BMC of the L2–L4 vertebrae develops proportionally to lean body mass in healthy adolescents. This complex system needs to be regulated by a concerted action of genes; also, it is very improbable that bone tissue homeostasis would be maintained by means of a single, simple mechanism. (2) There is a need for genetic mechanisms to be redundant, pleiotropic, and polygenic for safety reasons. 29 , 33
The spectrum of pleiotropic effects of a gene on a morphological trait may include both “direct” and “indirect” effects (through some intermediate mechanism), with a possible continuum in between. (38) It may be a single gene that can trigger changes in bone anatomy, and as a result, affect muscle architecture. For example, the genetically affected rate and/or duration of mitosis in the proliferative zones of femoral growth plates (39) or delayed mineralization of growth plate cartilage may lead to elongation of the femoral neck (with reduction of the neck shaft angle and height of the greater trochanter); both changes involve functional adaptations of soft tissues to maintain fitness in walking. (34) However, it is more plausible that slight changes of systemic control factors during development (e.g., small modulations of the growth hormone [GH] axis can generate fully coordinated morphological change). (34) For example, there is a plethora of indications that GH and related factors (such as insulin‐like growth factor 1 [IGF1]) may exert both direct anabolic effects on bones, as well as indirect effects, through influences on muscle (see Candidate genetic mechanisms for bone geometry and muscle mass).
Allometric shifts do not occur rapidly in an evolutionary and ontological sense; this is a long and involved process. Although bone has often been considered an optimal structure under given loading conditions, it cannot immediately attain an optimum under a varying load, because of the slow speed of adaptation. (40) Recent empiric and theoretical work suggests that there is no single optimal bone architecture; instead, many different architectural solutions produce adequate bone strength. 41 , 42 An interplay of genetic predisposition, environmental demands, and stochastic processes is thus expected to shape bones in vivo.
The mineral mass and lean mass content of the human body may also be functionally associated. (43) This has led to studies of functional (biomechanical) as well as genetically determined allometry. 32 , 44 If mineral mass and lean mass were functionally driven, morphologic traits would be related due to environmental stimuli. Indeed, and not surprisingly, body mass emerges as the strongest single predictor of femoral cross‐sectional geometry, explaining 82% of the variance in femoral second moments of area. (45) It is also possible that there are specific, local (i.e., nonallometric) increases in the size of the bones and joints as an isolated adaptation to load‐bearing. Such adaptations that have no antecedent differences in pattern formation and occur due to environmental stimuli, are probably epigenetic. (34) Bone at different locations in the skeleton maintains very dissimilar strain thresholds for initiating modeling. (41) The most widely exploited examples are the difference in size, shape, and moment of inertia between the dominant and nondominant arms in tennis players 46 , 47 and the asymmetry in metacarpal measurements due to hand dominance. (48) Even if there is an obvious environmental trigger, there should be a molecular substrate in bone responsible for recording and responding to the action of muscles; this susceptibility to outside triggers should be genetically determined.
EMPIRIC EVIDENCE (OBSERVATIONAL AND EXPERIMENTAL)
Inter‐relationship between muscle mass/strength and bone mass/geometry in adults
Studies of both humans (adults and children) and laboratory animals have documented a strong, positive correlation between muscle strength and bone mass. (49) According to in vitro studies, the ligamentous lumbar spine ex vivo, without assistance from muscles, buckles under compressive loads <90 N, whereas the in vivo spine can withstand loads >6000 N during daily tasks (50) and up to 18,000 N in competitive power lifters. (51) The ability of the in vivo spine to tolerate such high loads is mainly attributable to the dynamic stabilizing capacity of the trunk musculature. (19) Indeed, there are numerous slips of muscle, which either connect to individual lumbar vertebrae, ribs, or attach inferiorly to the pelvis and/or sacrum, or to the femora (psoas major). (52) Because so many morphological features interact to create the mechanical environment of the lumbar dorsal vertebra or proximal femur, analysis of its structure requires an integrated approach. (34)
Skeletal loads are dominated by muscle action, and the mechanostat theory postulates that bone strength adapts primarily to muscle forces. (28) In brief, the mechanostat theory proposes that loads that generate strains above some hypothetical threshold stimulate growth and inhibit haversian remodeling, whereas loads that generate strains below another threshold inhibit growth and stimulate loss of bone, thus helping bone to redistribute in space. Indeed, it was postulated that bone cross‐sectional properties mostly reflect shape adaptations to only the most vigorous forms of loading rather than to typical habitual activities. (2) However, there is evidence that the threshold of response varies with location in the bone (e.g., diaphysis versus epiphysis (53) ). Moreover, it has been hypothesized that extremely small strains, if induced at a sufficiently high frequency, are strong determinants of bone morphology. (54) In essence, this hypothesis has evolved from the demonstration that the spectrum of strain within functionally loaded bones can be characterized as having an inverse power‐law (1/f) relationship between the magnitude of strain events and the incidence of these events. (55) As such, the strain history of a bone is characterized by a very few large strain events (e.g., four events per 24 h that exceed 2000 microstrain (56) ), sloping linearly to 100,000s of very small strain events, each well below 10 microstrain, which arise through the dynamics of muscle contraction. This suggests that the bone tissue depends as much on the persistent, low magnitude strains that arise through dominant activities, such as standing, as it does on the relatively large, but rare strain events of intense (strenuous muscular) activity. From this perspective, the bone loss that accompanies aging or those subject to long‐term bed rest may result not only from diminished numbers of high strain magnitude cycles, but also diminished numbers of low strain magnitude cycles caused by the muscle wasting (sarcopenia) that parallels these conditions. (57)
The anatomical substrate for these actions of muscles on bones is not entirely clear. On one hand, tendons determine the translation of muscle force to the bone through the periosteum. (58) It is believed that muscle growth produces stretching of collagen fibers and periosteum at the muscle/bone interface, which stimulates local periosteal growth. (59) For example, inactivating mutations of the myostatin gene are associated with hypermuscularity, an increased size of the spinous processes, and expansion of entheses on both the femur and humerus. (60) On the other hand, specifically in entheses, the gradual transition between tissue types with distinctly different elastic moduli is thought to enhance the ability of tendons to dissipate force evenly during muscle contraction, thus resisting shear stresses at the bone surface. (61) Because blood flow to the limb is proportional to lean (muscle) mass, (62) one may expect that haversian perfusion will also benefit from well‐developed musculature.
As noted by Pearson and Lieberman, (2) most of the available data in both adults and children concern changes in BMD related to exercise and measures of lean mass; a smaller subset of experimental data illuminates the effects of exercise on the cross‐sectional geometry of long bones. 63 , 64 The study of the relationship between the muscle and bone morphology and especially cortical geometry probably will be even more important than the relation between the muscles and BMD/BMC from the biological and biomechanical perspective, because of the following reasons:
Measures of cross‐sectional geometry provide a robust way of assessing the summed effects of modeling on a bone and provide a good indication of the bone's ability to resist mechanical loads. (2)
Direct stimuli on the periosteum (independent of tendons/entheses) are more “proximal” to the muscles, meaning that shape adaptations to the effects of loading will be reflected there more clearly.
BMD is less useful than measures of cross‐sectional geometry, because it does not provide information about the surfaces or compartments in a bone that have grown or been resorbed in response to mechanical loading.
Trabecular architecture, which is equally important to bone geometry for the determination of bone strength, (65) is not well captured by DXA; therefore, the relevant knowledge is limited. We will thus focus most of the following arguments on cross‐sectional bone geometry.
Physical activity in older age
Aging is typically accompanied by declines in physical activity. Some relate it to energy deficiency with aging. (66) The general view may still be described as “increasing skepticism that exercise can add significant bone mass in adults, and mounting indirect evidence that the consequence of physical activity with regard to the adult skeleton is conservation, not acquisition.” (67)
Mechanical loads, greater than those habitually encountered by the skeleton, activate new bone formation on cortical and trabecular surfaces: strain can activate bone cells, which respond with gene activation, increased metabolism, growth factor production, and matrix synthesis. 28 , 68 In doing so, these loads increase bone strength and the resistance to fracture. (68) For example, mechanical stimulation of bone cells may induce elevated levels of IGF1, which promotes bone formation, and stimulate the differentiation of osteocytes from osteoblasts. (69) There are few studies that have examined whether discrete, localized loads such as those created by muscle contractions on attachment sites have local osteogenic effects. In one study, tensile forces induced the production of IGF1 and IGF1 receptors and proliferation of osteoblast‐like cells at the site of load application. (70) Thus, a leading contemporary theory suggests that both decreasing activity and muscle mass are the main causes of age‐related bone loss. (71) An example of a vicious circle has been suggested: less muscle force induces more bone resorption followed by more fractures, then bed rest/inactivity, decreased physical activity, and further decrease of muscular forces. (72) Age‐related decreases in the range of muscle contractions seem to represent a loss of a key regulatory stimulus. (73) Thus, an older inactive individual has neither high magnitude strain signals because of the lack of exercise nor low magnitude strain signals because of muscle fiber loss and limited standing activities.
It is well known that adult bone is less sensitive to exercise‐induced changes in peak muscle strain than younger, growing bone. (74) Thus, in a population‐based study of 375 women and 325 men (age, 21–97 yr), Melton et al. (75) failed to find a close correspondence between physical activity/muscle mass and changes in bone strength with aging. Moreover, interindividual variation in the strength‐to‐load ratios was substantial, (75) pointing out that individual intrinsic—probably genetic—factors must exist.
Evidence for genetic determination of bone and muscle mass/strength and genetic pleiotropy
Genetics of bone mass and geometry: Risk factors for osteoporosis (bone mass and structure/geometry) and sarcopenia (muscle mass and strength) are heritable. Genetic factors substantially contribute to variation in bone mass at different sites. (76) To better understand the genetic origins of hip and vertebral fractures, it is also important to examine heritable determinants of bone geometry. A wealth of studies have documented bone geometry to be under substantial genetic control with 30–70% heritability. 77 , 78 , 79 , 80 Genome‐wide linkage analyses of femoral geometry using plain radiographs 79 , 81 and DXA‐derived hip geometry traits 80 , 82 , 83 have identified several quantitative trait loci (QTLs) governing the variation in femoral neck geometry. Multiple studies of animals have also supported the above findings of high heritability of bone geometry. 84 , 85 For example, several studies using inbred mouse or rat strains have found distinct genetic contributions to vertebral area. 86 , 87 , 88 Several QTLs in mouse models 65 , 89 , 90 showed a convergence with syntenic loci in humans.
Genetics of muscle mass: Studies have shown that muscle strength and muscle mass are genetically determined. (91) Thus, in young brothers, Huygens et al. (23) found a heritability (h2) of muscle cross‐sectional area (CSA) and mass of ∼70–90%. Similarly, h2 up to 80% was found for DXA‐measured lean mass in female twin pairs. (92) In the Diabetes Heart Study families, h2 estimates of whole body lean mass (60%) and appendicular lean mass (66%) measured by DXA were similarly high, (93) suggesting that muscle strength/muscle mass are highly transmissible irrespective of measurement technology. Of note, heritability of lean body mass in mice has also been found to be in the same range, ∼60%. (94)
Evidence for shared genetics between bone and muscle mass
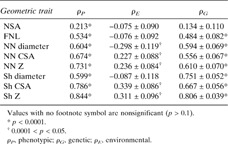
Given that muscle cells and osteoblasts derive from a common mesenchymal precursor and that muscle and bone are directly connected to each other, it is plausible that there would be potential genes determining both traits. High genetic correlation has been reported between femoral geometric parameters and total body lean mass in U.S. white adults from Nebraska, ranging from 0.28 to 0.69. (95) Furthermore, the most recent bivariate genome linkage analysis found in that same sample, two chromosomal regions, 5q35 and 10q24, with pleiotropic effects on these phenotypes. (96) Our preliminary results in the Framingham Osteoporosis Study showed high bivariate genetic correlations between leg lean mass and cross‐sectional femoral geometry, ranging from 0.56 to 0.81 (Table 1).
Table Table 1.
Correlations Between Age‐Adjusted Leg Lean Mass and Hip Geometry in the Framingham Osteoporosis Study Sample
|
|
Animal models confirm the above theoretical constructs and empirical observations. (97) Thus, inactivating mutations of the myostatin (growth differentiation factor 8 [GDF8]) gene induce a hypermuscular phenotype in cattle and mice. pQCT data showed that myostatin‐knockout mice have significantly greater cortical BMC at L5 than normal mice, (88) as well as an increased size of the spinous processes and expansion of entheses on both the femur and humerus. (60) In contrast, mice with the “mini‐muscle phenotype” (homozygous for a mendelian recessive allele that halves hindlimb muscle mass (98) ) exhibited significantly longer and thinner femora and tibia/fibulae, without a difference in bone mass. (38) Finally, QTLs have been identified for traits related to both bony carcass and meat quality in Scottish sheep (99) ; these traits were found to correlate with genes also in beef cattle. (100)
Implications for the genetic studies of osteoporosis: a need to consider pleiotropy
As substantiated above, there is abundant evidence suggesting that osteoporosis is largely determined by genetics. 101 , 102 As reviewed by Ioannidis et al., (103) by 2004, >60 QTLs had been identified from 20 human genome scans of BMD and/or fracture, covering all chromosomes except Y, but not many have been replicated in studies of independent groups or in a meta‐analysis. The relative lack of success in identifying genes for osteoporosis despite a plethora of recent studies may have resulted, in part, from an inadequate phenotype definition. Progress in the genetics of bone fragility is slow because the phenotype is poorly defined. (104) The risk of osteoporotic fracture can be viewed as a function of loading conditions and the ability of human bone to withstand the load. Risk of fracture is not entirely determined by intrinsic properties of bone, as other extrinsic factors are known to contribute (sarcopenia, fall‐related factors, including impaired cognition, and sensory input); there is thus an increasing realization that osteoporosis does not explain all low trauma fractures. (105) It is also important to remember that the genetic contribution to a risk factor may differ from the genetic contribution to the ultimate disease phenotype. For example, DXA‐derived aBMD, which has been commonly used as an endophenotype for fractures caused by osteoporosis, was found to share only modest genetic variance with osteoporotic fractures. 106 , 107 , 108 , 109 Therefore, neither aBMD alone or bone structure are perfect surrogates of the skeleton's ability to withstand the forces that produce fracture. Also, the most important clinical outcome of any anti‐osteoporosis intervention is a reduction in the risk of fractures. If this is achieved by increasing bone strength, this will be important; if this is done by decreasing fall rates, this will be even more beneficial for other outcomes of falls.
Phenotype definition is therefore an important consideration for any genetic epidemiological study. In addition, there is a need to take into account the multivariate etiology of osteoporosis. We postulate that to achieve success in the study of the genetics of osteoporosis (or genetics of sarcopenia), one needs to undertake a pleiotropic approach. There are several incentives for studying the contribution of genetics to both muscles and bones jointly, and there is both theoretical and empiric potential for success of this approach for several reasons. First, the “postgenomic” era provides the opportunity to use a technically advanced hypothesis‐free selection method to identify the major genetic variants. Second, the computational tools, exhaustive databases, and advanced database querying methods support the conduct of multivariate and multidisciplinary analyses. Comprehensive phenotyping will allow for broader exploration of contributing genes and should be particularly valuable for newly proposed analytic approaches, such as reverse phenotyping. 110 , 111 This approach, titled “applied phenomics,” would redefine a phenotype of a syndrome or condition based entirely on genetic covariance between the traits (which may not be obvious at the phenotypic level) and to embark on the theoretically increased power of performing joint analyses of genetically correlated traits. 112 , 113 , 114
Current approaches to determining genes that influence complex traits
There have been two different strategies to explore the genetic basis of complex traits (other than mendelian syndromes), each with its own advantages and pitfalls. The first strategy is genome‐wide, sometimes referred to as forward genetics. Because of recent technological advances in genome sequencing, the increased knowledge gained from the HapMap project, (115) and the refinement of bioinformatics, this strategy has been significantly facilitated with the use of microsatellite markers and at present, genome‐wide SNP chips. A newly available approach to reach that goal, genome‐wide association (GWA), analyzes SNPs across the whole genome 116 , 117 to provide robust de novo identification of chromosomal regions/SNPs that are linked and associated with the traits of interest. A second strategy (reverse genetics) is to pursue candidate genes through association studies. The candidate gene approach needs a strong prior hypothesis and has proven viable when there is a strong biological basis for inclusion of a gene as a plausible candidate. It seems that exploring several candidate genes belonging to the signaling pathways/gene regulation network with biological relevance to both bones and muscles will be a more promising strategy.
The majority of candidate genes traditionally come from basic studies in cell cultures and animal models. However, with the advent of genome‐wide scans, (96) expression experiments, (118) and bioinformatic tools, (119) new candidate genes may emerge for further pursuit.
Candidate genetic mechanisms for bone geometry and muscle mass
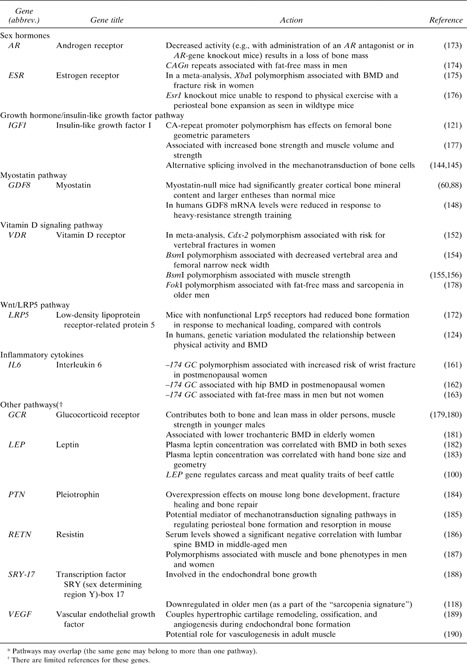
Numerous candidate genes for bone geometry have been proposed as contributors to osteoporosis risk based on bone biology. 120 , 121 , 122 Volkman et al. (123) proposed possible pathways through which genes may influence bone structure and geometry: (1) direct influence on bone size and shape (i.e., directed activity of osteoblasts and osteoclasts); (2) indirect effect on factors such as body weight, muscle strength, and activity level, which alter mechanical load on bones (124) ; and (3) effect on responsiveness of bone to applied mechanical loading (i.e., set points in a “mechanostat”‐like mechanism (28) ). We will focus on the latter two scenarios, which involve the key genetic pathways and single genes that apparently have a biologically plausible pleiotropic effect on both bone geometry and muscles. There are several parallel and partly complementary genetic systems involving intertwined hormonal functions for a muscle–bone interaction because these need to be both pleiotropic and multigenic for safety reasons. 29 , 33 In Table 2, we provide several pathways and single genes, known as candidates for osteoporosis, for which there are indications of pleiotropic actions also on lean mass/muscles. Of note, this is not a comprehensive review of the literature or a formal search of the relevant databases; these genes were identified by a PubMed search using keywords such as “osteoporosis,” “sarcopenia,” “gene,” or “polymorphism,” etc. Only those genes for which more than a single reference was available are listed in Table 2.
Table Table 2.
Candidate Genes and Pathways* for Pleiotropic Action on Bone and Muscle
|
|
Sex hormones: Sex hormones are obvious candidates for regulation of both bones and muscles because the sexual dimorphism in bone geometry/mass and muscle strength is pronounced. There are two primary groups of sex hormones: androgens and estrogens. The skeletal actions of androgens may result from direct activation of the androgen receptor (AR) or may alternatively depend on stimulation of the estrogen receptors (ESRs) after aromatization of androgens into estrogens that occurs in peripheral tissues (including bone); the enzyme, aromatase, catalyzes this conversion. These receptors are essential for normal skeletal growth and bone mineral acquisition, (125) as well as being important regulators of recovery from disuse atrophy. (126) Sex hormones or related factors could affect the threshold of the feedback system that controls bone remodeling to adapt bone structure to the strains derived from customary mechanical usage in each region of the skeleton (through a bone “mechanostat”). 29 , 43 , 127
Genetic polymorphisms: AR polymorphisms occur commonly in the population and are the best‐characterized genetic determinants of androgen responsiveness. The length of the trinucleotide repeat (CAGn) in exon 1 is inversely associated with transactivation activity because of reduced binding of AR co‐activators and can modulate physiological androgen effects, such as prostate size, sperm concentration, concentrations of lipids, insulin, and leptin, and BMD. (128) Polymorphisms in two estrogen receptors, ESR1 and ESR2, 129 , 130 , 131 as well as in the aromatase gene CYP19, 125 , 132 have been extensively studied. Studies suggested that some variants in ESR1 cause less sensitivity to estrogen, because of differential expression of the receptor. (133) The retinoblastoma‐interacting zinc finger protein (RIZ1) has been shown to function as a specific estrogen nuclear hormone receptor co‐activator and to strongly enhance the function of the ESR1 both in vivo and in vitro; a deletion polymorphism in the RIZ1 gene exhibits decreased response to estrogen in vitro. 134 , 135
Another candidate that has been studied with regard to bone and muscle effects is catechol‐O‐methyltransferase (COMT), an estrogen‐degrading enzyme, also involved in the degradation of catecholamines. The COMT val158met polymorphism was associated with peak BMD in young men, (136) and most recently, an interaction of COMT with physical activity was found in the same young men. (137) Also, CYP17A1, the cytochrome P450 family 17 subfamily A gene, has been considered as a strong candidate gene for osteoporosis, because of its role in coding for a key enzyme in the steroidogenic pathway that produces progestins, mineralocorticoids, glucocorticoids, estrogens, and androgens. (96)
IGF1: Systemic control hormones, GH and/or its related factors, are active during development and repair to generate morphological change in bones in a fully coordinated manner with the skeletal muscle. (34) They are also active during adult ontogenesis. GH activates IGF1 gene transcription in vivo; GH treatment upregulates the level of IGF1 expression. (138) IGF1 in turn plays a prominent role in the pathophysiology of osteoporotic fracture. Several reports 139 , 140 have documented a decrement in IGF1 plasma levels with age in humans. Mechanical stimulation on bone cells may induce elevated levels of IGF1, which in turn prompts the differentiation of osteocytes from osteoblasts and thus promotes bone formation, for example, in rat calvaria. (70) The relevance of IGF1 for cortical bone metabolism is also well established in the mouse. 141 , 142
A CA‐repeat promoter polymorphism in the IGF1 gene was found to be associated with circulating IGF1 levels. (143) Also, Goldspink et al. (144) cloned two different IGF1 isoforms that are expressed by skeletal muscle, and both are derived from the IGF1 gene by alternative splicing (reading frameshift change). One of these isoforms, called “mechanogrowth factor” (MGF), is expressed in response to physical activity and is apparently involved in the mechanotransduction of bone cells. (145) The other is similar to the systemic or liver type and is important as the provider of mature IGF1; its expression is also upregulated by exercise. It seems that these two forms of IGF1 have somewhat different actions and that they both are important regulators of muscle growth and therefore contribute to bone strength. Table 2 presents data on pleiotropic action of this and other polymorphisms. IGF1 induces muscle hypertrophy by activating the IGF1 receptor (IGF1R), which activates multiple signaling pathways, including the PI3K and MAPK pathways. In addition to IGF1, the IGF‐2 genotype also was associated with total body fat‐free mass in humans (146) and muscle growth in pigs. (147)
Myostatin: Another interesting candidate is myostatin (GDF8 gene), a member of the TGF‐β superfamily and a negative regulator of skeletal muscle mass. Inactivating mutations of the GDF8 gene induce a hypermuscular phenotype in cattle and mice. (60) Strength training suppresses myostatin expression, showing that downregulation of myostatin signaling is one mechanism by which resistance exercise increases muscle mass. (148) Findings in mdx mice (a model for Duchene muscular dystrophy) show that myostatin blockade can simulate the effects of strength training. Myostatin binds to an activin type II receptor and initiates a signaling cascade that involves the activation of Smad proteins (which is another possible pathway for the GDF8 involvement in the skeleton). Most recently, it was also indicated that myostatin may act upstream of Wnt pathway components; specifically, it repressed expression of Wnt4 in the skeletal muscle. (149) Table 2 presents data on pleiotropic action of the GDF8 on bone.
Vitamin D receptor
The vitamin D receptor (VDR) gene has long been targeted as one of the genetic determinants influencing bone status because it regulates bone homeostasis through the vitamin D endocrine system. There are a few studies that have examined the associations between VDR and muscle strength; Bischoff et al. 150 , 151 showed expression of the VDR in human skeletal muscle tissue, which decreases with age. Also, short‐term vitamin D supplementation does reduce body sway, in addition to an improvement in lower body strength, which is reduced in vitamin D deficiency. This reduction in the number of falls and fractures would indicate an alternative mechanism other than bone strength/BMD, and one option would be a relationship between VDR polymorphisms and muscle strength.
A number of SNPs within this well‐known and widely studied gene for osteoporosis have been studied, including the BsmI, ApaI, and TaqI polymorphisms located in the 3′end of the gene (152) or the Cdx‐2 transcription factor binding site, which is located within the promoter region. (153) Table 2 provides evidence for associations of VDR polymorphisms with bone geometry, (154) fractures, (152) and muscle mass or strength. 155 , 156
Low‐density lipoprotein receptor–related protein 5
Polymorphisms in the low‐density lipoprotein receptor–related protein 5 (LRP5), a Wnt co‐receptor, have been associated with BMD in men and women. 122 , 157 In mice, Akhter et al. (158) found that the Lrp5 G171V mutation results in a skeleton that has greater structural (femoral shaft, femoral neck, tibias, vertebral body) and apparent material (vertebral body) strength. They hypothesized that the denser and stiffer bones in G171V mice may represent greater sensitivity to normal mechanical stimuli, resulting in an overadaptation of the skeleton to weight‐related forces. More recent studies suggest that, whereas knockout mice lacking the protein can sense mechanical load correctly, they are unable to respond properly to it by forming new periosteal bone. (159) In our study, (124) genetic variation in polymorphisms of the LRP5 gene was found to modulate the relationship between physical activity and BMD in men, thus suggesting that LRP5 may play a role in the adaptation of bone to mechanical load in humans as well. Furthermore, Wnt signaling has been implicated in overload‐induced skeletal muscle hypertrophy in mice. (160) All things considered, this may suggest that Wnt signaling, and specifically, LRP5 may be involved in muscle/bone cross‐talk. As mentioned above, myostatin may act on both bone and muscle tissue upstream of Wnt signaling. (149)
Other genes with possible pleiotropic actions may exist. Actions of IL‐6 in bone and muscle loss also become apparent. (26) IL‐6 is a cytokine that has been shown to be produced at high levels by human skeletal muscle after endurance‐type exercise, having a role in the mobilization of substrates to support metabolism during exercise. The best studied with osteoporosis‐related traits is a SNP G/C at −174 bp, which was shown to be associated with increased risk of wrist fractures(161) and lower hip BMD in postmenopausal women(162) and fat‐free mass in men but not women.(163) In vitro studies showed that IL‐6 inhibits the secretion of IGF1; thus, the negative effect of IL‐6 on muscle function might be mediated through IGF1. (164)
In summary, Table 2 provides indications that genes from multiple pathways, including inflammatory, GH, and steroid metabolism, are candidates for pleiotropic regulation of bone and muscle. Other possible candidates such as leptin, transcription factor SRY‐box 17, pleiotrophin, vascular endothelial growth factor, and glucocorticoid receptor, are also shown in Table 2; for these genes, less is known about their pleiotropic action on bone and muscle. This list needs to be refined by bioinformatic search of genetic association and expression databases and confirmed through functional studies.
Additional considerations: gene‐by‐gene interactions on bone strength and muscle mass
Bone and muscle anabolism can be induced by multiple pathways. There are several parallel and partly complementary physiological feedback systems involving intertwined hormonal functions for the muscle–bone interaction. 29 , 33 Any list of the candidate genes may be either incomplete or redundant. We may thus expect that, in addition to pleiotropic effects of the genes, there are gene‐by‐gene interactions among these pathways; thus, epistatic pleiotropy may be involved.
Indeed, epistatic interactions between ESR1, ESR2, and nuclear receptor interacting protein 1 (NRIP1) genes were associated with osteoporosis in postmenopausal Spanish women. (165) Also, sex steroids interact with the GH/IGF1 axis during puberty, (166) which may be caused by utilization of the same signal transduction pathways, including MAPKs and PI3K. There is an indication that testosterone increases concentrations of IGF1 and reduces concentrations of IGF1 inhibitory proteins. (167) Alternatively, systemic IGF1 and AR‐signaling may stimulate periosteal growth independently. (168) Also, Rivadeneira et al. (169) found that variants of ESR2 in interaction with ESR1 and IGF1 influenced femoral neck cortical thickness and section modulus in postmenopausal women.
These gene interactions add a degree of complexity to the relationships among the genes in the anabolic pathways chosen above as examples. This also strongly suggests a need to explore the interactions, at least between the genes belonging to the same pathway. The question of interactions between multiple genes is thus an intriguing one that requires more elaborate study designs.
SUMMARY AND RECOMMENDATIONS.
In conclusion, there are many more questions than answers. It is not fully understood how bone senses and responds to muscle actions, whether it loses its mechanosensitivity with aging, or which cells are responsible for this ability. Currently not much is known of the role endogenous estrogens and administration of estrogen may play in the management of sarcopenia; estrogen replacement therapy (ERT) did not prevent loss of muscle composition and strength with aging. 170 , 171 Similarly, the role of aromatization of testosterone into estrogen in mediating the testosterone effects on body composition/muscle mass is poorly understood.
A joint approach focusing on common genetic determination of both skeletal geometry and muscular mass will identify new signaling pathways, which in turn will pinpoint novel biological mechanisms. The study of model organisms and whole genome expression and candidate gene association that deals with two outcomes, muscular and osseous, should prove helpful in this task.
It is important to consider dose‐response depending on a threshold of exercise necessary to maintain the muscle and bone. Future work should attempt to document whether such a threshold exists and, if it does, to determine the loads necessary to induce osteogenesis. (53) There is a need to create a drug/intervention able to reverse the aging of bone, to make bones more susceptible to beneficial effects of exercise, muscle load, and stimulation through vibration, comparable of that of young subjects. This type of therapy could be used to reverse sarcopenia in the elderly, to prevent muscle loss that occurs in astronauts, (172) and to combat falls and fractures. The progress in these studies may translate into new approaches to the prevention of hip and vertebral fractures as well as muscle wasting; moreover, it may ultimately help understand broader concepts, such as frailty.
We postulate that to achieve success in the study of osteoporosis (as well as sarcopenia), there is a need to take into account the multivariate nature of the complex diseases and to capitalize on benefits of the pleiotropic approach. Identifying significant genetic variants underlying both bones and muscles, measured with state‐of‐art technology and replicated in large human cohorts and animal experiments, will provide valuable insight into important potential targets for risk stratification, as well as pharmacogenetic applications. Knowledge of the genetic machinery underlying an interaction between bones and muscles may ultimately identify targets for specific interventions aimed at increasing bone strength, muscle strength, or both.
GLOSSARY
Allometric: relates to the proportions of various parts of an organism as a consequence of growth and development, for example, the change of body parts in direct proportion to body size
Alternative splicing: a regulatory mechanism by which different forms of mature mRNA are generated from the same gene to produce more than one related protein (isoform), by variations in the incorporation of the exons, or coding regions, into mRNA
Endophenotype: a measurable trait that is more proximate to the pathophysiologic effects of the gene. For example, in the case of osteoporosis, endophenotypes will be bone turnover markers, whereas BMD, muscle mass, etc., are “intermediate” phenotypes
Epigenetic: any factor that indirectly influences the expression of the genome without directly affecting its DNA, for example, DNA methylation and chromatin remodeling
Epistasis: two or more genes interacting with one another in a multiplicative fashion
Gene expression: the process of converting the genetic information encoded in the DNA into the final gene product
Heritability(h2): In the narrow sense, h2 is defined as the proportion of the total phenotypic variance in a trait that is caused by the additive effects of genes, when environmental factors are excluded. In the broad sense, heritability is proportion of the total phenotypic variance of a trait that is caused by all genetic effects, including additive and dominance effects.
Knockout mice: mice in which a gene has been deleted or inactivated in both the somatic and the germ cells so that the animals produce no functional gene product
Phenotype: observed physical, biochemical, structural, or physiological manifestation of a genotype
Pleiotropy: one gene leading to many different phenotypic expressions
Polymorphism: any locus at which at least two alleles are available, such that at least 1% of a population carries the rarer genotype
Quantitative trait: a phenotype that varies in a quantitative manner, which can be caused by combinations of genetic and environmental factors, as well as a measurement error. Quantitative traits are often considered to be polygenic traits.
Reverse phenotyping: a computational process by which genetic data used to drive new phenotype definitions (in silico)
Strain: deformation of a material divided by a corresponding undEFormed dimension (e.g., 1 microstrain = 10−6 m/m or in/in)
The authors state that they have no conflicts of interest.
REFERENCES
- 1. Karasik D, Hannan MT, Cupples LA, Felson DT, Kiel DP 2004. Genetic contribution to biological aging: The Framingham Study. J Gerontol A Biol Sci Med Sci 59: 218–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pearson OM, Lieberman DE 2004. The aging of Wolff's “law”: Ontogeny and responses to mechanical loading in cortical bone. Am J Phys Anthropol Suppl 39: 63–99. [DOI] [PubMed]
- 3. Manolagas SC 2000. Birth and death of bone cells: Basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr Rev 21: 115–137. [DOI] [PubMed] [Google Scholar]
- 4. Kiel DP 2000. Adult bone maintenance In: Marcus R. (ed.) Atlas of Clinical Endocrinology. III. Osteoporosis. Current Medicine, Philadelphia, PA, USA, 29–39. [Google Scholar]
- 5. Buckey JC 2006. Space Physiology. University Press, Oxford, UK. [Google Scholar]
- 6. Zethraeus N, Borgstrom F, Strom O, Kanis JA, Jonsson B 2007. Cost‐effectiveness of the treatment and prevention of osteoporosis–a review of the literature and a reference model. Osteoporos Int 18: 9–23. [DOI] [PubMed] [Google Scholar]
- 7. Faulkner KG, Wacker WK, Barden HS, Simonelli C, Burke PK, Ragi S, Del Rio L 2006. Femur strength index predicts hip fracture independent of bone density and hip axis length. Osteoporos Int 17: 593–599. [DOI] [PubMed] [Google Scholar]
- 8. Faulkner KG, Cummings SR, Black D, Palermo L, Gluer CC, Genant HK 1993. Simple measurement of femoral geometry predicts hip fracture: The study of osteoporotic fractures. J Bone Miner Res 8: 1211–1217. [DOI] [PubMed] [Google Scholar]
- 9. Melton LJ III, Riggs BL, van Lenthe GH, Achenbach SJ, Muller R, Bouxsein ML, Amin S, Atkinson EJ, Khosla S 2007. Contribution of in vivo structural measurements and load/strength ratios to the determination of forearm fracture risk in postmenopausal women. J Bone Miner Res 22: 1442–1448. [DOI] [PubMed] [Google Scholar]
- 10. Lauretani F, Bandinelli S, Bartali B, Iorio AD, Giacomini V, Corsi AM, Guralnik JM, Ferrucci L 2006. Axonal degeneration affects muscle density in older men and women. Neurobiol Aging 27: 1145–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tan RS 2005. Aging Men's Health: A Case‐Based Approach Thieme, New York, NY, USA. [Google Scholar]
- 12. Frontera WR, Hughes VA, Fielding RA, Fiatarone MA, Evans WJ, Roubenoff R 2000. Aging of skeletal muscle: A 12‐yr longitudinal study. J Appl Physiol 88: 1321–1326. [DOI] [PubMed] [Google Scholar]
- 13. Frederiksen H, Gaist D, Petersen HC, Hjelmborg J, McGue M, Vaupel JW, Christensen K 2002. Hand grip strength: A phenotype suitable for identifying genetic variants affecting mid‐ and late‐life physical functioning. Genet Epidemiol 23: 110–122. [DOI] [PubMed] [Google Scholar]
- 14. Rantanen T, Volpato S, Ferrucci L, Heikkinen E, Fried LP, Guralnik JM 2003. Handgrip strength and cause‐specific and total mortality in older disabled women: Exploring the mechanism. J Am Geriatr Soc 51: 636–641. [DOI] [PubMed] [Google Scholar]
- 15. Frontera WR, Suh D, Krivickas LS, Hughes VA, Goldstein R, Roubenoff R 2000. Skeletal muscle fiber quality in older men and women. Am J Physiol Cell Physiol 279: C611–C618. [DOI] [PubMed] [Google Scholar]
- 16. Goodpaster BH, Kelley DE, Thaete FL, He J, Ross R 2000. Skeletal muscle attenuation determined by computed tomography is associated with skeletal muscle lipid content. J Appl Physiol 89: 104–110. [DOI] [PubMed] [Google Scholar]
- 17. Goodpaster BH, Thaete FL, Kelley DE 2000. Composition of skeletal muscle evaluated with computed tomography. Ann N Y Acad Sci 904: 18–24. [DOI] [PubMed] [Google Scholar]
- 18. Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, Tylavsky FA 2005. Trunk muscle composition as a predictor of reduced functional capacity in the health, aging and body composition study: The moderating role of back pain. J Gerontol A Biol Sci Med Sci 60: 1420–1424. [DOI] [PubMed] [Google Scholar]
- 19. Hicks GE, Simonsick EM, Harris TB, Newman AB, Weiner DK, Nevitt MA, Tylavsky FA 2005. Cross‐sectional associations between trunk muscle composition, back pain, and physical function in the health, aging and body composition study. J Gerontol A Biol Sci Med Sci 60: 882–887. [DOI] [PubMed] [Google Scholar]
- 20. Prendergast P, van der Helm F, Duda G 2005. Analysis of muscles and joint loads In: Mow V, Huiskes R. (eds.) Basic Orthopaedic Biomechanics and Mechano‐Biology, 3rd ed Lippincott Williams & Wilkins, Philadelphia, PA, USA, 29–89. [Google Scholar]
- 21. Visser M, Kritchevsky SB, Goodpaster BH, Newman AB, Nevitt M, Stamm E, Harris TB 2002. Leg muscle mass and composition in relation to lower extremity performance in men and women aged 70 to 79: The health, aging and body composition study. J Am Geriatr Soc 50: 897–904. [DOI] [PubMed] [Google Scholar]
- 22. Broadwin J, Goodman‐Gruen D, Slymen D 2001. Ability of fat and fat‐free mass percentages to predict functional disability in older men and women. J Am Geriatr Soc 49: 1641–1645. [DOI] [PubMed] [Google Scholar]
- 23. Huygens W, Thomis MA, Peeters MW, Vlietinck RF, Beunen GP 2004. Determinants and upper‐limit heritabilities of skeletal muscle mass and strength. Can J Appl Physiol 29: 186–200. [DOI] [PubMed] [Google Scholar]
- 24. Visser M, Harris TB, Langlois J, Hannan MT, Roubenoff R, Felson DT, Wilson PW, Kiel DP 1998. Body fat and skeletal muscle mass in relation to physical disability in very old men and women of the Framingham Heart Study. J Gerontol A Biol Sci Med Sci 53: M214–M221. [DOI] [PubMed] [Google Scholar]
- 25. Visser M, Deeg DJ, Lips P, Harris TB, Bouter LM 2000. Skeletal muscle mass and muscle strength in relation to lower‐extremity performance in older men and women. J Am Geriatr Soc 48: 381–386. [DOI] [PubMed] [Google Scholar]
- 26. Solomon AM, Bouloux PM 2006. Modifying muscle mass—the endocrine perspective. J Endocrinol 191: 349–360. [DOI] [PubMed] [Google Scholar]
- 27. Frost HM, Schonau E 2000. The “muscle‐bone unit” in children and adolescents: A 2000 overview. J Pediatr Endocrinol Metab 13: 571–590. [DOI] [PubMed] [Google Scholar]
- 28. Frost HM 2003. Bone's mechanostat: A 2003 update. Anat Rec A Discov Mol Cell Evol Biol 275: 1081–101. [DOI] [PubMed] [Google Scholar]
- 29. Sievänen H 2005. Hormonal influences on the muscle‐bone feedback system: A perspective. J Musculoskelet Neuronal Interact 5: 255–261. [PubMed] [Google Scholar]
- 30. Orestes‐Cardoso SM, Nefussi JR, Hotton D, Mesbah M, Orestes‐Cardoso MD, Robert B, Berdal A 2001. Postnatal Msx1 expression pattern in craniofacial, axial, and appendicular skeleton of transgenic mice from the first week until the second year. Dev Dyn 221: 1–13. [DOI] [PubMed] [Google Scholar]
- 31. Matsuoka T, Ahlberg PE, Kessaris N, Iannarelli P, Dennehy U, Richardson WD, McMahon AP, Koentges G 2005. Neural crest origins of the neck and shoulder. Nature 436: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Churchill SE 1996. Particulate versus integrated evolution of the upper body in late pleistocene humans: A test of two models. Am J Phys Anthropol 100: 559–583. [DOI] [PubMed] [Google Scholar]
- 33. Wolf JB, Pomp D, Eisen EJ, Cheverud JM, Leamy LJ 2006. The contribution of epistatic pleiotropy to the genetic architecture of covariation among polygenic traits in mice. Evol Dev 8: 468–476. [DOI] [PubMed] [Google Scholar]
- 34. Lovejoy CO, Meindl RS, Ohman JC, Heiple KG, White TD 2002. The Maka femur and its bearing on the antiquity of human walking: Applying contemporary concepts of morphogenesis to the human fossil record. Am J Phys Anthropol 119: 97–133. [DOI] [PubMed] [Google Scholar]
- 35. Carter DR, Orr TE 1992. Skeletal development and bone functional adaptation. J Bone Miner Res 7 (Suppl 2): S389–S395. [DOI] [PubMed] [Google Scholar]
- 36. Nevill AM, Holder RL, Stewart AD 2003. Modeling elite male athletes' peripheral bone mass, assessed using regional dual x‐ray absorptiometry. Bone 32: 62–68. [DOI] [PubMed] [Google Scholar]
- 37. Nevill AM, Holder RL, Maffulli N, Cheng JC, Leung SS, Lee WT, Lau JT 2002. Adjusting bone mass for differences in projected bone area and other confounding variables: An allometric perspective. J Bone Miner Res 17: 703–708. [DOI] [PubMed] [Google Scholar]
- 38. Kelly SA, Czech PP, Wight JT, Blank KM, Garland T Jr 2006. Experimental evolution and phenotypic plasticity of hindlimb bones in high‐activity house mice. J Morphol 267: 360–374. [DOI] [PubMed] [Google Scholar]
- 39. Wilsman NJ, Farnum CE, Leiferman EM, Fry M, Barreto C 1996. Differential growth by growth plates as a function of multiple parameters of chondrocytic kinetics. J Orthop Res 14: 927–936. [DOI] [PubMed] [Google Scholar]
- 40. Bagge M 2000. A model of bone adaptation as an optimization process. J Biomech 33: 1349–1357. [DOI] [PubMed] [Google Scholar]
- 41. Turner CH 2002. Biomechanics of bone: Determinants of skeletal fragility and bone quality. Osteoporos Int 13: 97–104. [DOI] [PubMed] [Google Scholar]
- 42. Nowlan NC, Prendergast PJ 2005. Evolution of mechanoregulation of bone growth will lead to non‐optimal bone phenotypes. J Theor Biol 235: 408–418. [DOI] [PubMed] [Google Scholar]
- 43. Ferretti JL, Capozza RF, Cointry GR, Garcia SL, Plotkin H, Alvarez Filgueira ML, Zanchetta JR 1998. Gender‐related differences in the relationship between densitometric values of whole‐body bone mineral content and lean body mass in humans between 2 and 87 years of age. Bone 22: 683–690. [DOI] [PubMed] [Google Scholar]
- 44. Washburn RA, Smith KW, Jette AM, Janney CA 1993. The Physical Activity Scale for the Elderly (PASE): Development and evaluation. J Clin Epidemiol 46: 153–162. [DOI] [PubMed] [Google Scholar]
- 45. van der Meulen MC, Carter DR 1995. Developmental mechanics determine long bone allometry. J Theor Biol 172: 323–327. [DOI] [PubMed] [Google Scholar]
- 46. Haapasalo H, Kontulainen S, Sievanen H, Kannus P, Jarvinen M, Vuori I 2000. Exercise‐induced bone gain is due to enlargement in bone size without a change in volumetric bone density: A peripheral quantitative computed tomography study of the upper arms of male tennis players. Bone 27: 351–357. [DOI] [PubMed] [Google Scholar]
- 47. Bass SL, Saxon L, Daly RM, Turner CH, Robling AG, Seeman E, Stuckey S 2002. The effect of mechanical loading on the size and shape of bone in pre‐, peri‐, and postpubertal girls: A study in tennis players. J Bone Miner Res 17: 2274–2280. [DOI] [PubMed] [Google Scholar]
- 48. Plato CC, Wood JL, Norris AH 1980. Bilateral asymmetry in bone measurements of the hand and lateral hand dominance. Am J Phys Anthropol 52: 27–31. [DOI] [PubMed] [Google Scholar]
- 49. Gilsanz V, Wren TA, Sanchez M, Dorey F, Judex S, Rubin C 2006. Low‐level, high‐frequency mechanical signals enhance musculoskeletal development of young women with low BMD. J Bone Miner Res 21: 1464–1474. [DOI] [PubMed] [Google Scholar]
- 50. McGill SM, Norman RW 1986. Partitioning of the L4‐L5 dynamic moment into disc, ligamentous, and muscular components during lifting. Spine 11: 666–678. [DOI] [PubMed] [Google Scholar]
- 51. Cholewicki J, McGill SM 1996. Mechanical stability of the in vivo lumbar spine: Implications for injury and chronic low back pain. Clin Biomech (Bristol, Avon) 11: 1–15. [DOI] [PubMed] [Google Scholar]
- 52. Stokes IA, Gardner‐Morse M 1999. Quantitative anatomy of the lumbar musculature. J Biomech 32: 311–316. [DOI] [PubMed] [Google Scholar]
- 53. Zumwalt A 2006. The effect of endurance exercise on the morphology of muscle attachment sites. J Exp Biol 209: 444–454. [DOI] [PubMed] [Google Scholar]
- 54. Rubin CT, Sommerfeldt DW, Judex S, Qin Y 2001. Inhibition of osteopenia by low magnitude, high‐frequency mechanical stimuli. Drug Discov Today 6: 848–858. [DOI] [PubMed] [Google Scholar]
- 55. Fritton SP, McLeod KJ, Rubin CT 2000. Quantifying the strain history of bone: Spatial uniformity and self‐similarity of low‐magnitude strains. J Biomech 33: 317–325. [DOI] [PubMed] [Google Scholar]
- 56. Adams DJ, Spirt AA, Brown TD, Fritton SP, Rubin CT, Brand RA 1997. Testing the daily stress stimulus theory of bone adaptation with natural and experimentally controlled strain histories. J Biomech 30: 671–678. [DOI] [PubMed] [Google Scholar]
- 57. Desplanches D, Mayet MH, Ilyina‐Kakueva EI, Sempore B, Flandrois R 1990. Skeletal muscle adaptation in rats flown on Cosmos 1667. J Appl Physiol 68: 48–52. [DOI] [PubMed] [Google Scholar]
- 58. Daly RM, Bass SL 2006. Lifetime sport and leisure activity participation is associated with greater bone size, quality and strength in older men. Osteoporos Int 17: 1258–1267. [DOI] [PubMed] [Google Scholar]
- 59. Herring S 1994. Development of functional interactions between skeletal and muscular systems In: Hall B. (ed.) Bone, vol 9 CRC Press, Boca Raton, FL, USA, 165–191. [Google Scholar]
- 60. Hamrick MW 2003. Increased bone mineral density in the femora of GDF8 knockout mice. Anat Rec A Discov Mol Cell Evol Biol 272: 388–391. [DOI] [PubMed] [Google Scholar]
- 61. Benjamin M, Rufai A, Ralphs J 2000. The mechanism of formation of bony spurs (enthesophytes) in the Achilles tendon. Arthritis Rheum 43: 576–583. [DOI] [PubMed] [Google Scholar]
- 62. Hamrick MW, Pennington C, Newton D, Xie D, Isales C 2004. Leptin deficiency produces contrasting phenotypes in bones of the limb and spine. Bone 34: 376–383. [DOI] [PubMed] [Google Scholar]
- 63. Uusi‐Rasi K, Sievanen H, Heinonen A, Beck TJ, Vuori I 2004. Determinants of changes in bone mass and femoral neck structure, and physical performance after menopause: A 9‐year follow‐up of initially peri‐menopausal women. Osteoporos Int 15: 909–917. [DOI] [PubMed] [Google Scholar]
- 64. Petit MA, Beck TJ, Lin HM, Bentley C, Legro RS, Lloyd T 2004. Femoral bone structural geometry adapts to mechanical loading and is influenced by sex steroids: The Penn State Young Women's Health Study. Bone 35: 750–759. [DOI] [PubMed] [Google Scholar]
- 65. Bouxsein ML, Uchiyama T, Rosen CJ, Shultz KL, Donahue LR, Turner CH, Sen S, Churchill GA, Muller R, Beamer WG 2004. Mapping quantitative trait loci for vertebral trabecular bone volume fraction and microarchitecture in mice. J Bone Miner Res 19: 587–599. [DOI] [PubMed] [Google Scholar]
- 66. Wallace DC 2005. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu Rev Genet 39: 359–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Forwood MR, Burr DB 1993. Physical activity and bone mass: Exercises in futility? Bone Miner 21: 89–112. [DOI] [PubMed] [Google Scholar]
- 68. Forwood MR 2001. Mechanical effects on the skeleton: Are there clinical implications? Osteoporos Int 12: 77–83. [DOI] [PubMed] [Google Scholar]
- 69. Schmid K, McSharry W, Pameijer C, Binette J 1980. Chemical and physiochemical studies on the mineral deposits of the human atherosclerotic aorta. Atherosclerosis 37: 199–210. [DOI] [PubMed] [Google Scholar]
- 70. Hirukawa K, Miyazawa K, Maeda H, Kameyama Y, Goto S, Togari A 2005. Effect of tensile force on the expression of IGF‐I and IGF‐I receptor in the organ‐cultured rat cranial suture. Arch Oral Biol 50: 367–372. [DOI] [PubMed] [Google Scholar]
- 71. Kaptoge S, Dalzell N, Jakes RW, Wareham N, Day NE, Khaw KT, Beck TJ, Loveridge N, Reeve J 2003. Hip section modulus, a measure of bending resistance, is more strongly related to reported physical activity than BMD. Osteoporos Int 14: 941–949. [DOI] [PubMed] [Google Scholar]
- 72. Fricke O, Schoenau E 2007. The ‘Functional Muscle‐Bone Unit’: Probing the relevance of mechanical signals for bone development in children and adolescents. Growth Horm IGF Res 17: 1–9. [DOI] [PubMed] [Google Scholar]
- 73. Huang RP, Rubin CT, McLeod KJ 1999. Changes in postural muscle dynamics as a function of age. J Gerontol A Biol Sci Med Sci 54: B352–B357. [DOI] [PubMed] [Google Scholar]
- 74. Turner CH, Takano Y, Owan I 1995. Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res 10: 1544–1549. [DOI] [PubMed] [Google Scholar]
- 75. Melton LJ III, Riggs BL, Achenbach SJ, Amin S, Camp JJ, Rouleau PA, Robb RA, Oberg AL, Khosla S 2006. Does reduced skeletal loading account for age‐related bone loss? J Bone Miner Res 21: 1847–1855. [DOI] [PubMed] [Google Scholar]
- 76. Karasik D, Cupples LA, Hannan MT, Kiel DP 2004. Genome screen for a combined bone phenotype using principal component analysis: The Framingham study. Bone 34: 547–556. [DOI] [PubMed] [Google Scholar]
- 77. Looker AC, Beck TJ 2002. Maternal history of osteoporosis and femur geometry. 75: 277–285. [DOI] [PubMed] [Google Scholar]
- 78. Karasik D, Ginsburg E, Livshits G, Pavlovsky O, Kobyliansky E 2000. Evidence on major gene control of cortical bone loss in human population. Genet Epidemiol 19: 410–421. [DOI] [PubMed] [Google Scholar]
- 79. Koller DL, Liu G, Econs MJ, Hui SL, Morin PA, Joslyn G, Rodriguez LA, Conneally PM, Christian JC, Johnston CC, Foroud T, Peacock M 2001. Genome screen for quantitative trait loci underlying normal variation in femoral structure. J Bone Miner Res 16: 985–991. [DOI] [PubMed] [Google Scholar]
- 80. Xiong DH, Shen H, Xiao P, Guo YF, Long JR, Zhao LJ, Liu YZ, Deng HY, Li JL, Recker RR, Deng, HW 2006. Genome‐wide scan identified QTLs underlying femoral neck cross‐sectional geometry that are novel studied risk factors of osteoporosis. J Bone Miner Res 21: 424–437. [DOI] [PubMed] [Google Scholar]
- 81. Peacock M, Koller DL, Lai D, Hui S, Foroud T, Econs MJ 2005. Sex‐specific quantitative trait loci contribute to normal variation in bone structure at the proximal femur in men. Bone 37: 467–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Shen H, Long JR, Xiong DH, Liu YJ, Liu YZ, Xiao P, Zhao LJ, Dvornyk V, Zhang YY, Rocha‐Sanchez S, Liu PY, Li JL, Deng HW 2005. Mapping quantitative trait loci for cross‐sectional geometry at the femoral neck. J Bone Miner Res 20: 1973–1982. [DOI] [PubMed] [Google Scholar]
- 83. Demissie S, Dupuis J, Cupples LA, Beck TJ, Kiel DP, Karasik D 2007. Proximal hip geometry is linked to several chromosomal regions: Genome‐wide linkage results from the Framingham Osteoporosis Study. Bone 40: 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F 2003. High bone mass in mice expressing a mutant LRP5 gene. J Bone Miner Res 18: 960–974. [DOI] [PubMed] [Google Scholar]
- 85. Volkman SK, Galecki AT, Burke DT, Miller RA, Goldstein SA 2004. Quantitative trait loci that modulate femoral mechanical properties in a genetically heterogeneous mouse population. J Bone Miner Res 19: 1497–1505. [DOI] [PubMed] [Google Scholar]
- 86. Turner CH, Roeder RK, Wieczorek A, Foroud T, Liu G, Peacock M 2001. Variability in skeletal mass, structure, and biomechanical properties among inbred strains of rats. J Bone Miner Res 16: 1532–1539. [DOI] [PubMed] [Google Scholar]
- 87. Klein OF, Carlos AS, Vartanian KA, Chambers VK, Turner EJ, Phillips TJ, Belknap JK, Orwoll ES 2001. Confirmation and fine mapping of chromosomal regions influencing peak bone mass in mice. J Bone Miner Res 16: 1953–1961. [DOI] [PubMed] [Google Scholar]
- 88. Hamrick MW, Pennington C, Byron CD 2003. Bone architecture and disc degeneration in the lumbar spine of mice lacking GDF‐8 (myostatin). J Orthop Res 21: 1025–1032. [DOI] [PubMed] [Google Scholar]
- 89. Masinde GL, Wergedal J, Davidson H, Mohan S, Li R, Li X, Baylink DJ 2003. Quantitative trait loci for periosteal circumference (PC): Identification of single loci and epistatic effects in F2 MRL/SJL mice. Bone 32: 554–560. [DOI] [PubMed] [Google Scholar]
- 90. Klein RF, Turner RJ, Skinner LD, Vartanian KA, Serang M, Carlos AS, Shea M, Belknap JK, Orwoll ES 2002. Mapping quantitative trait loci that influence femoral cross‐sectional area in mice. J Bone Miner Res 17: 1752–1760. [DOI] [PubMed] [Google Scholar]
- 91. Zhai G, Ding C, Stankovich J, Cicuttini F, Jones G 2005. The genetic contribution to longitudinal changes in knee structure and muscle strength: A sibpair study. Arthritis Rheum 52: 2830–2834. [DOI] [PubMed] [Google Scholar]
- 92. Seeman E, Hopper JL, Young NR, Formica C, Goss P, Tsalamandris C 1996. Do genetic factors explain associations between muscle strength, lean mass, and bone density? A twin study. Am J Physiol 270: E320–E327. [DOI] [PubMed] [Google Scholar]
- 93. Hsu FC, Lenchik L, Nicklas BJ, Lohman K, Register TC, Mychaleckyj J, Langefeld CD, Freedman BI, Bowden DW, Carr JJ 2005. Heritability of body composition measured by DXA in the diabetes heart study. Obes Res 13: 312–319. [DOI] [PubMed] [Google Scholar]
- 94. Li X, Masinde G, Gu W, Wergedal J, Mohan S, Baylink DJ 2002. Genetic dissection of femur breaking strength in a large population (MRL/MpJ × SJL/J) of F2 Mice: Single QTL effects, epistasis, and pleiotropy. Genomics 79: 734–740. [DOI] [PubMed] [Google Scholar]
- 95. Sun X, Lei SF, Deng FY, Wu S, Papacian C, Hamilton J, Recker RR, Deng HW 2006. Genetic and environmental correlations between bone geometric parameters and body compositions. Calcif Tissue Int 79: 43–49. [DOI] [PubMed] [Google Scholar]
- 96. Deng FY, Xiao P, Lei SF, Zhang L, Yang F, Tang ZH, Liu PY, Liu YJ, Recker RR, Deng HW 2007. Bivariate whole genome linkage analysis for femoral neck geometric parameters and total body lean mass. J Bone Miner Res 22: 808–816. [DOI] [PubMed] [Google Scholar]
- 97. Li R, Tsaih SW, Shockley K, Stylianou IM, Wergedal J, Paigen B, Churchill GA 2006. Structural model analysis of multiple quantitative traits. PLoS Genet 2: e114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Garland T Jr, Morgan MT, Swallow JG, Rhodes JS, Girard I, Belter JG, Carter PA 2002. Evolution of a small‐muscle polymorphism in lines of house mice selected for high activity levels. Evolution Int J Org Evolution 56: 1267–1275. [DOI] [PubMed] [Google Scholar]
- 99. Karamichou E, Richardson RI, Nute GR, Gibson KP, Bishop SC 2006. Genetic analyses and quantitative trait loci detection, using a partial genome scan, for intramuscular fatty acid composition in Scottish Blackface sheep. J Anim Sci 84: 3228–3238. [DOI] [PubMed] [Google Scholar]
- 100. Schenkel FS, Miller SP, Ye X, Moore SS, Nkrumah JD, Li C, Yu J, Mandell IB, Wilton JW, Williams JL 2005. Association of single nucleotide polymorphisms in the leptin gene with carcass and meat quality traits of beef cattle. J Anim Sci 83: 2009–2020. [DOI] [PubMed] [Google Scholar]
- 101. Eisman JA 1999. Genetics of osteoporosis. Endocr Rev 20: 788–804. [DOI] [PubMed] [Google Scholar]
- 102. Ralston SH 2002. Genetic control of susceptibility to osteoporosis. J Clin Endocrinol Metab 87: 2460–2466. [DOI] [PubMed] [Google Scholar]
- 103. Ioannidis JP, Ng MY, Sham PC, Zintzaras E, Lewis CM, Deng HW, Econs MJ, Karasik D, Devoto M, Kammerer CM, Spector T, Andrew T, Cupples LA, Duncan EL, Foroud T, Kiel DP, Koller D, Langdahl B, Mitchell BD, Peacock M, Recker R, Shen H, Sol‐Church K, Spotila LD, Uitterlinden AG, Wilson SG, Kung AW, Ralston SH 2007. Meta‐analysis of genome‐wide scans provides evidence for sex‐ and site‐specific regulation of bone mass. J Bone Miner Res 22: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Seeman E 2003. Invited Review: Pathogenesis of osteoporosis. J Appl Physiol 95: 2142–2151. [DOI] [PubMed] [Google Scholar]
- 105. Kanis JA 2002. Diagnosis of osteoporosis and assessment of fracture risk. Lancet 359: 1929–1936. [DOI] [PubMed] [Google Scholar]
- 106. Deng HW, Mahaney MC, Williams JT, Li J, Conway T, Davies KM, Li JL, Deng H, Recker RR 2002. Relevance of the genes for bone mass variation to susceptibility to osteoporotic fractures and its implications to gene search for complex human diseases. Genet Epidemiol 22: 12–25. [DOI] [PubMed] [Google Scholar]
- 107. Andrew T, Antioniades L, Scurrah KJ, Macgregor AJ, Spector TD 2005. Risk of wrist fracture in women is heritable and is influenced by genes that are largely independent of those influencing BMD. J Bone Miner Res 20: 67–74. [DOI] [PubMed] [Google Scholar]
- 108. van Meurs JB, Schuit SC, Weel AE, van der Klift M, Bergink AP, Arp PP, Colin EM, Fang Y, Hofman A, van Duijn CM, van Leeuwen JP, Pols HA, Uitterlinden AG 2003. Association of 5′ estrogen receptor alpha gene polymorphisms with bone mineral density, vertebral bone area and fracture risk. Hum Mol Genet 12: 1745–1754. [DOI] [PubMed] [Google Scholar]
- 109. Villadsen MM, Bunger MH, Carstens M, Stenkjaer L, Langdahl BL 2005. Methylenetetrahydrofolate reductase (MTHFR) C677T polymorphism is associated with osteoporotic vertebral fractures, but is a weak predictor of BMD. Osteoporos Int 16: 411–416. [DOI] [PubMed] [Google Scholar]
- 110. Schulze TG, McMahon FJ 2004. Defining the phenotype in human genetic studies: Forward genetics and reverse phenotyping. Hum Hered 58: 131–138. [DOI] [PubMed] [Google Scholar]
- 111. Harris TB, Launer LJ, Eiriksdottir G, Kjartansson O, Jonsson PV, Sigurdsson G, Thorgeirsson G, Aspelund T, Garcia ME, Cotch MF, Hoffman HJ, Gudnason V 2007. Age, Gene/Environment Susceptibility‐Reykjavik Study: Multidisciplinary applied phenomics. Am J Epidemiol 165: 1076–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Almasy L, Dyer TD, Blangero J 1997. Bivariate quantitative trait linkage analysis: Pleiotropy versus co‐ incident linkages. Genet Epidemiol 14: 953–958. [DOI] [PubMed] [Google Scholar]
- 113. Williams JT, Van Eerdewegh P, Almasy L, Blangero J 1999. Joint multipoint linkage analysis of multivariate qualitative and quantitative traits. I. Likelihood formulation and simulation results. Am J Hum Genet 65: 1134–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Havill LM, Rogers J, Cox LA, Mahaney MC 2006. QTL with pleiotropic effects on serum levels of bone‐specific alkaline phosphatase and osteocalcin maps to the baboon ortholog of human chromosome 6p23‐21.3. J Bone Miner Res 21: 1888–1896. [DOI] [PubMed] [Google Scholar]
- 115. de Bakker PI, Yelensky R, Pe'er I, Gabriel SB, Daly MJ, Altshuler D 2005. Efficiency and power in genetic association studies. Nat Genet 37: 1217–1223. [DOI] [PubMed] [Google Scholar]
- 116. Hirschhorn JN, Daly MJ 2005. Genome‐wide association studies for common diseases and complex traits. Nat Rev Genet 6: 95–108. [DOI] [PubMed] [Google Scholar]
- 117. Kiel D, Demissie S, Cupples L, Dupuis J, Lunetta K, Murabito JM, Karasik D 2007. Genome‐wide association with bone mass and geometry in the Framingham Heart Study. BMC Med Genet 8 (Suppl I): S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Giresi PG, Stevenson EJ, Theilhaber J, Koncarevic A, Parkington J, Fielding RA, Kandarian SC 2005. Identification of a molecular signature of sarcopenia. Physiol Genomics 21: 253–263. [DOI] [PubMed] [Google Scholar]
- 119. Gajendran VK, Lin JR, Fyhrie DP 2007. An application of bioinformatics and text mining to the discovery of novel genes related to bone biology. Bone 40: 1378–1388. [DOI] [PubMed] [Google Scholar]
- 120. Qureshi A, McGuigan F, Seymour D, Hutchison J, Reid D, Ralston S 2001. Association between COLIA1 Sp1 alleles and femoral neck geometry. Calcif Tissue Int 69: 67–72. [DOI] [PubMed] [Google Scholar]
- 121. Rivadeneira F, Houwing‐Duistermaat JJ, Beck TJ, Janssen JA, Hofman A, Pols HA, Van Duijn CM, Uitterlinden AG 2004. The influence of an insulin‐like growth factor I gene promoter polymorphism on hip bone geometry and the risk of nonvertebral fracture in the elderly: The Rotterdam Study. J Bone Miner Res 19: 1280–1290. [DOI] [PubMed] [Google Scholar]
- 122. van Meurs JB, Rivadeneira F, Jhamai M, Hugens W, Hofman A, van Leeuwen JP, Pols HA, Uitterlinden AG 2006. Common genetic variation of the low‐density lipoprotein receptor‐related protein 5 and 6 genes determines fracture risk in elderly white men. J Bone Miner Res 21: 141–150. [DOI] [PubMed] [Google Scholar]
- 123. Volkman SK, Galecki AT, Burke DT, Paczas MR, Moalli MR, Miller RA, Goldstein SA 2003. Quantitative trait loci for femoral size and shape in a genetically heterogeneous mouse population. J Bone Miner Res 18: 1497–1505. [DOI] [PubMed] [Google Scholar]
- 124. Kiel DP, Ferrari SL, Cupples LA, Karasik D, Manen D, Imamovic A, Herbert AG, Dupuis J 2007. Genetic variation at the low‐density lipoprotein receptor‐related protein 5 (LRP5) locus modulates Wnt signaling and the relationship of physical activity with bone mineral density in men. Bone 40: 587–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Gennari L, Masi L, Merlotti D, Picariello L, Falchetti A, Tanini A, Mavilia C, Del Monte F, Gonnelli S, Lucani B, Gennari C, Brandi ML 2004. A polymorphic CYP19 TTTA repeat influences aromatase activity and estrogen levels in elderly men: Effects on bone metabolism. J Clin Endocrinol Metab 89: 2803–2810. [DOI] [PubMed] [Google Scholar]
- 126. McClung JM, Davis JM, Wilson MA, Goldsmith EC, Carson JA 2006. Estrogen status and skeletal muscle recovery from disuse atrophy. J Appl Physiol 100: 2012–2023. [DOI] [PubMed] [Google Scholar]
- 127. Frost HM 1999. On the estrogen‐bone relationship and postmenopausal bone loss: A new model. J Bone Miner Res 14: 1473–1477. [DOI] [PubMed] [Google Scholar]
- 128. Herbst KL, Bhasin S 2004. Testosterone action on skeletal muscle. Curr Opin Clin Nutr Metab Care 7: 271–277. [DOI] [PubMed] [Google Scholar]
- 129. Shearman AM, Cupples LA, Demissie S, Peter I, Schmid CH, Karas RH, Mendelsohn ME, Housman DE, Levy D 2003. Association between estrogen receptor alpha gene variation and cardiovascular disease. JAMA 290: 2263–2270. [DOI] [PubMed] [Google Scholar]
- 130. Shearman AM, Karasik D, Gruenthal KM, Demissie S, Cupples LA, Housman DE, Kiel DP 2004. Estrogen receptor Beta polymorphisms are associated with bone mass in women and men: The Framingham study. J Bone Miner Res 19: 773–781. [DOI] [PubMed] [Google Scholar]
- 131. Grundberg E, Ribom EL, Brandstrom H, Ljunggren O, Mallmin H, Kindmark A 2005. A TA‐repeat polymorphism in the gene for the estrogen receptor alpha does not correlate with muscle strength or body composition in young adult Swedish women. Maturitas 50: 153–160. [DOI] [PubMed] [Google Scholar]
- 132. Karasik D, Shearman A, Cupples L, Demissie S, Gruenthal K, Housman D, Kiel DP 2003. Association of Aromatase Gene Polymorphisms with Bone Mineral Density in Men and Women: The Framingham Offspring Study. J Bone Miner Res 18: S325. [Google Scholar]
- 133. Herrington DM, Howard TD, Brosnihan KB, McDonnell DP, Li X, Hawkins GA, Reboussin DM, Xu J, Zheng SL, Meyers DA, Bleecker ER 2002. Common estrogen receptor polymorphism augments effects of hormone replacement therapy on E‐selectin but not C‐reactive protein. Circulation 105: 1879–1882. [DOI] [PubMed] [Google Scholar]
- 134. Carling T, Kim KC, Yang XH, Gu J, Zhang XK, Huang S 2004. A histone methyltransferase is required for maximal response to female sex hormones. Mol Cell Biol 24: 7032–7042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Grundberg E, Carling T, Brandstrom H, Huang S, Ribom EL, Ljunggren O, Mallmin H, Kindmark A 2004. A deletion polymorphism in the RIZ gene, a female sex steroid hormone receptor coactivator, exhibits decreased response to estrogen in vitro and associates with low bone mineral density in young Swedish women. J Clin Endocrinol Metab 89: 6173–6178. [DOI] [PubMed] [Google Scholar]
- 136. Lorentzon M, Eriksson AL, Mellstrom D, Ohlsson C 2004. The COMT val158met polymorphism is associated with peak BMD in men. J Bone Miner Res 19: 2005–2011. [DOI] [PubMed] [Google Scholar]
- 137. Lorentzon M, Eriksson AL, Nilsson S, Mellstrom D, Ohlsson C 2007. Association between physical activity and BMD in young men is modulated by catechol‐O‐methyltransferase (COMT) genotype: The GOOD study. J Bone Miner Res 22: 1165–1172. [DOI] [PubMed] [Google Scholar]
- 138. Goldspink G 2004. Age‐related muscle loss and progressive dysfunction in mechanosensitive growth factor signaling. Ann N Y Acad Sci 1019: 294–298. [DOI] [PubMed] [Google Scholar]
- 139. Langlois JA, Rosen CJ, Visser M, Hannan MT, Harris T, Wilson PW, Kiel DP 1998. Association between insulin‐like growth factor I and bone mineral density in older women and men: The Framingham Heart Study. J Clin Endocrinol Metab 83: 4257–4262. [DOI] [PubMed] [Google Scholar]
- 140. Karasik D, Rosen CJ, Hannan MT, Broe KE, Dawson‐Hughes B, Gagnon DR, Wilson PW, Visser M, Langlois JA, Mohan S, Kiel DP 2002. Insulin‐like growth factor binding proteins 4 and 5 and bone mineral density in elderly men and women. Calcif Tissue Int 71: 323–328. [DOI] [PubMed] [Google Scholar]
- 141. Lupu F, Terwilliger JD, Lee K, Segre GV, Efstratiadis A 2001. Roles of growth hormone and insulin‐like growth factor 1 in mouse postnatal growth. Dev Biol 229: 141–162. [DOI] [PubMed] [Google Scholar]
- 142. Rosen CJ, Ackert‐Bicknell CL, Adamo ML, Shultz KL, Rubin J, Donahue LR, Horton LG, Delahunty KM, Beamer WG, Sipos J, Clemmons D, Nelson T, Bouxsein ML, Horowitz M 2004. Congenic mice with low serum IGF‐I have increased body fat, reduced bone mineral density, and an altered osteoblast differentiation program. Bone 35: 1046–1058. [DOI] [PubMed] [Google Scholar]
- 143. Vaessen N, Heutink P, Janssen JA, Witteman JC, Testers L, Hofman A, Lamberts SW, Oostra BA, Pols HA, van Duijn CM 2001. A polymorphism in the gene for IGF‐I: Functional properties and risk for type 2 diabetes and myocardial infarction. Diabetes 50: 637–642. [DOI] [PubMed] [Google Scholar]
- 144. Goldspink G, Yang SY 2004. The splicing of the IGF‐I gene to yield different muscle growth factors. Adv Genet 52: 23–49. [DOI] [PubMed] [Google Scholar]
- 145. Tang LL, Wang YL, Sun CX 2004. The stress reaction and its molecular events: Splicing variants. Biochem Biophys Res Commun 320: 287–291. [DOI] [PubMed] [Google Scholar]
- 146. Schrager MA, Roth SM, Ferrell RE, Metter EJ, Russek‐Cohen E, Lynch NA, Lindle RS, Hurley BF 2004. Insulin‐like growth factor‐2 genotype, fat‐free mass, and muscle performance across the adult life span. J Appl Physiol 97: 2176–2183. [DOI] [PubMed] [Google Scholar]
- 147. Van Laere AS, Nguyen M, Braunschweig M, Nezer C, Collette C, Moreau L, Archibald AL, Haley CS, Buys N, Tally M, Andersson G, Georges M, Andersson L 2003. A regulatory mutation in IGF2 causes a major QTL effect on muscle growth in the pig. Nature 425: 832–836. [DOI] [PubMed] [Google Scholar]
- 148. Roth SM, Martel GF, Ferrell RE, Metter EJ, Hurley BF, Rogers MA 2003. Myostatin gene expression is reduced in humans with heavy‐resistance strength training: A brief communication. Exp Biol Med (Maywood) 228: 706–709. [DOI] [PubMed] [Google Scholar]
- 149. Steelman CA, Recknor JC, Nettleton D, Reecy JM 2006. Transcriptional profiling of myostatin‐knockout mice implicates Wnt signaling in postnatal skeletal muscle growth and hypertrophy. FASEB J 20: 580–582. [DOI] [PubMed] [Google Scholar]
- 150. Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, Dick W 2001. In situ detection of 1,25‐dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J 33: 19–24. [DOI] [PubMed] [Google Scholar]
- 151. Bischoff‐Ferrari HA, Borchers M, Gudat F, Durmuller U, Stahelin HB, Dick W 2004. Vitamin D receptor expression in human muscle tissue decreases with age. J Bone Miner Res 19: 265–269. [DOI] [PubMed] [Google Scholar]
- 152. Uitterlinden AG, Ralston SH, Brandi ML, Carey AH, Grinberg D, Langdahl BL, Lips P, Lorenc R, Obermayer‐Pietsch B, Reeve J, Reid DM, Amedei A, Bassiti A, Bustamante M, Husted LB, Diez‐Perez A, Dobnig H, Dunning AM, Enjuanes A, Fahrleitner‐Pammer A, Fang Y, Karczmarewicz E, Kruk M, van Leeuwen JP, Mavilia C, van Meurs JB, Mangion J, McGuigan FE, Pols HA, Renner W, Rivadeneira F, van Schoor NM, Scollen S, Sherlock RE, Ioannidis JP 2006. The association between common vitamin D receptor gene variations and osteoporosis: A participant‐level meta‐analysis. Ann Intern Med 145: 255–264. [DOI] [PubMed] [Google Scholar]
- 153. Arai H, Miyamoto KI, Yoshida M, Yamamoto H, Taketani Y, Morita K, Kubota M, Yoshida S, Ikeda M, Watabe F, Kanemasa Y, Takeda E 2001. The polymorphism in the caudal‐related homeodomain protein Cdx‐2 binding element in the human vitamin D receptor gene. J Bone Miner Res 16: 1256–1264. [DOI] [PubMed] [Google Scholar]
- 154. Fang Y, van Meurs JB, Rivadeneira F, van Schoor NM, van Leeuwen JP, Lips P, Pols HA, Uitterlinden AG 2007. Vitamin D receptor gene haplotype is associated with body height and bone size. J Clin Endocrinol Metab 92: 1491–1501. [DOI] [PubMed] [Google Scholar]
- 155. Windelinckx A, De Mars G, Beunen G, Aerssens J, Delecluse C, Lefevre J, Thomis MA 2007. Polymorphisms in the vitamin D receptor gene are associated with muscle strength in men and women. Osteoporos Int 18: 1235–1242. [DOI] [PubMed] [Google Scholar]
- 156. Grundberg E, Brandstrom H, Ribom EL, Ljunggren O, Mallmin H, Kindmark A 2004. Genetic variation in the human vitamin D receptor is associated with muscle strength, fat mass and body weight in Swedish women. Eur J Endocrinol 150: 323–328. [DOI] [PubMed] [Google Scholar]
- 157. Ferrari SL, Deutsch S, Choudhury U, Chevalley T, Bonjour JP, Dermitzakis ET, Rizzoli R, Atonarakis SE 2004. Polymorphisms in the low‐density lipoprotein receptor‐related protein 5 (LRP5) gene are associated with variation in vertebral bone mass, vertebral bone size, and stature in whites. Am J Hum Genet 74: 866–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, Babij P, Allen KM, Yaworsky PJ, Bex F, Recker RR 2004. Bone biomechanical properties in LRP5 mutant mice. Bone 35: 162–169. [DOI] [PubMed] [Google Scholar]
- 159. Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH 2006. The Wnt co‐receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 281: 23698–23711. [DOI] [PubMed] [Google Scholar]
- 160. Armstrong DD, Esser KA 2005. Wnt/beta‐catenin signaling activates growth‐control genes during overload‐induced skeletal muscle hypertrophy. Am J Physiol Cell Physiol 289: C853–C859. [DOI] [PubMed] [Google Scholar]
- 161. Nordstrom A, Gerdhem P, Brandstrom H, Stiger F, Lerner UH, Lorentzon M, Obrant K, Nordstrom P, Akesson K 2004. Interleukin‐6 promoter polymorphism is associated with bone quality assessed by calcaneus ultrasound and previous fractures in a cohort of 75‐year‐old women. Osteoporos Int 15: 820–826. [DOI] [PubMed] [Google Scholar]
- 162. Ferrari SL, Karasik D, Liu J, Karamohamed S, Herbert AG, Cupples LA, Kiel DP 2004. Interactions of interleukin‐6 promoter polymorphisms with dietary and lifestyle factors and their association with bone mass in men and women from the framingham osteoporosis study. J Bone Miner Res 19: 552–559. [DOI] [PubMed] [Google Scholar]
- 163. Roth SM, Schrager MA, Lee MR, Metter EJ, Hurley BF, Ferrell RE 2003. Interleukin‐6 (IL6) genotype is associated with fat‐free mass in men but not women. J Gerontol A Biol Sci Med Sci 58: B1085–B1088. [DOI] [PubMed] [Google Scholar]
- 164. Barbieri M, Ferrucci L, Ragno E, Corsi A, Bandinelli S, Bonafe M, Olivieri F, Giovagnetti S, Franceschi C, Guralnik JM, Paolisso G 2003. Chronic inflammation and the effect of IGF‐I on muscle strength and power in older persons. Am J Physiol Endocrinol Metab 284: E481–E487. [DOI] [PubMed] [Google Scholar]
- 165. Moron FJ, Mendoza N, Vazquez F, Molero E, Quereda F, Salinas A, Fontes J, Martinez‐Astorquiza T, Sanchez‐Borrego R, Ruiz A 2006. Multilocus analysis of estrogen‐related genes in Spanish postmenopausal women suggests an interactive role of ESR1, ESR2 and NRIP1 genes in the pathogenesis of osteoporosis. Bone 39: 213–221. [DOI] [PubMed] [Google Scholar]
- 166. Szulc P, Delmas PD 2007. Bone width is correlated positively with the upper to the lower segment ratio in elderly men–the MINOS study. Bone 40: 194–199. [DOI] [PubMed] [Google Scholar]
- 167. Kamel HK, Maas D, Duthie EH Jr 2002. Role of hormones in the pathogenesis and management of sarcopenia. Drugs Aging 19: 865–877. [DOI] [PubMed] [Google Scholar]
- 168. Vanderschueren D, Venken K, Ophoff J, Bouillon R, Boonen S 2006. Clinical Review: Sex steroids and the periosteum–reconsidering the roles of androgens and estrogens in periosteal expansion. J Clin Endocrinol Metab 91: 378–382. [DOI] [PubMed] [Google Scholar]
- 169. Rivadeneira F, van Meurs JB, Kant J, Zillikens MC, Stolk L, Beck TJ, Arp P, Schuit SC, Hofman A, Houwing‐Duistermaat JJ, van Duijn CM, van Leeuwen JP, Pols HA, Uitterlinden AG 2006. Estrogen receptor beta (ESR2) polymorphisms in interaction with estrogen receptor alpha (ESR1) and insulin‐like growth factor I (IGF1) variants influence the risk of fracture in postmenopausal women. J Bone Miner Res 21: 1443–1456. [DOI] [PubMed] [Google Scholar]
- 170. Kenny AM, Dawson L, Kleppinger A, Iannuzzi‐Sucich M, Judge JO 2003. Prevalence of sarcopenia and predictors of skeletal muscle mass in nonobese women who are long‐term users of estrogen‐replacement therapy. J Gerontol A Biol Sci Med Sci 58: M436–M440. [DOI] [PubMed] [Google Scholar]
- 171. Taaffe DR, Newman AB, Haggerty CL, Colbert LH, de Rekeneire N, Visser M, Goodpaster BH, Nevitt MC, Tylavsky FA, Harris TB 2005. Estrogen replacement, muscle composition, and physical function: The Health ABC Study. Med Sci Sports Exerc 37: 1741–1747. [DOI] [PubMed] [Google Scholar]
- 172. Turner CH 2007. Molecular mechanisms of exercise in bone and muscle: The search for an exercise pill In: Cavanaugh P. (ed.) Bone Loss During Spaceflight: Etiology, Countermeasures, and Implications for Bone Health on Earth. Cleveland Clinic Press, Cleveland, OH, USA, 1–7. [Google Scholar]
- 173. Bhasin S, Buckwalter JG 2001. Testosterone supplementation in older men: A rational idea whose time has not yet come. J Androl 22: 718–731. [PubMed] [Google Scholar]
- 174. Walsh S, Zmuda JM, Cauley JA, Shea PR, Metter EJ, Hurley BF, Ferrell RE, Roth SM 2005. Androgen receptor CAG repeat polymorphism is associated with fat‐free mass in men. J Appl Physiol 98: 132–137. [DOI] [PubMed] [Google Scholar]
- 175. Ioannidis JP, Stavrou I, Trikalinos TA, Zois C, Brandi ML, Gennari L, Albagha O, Ralston SH, Tsatsoulis A 2002. Association of polymorphisms of the estrogen receptor alpha gene with bone mineral density and fracture risk in women: A meta‐analysis. J Bone Miner Res 17: 2048–2060. [DOI] [PubMed] [Google Scholar]
- 176. Lee K, Jessop H, Suswillo R, Zaman G, Lanyon L 2003. Endocrinology: Bone adaptation requires oestrogen receptor‐alpha. Nature 424: 389. [DOI] [PubMed] [Google Scholar]
- 177. Kostek MC, Delmonico MJ, Reichel JB, Roth SM, Douglass L, Ferrell RE, Hurley BF 2005. Muscle strength response to strength training is influenced by insulin‐like growth factor 1 genotype in older adults. J Appl Physiol 98: 2147–2154. [DOI] [PubMed] [Google Scholar]
- 178. Roth SM, Zmuda JM, Cauley JA, Shea PR, Ferrell RE 2004. Vitamin D receptor genotype is associated with fat‐free mass and sarcopenia in elderly men. J Gerontol A Biol Sci Med Sci 59: 10–15. [DOI] [PubMed] [Google Scholar]
- 179. van Rossum EF, Koper JW, van den Beld AW, Uitterlinden AG, Arp P, Ester W, Janssen JA, Brinkmann AO, de Jong FH, Grobbee DE, Pols HA, Lamberts SW 2003. Identification of the BclI polymorphism in the glucocorticoid receptor gene: Association with sensitivity to glucocorticoids in vivo and body mass index. Clin Endocrinol (Oxf) 59: 585–592. [DOI] [PubMed] [Google Scholar]
- 180. van Rossum EF, Voorhoeve PG, te Velde SJ, Koper JW, de Delemarre‐van Waal HA, Kemper HC, Lamberts SW 2004. The ER22/23EK polymorphism in the glucocorticoid receptor gene is associated with a beneficial body composition and muscle strength in young adults. J Clin Endocrinol Metab 89: 4004–4009. [DOI] [PubMed] [Google Scholar]
- 181. van Schoor NM, Dennison E, Lips P, Uitterlinden AG, Cooper C 2007. Serum fasting cortisol in relation to bone, and the role of genetic variations in the glucocorticoid receptor. Clin Endocrinol (Oxf) 67: 871–878. [DOI] [PubMed] [Google Scholar]
- 182. Dennison EM, Syddall HE, Fall CH, Javaid MK, Arden NK, Phillips DI, Cooper C 2004. Plasma leptin concentration and change in bone density among elderly men and women: The Hertfordshire Cohort Study. Calcif Tissue Int 74: 401–406. [DOI] [PubMed] [Google Scholar]
- 183. Livshits G, Pantsulaia I, Trofimov S, Kobyliansky E 2003. Genetic variation of circulating leptin is involved in genetic variation of hand bone size and geometry. Osteoporos Int 14: 476–483. [DOI] [PubMed] [Google Scholar]
- 184. Li G, Bunn JR, Mushipe MT, He Q, Chen X 2005. Effects of pleiotrophin (PTN) over‐expression on mouse long bone development, fracture healing and bone repair. Calcif Tissue Int 76: 299–306. [DOI] [PubMed] [Google Scholar]
- 185. Xing W, Baylink D, Kesavan C, Hu Y, Kapoor S, Chadwick RB, Mohan S 2005. Global gene expression analysis in the bones reveals involvement of several novel genes and pathways in mediating an anabolic response of mechanical loading in mice. J Cell Biochem 96: 1049–1060. [DOI] [PubMed] [Google Scholar]
- 186. Oh KW, Lee WY, Rhee EJ, Baek KH, Yoon KH, Kang MI, Yun EJ, Park CY, Ihm SH, Choi MG, Yoo HJ, Park SW 2005. The relationship between serum resistin, leptin, adiponectin, ghrelin levels and bone mineral density in middle‐aged men. Clin Endocrinol (Oxf) 63: 131–138. [DOI] [PubMed] [Google Scholar]
- 187. Pistilli EE, Gordish‐Dressman H, Seip RL, Devaney JM, Thompson PD, Price TB, Angelopoulos TJ, Clarkson PM, Moyna NM, Pescatello LS, Visich PS, Zoeller RF, Hoffman EP, Gordon PM 2007. Resistin polymorphisms are associated with muscle, bone, and fat phenotypes in white men and women. Obesity (Silver Spring) 15: 392–402. [DOI] [PubMed] [Google Scholar]
- 188. Agoston H, Khan S, James CG, Gillespie JR, Serra R, Stanton LA, Beier F 2007. C‐type natriuretic peptide regulates endochondral bone growth through p38 MAP kinase‐dependent and ‐independent pathways. BMC Dev Biol 7: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Gerber HP, Vu TH, Ryan AM, Kowalski J, Werb Z, Ferrara N 1999. VEGF couples hypertrophic cartilage remodeling, ossification and angiogenesis during endochondral bone formation. Nat Med 5: 623–628. [DOI] [PubMed] [Google Scholar]
- 190. Springer ML, Chen AS, Kraft PE, Bednarski M, Blau HM 1998. VEGF gene delivery to muscle: Potential role for vasculogenesis in adults. Mol Cell 2: 549–558. [DOI] [PubMed] [Google Scholar]