Abstract
Acute kidney injury occurs with kidney transplantation and too frequently progresses to the clinical diagnosis of delayed graft function (DGF). Poor kidney function in the first week of graft life is detrimental to the longevity of the allograft. Challenges to understand the root cause of DGF include several pathologic contributors derived from the donor (ischemic injury, inflammatory signaling) and recipient (reperfusion injury, the innate immune response, and the adaptive immune response). Progressive demand for renal allografts has generated new organ categories which continue to carry high risk for DGF for deceased donor organ transplantation. New therapies seek to subdue the inflammatory response in organs with high likelihood to benefit from intervention. Future success in suppressing the development of DGF will require a concerted effort to anticipate and treat tissue injury throughout the arc of the transplantation process.
Introduction
Delayed graft function (DGF) is a manifestation of acute kidney injury (AKI) with attributes unique to the transplant process. For native kidneys, acute kidney injury is defined as an increase in serum creatinine within 48 hours of an inciting event. In the transplant, timing is less straightforward. The diagnosis of DGF is complicated by a variety of definitions based on a range of clinical criteria dependent on the local transplant center, region, and country (2–4). There are over 10 definitions of DGF recorded in the literature (5–7). In 69% of studies reviewed between 1984 and 2007 DGF was defined as the use of dialysis within seven days of the transplant (8). The criterion has shortfalls as dialysis may be used in the first week after transplant without confirmation of kidney damage (8–10). Nevertheless, this definition offers a standard by which transplant centers can pragmatically report outcomes. Its simplicity offers transparent epidemiologic analyses and inter-center comparisons. Challenges remain to address the mechanism of transplant AKI and potential treatment of DGF directly.
The reported incidence of DGF in deceased donors has increased over time despite the progress in acute rejection treatment and translates to a 40% decrease in long-term graft survival (11, 12). Between 1985 and 1992 the rate of DGF in U.S. scientific registries was 14.7% (13). The incidence rose to 23% in 1998–2004 (3). In the most recent reports DGF occurred in 2,409 patients of all U.S. patients transplanted in 2008 (21.3%) (14). The increase has been contemporaneous with the use of expanded criteria donors (ECD) and donation after cardiac death (DCD). Whether long-term outcomes in the next decade will be negatively influenced by the increased rate of DGF remains to be determined.
DGF is a major obstacle for allograft survival as it can be compounded by acute rejection and chronic allograft nephropathy (CAN). Patients with both DGF and acute rejection had a 5-year survival rate of 34% in U.S. transplant patients between 1985 and 1992 (13). A meta-analysis of 34 studies from 1988 through 2007 concluded that patients with DGF had a 49% pooled incidence of acute rejection compared to 35% incidence in non-DGF patients (12). Initial associations have also been made at single centers that identify DGF as one of the strongest risk factors for CAN (RR 6.1) with greater risk than pre-transplant diabetes (RR 5.8) or pre-transplant hypertension (RR=3.1) (15). The complex relationship between DGF and allograft durability is still poorly understood due to the time lapse between inciting event and outcome.
In this review we explore the risk factors for DGF proceeding from the identification of a donor through the postoperative period and beyond. We describe the substantive mechanisms of ischemic and immunologic kidney injury that have direct reference to transplant patients. Finally, DGF prevention strategies are reviewed with emphasis on therapeutic targets that relieve the ischemic condition and diminish immunologic responses.
The pre-procurement period
Mechanism of ischemia
From the time a patient is identified as a potential organ donor it is critical to maintain adequate organ perfusion and avoid hypoxemia. Maintenance of intracellular oxygen content is dependent on hemoglobin delivery to the renal microvascular space. Ischemic kidney injury occurs after failure of a cadre of physiologic responses including arteriolar vasoconstriction, xanthine dehydrogenase activation (XO), and heme oxygenase-1 (HO-1) (Figure 1). In times of decreased perfusion the kidney’s afferent arteriole acts as a baro-detector distinct from the sympathetic nervous system (16). Decreased vascular wall tension activates renin synthesis in the macula densa. The concentration of ligands that bind to transmembrane G protein coupled receptors (GPCR), including thromboxane A2, angiotensin II and endothelin-1 increase to maintain intravascular perfusion pressure (17, 18). Calcium is released from the sarcoplasmic reticulum promoting actin myosin coupling. In a hypothermic state, renal tubular cells avoid intracellular Ca2+ accumulation due to their low membrane permeability (19, 20).
Figure 1. Mechanism of Injury in the Kidney Transplant Process.
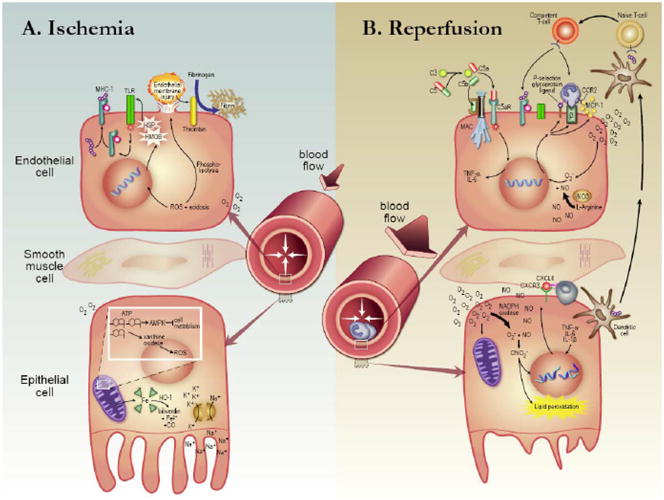
(A) Ischemia. Microvascular tone increases in response to a decrease in blood pressure. Ischemia initiates signaling events on the vascular endothelial cell surface. Heat shock proteins and High-mobility-group B-1 activate Toll-like receptors which stimulate synthesis of MHC-1 molecules. Reactive oxygen species and an acidotic milieu result in phospholipolysis, endothelial membrane injury and thrombin-mediated fibrin deposition. In the tubular epithelial cell oxygen supply is depleted. ATP degrades forming superoxide among its byproducts. Excess adenosine nucleotides signal AMPK activation which limits the cell’s metabolic rate. Oxygen-carrying metalloproteins are degraded via heme oxygenase-1 (HO-1). Without ATP, Na/K ATPase exchangers cannot function leading to intracellular K+ and extracellular Na+ retention. (B) Reperfusion. Injury begins with complement activation, C5b binding to the membrane attack complex, and C5a to the C5a anaphylotoxin receptor. In response to ROS-mediated signaling leukocytes localize to the endothelial cell. Cell stress upregulates P-selectin surface receptor, β-integrins and chemokines such as monocyte chemoattractant protein-1. Dendritic cells present exogenous antigens to recipient secondary lymphoid tissue which transform T-cells from a naïve to a competent phenotype. Excess oxygen can not be utilized efficiently in mitochondria deficient of nucleoside precursors. NADPH oxidase is upregulated instead forming superoxide. In combination with an excess in nitric oxide, peroxynitrate leads to DNA strand rupture, lipid peroxidation, programmed cell death (apoptosis) and unprogrammed cell death (necrosis).
As aerobic metabolism is turned off, volatile iron-containing cytochromes are catabolized by HO-1 (21). In severe injury these cytochromes spill from the mitochondrion’s inner membrane and overwhelm the capacity of HO-1 to convert the cytochromes to more inert compounds. Iron-containing molecules saturate the cytosol and exacerbate oxygen free radical generation. Further, ferroportin loses efficiency in shuttling free iron out of the cytosol (22). Free iron then non-specifically catalyzes redox reactions to generate hydroxyl radicals. Collectively oxygen free radicals disrupt intracellular metabolic processes, transcellular solute gradients, and the proximal tubular cell super structure (23).
Without blood flow an accumulation of reactive oxygen species (Figure 1) is diverted by xanthine dehydrogenase (XD). The enzyme plays an anti-inflammatory role by generating reduced nicotinamide adenine dinucleotide (NADH) and urate. In contrast xanthine oxidase, derived from the inactive xanthine oxidoreductase (XOR) enzyme, catabolizes adenine nucleotides (ATP, -DP, -MP) which are no longer in high demand during anaerobic respiration. Xanthine oxidase has a pro-inflammatory effect. Indeed, a cohort of 69 patients deemed high risk for DGF (hazard ratio >1.5 vs. controls) had less XD compared to total XOR with the same relationship maintained in those with slow graft function as defined by sCr >3.0mg/dL on postoperative day 5 (24). While each of the above events occur in a deceased donor special considerations need to be kept in mind for each donor subgroup.
Donor subtype: donation after brain death
Brain death induces an inflammatory state likely initiated by derangements in vascular tone governed by the sympathetic nervous system (25, 26). These inflammatory immunologic signals are often initiated long before organ recovery (27, 28). Empirically, hyaline deposits rich in complement inhibitor iC3b, a product of the complement cascade, are often found in donors with brain death (DBD) prior to procurement. These are also seen with disseminated intravascular coagulation.
In concert with autocrine stimulation endothelial cells upregulate cell adhesion molecules such as inter-cellular adhesion molecule-1 (ICAM-1) and P-selectin to facilitate innate immune cell localization and diapedesis (29–31). Beta-2 integrins on the leukocyte cell surface such as lymphocyte functioning antigen-1 (LFA-1), macrophage-1 (Mac-1), and very late antigen-4 (VLA-4) bind to endothelial ligands ICAM-1, ICAM-2 and vascular cell adhesion molecule-1 (VCAM-1) respectively. Thrombin, which is released from injured tissues, binds to the endothelial cell surface causing P-selectin to translocate to the intravascular space and enhance neutrophil localization (30). Evidence of these activities in transplant patients was identified by Koo and colleagues. Renal biopsies from 55 deceased-donor renal allografts (DDRA) were analyzed for expression of P-selectin and compared to 11 living-related donor renal allografts (LRDRA). P-selectin localized to the inter-tubular capillaries of 20/24 (83%) of DDRA and 1/11 (9%) of LRDRA. P-selectin expression correlated with neutrophil infiltration (86% of DDRA vs. 0% LRDRA) and DGF (32).
With brain death, HO-1 also plays a key role as antagonist to free radical generation which is associated with a clinical reduction in DGF rates. In a cohort of 254 renal transplant recipients, dopamine, an HO-1 inducer, was given to donors prior to organ procurement. The incidence of DGF was reduced and after a mean follow up of 4.8 years graft survival was prolonged in organs receiving dopamine (50% vs. 29%) (33). This study was followed by a randomized controlled trial of 264 donors and 487 recipients with the donors in the experimental arm receiving 4 μg/kg/min of dopamine for a mean of 344 minutes until surgical cross-clamp of the organ. Delayed graft function defined as >1 acute hemodialysis treatment was reduced (35.4% [92/260] vs. 24.7% [56/227]) when donors received dopamine and organ cold ischemia time was 17–24 hours (34).
Donor treatment using hormone supplementation with steroids, vasopressin and levothyroxine increased organ procurement yield compared to treatment of the donor with epinephrine (35–37). Other key factors associated with DGF include the duration of brain death (>24 hrs) and the length of stay in the intensive care unit (>40hrs) (35, 38).
Anticoagulation is routinely used during organ recovery from donors diagnosed with brain death; whereas the same treatment remains ethically controversial for DCD donors because anticoagulation does not relieve suffering but may hasten death if the patient is at risk of hemorrhage (39). Macroscopic thrombosis at time of procurement is a rare event. The prevalence of microscopic allograft thrombosis, however, may be underappreciated and underreported (40). Melagatran is an anticoagulant which inhibits fibrin deposition and has the potential to prevent microthrombi formation. In a pig model of DCD donors treated with the thrombin inhibitor melagatran prior to infusion of University of Wisconsin (UW), graft survival and function were improved when followed up to three months (41). Treatment was associated with reduced P-selectin and C3 mRNA transcripts indicating less endothelial activation. In another study, the same investigators found treatment with melagatran reduced expression of oxidative stress mediators including Nox2 and iNOS. In summary, the benefit of thrombin inhibition likely derives from its influence on both the coagulation cascade and oxidative metabolism (42).
Donor subtype: Donation after cardiac death
Historic rates of DGF are highest in patients receiving organs from donors undergoing controlled withdrawal of care known as donation after cardiac death (DCD). Central to the risk identified in this donor population is the duration of perfused ischemic time between extubation and asystole commonly known as warm ischemic time. This period leaves the kidney susceptible to sustained anaerobic metabolism manifest in the post implant biopsy as acute tubular necrosis (ATN) (Figure 2) (43). As the renal cortex is reperfused, perivascular edema of capillaries perpetuates ischemia at the level of the corticomedullary junction. Medullary warm ischemia results in the condition of “microvascular no-reflow” (44, 45). If this period of time is prolonged the risk of primary nonfunction escalates. In procurement protocols that limit warm ischemic time to <45 minutes DCD organs appear to have long term function rates equivalent to DBD organs along with equivalent DGF rates (46, 47). However, even with limited warm ischemic time primary nonfunction is a frequent occurrence (47).
Figure 2. Gradations of Cellular Damage in the Kidney from Ischemia-reperfusion Injury 48–72hrs post-implantation.
(A,B) Allograft, living donor (LD) (Periodic acid-Schiff). (A) Apical membrane of the proximal tubules is breached in isolated sections (black arrow) while the basement membrane remains adherent. Interstitial infiltrates are mild with few inflammatory cells (black triangle). Bowman’s space is present with little compression of the glomerulus (asterisk) (100x). (B) Mild cellular edema is present in the proximal tubule (arrow) with none in the thin segment of the loop of Henle (asterisk) (300x). (C,D) Allograft, donation after cardiac death (DCD) (Periodic acid-Schiff). (C) Proximal and distal tubule cell damage is evident with basement membrane separation (black arrow). (D) Rich interstitial leukocyte infiltration is present with little invasion of the tubular basement membrane (triangles). Tubular cells in the thin (T) and thick (Th) segments of the loop of Henle have separated from the basement membrane. Cellular edema is present (asterisk). (E,F) Native kidney ATN (Periodic acid-Schiff). (E) Proximal and distal segments of the tubule are damaged with extensive denudation of the basement membrane (black arrow). The Bowman’s capsule is expanded with debris in the urinary space (asterisk) and breakdown of basement membrane but (F) relatively less transmigration of leukocytes into the interstitium (black outline).
Donor subtype: Expanded criteria donor
With the knowledge that age alone is a determinant of DGF it is expected that ECD organs are at higher risk for complications than SCD organs (48). Unexpected however are the equivalent benefits between standard criteria donor (SCD) and ECD organs using preservation techniques such as machine perfusion (49). Gallinat and colleagues found in retrospective analysis that even organs from very old donors (>75yo) with short cold ischemia time and an absence of comorbidities such as coronary artery disease and malignancy carry a reduced risk of DGF and nonfunction (50). To assist in identifying at risk ECD organs pre-implantation biopsies can be useful when disease chronicity scoring systems are used to weight histologic evidence of vascular disease and glomerular sclerosis (51–53). Commonly ECD organs are avoided if >20% of glomeruli are sclerosed.
Composite donor risk
Many donor-specific risk factors for DGF in renal transplantation have been identified, including both immunologic (e.g. absence of T-cell antibody induction therapy, female donor gender) and nonimmunologic (donor age, weight, prolonged cold/warm ischemia time) origin (13, 48, 54–56). A donor scoring system based on a small cohort of patients was developed to quantify some of these risks to determine early (day 30) graft function and assist in “marginal” donor allocation (57). Other investigators developed a nomogram that quantified a large number of risk factors, each of which demonstrated predictive discrimination and were independently correlated with DGF (58). This nomogram was further refined in 2010 with more recent data reported to the United Network for Organ Sharing (UNOS)/Organ Procurement and Transplantation Network (OPTN)(59).
Composite recipient risk
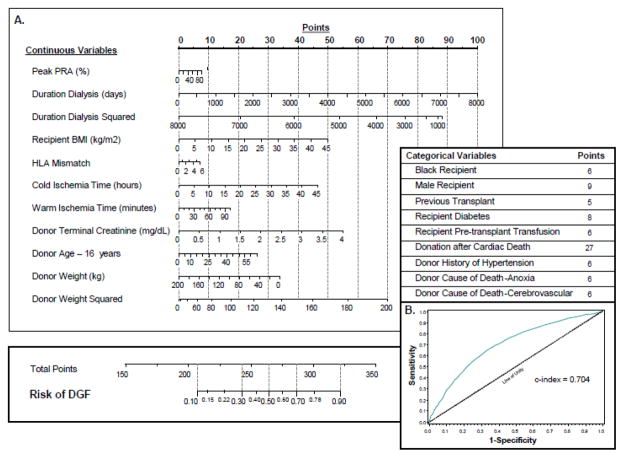
Distinct patient characteristics of the recipient place him or her at risk of DGF. Maintenance dialysis prior to transplant is perhaps the single greatest contributor (60). Obesity and diabetes add significant risk and were shown to add 57.9% and 36.3% higher rates respectively of DGF compared to mate kidneys in a 5,382 patient cohort (61, 62). Other factors that add independent risk include age >55yo, male sex, African-American race, prolonged wait period, immune sensitization events, and small-for-size organs (38). Many of these factors have also been included in the nomogram generated by Irish and colleagues to create a composite risk assessment based on both donor and recipient characteristics prior to the initiation of implantation surgery (59) (Figure 3).
Figure 3. Delayed Graft Function risk calculator.
Donor, recipient, and surgical factors are included in a functional nomogram. The information includes (A) continuous and categorical variables that have the highest likelihood of contributing to the development of DGF. (B) Patient characteristics are additive and correlate with a risk of DGF between 10–90%.
Procurement: organ preservation techniques
Once a donor is selected and the organ is recovered, metabolic homeostasis can be prolonged to offer a window of time to transport the organ without incurring permanent tissue damage. Cold preservation alone decreases cellular metabolic rate in the ischemic phase of injury (63). In low-metabolic states adenine nucleotides spontaneously disassemble by hydrolysis and deplete substrate for ATP synthesis at time of reperfusion. Therefore, adenosine-containing cold preservative buffer may mitigate reperfusion injury by directing an influx of oxygen toward adenine nucleotide synthesis instead of free radical generation (64).
Preservation buffers are designed with two common objectives 1) reduce osmotic injury to the cell and 2) delay acidosis with H+ buffer systems. The University of Wisconsin lactobionate/raffinose (UW) solution and Histidine-Tryptophan Ketoglutarate (HTK) solutions are among the most commonly used. Both have similar osmolarity and contain glucose alternatives to reduce cellular edema (65, 66). Histidine-Tryptophan Ketoglutarate exclusively offers a low potassium content favorable to recipients in systemic potassium excess. It is favored in cardiac transplantation for cardioplegia induction and relaxation of coronary vascular tone (67, 68). In comparison, the unique constituents of UW solution include adenosine (5mM) and allopurinol (1mM). Adenosine provides substrate for aerobic metabolism upon reperfusion. UW solution also contains the additive allopurinol (1mM) to inhibit xanthine oxidase. UW and HTK preparations have equivalent effect in most kidney donor types (6, 69). Organs subject to >24 hours cold storage have less DGF when perfused with UW (70). This is in part explained by adenine deficiency exacerbated by extended cold storage (70, 71).
Organ storage techniques appear to influence DGF in marginal donors when considered separately from the preservation buffer. Wight and colleagues in 2003 summarized several studies and determined that transported organs may benefit from machine perfusion (72). Current, more robust, studies diverge as to the benefit of active perfusion over static perfusion (Table 1) (49, 73, 74). DBD organs respond positively to machine perfusion for <16hrs compared to cold storage over the same time period (49). Treckman and colleagues identified benefits of machine perfusion in ECD kidneys using endpoints that included the incidence of DGF, primary nonfunction and 1 year graft survival (75). Results from studies that focus on the DCD donor type are conflicting. Although these positive findings were expected to be amplified in the DCD donorsThe study by Watson and colleagues was closed early due to the lack of efficacy of machine perfusion in DCD donors (74, 76). The benefit of machine perfusion in DCD’s demonstrated by Jochman and colleagues was blunted by a persistent DGF rate of >50% in treated organs (73).
Table 1.
Efficacy of Treatment for Delayed Graft Function
| Mechanism/Technique | Study group | Intervention | DGF incidence vs. control (%) | Treatment effect |
|---|---|---|---|---|
| organ perfusion | Watson et al.* | PMP1 in DCD | 26(58)/25(56) | none2 |
| organ perfusion | Treckmann et al.* | PMP in DCD | 20(22)/27(30) | positive |
| organ perfusion | Jochmans et al.* | PMP in DCD | 44(54)/57(70) | positive |
| organ perfusion | Moers et al.* | PMP in SCD | 70(21)/89(27) | positive |
| preservation buffer | de Boer et al.* | HTK3 | 105(33)/99(33)4 | none |
| preservation buffer | Stevens et al. | UW5 | 19.5(30)/9(11) | none |
| HO-1 induction | Schnuelle et al. | dopamine | 56(25)/92(35) | positive |
| CAM-ab6 | Salmela et al. | enlimomab | 41(31)/34(26) | none |
| CAM-ab | Gaber et al. | rPSGL-Ig | 29(41)/6(20) | none |
| CSF7 | Martinez et al. | erythropoietin | 16(32)/21(39) | positive |
| anticoagulant | Kikic et al. | citrate | 9(21)/20(30) | positive |
| apoptosis inhibitor | Cooper et al. | annexin V | 50 | NA8 |
| siRNA | Gaber et al. | QPI-1002 | 10(25) | NA9 |
randomized control trial
pulsatile machine perfusion
p≤0.05 = positive treatment effect
histidine-tiyptophan-α-ketoglutarate
reported rates include both initial non function and delayed graft function
University of Wisconsin solution
cell adhesion molecule
colony stimulating factor
not applicable in this Phase 1 trial
DGF event rates in trial arms not available
At this time it remains unclear if the dynamic settings for machine perfusion influence the technique’s efficacy. This is in part due to the small cohorts from which the parameters derive (77, 78). Protocols to assess machine perfusion settings often include directives for organ discard if perfusion flow is <0.4mL/min·gram of tissue, resistance is >80mmHg/mL/[min·gram of tissue] and resistive index (mean perfusion pressure/perfusate flow rate) is ≥0.5mmHg /[mL/min/100grams of tissue]) (77, 79–81). Patients receiving ECD and DCD kidneys may benefit from a resistive index calculation to prevent primary nonfunction; however controlled studies to address this topic have not been performed (75, 82, 83).
Donor organ oxygen supplementation was one technique used in Collins and Terasaki’s first organ storage experiments (84). Cost-effectiveness is difficult to justify clinically but experimentally, oxygenated machine perfusion in a pig model appears to improve immediate graft function after cold storage (85, 86). In a small animal model, perfluorocarbon-based oxygen delivery caused an overexpression of HO-1 and suppression of inducible NO-synthetase suggesting aerobic metabolism is shifted to a relative antioxidant state (87). Similarly, normothermic extracorporeal membrane oxygenation (ECMO) is a promising technique to reduce DGF. Retrospective observations calculate DGF rates of 8–42% in small cohorts of 32 patients or less (88–90). From these initial reports it appears important to limit ECMO support to <50 min for it to be considered a viable method of expanding the DCD donor pool.
The peri- and post operative period
Intraoperative volume balance
Intraoperative fluid balance during renal transplantation is an exception to most circumstances involving patients with severe kidney disease. Rather than restricting intraoperative hydration to avoid respiratory compromise, aggressive hydration has proven effective in avoiding DGF. Complications are often avoided since the allograft populations studied include an array of organs from living, DBD, DCD, and ECD donors which as a single group display immediate function after implantation (91). Maintaining central venous pressure ≥8mmHg appears to reduce the incidence of DGF without inducing respiratory or cardiac compromise although these techniques have not been tested rigorously (92–94).
Reperfusion: Vascular contractility
Once implantation is complete the allograft responds to reperfusion in several ways. Due to preceding ischemic injury pathologic vasoconstriction occurs with the activation of G protein coupled receptors (95). Damaged vascular endothelium release endothelin (ET-1), a cognate ligand for vascular- specific G protein coupled receptors (GPCR), and may lead to hyperactivation of ET-1A GPCR’s (96–98). This is exacerbated by increased endothelin levels in the blood that have also been detected in patients with DGF (99).
Renal nitric oxide (NO) content increases with reperfusion injury to balance the vasoconstrictive effect of endothelin. ET-1B receptor signaling leads to NO synthesis which has multiple functions in ischemia-reperfusion (IR) injury. Classically, NO induces vasodilation at low concentrations; however, at higher concentrations, NO reacts with superoxide to produce peroxynitrite, a reactive oxygen species (100, 101). Endothelium-derived NOS (eNOS) generates basal levels of NO that sustain a vasodilated state and are protective (102). Gene polymorphism of intron 4 in human endothelium-derived NOS (NOS3) was associated with 40.0%–51.7% DGF rate compared to control patients with a similar rate of DGF (31.3%) (103).
Reperfusion: Innate immune response
The host inflammatory response is the basis of sustained reperfusion injury. A cluster of cell types contributes to the resultant pathophysiology. Neutrophils and macrophages have been studied extensively and likely contribute clinically. Migration of these cells into the transplant has been identified to occur within 6 hours of reperfusion (104, 105). Macrophages may stimulate chemokine synthesis in resident dendritic cells which then activate T lymphocytes and recruit adaptive immune cells without involving allograft antigen presentation (106, 107). Invading cells are guided by chemotactic signals released by the endothelial cells such as macrophage chemoattractant protein-1 (MCP-1) which binds to the C-X-C motif chemokine 2 receptor (CXCR2) found on the leukocyte cell surface (108). Proximal tubule cells secrete pro-inflammatory cytokines (TNF-α, IL-6, IL-1β) and upregulate the density of the C-X-C motif chemokine 3 receptor (CXCR3) on their basement membrane to facilitate the penetration of inflammatory cells beyond the microvascular cuff. Notably, renal transplant recipients with ligands to CXCR3 detected in the urine are at higher risk for DGF post-transplant (109). Once leukocytes penetrate proximal tubule cells, myeloperoxidase in neutrophils and nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in macrophages contribute to local free radical production.
Damaged epithelial cells then release warning signals that include heat-shock proteins (HSPs), nonhistone chromatin-binding protein high-mobility group box 1 (HMGB1), and heparin sulfate biglycan that activate Toll-like receptors (TLR) such as TLR2, TLR3, and TLR4 (110–112). TLR’s are highly conserved receptors that classically bind to foreign microorganisms through recognition of molecular motifs and supplement the antimicrobial action of the complement system. In response to ischemic kidney injury however, tubular epithelial cells and mesangial cells express TLR’s without direct contribution from the complement system (111, 112). Kruger and colleagues identified a clinical link between TLR expression and 265 transplant recipients. Delayed graft function was associated with a TLR3 single nucleotide polymorphism in 21.4% of patients compared to 12% in those without the polymorphism (113).
During reperfusion the complement pathway interfaces with the above cellular immune reactions to activate cell lysis in the kidney allograft with either complement-fixing antibodies or direct binding to endothelial receptors (114, 115). Intrinsic renal synthesis of the third complement component C3 (C3) was first shown in an animal model to contribute to acute rejection by priming a T-cell-mediated response (116). In brain dead donors, local renal C3 levels are higher at procurement and inversely related to renal function 14 days after transplant (117). The importance of C3 polymorphisms in AKI and DGF is unclear (118, 119).
Reperfusion: Adaptive immune response
Allograft reperfusion is the only subtype of renal reperfusion injury where the donor’s adaptive immune cell response can potentially modify syngeneic major histocompatibility complexes (MHC) while the recipient’s immune system responds to these and other allogeneic MHCs. Although MHC’s are the basis of transplant immunology, ischemia of the kidney in non-transplant models can initiate MHC receptor expression through interferon gamma (IFN-γ) signaling (120–122). In one such animal model class I MHC was increased 3–6 fold in tubular epithelial cells three days after pedicle clamping compared to baseline (120). Additionally, MHC expression can be upregulated by TLR-induced production of cytokines, chemokines, and costimulatory molecules (111). In contrast, HO-1 induction appears to decrease both MHC class expression and co-stimulatory molecules to suppress an alloantigen response (123).
The adaptive immune system further participates in early kidney injury through direct allorecognition. Dendritic cells may initiate the process prior to implantation through stimuli such as hypoxia, hypotension and sepsis (124, 125). Upon reperfusion, donor dendritic cells migrate out of the allograft and return not to the host of origin but to the recipient’s secondary lymphoid tissue (lymph nodes, spleen). Dendritic cells present antigen to the recipient’s naïve T cells which undergo transformation into competent T cells. Expression of various cell adhesion molecules facilitates an adaptive immune response in the allograft (126, 127)
Clearly T lymphocytes alone play a role in early allograft rejection although the explicit role in reperfusion injury is difficult to isolate. It was found that CD4+/CD8+ knockout mice are resistant to IR injury. This is in distinction to recombination activating gene (RAG-1) knockout mice lacking both B and T cell activity which have no resistance to IR injury (128, 129). Even so Loverre and colleagues have demonstrated in patients diagnosed with DGF that a T helper lymphocyte 1 (Th1) population is predominant (130).
Once activated, the large influx of competent lymphocytes, along with leukocytes in general, lead to vascular congestion, activation of the complement and clotting cascades, and thrombosis. Hypoxia upregulates synthesis of thrombokinase (tissue factor) present in endothelial cells, while platelets and leukocytes and can cause microthrombi in the renal allograft (131). As a serine protease, thrombin converts soluble fibrinogen to insoluble fibrin struts. D-dimer, a fibrin degradation product, is released by allografts immediately upon reperfusion and is elevated in recipients diagnosed with DGF (40). Taken together, the innate and adaptive immune responses have the potential to cause devastating injury to the new kidney allograft soon after the commencement of reperfusion.
Treating DGF
Opportunities in nonbiased profiling techniques
Biomarker discovery tools have the potential to avoid DGF in patients susceptible to the condition and offer new therapeutic targets for prophylaxis or treatment. Genomic studies focus on single nucleotide polymorphisms (SNP’s), variant gene expression and mRNA expression profiles (132–135). Particular SNP’s infer a genetic predisposition to acute rejection and DGF. Along with TLR3 polymorphisms discussed above, polymorphisms in the tumor necrosis factor (TNF) gene have been identified in patients with DGF (136). A variant in the multidrug resistance-related protein 2 (MRP2) gene from donor organs was associated with DGF in a cohort of 98 patients if downstream point mutations were present at nucleotide positions 3563 and 4544 (137). Knowledge of this mutation has not yet been used in a prospective clinical application.
Screening cDNA microarrays have identified thousands of modulated mRNA transcripts in the setting of DGF. With this technique upregulation of C-C motif chemokine receptor ligand 19 (CCL19) and 21 (CCL21) were identified in allografts diagnosed with DGF (138). Gene cluster analysis using 7376 standardized RNA probes including those to CCL19 and CCL21 identified a subpopulation of at-risk deceased donor allografts. Genes coding for collagens, integrins, chemokines, TLRs, and antigen processing were more highly expressed in these patients (134). Microarray analysis has also identified transcriptional patterns in the allograft repair phase. Transcripts for calcium-binding protein A4 (S100A4) and matrix Gla protein (MGP) remain upregulated during the recovery phase of acute kidney injury in small animal models (29). S100A4 was reduced in a pig model of chronic kidney graft fibrosis that linked thrombin inhibition with prevention of chronic allograft damage (42).
Small inhibitory microRNAs (siRNA’s) are powerful regulatory tools that modulate gene transcription in IR injury. They can be studied with microarray techniques similar to mRNA expression profiling. We now know that tubular epithelial cells robustly upregulate microRNA 21 (mir21) expression after renal ischemia in response to TGF-β signaling. In turn mir21 expression upregulates B-cell lymphoma 2 (Bcl-2) expression, an anti-apoptotic protein (139). Clinical trials using p53 siRNA will be discussed below.
The search for biomarkers in the area of proteomics has yielded molecules relevant to the diagnosis of AKI in cardiac surgery, radiocontrast exposure, and recently in renal transplantation (140). Urinary neutrophil gelatinase-associated lipocalin (uNGAL) and serum interleukin-18 (IL-18) collected on postoperative days 1–3 independently predicted the need for hemodialysis by postoperative day 7 in a small cohort of moderate risk patients. Receiver operator curve (ROC) characteristics are similar for uNGAL and IL-18, on postoperative day 1, (AUC 0.82 [CI 0.72–0.92]) (141). Kidney injury molecule-1(KIM-1) continues to be investigated in the post-transplant period but has limited use in the prediction of DGF prior to graft implantation (142). Current advances in biomarker discovery are likely to help the physician anticipate development of DGF and treat DGF earlier in its course.
Recipient-directed therapies
Treatment opportunities can be offered to the recipient prior to, during, and after organ recovery. Current considerations for the recipient focus on the following types of intervention: recipient pre-treatment, allograft vasodilation, inflammatory cascade antagonism, and allograft immunosuppression (Figure 4) (Table 1,2).
Figure 4. Treatment of Early Transplant Kidney Injury.
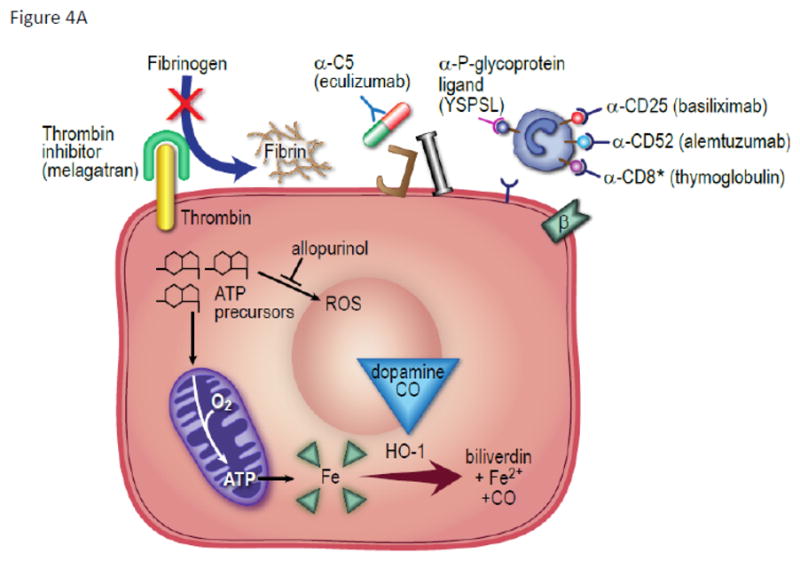
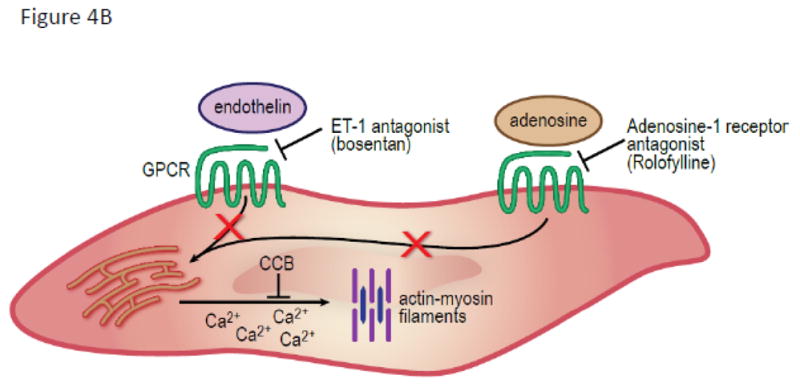
(A) On endothelial cells the thrombin inhibitors (melagatran) prevent fibrinogen conversion to fibrin deposit. Anti-complement factor 5 antibody (eculizumab) prevents MAC formation and anaphylotoxin signaling. Inflammatory cell signaling is targeted by biologic agents that bind to α-P-glycoprotein ligand (YSPSL), CD-25 receptor (basiliximab) and CD-52 receptor (alemtuzumab). Multiple receptors are targeted with anti-thymocyte globulin including CD-8. Dopamine and carbon monoxide upregulate heme oxygenase-1 activity to convert metalloproteins to the more inert byproducts of biliverdin, Fe2+, and carbon monoxide.(B) In vascular smooth muscle cells vasospasm is relieved by inhibition of calcium binding to the actin myofilament. Endothelin-1A (bosentan) and adenosine receptor-1A antagonists (rolofylline) inhibit G protein coupled receptors which open inositol triphosphate-dependent calcium channels. Calcium channel blockers prevent calcium release through voltage-dependent channels.
Table 2.
Incidence of Delayed Graft Function with Induction Immunosuppressants
| Antibody | Study group | Experimental Drug | Control Drug | Experimental Arm (%) | Control Arm (induction) (%) | Control Arm (no induction) (%) |
|---|---|---|---|---|---|---|
| CD-25 | Hernandez et al.* | Basiliximab | Thymoglobulin | 33–40 | 28 | |
| Lebranchu et al.* | Basiliximab | Thymoglobulin | 18 | 22 | ||
| Vincenti et al.* | Daclizumab | 21 | 29 | |||
| Ginny et al. | Daclizumab | 15 | 26 | |||
| Kandus et al. | Daclizumab | 27 | ||||
| Thymocyte | Brennan et al. (2006)* | Thymoglobulin | Basiliximab | 40 | 45 | |
| Noel et al.* | Thymoglobulin | Daclizumab | 32 | 45 | ||
| Ciancio et al. (2005)* | Thymoglobulin | Alemtuzumab/daclizumab | 13 | 7/7 | ||
| Goggins et al.* | Thymoglobulin (intraop.) | Thymoglobulin (postop.) | 15 | 36 | ||
| Brennan et al. (1999)* | Thymoglobulin | Atgam | 1 | 0 | ||
| Hardinger et al. (2004)* | Thymoglobulin | Atgam | 1 | 0 | ||
| Hardinger et al. (2005)* | Thymoglobulin (+CsA) | Thymoglobulin (+Tacro.) | 5 | 5 | ||
| Malinow et al. | Atgam (equine) | muromonab | 22 | 40 | ||
| Mourad et al. | Thymoglobulin | 18 | 24 | |||
| Thymocyte + CD-25 | Ciancio et al (2008). | Thymoglobulin + daclizumab | Thymoglobulin + daclizumab | 1 | 4 | |
| CD-52 | Knechtle et al. | Alemtuzumab | Basiliximab/thymoglobulin | 18 | 16/26 | |
| Farney et al. | Alemtuzumab | 17 | 15 | |||
| Pascual et al. | Alemtuzumab | 20 | ||||
| Vathsala et al.* | Alemtuzumab | 20 | 10 | |||
| CD-3 | Flechner et al.* | Muromonab (steroids before) | Muromonab (steroids after) | 28 | 33 | |
| Lacha et al. | muromonab | Daclizumab | 45 | 57 |
randomized trials comparing at least two induction strategies
CsA = cyclosporine Tacro. = tacrolimus
Recipient-directed therapies: ischemic pre-conditioning
Ischemic pre-conditioning has been studied extensively for the purpose of shifting metabolism to a conservational mode and to avoid apoptosis. Recipient pre-treatment with carbon monoxide (CO) has been studied for its ability to avert apoptosis and induce heme oxygenase-1 (143). In a large animal model CO was given to recipients 1 hour prior to surgery. Allografts undergoing 45 minutes of warm ischemia time showed greatest relative benefit when transplanted into recipients receiving CO. Renal function was maintained, acute tubular necrosis reduced and pro-inflammatory mRNA transcripts downregulated. This technique has yet to be clinically evaluated for the treatment of DGF.
Recipient-directed therapies: vasodilatory agents
Drug therapy for vasoconstriction in transplantation is appealing because it can be offered in a closely monitored setting to avoid hypotension. Agents investigated include endothelin receptor antagonists, calcium channel blockers and adenosine A1 receptor antagonists.
In a small animal transplantation model an endothelin receptor antagonist was given prophylactically one day prior to and post-operatively for 7 days (144). Treatment accelerated renal function recovery and avoided acute tubular necrosis. Similar improvement in renal function occurred when an endothelin receptor antagonist was added to cold perfusate prior to implantation (96). These results have yet to be adapted to human trials (145). Endothelin antagonists may have an additional role in those transplant candidates with pulmonary hypertension. There is direct association between pre-transplant pulmonary hypertension and subsequent DGF (146, 147). Therefore treatment with endothelin antagonists or phosphodiesterase inhibitors that increase NO and antagonize endothelin may be helpful in treating vasoconstriction of the pulmonary vascular bed in the transplant candidate.
Calcium channel blockers (CCB) have been used extensively to prevent renal vasoconstriction in transplant recipients (148). More recently, patients maintained on dihydropyridine therapy for two years post-transplant had higher iohexol renal clearances than those receiving a calcineurin-inhibitor without the dihydropyridine, lacidipine (149). The equivalence in acute rejection rates between groups infers lacidipine’s action was likely non-immunologic although it is recognized that some dihydropyridines, but not lacidipine, may decrease the rate of calcineurin inhibitor metabolism. This effect is much reduced compared to non-dihydropyridines (150).
Adenosine A1 receptor antagonists directly affect the renal afferent arteriole. Rolofylline, a selective adenosine A1 receptor antagonist, inhibits adenylate cyclase activity. This results in afferent arteriole dilation and increased glomerular filtration rate. Rolofylline was initially studied in heart failure-induced AKI without benefit, but may have benefit in patients susceptible to AKI with stable cardiac function (151, 152).
Recipient-directed therapies: anti-inflammatory agents
Inhibition of leukocyte activation has the potential to prohibit the migration of cell types involved in both the innate and adaptive immune response leveled against the allograft. Several potential agents are being considered. 1) Recombinant P-selectin glycoprotein ligand IgG fusion protein, rPSGL-Ig (YSPSL) offers one solution to reduce the rate of leukocyte transmigration by binding to and decreasing the number of available P-selectin ligands. YSPSL was evaluated in a 59 patient Phase 2a trial (139). Fewer patients receiving YSPSL had a serum creatinine >6mg/dL on post-operative day 5 (26% vs. 55%). However, the high rate of study-defined DGF in the control arm confounds interpretation of the results (153). 2) In a Phase 1 trial of an siRNA inhibiting p53 activation (QPI-1002), and therefore apoptotic signaling, 40 patients were enrolled with a 25% (10/40) reported DGF rate (154). Phase 2 trials with QPI-1002 are anticipated. 3) Erythropoietin’s anti-apoptotic attributes have also been investigated with the express purpose of preventing DGF. Martinez and colleagues gave high doses of erythropoietin (30,000U) to new transplant recipients 1 day before surgery and 3 doses after surgery (12hr, 7d, 14d). The incidence of DGF was equivalent between experimental and placebo arms (32% vs. 38.8% respectively, p= 0.77) (155). 4) Recombinant human annexin V homodimer (Diannexin) inhibits apoptotic signaling at a point when cell death and inflammatory homing may be averted. The drug was evaluated in 42 randomized patients who received organs with prolonged ischemia as a part of a Phase 2 drug safety trial. Those receiving the 400 μg/kg dose had both a lower incidence and shorter period of dialysis therapy through post-operative day 29 (156). 5) Chemokine C-X motif receptor (CXCR) inhibitors such as meraxin and repertaxin have shown success in small animal transplantation models while adaptation to target patient groups remains challenging (157–159).
Recipient-directed therapies: induction immunosuppressants
Induction immunosuppression can have the secondary effect of decreasing DGF rates by suppressing leukocyte-rich vascular congestion and endothelial injury. DGF rates fluctuate between study groups; however, rates in induction-free control arms are systematically higher across patient strata. The most common induction agents include anti-CD25, anti-thymocyte, anti-CD52, and anti-CD3 immunoglobulins. DGF rates per immunosuppressant vary widely dependent on the randomized study’s donor type (58, 160–180). A non-critical list of trials of induction or no induction where DGF was reported is shown in Table 2.
Randomized control trials comparing induction immunosuppression regimes that address DGF incidence most often compare anti-thymocyte globulin to anti-CD25 antibody induction. A summary of 8 RCTs concluded an equivalent rate of DGF in the patients treated with a standard (20mg) two dose basiliximab regimen compared to cumulative thymoglobulin dose of 6–9mg/kg (181). DGF in patients given anti-thymocyte globulin (ATG) appears to be dose dependent. Rabbit-ATG (Thymoglobulin, Sanofi, Cambridge, MA) given at a dose of 7.5mg/kg showed no significant difference in DGF rate between patients induced with r-ATG (40.4%) compared to basiliximab (44.5%) in a randomized control trial of deceased donor transplant recipients (182). The same r-ATG formulation was studied in relation to the anti-CD25 immunoglobulin, daclizumab, in a randomized control trial of 227 high risk patients. Total ATG dose was 8.75mg/kg and total daclizumab dose was 5mg/kg with lower DGF rates among the ATG-treated patients, 31.5% vs. 44.6% respectively (183). Anti-CD52 antibody (alemtuzumab) may protect against DGF but has not been analyzed in studies with high DGF control rates (>20%) (58, 161, 164).
When addressing chronic immunosuppression there is receding evidence to support delayed introduction, avoidance or withdrawal of calcineurin inhibitors (CNI). CNIs have recognized vasoconstrictive properties and their discontinuation in presence of DGF appears intuitive but may be nonbeneficial or even harmful (184, 185). Recently, 254 patients, average age 66 years old, were enrolled in a randomized open label trial to study early post-transplant tacrolimus dosing strategies (169). Investigators withheld oral tacrolimus in the study’s experimental arm until post-operative day 7. DGF was numerically higher in those with delayed tacrolimus dosing (30.3% vs. 23.8% respectively) (184, 186). A contrasting study observed that trough levels < 9ng/mL on post-operative day 5 in patients given anti-CD25 induction immunosuppression were associated with a higher rate of acute rejection (187). In a second randomized control trial 197 patients with low immunogenic risk and high DGF risk were studied. A majority of patients (83%) received anti-CD25 induction immunosuppression whereas 97 (49.2%) received their first dose of cyclosporine on postoperative day (POD) 0 and 100 (49.8%) received their first dose of cyclosporine on POD 6. Delayed graft function rates were equivalent in both arms of the study (26.8% vs. 23.0% respectively) (188). Therefore, current outcome studies support that administration of maintenance CNI administration throughout the period of early graft recovery does not perpetuate DGF or impair recovery from DGF.
Management of established delayed graft function
After competing pre-renal, renal and post-renal diagnoses are excluded, patients suspected of DGF should be biopsied to determine the presence of acute rejection and ATN.
In the absence of acute rejection specific supportive parameters remain largely unknown as to their influence on reducing the duration of DGF. Whereas pre-operative hemodialysis therapy should avoid ultrafiltration, post-transplant hemodialysis should be offered when clinically indicated (189–191). Hemodynamic instability and nephrotoxins should be avoided in any patient diagnosed with AKI. These guidelines also apply to radiologic imaging technique and pharmacokinetic drug interactions (192). As above delaying the introduction of the CNI or replacing it with sirolimus or everolimus is not warranted. Use of anti-lymphocyte therapy after DGF is established may not treat the DGF, but use of anti-lymphocyte therapy will reduce the rejection rate and minimize the negative impact of acute rejection in association with DGF (193).
Diverse approaches have been used to understand the development from DGF to long term allograft function and to potentially block this transition. Some of the most substantive work with clinical application has focused on mitochondrial metabolism and cilial repair (194). Of the few large animal studies following long-term renal allograft performance post-transplantation, pig kidneys were procured and kept in cold storage for 24–72hrs. Trimetazidine (TMZ) is one of the few drugs studied over a period of extended follow up in a large animal model. As an inhibitor of mitochondrial 3-ketoacyl coenzyme A thiolase it is theorized to prevent interstitial fibrosis and tubular atrophy through a switch from fatty acid to glucose oxidation. The effect of TMZ persisted for 16 weeks in an allogeneic pig kidney model despite the lack of postoperative drug administration (195).
A delay in the evolution of cilia length in patients’ renal allograft tubules may also contribute to prolonged injury to the tubular epithelia via impaired Wingless- int-1 (Wnt) signaling. The renal primary cilia which reside on the nephron’s apical surface are highly sensitive to IR injury. Wnt signaling contributes to repair of the terminally differentiated kidney through regulation of cell proliferation and differentiation. The proximal tubule cells have remarkable potential for self-renewal mediated by canonical Wnt signaling and when this pathway is interrupted, the rate of renal fibrosis accelerates (196, 197). In post-transplant renal biopsies (<post-operative day 84) renal primary cilia loss was coincident with the diagnosis of ATN (194). Following biopsies serially, cilia length doubled after one week and then decreased in length as recovery progressed. After 84 days cilia length approached control measures (2 μm). Altering the Wnt signaling pathway remains a vigorous area of therapeutic research.
Conclusion
Delayed graft function remains a vexing complication of kidney transplantation with detrimental effects for both graft life and patient survival. Over the past decade a consensus definition has developed to provide greater clarity in to the study of DGF. Research into the molecular mechanisms of oxidant stress, vasospasm, cytokine signaling, endothelial cell injury, epithelial cell injury, innate immunity and adaptive immunity have improved our understanding of DGF. The complexity of pathologic mechanisms that cause DGF offers many potential targets for therapy to inhibit oxidant stress, encourage vasodilation, and blunt the immune response.
Several targets are currently being explored in 17 separate clinical trials with the primary endpoint of DGF (198). The most promising therapies take advantage of an anticipated surgical process and offer early intervention to preserve organ function. Over the next decade we anticipate management strategies will evolve to provide physicians with centralized access to real-time information that will individualize therapeutic decisions for each step of the transplantation process to further reduce the clinical burden of DGF.
Acknowledgments
This work was supported in part by the George M. Obrien Center at Washington University NIHDDK P30-DK079333 and K08DK089002 (A.M.S.) from the NIDDK. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIDDK. We would like to extend our appreciation to Dr. Helen Liapis and Dr. Joseph Gaut for their assistance in obtaining histologic materials; and Marcy H. Hartstein, MedPIC, Washington University School of Medicine, and Dr. Matthew S. Chin for their expertise in graphic design. We appreciate the editorial support of Kimberly L. Knolhoff.
Footnotes
Disclosure
The authors of this manuscript have no conflicts of interest to disclose as described by the American Journal of Transplantation.
References
- 1.KDIGO Nephrology Guideline Database. Available from: http://www.kdigo.org/nephrology_guideline_database/guideline_summaries_by_topic.php.
- 2.Akkina SK, Connaire JJ, Israni AK, Snyder JJ, Matas AJ, Kasiske BL. Similar outcomes with different rates of delayed graft function may reflect center practice, not center performance. Am J Transplant. 2009;9(6):1460–1466. doi: 10.1111/j.1600-6143.2009.02651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tapiawala SN, Tinckam KJ, Cardella CJ, Schiff J, Cattran DC, Cole EH, et al. Delayed graft function and the risk for death with a functioning graft. J Am Soc Nephrol. 2010;21(1):153–161. doi: 10.1681/ASN.2009040412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang SH, Russ GR, Chadban SJ, Campbell SB, McDonald SP. Trends in kidney transplantation in Australia and New Zealand, 1993–2004. Transplantation. 2007;84(5):611–618. doi: 10.1097/01.tp.0000280553.23898.ef. [DOI] [PubMed] [Google Scholar]
- 5.Halloran PF, Aprile MA, Farewell V, Ludwin D, Smith EK, Tsai SY, et al. Early function as the principal correlate of graft survival. A multivariate analysis of 200 cadaveric renal transplants treated with a protocol incorporating antilymphocyte globulin and cyclosporine. Transplantation. 1988;46(2):223–228. [PubMed] [Google Scholar]
- 6.Agarwal A, Murdock P, Fridell JA. Comparison of histidine-tryptophan ketoglutarate solution and University of Wisconsin solution in prolonged cold preservation of kidney allografts. Transplantation. 2006;81(3):480–482. doi: 10.1097/01.tp.0000196724.89757.79. [DOI] [PubMed] [Google Scholar]
- 7.Roels L, Coosemans W, Donck J, Maes B, Peeters J, Vanwalleghem J, et al. Inferior outcome of cadaveric kidneys preserved for more than 24 hr in histidine-tryptophan-ketoglutarate solution. Leuven Collaborative Group for Transplantation. Transplantation. 1998;66(12):1660–1664. doi: 10.1097/00007890-199812270-00015. [DOI] [PubMed] [Google Scholar]
- 8.Yarlagadda SG, Coca SG, Garg AX, Doshi M, Poggio E, Marcus RJ, et al. Marked variation in the definition and diagnosis of delayed graft function: a systematic review. Nephrol Dial Transplant. 2008;23(9):2995–3003. doi: 10.1093/ndt/gfn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peeters P, Terryn W, Vanholder R, Lameire N. Delayed graft function in renal transplantation. Curr Opin Crit Care. 2004;10(6):489–498. doi: 10.1097/01.ccx.0000146119.46547.05. [DOI] [PubMed] [Google Scholar]
- 10.Shoskes DA, Shahed AR, Kim S. Delayed graft function. Influence on outcome and strategies for prevention. Urol Clin North Am. 2001;28(4):721–732. [PubMed] [Google Scholar]
- 11.Hariharan S, McBride MA, Cherikh WS, Tolleris CB, Bresnahan BA, Johnson CP. Post-transplant renal function in the first year predicts long-term kidney transplant survival. Kidney Int. 2002;62(1):311–318. doi: 10.1046/j.1523-1755.2002.00424.x. [DOI] [PubMed] [Google Scholar]
- 12.Yarlagadda SG, Coca SG, Formica RN, Jr, Poggio ED, Parikh CR. Association between delayed graft function and allograft and patient survival: a systematic review and meta-analysis. Nephrol Dial Transplant. 2009;24(3):1039–1047. doi: 10.1093/ndt/gfn667. [DOI] [PubMed] [Google Scholar]
- 13.Ojo AO, Wolfe RA, Held PJ, Port FK, Schmouder RL. Delayed graft function: risk factors and implications for renal allograft survival. Transplantation. 1997;63(7):968–974. doi: 10.1097/00007890-199704150-00011. [DOI] [PubMed] [Google Scholar]
- 14. [accessed 02/10/2011];United Network of Organ Sharing database. Available from: www.unos.org.
- 15.Khalkhali HR, Ghafari A, Hajizadeh E, Kazemnejad A. Risk factors of long-term graft loss in renal transplant recipients with chronic allograft dysfunction. Exp Clin Transplant. 2010;8(4):277–282. [PubMed] [Google Scholar]
- 16.Wagner C, Jobs A, Schweda F, Kurtz L, Kurt B, Lopez ML, et al. Selective deletion of Connexin 40 in renin-producing cells impairs renal baroreceptor function and is associatedwith arterial hypertension. Kidney Int. 2010;78(8):762–768. doi: 10.1038/ki.2010.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loutzenhiser R, Bidani A, Chilton L. Renal myogenic response: kinetic attributes and physiological role. Circ Res. 2002;90(12):1316–1324. doi: 10.1161/01.res.0000024262.11534.18. [DOI] [PubMed] [Google Scholar]
- 18.Chen J, Sgouralis I, Moore LC, Layton HE, Layton AT. A Mathematical Model of the Myogenic Response to Systolic Pressure in the Afferent Arteriole. Am J Physiol Renal Physiol. 2011;300:F669. doi: 10.1152/ajprenal.00382.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hochachka PW. Defense strategies against hypoxia and hypothermia. Science. 1986;231(4735):234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- 20.D’Alessandro AM, Southard JH, Love RB, Belzer FO. Organ preservation. Surg Clin North Am. 1994;74(5):1083–1095. [PubMed] [Google Scholar]
- 21.Ollinger R, Pratschke J. Role of heme oxygenase-1 in transplantation. Transpl Int. 2010;23(11):1071–1081. doi: 10.1111/j.1432-2277.2010.01158.x. [DOI] [PubMed] [Google Scholar]
- 22.Smith CP, Thevenod F. Iron transport and thekidney. Biochim Biophys Acta. 2009;1790(7):724–730. doi: 10.1016/j.bbagen.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Ganz T. Molecular control of iron transport. J Am Soc Nephrol. 2007;18(2):394–400. doi: 10.1681/ASN.2006070802. [DOI] [PubMed] [Google Scholar]
- 24.Dolegowska B, Blogowski W, Domanski L. Clinical evidence of the association between serum perioperative changes inxanthine metabolizing enzymes activity and early post-transplant kidney allograft function. J Am Coll Surg. 2010;211(5):587–595. doi: 10.1016/j.jamcollsurg.2010.06.391. [DOI] [PubMed] [Google Scholar]
- 25.Powner DJ, Hendrich A, Nyhuis A, Strate R. Changes in serum catecholamine levels in patients who are brain dead. J Heart Lung Transplant. 1992;11(6):1046–1053. [PubMed] [Google Scholar]
- 26.Gramm HJ, Zimmermann J, Meinhold H, Dennhardt R, Voigt K. Hemodynamic responses to noxious stimuli in brain-dead organ donors. Intensive Care Med. 1992;18(8):493–495. doi: 10.1007/BF01708589. [DOI] [PubMed] [Google Scholar]
- 27.Koo DD, Welsh KI, McLaren AJ, Roake JA, Morris PJ, Fuggle SV. Cadaver versus living donor kidneys: impact of donor factors on antigen induction before transplantation. Kidney Int. 1999;56(4):1551–1559. doi: 10.1046/j.1523-1755.1999.00657.x. [DOI] [PubMed] [Google Scholar]
- 28.van der Hoeven JA, Molema G, Ter Horst GJ, Freund RL, Wiersema J, van Schilfgaarde R, et al. Relationship between duration of brain death and hemodynamic (in)stability on progressive dysfunction and increased immunologic activation of donor kidneys. Kidney Int. 2003;64(5):1874–1882. doi: 10.1046/j.1523-1755.2003.00272.x. [DOI] [PubMed] [Google Scholar]
- 29.Devarajan P. Update on mechanisms of ischemic acute kidney injury. J Am Soc Nephrol. 2006;17(6):1503–1520. doi: 10.1681/ASN.2006010017. [DOI] [PubMed] [Google Scholar]
- 30.Singbartl K, Green SA, Ley K. Blocking P-selectin protects from ischemia/reperfusion-induced acute renal failure. FASEB J. 2000;14(1):48–54. doi: 10.1096/fasebj.14.1.48. [DOI] [PubMed] [Google Scholar]
- 31.Salmela K, Wramner L, Ekberg H, Hauser I, Bentdal O, Lins LE, et al. Arandomized multicenter trial of the anti-ICAM-1 monoclonal antibody (enlimomab) for the prevention of acute rejection and delayed onset of graft function in cadaveric renal transplantation: a report of the European Anti-ICAM-1 Renal Transplant Study Group. Transplantation. 1999;67(5):729–736. doi: 10.1097/00007890-199903150-00015. [DOI] [PubMed] [Google Scholar]
- 32.Koo DD, Welsh KI, Roake JA, Morris PJ, Fuggle SV. Ischemia/reperfusion injury in human kidney transplantation: an immunohistochemical analysis of changes after reperfusion. Am J Pathol. 1998;153(2):557–566. doi: 10.1016/S0002-9440(10)65598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schnuelle P, Yard BA, Braun C, Dominguez-Fernandez E, Schaub M, Birck R, et al. Impact of donor dopamine on immediate graft function after kidney transplantation. Am J Transplant. 2004;4(3):419–426. doi: 10.1111/j.1600-6143.2004.00331.x. [DOI] [PubMed] [Google Scholar]
- 34.Schnuelle P, Gottmann U, Hoeger S, Boesebeck D, Lauchart W, Weiss C, et al. Effects of donor pretreatment with dopamine on graft function after kidney transplantation: a randomized controlled trial. JAMA. 2009;302(10):1067–1075. doi: 10.1001/jama.2009.1310. [DOI] [PubMed] [Google Scholar]
- 35.Blasco V, Leone M, Bouvenot J, Geissler A, Albanese J, Martin C. Impact of intensivecare on renal function before graft harvest: results of a monocentric study. Crit Care. 2007;11(5):R103. doi: 10.1186/cc6120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rosendale JD, Kauffman HM, McBride MA, Chabalewski FL, Zaroff JG, Garrity ER, et al. Aggressive pharmacologic donor management results in more transplanted organs. Transplantation. 2003;75(4):482–487. doi: 10.1097/01.TP.0000045683.85282.93. [DOI] [PubMed] [Google Scholar]
- 37.DuBose J, Salim A. Aggressive organ donor management protocol. J Intensive Care Med. 2008;23(6):367–375. doi: 10.1177/0885066608324208. [DOI] [PubMed] [Google Scholar]
- 38.Giral M, Bertola JP, Foucher Y, Villers D, Bironneau E, Blanloeil Y, et al. Effect of brain-dead donor resuscitation on delayed graft function: results of a monocentric analysis. Transplantation. 2007;83(9):1174–1181. doi: 10.1097/01.tp.0000259935.82722.11. [DOI] [PubMed] [Google Scholar]
- 39.Souter M, Van Norman G. Ethical controversies at end of life after traumatic brain injury: defining death and organ donation. Critical care medicine. 2010;38(9 Suppl):S502–509. doi: 10.1097/CCM.0b013e3181ec5354. [DOI] [PubMed] [Google Scholar]
- 40.Turunen AJ, Lindgren L, Salmela KT, Kyllonen LE, Petaja J, Pesonen EJ. Intragraft coagulation events and delayed graft function in clinical renal transplantation. Transplantation. 2008;85(5):693–699. doi: 10.1097/TP.0b013e31816615d8. [DOI] [PubMed] [Google Scholar]
- 41.Thuillier R, Favreau F, Celhay O, Macchi L, Milin S, Hauet T. Thrombin inhibition during kidney ischemia-reperfusion reduces chronic graft inflammation and tubular atrophy. Transplantation. 90(6):612–621. doi: 10.1097/tp.0b013e3181d72117. [DOI] [PubMed] [Google Scholar]
- 42.Favreau F, Thuillier R, Cau J, Milin S, Manguy E, Mauco G, et al. Anti-thrombin therapy during warm ischemia and cold preservation prevents chronic kidney graft fibrosis in a DCD model. Am J Transplant. 2010;10(1):30–39. doi: 10.1111/j.1600-6143.2009.02924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rojas-Pena A, Reoma JL, Krause E, Boothman EL, Padiyar NP, Cook KE, et al. Extracorporeal support: improves donor renal graft function after cardiac death. Am J Transplant. 2010;10(6):1365–1374. doi: 10.1111/j.1600-6143.2010.03063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ames A, 3rd, Wright RL, Kowada M, Thurston JM, Majno G. Cerebral ischemia. II. The no-reflow phenomenon. Am J Pathol. 1968;52(2):437–453. [PMC free article] [PubMed] [Google Scholar]
- 45.Anaise D, Ramsammy L, Lane B, Waltzer WC, Rapaport FT. The pathophysiology of the no-reflow phenomenon in cold-stored kidneys. Transplant Proc. 1987;19(1 Pt 2):1348–1352. [PubMed] [Google Scholar]
- 46.Summers DM, Johnson RJ, Allen J, Fuggle SV, Collett D, Watson CJ, et al. Analysis of factors that affect outcome after transplantation of kidneys donated after cardiac death in the UK: a cohort study. Lancet. 2010;376(9749):1303–1311. doi: 10.1016/S0140-6736(10)60827-6. [DOI] [PubMed] [Google Scholar]
- 47.Snoeijs MG, Winkens B, Heemskerk MB, Hoitsma AJ, Christiaans MH, Buurman WA, et al. Kidney transplantation from donors after cardiac death: a 25-year experience. Transplantation. 2010;90(10):1106–1112. doi: 10.1097/TP.0b013e3181f83b0b. [DOI] [PubMed] [Google Scholar]
- 48.Koning OH, Ploeg RJ, van Bockel JH, Groenewegen M, van der Woude FJ, Persijn GG, et al. Risk factors for delayed graft function in cadaveric kidney transplantation: a prospective study of renal function and graft survival after preservation with University of Wisconsin solution in multi-organ donors. European Multicenter Study Group. Transplantation. 1997;63(11):1620–1628. doi: 10.1097/00007890-199706150-00015. [DOI] [PubMed] [Google Scholar]
- 49.Moers C, Smits JM, Maathuis MH, Treckmann J, van Gelder F, Napieralski BP, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. N Engl J Med. 2009;360(1):7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 50.Gallinat A, Feldkamp T, Schaffer R, Radunz S, Treckmann JW, Minor T, et al. Single-center experience with kidney transplantation using deceased donors older than 75 years. Transplantation. 2011;92(1):76–81. doi: 10.1097/TP.0b013e31821d2687. [DOI] [PubMed] [Google Scholar]
- 51.Karpinski J, Lajoie G, Cattran D, Fenton S, Zaltzman J, Cardella C, et al. Outcome of kidney transplantation from high-risk donors is determined by both structure and function. Transplantation. 1999;67(8):1162–1167. doi: 10.1097/00007890-199904270-00013. [DOI] [PubMed] [Google Scholar]
- 52.Snoeijs MG, Buurman WA, Christiaans MH, van Hooff JP, Goldschmeding R, van Suylen RJ, et al. Histological assessment of preimplantation biopsies may improve selection of kidneys from old donors after cardiac death. Am J Transplant. 2008;8(9):1844–1851. doi: 10.1111/j.1600-6143.2008.02318.x. [DOI] [PubMed] [Google Scholar]
- 53.Snoeijs MG, Boonstra LA, Buurman WA, Goldschmeding R, van Suylen RJ, van Heurn LW, et al. Histological assessment of pre-transplant kidney biopsies is reproducible and representative. Histopathology. 2010;56(2):198–202. doi: 10.1111/j.1365-2559.2009.03469.x. [DOI] [PubMed] [Google Scholar]
- 54.Shoskes DA, Cecka JM. Deleterious effects of delayed graft function in cadaveric renal transplant recipients independent of acute rejection. Transplantation. 1998;66(12):1697–1701. doi: 10.1097/00007890-199812270-00022. [DOI] [PubMed] [Google Scholar]
- 55.Shoskes DA, Cecka JM. Effect ofdelayed graft function on short-and long-term kidney graft survival. Clin Transpl. 1997:297–303. [PubMed] [Google Scholar]
- 56.McLaren AJ, Jassem W, Gray DW, Fuggle SV, Welsh KI, Morris PJ. Delayed graft function: risk factors and the relative effects of early function and acute rejection on long-term survival in cadaveric renal transplantation. Clin Transplant. 1999;13(3):266–272. doi: 10.1034/j.1399-0012.1999.130308.x. [DOI] [PubMed] [Google Scholar]
- 57.Nyberg SL, Matas AJ, Rogers M, Harmsen WS, Velosa JA, Larson TS, et al. Donor scoring system for cadaveric renal transplantation. Am J Transplant. 2001;1(2):162–170. [PubMed] [Google Scholar]
- 58.Ciancio G, Burke GW, Gaynor JJ, Roth D, Kupin W, Rosen A, et al. A randomized trial of thymoglobulin vs. alemtuzumab (with lower dose maintenance immunosuppression) vs. daclizumab in renal transplantation at 24 months of follow-up. Clin Transplant. 2008;22(2):200–210. doi: 10.1111/j.1399-0012.2007.00774.x. [DOI] [PubMed] [Google Scholar]
- 59.Irish WD, Ilsley JN, Schnitzler MA, Feng S, Brennan DC. A risk prediction model for delayed graft function in the current era of deceased donor renal transplantation. Am J Transplant. 2010;10(10):2279–2286. doi: 10.1111/j.1600-6143.2010.03179.x. [DOI] [PubMed] [Google Scholar]
- 60.Doshi MD, Garg N, Reese PP, Parikh CR. Recipient risk factors associated with delayed graft function: a paired kidney analysis. Transplantation. 2011;91(6):666–671. doi: 10.1097/TP.0b013e318209f22b. [DOI] [PubMed] [Google Scholar]
- 61.Meier-Kriesche HU, Arndorfer JA, Kaplan B. The impact of body mass index on renal transplant outcomes: a significant independent risk factor for graft failure and patient death. Transplantation. 2002;73(1):70–74. doi: 10.1097/00007890-200201150-00013. [DOI] [PubMed] [Google Scholar]
- 62.Parekh J, Bostrom A, Feng S. Diabetes mellitus: a risk factor for delayed graft function after deceased donor kidney transplantation. Am J Transplant. 2010;10(2):298–303. doi: 10.1111/j.1600-6143.2009.02936.x. [DOI] [PubMed] [Google Scholar]
- 63.Belzer FO, Southard JH. Principles of solid-organ preservation by cold storage. Transplantation. 1988;45(4):673–676. doi: 10.1097/00007890-198804000-00001. [DOI] [PubMed] [Google Scholar]
- 64.Mangino MJ, Ametani M, Szabo C, Southard JH. Poly(ADP-ribose) polymerase and renal hypothermic preservation injury. Am J Physiol Renal Physiol. 2004;286(5):F838–847. doi: 10.1152/ajprenal.00230.2003. [DOI] [PubMed] [Google Scholar]
- 65.Ploeg RJ, Goossens D, McAnulty JF, Southard JH, Belzer FO. Successful 72-hour cold storage of dog kidneys with UW solution. Transplantation. 1988;46(2):191–196. doi: 10.1097/00007890-198808000-00002. [DOI] [PubMed] [Google Scholar]
- 66.Wahlberg JA, Southard JH, Belzer FO. Development of a cold storage solution for pancreas preservation. Cryobiology. 1986;23(6):477–482. doi: 10.1016/0011-2240(86)90056-8. [DOI] [PubMed] [Google Scholar]
- 67.Yang Q, Zhang RZ, Yim AP, He GW. Histidine-tryptophan-ketoglutarate solution maximally preserves endothelium-derived hyperpolarizing factor-mediated function during heart preservation: comparison with University of Wisconsin solution. J Heart Lung Transplant. 2004;23(3):352–359. doi: 10.1016/S1053-2498(03)00187-6. [DOI] [PubMed] [Google Scholar]
- 68.Reichenspurner H, Russ C, Uberfuhr P, Nollert G, Schluter A, Reichart B, et al. Myocardial preservation using HTK solution for heart transplantation. A multicenter study. Eur J Cardiothorac Surg. 1993;7(8):414–419. doi: 10.1016/1010-7940(93)90005-v. [DOI] [PubMed] [Google Scholar]
- 69.de Boer J, De Meester J, Smits JM, Groenewoud AF, Bok A, van der Velde O, et al. Eurotransplant randomized multicenter kidney graft preservation study comparing HTK with UW and Euro-Collins. Transpl Int. 1999;12(6):447–453. doi: 10.1007/s001470050256. [DOI] [PubMed] [Google Scholar]
- 70.Stevens RB, Skorupa JY, Rigley TH, Yannam GR, Nielsen KJ, Schriner ME, et al. Increased primary non-function in transplanted deceased-donor kidneys flushed with histidine-tryptophan-ketoglutarate solution. Am J Transplant. 2009;9(5):1055–1062. doi: 10.1111/j.1600-6143.2009.02624.x. [DOI] [PubMed] [Google Scholar]
- 71.Semmelmann A, Neeff H, Sommer O, Thomusch O, Hopt UT, von Dobschuetz E. Evaluation of preservation solutions by ESR-spectroscopy: superior effects of University of Wisconsin over Histidine-Tryptophan-Ketoglutarate in reducing renal reactive oxygen species. Kidney Int. 2007;71(9):875–881. doi: 10.1038/sj.ki.5002129. [DOI] [PubMed] [Google Scholar]
- 72.Wight JP, Chilcott JB, Holmes MW, Brewer N. Pulsatile machine perfusion vs. cold storage of kidneys for transplantation: a rapid and systematic review. Clin Transplant. 2003;17(4):293–307. doi: 10.1034/j.1399-0012.2003.00077.x. [DOI] [PubMed] [Google Scholar]
- 73.Jochmans I, Moers C, Smits JM, Leuvenink HG, Treckmann J, Paul A, et al. Machine perfusion versus cold storage for the preservation of kidneys donated after cardiac death: a multicenter, randomized, controlled trial. Ann Surg. 2010;252(5):756–764. doi: 10.1097/SLA.0b013e3181ffc256. [DOI] [PubMed] [Google Scholar]
- 74.Watson CJ, Wells AC, Roberts RJ, Akoh JA, Friend PJ, Akyol M, et al. Cold machine perfusion versus static cold storage of kidneys donated after cardiac death: a UK multicenter randomized controlled trial. Am J Transplant. 2010;10(9):1991–1999. doi: 10.1111/j.1600-6143.2010.03165.x. [DOI] [PubMed] [Google Scholar]
- 75.Treckmann J, Moers C, Smits JM, Gallinat A, Maathuis MH, van Kasterop-Kutz M, et al. Machine perfusion versus cold storage for preservation of kidneys from expanded criteria donors after brain death. Transpl Int. 2011;24(6):548–554. doi: 10.1111/j.1432-2277.2011.01232.x. [DOI] [PubMed] [Google Scholar]
- 76.Irish WD, Katz E. Cold machine perfusion or static cold storage of kidneys: why the debate continues. Am J Transplant. 2010;10(9):1955–1956. doi: 10.1111/j.1600-6143.2010.03217.x. [DOI] [PubMed] [Google Scholar]
- 77.Kozaki K, Sakurai E, Kubota K, Iwamoto H, Hama K, Narumi Y, et al. Prediction of kidney nonfunction after transplantation with machine perfusion preservation. Transplant Proc. 2000;32(2):275–276. doi: 10.1016/s0041-1345(99)00955-0. [DOI] [PubMed] [Google Scholar]
- 78.Matsuno N, Kozaki K, Degawa H, Narumi Y, Suzuki N, Kikuchi K, et al. A useful predictor in machine perfusion preservation for kidney transplantation from non-heart-beating donors. Transplant Proc. 2000;32(1):173–174. doi: 10.1016/s0041-1345(99)00919-7. [DOI] [PubMed] [Google Scholar]
- 79.St Peter SD, Imber CJ, Friend PJ. Liver and kidney preservation by perfusion. Lancet. 2002;359(9306):604–613. doi: 10.1016/S0140-6736(02)07749-8. [DOI] [PubMed] [Google Scholar]
- 80.Nyberg SL, Baskin-Bey ES, Kremers W, Prieto M, Henry ML, Stegall MD. Improving the prediction of donor kidney quality: deceased donor score and resistive indices. Transplantation. 2005;80(7):925–929. doi: 10.1097/01.tp.0000173798.04043.af. [DOI] [PubMed] [Google Scholar]
- 81.Mozes MF, Skolek RB, Korf BC. Use of perfusion parameters in predicting outcomes of machine-preserved kidneys. Transplant Proc. 2005;37(1):350–351. doi: 10.1016/j.transproceed.2005.01.058. [DOI] [PubMed] [Google Scholar]
- 82.Domagala P, Kwiatkowski A, Wszola M, Czerwinski J, Cybula K, Trzebicki J, et al. Complications of transplantation of kidneys from expanded-criteria donors. Transplant Proc. 2009;41(8):2970–2971. doi: 10.1016/j.transproceed.2009.07.085. [DOI] [PubMed] [Google Scholar]
- 83.Hoogland ER, Snoeijs MG, Winkens B, Christaans MH, van Heurn LW. Kidney Transplantation from Donors after Cardiac Death: Uncontrolled versus Controlled Donation. Am J Transplant. 2011;11(7):1427–1434. doi: 10.1111/j.1600-6143.2011.03562.x. [DOI] [PubMed] [Google Scholar]
- 84.Collins GM, Bravo-Shugarman M, Terasaki PI. Kidney preservation for transportation. Initial perfusion and 30 hours’ ice storage. Lancet. 1969;2(7632):1219–1222. doi: 10.1016/s0140-6736(69)90753-3. [DOI] [PubMed] [Google Scholar]
- 85.Koetting M, Frotscher C, Minor T. Hypothermic reconditioning after cold storage improves postischemic graft function in isolated porcine kidneys. Transpl Int. 2010;23(5):538–542. doi: 10.1111/j.1432-2277.2009.01014.x. [DOI] [PubMed] [Google Scholar]
- 86.Manekeller S, Leuvenink H, Sitzia M, Minor T. Oxygenated machine perfusion preservation of predamaged kidneys with HTK and Belzer machine perfusion solution: an experimental study in pigs. Transplant Proc. 2005;37(8):3274–3275. doi: 10.1016/j.transproceed.2005.09.034. [DOI] [PubMed] [Google Scholar]
- 87.Maluf DG, Mas VR, Yanek K, Stone JJ, Weis R, Massey D, et al. Molecular markers in stored kidneys using perfluorocarbon-based preservation solution: preliminary results. Transplant Proc. 2006;38(5):1243–1246. doi: 10.1016/j.transproceed.2006.02.109. [DOI] [PubMed] [Google Scholar]
- 88.Gravel MT, Arenas JD, Chenault R, 2nd, Magee JC, Rudich S, Maraschio M, et al. Kidney transplantation from organ donors following cardiopulmonary death using extracorporeal membrane oxygenation support. Ann Transplant. 2004;9(1):57–58. [PubMed] [Google Scholar]
- 89.Magliocca JF, Magee JC, Rowe SA, Gravel MT, Chenault RH, 2nd, Merion RM, et al. Extracorporeal support for organ donation after cardiac death effectively expands the donor pool. J Trauma. 2005;58(6):1095–1101. doi: 10.1097/01.ta.0000169949.82778.df. discussion 1101–1092. [DOI] [PubMed] [Google Scholar]
- 90.Lee CY, Tsai MK, Ko WJ, Chang CJ, Hu RH, Chueh SC, et al. Expanding the donor pool: use of renal transplants from non-heart-beatingdonors supported with extracorporeal membrane oxygenation. Clin Transplant. 2005;19(3):383–390. doi: 10.1111/j.1399-0012.2005.00358.x. [DOI] [PubMed] [Google Scholar]
- 91.Othman MM, Ismael AZ, Hammouda GE. The impact of timing of maximal crystalloid hydration on early graft function during kidney transplantation. Anesthesia and analgesia. 2010;110(5):1440–1446. doi: 10.1213/ANE.0b013e3181d82ca8. [DOI] [PubMed] [Google Scholar]
- 92.Bacchi G, Buscaroli A, Fusari M, Neri L, Cappuccilli ML, Carretta E, et al. The influence of intraoperative central venous pressure on delayed graft function in renal transplantation: a single-center experience. Transplant Proc. 2010;42(9):3387–3391. doi: 10.1016/j.transproceed.2010.08.042. [DOI] [PubMed] [Google Scholar]
- 93.Zukowski M, Bohatyrewicz R, Krawczyk AA. Influence of selected factors on occurrence of delayed kidney graft function: a multivariate analysis. Transplant Proc. 2007;39(9):2704–2706. doi: 10.1016/j.transproceed.2007.09.016. [DOI] [PubMed] [Google Scholar]
- 94.Snoeijs MG, Wiermans B, Christiaans MH, van Hooff JP, Timmerman BE, Schurink GW, et al. Recipient hemodynamics during non-heart-beating donor kidney transplantation are major predictors of primary nonfunction. Am J Transplant. 2007;7(5):1158–1166. doi: 10.1111/j.1600-6143.2007.01744.x. [DOI] [PubMed] [Google Scholar]
- 95.Conger JD, Robinette JB, Hammond WS. Differences in vascular reactivity in models of ischemic acute renal failure. Kidney Int. 1991;39(6):1087–1097. doi: 10.1038/ki.1991.138. [DOI] [PubMed] [Google Scholar]
- 96.Inman SR, Plott WK, Pomilee RA, Antonelli JA, Lewis RM. Endothelin-receptor blockade mitigates the adverse effect of preretrieval warm ischemiaon posttransplantation renal function in rats. Transplantation. 2003;75(10):1655–1659. doi: 10.1097/01.TP.0000063127.02261.E4. [DOI] [PubMed] [Google Scholar]
- 97.Siedlecki A, Anderson JR, Jin X, Garbow JR, Lupu TS, Muslin AJ. RGS4 controls renal blood flow and inhibits cyclosporine-mediated nephrotoxicity. Am J Transplant. 2010;10(2):231–241. doi: 10.1111/j.1600-6143.2009.02930.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Siedlecki A, Jin X, Thomas W, Muslin A. RGS4 Prevents the Development of Renal Dysfunction in Response to Ischemia/Reperfusion Injury. Kidney Int. 2011 In press. [Google Scholar]
- 99.Schilling M, Holzinger F, Friess H, Seiler C, Buchler MW. Pathogenesisof delayed kidney graft function: role of endothelin-1, thromboxane B2, and leukotriene B4. Transplant Proc. 1996;28(1):304–305. [PubMed] [Google Scholar]
- 100.Chatterjee PK, Patel NS, Sivarajah A, Kvale EO, Dugo L, Cuzzocrea S, et al. GW274150, a potent and highly selective inhibitor of iNOS, reduces experimental renal ischemia/reperfusion injury. Kidney Int. 2003;63(3):853–865. doi: 10.1046/j.1523-1755.2003.00802.x. [DOI] [PubMed] [Google Scholar]
- 101.Khanafer A, Ilham MA, Namagondlu GS, Janzic A, Sikas N, Smith D, et al. Increased nitric oxide production during acute rejection in kidney transplantation: a useful marker to aid in the diagnosis of rejection. Transplantation. 2007;84(5):580–586. doi: 10.1097/01.tp.0000278120.55796.42. [DOI] [PubMed] [Google Scholar]
- 102.Alonso D, Radomski MW. The nitric oxide-endothelin-1 connection. Heart Fail Rev. 2003;8(1):107–115. doi: 10.1023/a:1022155206928. [DOI] [PubMed] [Google Scholar]
- 103.Dutkiewicz G, Domanski L, Binczak-Kuleta A, Pawlik A, Safranow K, Ciechanowicz A, et al. The association between eNOS intron 4 VNTR polymorphism and delayed graft function of kidney allografts. Clin Transplant. 2010;24(5):E130–136. doi: 10.1111/j.1399-0012.2009.01187.x. [DOI] [PubMed] [Google Scholar]
- 104.Neto JS, Nakao A, Kimizuka K, Romanosky AJ, Stolz DB, Uchiyama T, et al. Protection of transplant-induced renal ischemia-reperfusion injury with carbon monoxide. Am J Physiol Renal Physiol. 2004;287(5):F979–989. doi: 10.1152/ajprenal.00158.2004. [DOI] [PubMed] [Google Scholar]
- 105.Li L, Okusa MD. Macrophages, dendritic cells, and kidney ischemia-reperfusion injury. Seminars in nephrology. 2010;30(3):268–277. doi: 10.1016/j.semnephrol.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jang HR, Ko GJ, Wasowska BA, Rabb H. The interaction between ischemia-reperfusion and immune responses in the kidney. J Mol Med. 2009;87(9):859–864. doi: 10.1007/s00109-009-0491-y. [DOI] [PubMed] [Google Scholar]
- 107.Dong X, Swaminathan S, Bachman LA, Croatt AJ, Nath KA, Griffin MD. Resident dendriticcells are the predominant TNF-secreting cell in early renal ischemia-reperfusion injury. Kidney Int. 2007;71(7):619–628. doi: 10.1038/sj.ki.5002132. [DOI] [PubMed] [Google Scholar]
- 108.Furuichi K, Wada T, Iwata Y, Kitagawa K, Kobayashi K, Hashimoto H, et al. CCR2 signaling contributes to ischemia-reperfusion injuryin kidney. J Am Soc Nephrol. 2003;14(10):2503–2515. doi: 10.1097/01.asn.0000089563.63641.a8. [DOI] [PubMed] [Google Scholar]
- 109.Hu H, Aizenstein BD, Puchalski A, Burmania JA, Hamawy MM, Knechtle SJ. Elevation of CXCR3-binding chemokines in urine indicates acute renal-allograft dysfunction. Am J Transplant. 2004;4(3):432–437. doi: 10.1111/j.1600-6143.2004.00354.x. [DOI] [PubMed] [Google Scholar]
- 110.Pedregosa JF, Haidar AA, Hirata AE, Franco M, Gomes GN, Bueno V. TLR2 and TLR4 expression after kidney ischemia and reperfusion injury in mice treated with FTY720. International immunopharmacology. 2011 doi: 10.1016/j.intimp.2011.04.014. [DOI] [PubMed] [Google Scholar]
- 111.Anders HJ, Banas B, Schlondorff D. Signalingdanger: toll-like receptors and their potential roles in kidney disease. J Am Soc Nephrol. 2004;15(4):854–867. doi: 10.1097/01.asn.0000121781.89599.16. [DOI] [PubMed] [Google Scholar]
- 112.Wu H, Chen G, Wyburn KR, Yin J, Bertolino P, Eris JM, et al. TLR4 activation mediates kidney ischemia/reperfusion injury. J Clin Invest. 2007;117(10):2847–2859. doi: 10.1172/JCI31008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kruger B, Banas MC, Walberer A, Boger CA, Farkas S, Hoffmann U, et al. A comprehensive genotype-phenotype interaction of different Toll-like receptor variations in a renal transplant cohort. Clin Sci (Lond) 2010;119(12):535–544. doi: 10.1042/CS20100190. [DOI] [PubMed] [Google Scholar]
- 114.Zhou W, Medof ME, Heeger PS, Sacks S. Graft-derived complement as a mediator of transplant injury. Curr Opin Immunol. 2007;19(5):569–576. doi: 10.1016/j.coi.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 115.Bao L, Wang Y, Chang A, Minto AW, Zhou J, Kang H, et al. Unrestricted C3 activation occurs in Crry-deficient kidneys and rapidly leads to chronic renal failure. J Am Soc Nephrol. 2007;18(3):811–822. doi: 10.1681/ASN.2006101176. [DOI] [PubMed] [Google Scholar]
- 116.Pratt JR, Basheer SA, Sacks SH. Local synthesis of complement component C3 regulates acute renal transplant rejection. Nat Med. 2002;8(6):582–587. doi: 10.1038/nm0602-582. [DOI] [PubMed] [Google Scholar]
- 117.Damman J, Nijboer WN, Schuurs TA, Leuvenink HG, Morariu AM, Tullius SG, et al. Local renal complement C3 induction by donor brain death is associated with reduced renal allograft function after transplantation. Nephrol Dial Transplant. 2010;26(7):2345. doi: 10.1093/ndt/gfq717. [DOI] [PubMed] [Google Scholar]
- 118.Brown KM, Kondeatis E, Vaughan RW, Kon SP, Farmer CK, Taylor JD, et al. Influence of donor C3 allotype on late renal-transplantation outcome. N Engl J Med. 2006;354(19):2014–2023. doi: 10.1056/NEJMoa052825. [DOI] [PubMed] [Google Scholar]
- 119.Varagunam M, Yaqoob MM, Dohler B, Opelz G. C3 polymorphisms and allograft outcome in renal transplantation. N Engl J Med. 2009;360(9):874–880. doi: 10.1056/NEJMoa0801861. [DOI] [PubMed] [Google Scholar]
- 120.Shoskes DA, Parfrey NA, Halloran PF. Increased major histocompatibility complex antigen expression in unilateral ischemic acute tubular necrosis in the mouse. Transplantation. 1990;49(1):201–207. doi: 10.1097/00007890-199001000-00045. [DOI] [PubMed] [Google Scholar]
- 121.Shackleton CR, Ettinger SL, McLoughlin MG, Scudamore CH, Miller RR, Keown PA. Effect of recovery from ischemic injury on class I and class II MHC antigen expression. Transplantation. 1990;49(3):641–644. [PubMed] [Google Scholar]
- 122.Goes N, Urmson J, Vincent D, Halloran PF. Acuterenal injury in the interferon-gamma gene knockout mouse: effect on cytokine gene expression. Transplantation. 1995;60(12):1560–1564. doi: 10.1097/00007890-199560120-00031. [DOI] [PubMed] [Google Scholar]
- 123.Kotsch K, Martins PN, Klemz R, Janssen U, Gerstmayer B, Dernier A, et al. Heme oxygenase-1 ameliorates ischemia/reperfusion injury by targeting dendritic cell maturation and migration. Antioxid Redox Signal. 2007;9(12):2049–2063. doi: 10.1089/ars.2007.1801. [DOI] [PubMed] [Google Scholar]
- 124.Jantsch J, Chakravortty D, Turza N, Prechtel AT, Buchholz B, Gerlach RG, et al. Hypoxia and hypoxia-inducible factor-1 alpha modulate lipopolysaccharide-induced dendritic cell activation and function. J Immunol. 2008;180(7):4697–4705. doi: 10.4049/jimmunol.180.7.4697. [DOI] [PubMed] [Google Scholar]
- 125.Kerschen E, Hernandez I, Zogg M, Jia S, Hessner MJ, Fernandez JA, et al. Activated protein C targets CD8+ dendritic cells to reduce the mortality of endotoxemia in mice. J Clin Invest. 2010;120(9):3167–3178. doi: 10.1172/JCI42629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Maltzman JS, Haase VH. Low oxygen stimulates the immune system. Kidney Int. 2008;73(7):797–799. doi: 10.1038/ki.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lu CY, Penfield JG, Kielar ML, Vazquez MA, Jeyarajah DR. Hypothesis: is renal allograft rejection initiated by the response to injury sustained during the transplant process? Kidney Int. 1999;55(6):2157–2168. doi: 10.1046/j.1523-1755.1999.00491.x. [DOI] [PubMed] [Google Scholar]
- 128.Rabb H, Daniels F, O’Donnell M, Haq M, Saba SR, Keane W, et al. Pathophysiological role of T lymphocytes in renal ischemia-reperfusion injury in mice. Am J Physiol Renal Physiol. 2000;279(3):F525–531. doi: 10.1152/ajprenal.2000.279.3.F525. [DOI] [PubMed] [Google Scholar]
- 129.Park P, Haas M, Cunningham PN, Bao L, Alexander JJ, Quigg RJ. Injury in renal ischemia-reperfusion is independent from immunoglobulins and T lymphocytes. Am J Physiol Renal Physiol. 2002;282(2):F352–357. doi: 10.1152/ajprenal.00160.2001. [DOI] [PubMed] [Google Scholar]
- 130.Loverre A, Divella C, Castellano G, Tataranni T, Zaza G, Rossini M, et al. T helper 1, 2 and 17 cell subsets in renal transplant patients with delayed graft function. Transpl Int. 2011;24(3):233–242. doi: 10.1111/j.1432-2277.2010.01157.x. [DOI] [PubMed] [Google Scholar]
- 131.Yan SF, Lawson CA, Stern DM, Pinsky DJ. Hypoxia-mediated modulation of vascular function--implications for organ preservation and thrombogenesis: Roger S. Mitchell lecture Chest. 1998;114(1 Suppl):46S–50S. doi: 10.1378/chest.114.1_supplement.46s. [DOI] [PubMed] [Google Scholar]
- 132.Sarwal M, Chua MS, Kambham N, Hsieh SC, Satterwhite T, Masek M, et al. Molecular heterogeneity in acute renal allograft rejection identified by DNA microarray profiling. N Engl J Med. 2003;349(2):125–138. doi: 10.1056/NEJMoa035588. [DOI] [PubMed] [Google Scholar]
- 133.Reeve J, Einecke G, Mengel M, Sis B, Kayser N, Kaplan B, et al. Diagnosing rejection in renal transplants: a comparison of molecular-andhistopathology-based approaches. Am J Transplant. 2009;9(8):1802–1810. doi: 10.1111/j.1600-6143.2009.02694.x. [DOI] [PubMed] [Google Scholar]
- 134.Mueller TF, Reeve J, Jhangri GS, Mengel M, Jacaj Z, Cairo L, et al. The transcriptome of the implant biopsy identifies donor kidneys at increased risk of delayed graft function. Am J Transplant. 2008;8(1):78–85. doi: 10.1111/j.1600-6143.2007.02032.x. [DOI] [PubMed] [Google Scholar]
- 135.Perianayagam MC, Liangos O, Kolyada AY, Wald R, MacKinnon RW, Li L, et al. NADPH oxidase p22phox and catalase gene variants are associated with biomarkers of oxidative stress and adverse outcomes in acute renal failure. J Am Soc Nephrol. 2007;18(1):255–263. doi: 10.1681/ASN.2006070806. [DOI] [PubMed] [Google Scholar]
- 136.Israni AK, Li N, Cizman BB, Snyder J, Abrams J, Joffe M, et al. Association of donor inflammation-and apoptosis-related genotypes and delayed allograft function after kidney transplantation. Am J Kidney Dis. 2008;52(2):331–339. doi: 10.1053/j.ajkd.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Grisk O, Steinbach AC, Ciecholewski S, Schluter T, Kloting I, Schmidt H, et al. Multidrug resistance-related protein 2 genotype of the donor affects kidney graft function. Pharmacogenet Genomics. 2009;19(4):276–288. doi: 10.1097/FPC.0b013e328328d4e9. [DOI] [PubMed] [Google Scholar]
- 138.Kotsch K, Kunert K, Merk V, Reutzel-Selke A, Pascher A, Fritzsche F, et al. Novel markers in zero-hour kidney biopsies indicate graft quality and clinical outcome. Transplantation. 2010;90(9):958–965. doi: 10.1097/TP.0b013e3181f546e8. [DOI] [PubMed] [Google Scholar]
- 139.Godwin JG, Ge X, Stephan K, Jurisch A, Tullius SG, Iacomini J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc Natl Acad Sci U S A. 2010;107(32):14339–14344. doi: 10.1073/pnas.0912701107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Coca SG, Yalavarthy R, Concato J, Parikh CR. Biomarkers for the diagnosis and risk stratification of acute kidney injury: a systematic review. Kidney Int. 2008;73(9):1008–1016. doi: 10.1038/sj.ki.5002729. [DOI] [PubMed] [Google Scholar]
- 141.Hall IE, Yarlagadda SG, Coca SG, Wang Z, Doshi M, Devarajan P, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21(1):189–197. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Schroppel B, Kruger B, Walsh L, Yeung M, Harris S, Garrison K, et al. Tubular expression of KIM-1 does not predict delayed function after transplantation. J Am Soc Nephrol. 2009;21(3):536–542. doi: 10.1681/ASN.2009040390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Hanto DW, Maki T, Yoon MH, Csizmadia E, Chin BY, Gallo D, et al. Intraoperative administration of inhaled carbon monoxide reduces delayed graft function in kidney allografts in Swine. Am J Transplant. 2010;10(11):2421–2430. doi: 10.1111/j.1600-6143.2010.03289.x. [DOI] [PubMed] [Google Scholar]
- 144.Herrero I, Torras J, Riera M, Condom E, Coll O, Cruzado JM, et al. Prevention of cold ischaemia-reperfusion injury by an endothelin receptor antagonist in experimental renal transplantation. Nephrol Dial Transplant. 1999;14(4):872–880. doi: 10.1093/ndt/14.4.872. [DOI] [PubMed] [Google Scholar]
- 145.Neuhofer W, Pittrow D. Role of endothelin and endothelin receptor antagonists in renal disease. Eur J Clin Invest. 2006;36 (Suppl 3):78–88. doi: 10.1111/j.1365-2362.2006.01689.x. [DOI] [PubMed] [Google Scholar]
- 146.Issa N, Krowka MJ, Griffin MD, Hickson LJ, Stegall MD, Cosio FG. Pulmonary hypertension is associated with reduced patient survival after kidney transplantation. Transplantation. 2008;86(10):1384–1388. doi: 10.1097/TP.0b013e318188d640. [DOI] [PubMed] [Google Scholar]
- 147.Zlotnick DM, Axelrod DA, Chobanian MC, Friedman S, Brown J, Catherwood E, et al. Non-invasive detection of pulmonary hypertension prior to renal transplantation is a predictor of increased risk for early graft dysfunction. Nephrol Dial Transplant. 2010;25(9):3090–3096. doi: 10.1093/ndt/gfq141. [DOI] [PubMed] [Google Scholar]
- 148.Neumayer HH, Kunzendorf U, Schreiber M. Protective effects of calcium antagonists in human renal transplantation. Kidney Int Suppl. 1992;36:S87–93. [PubMed] [Google Scholar]
- 149.Kuypers DR, Neumayer HH, Fritsche L, Budde K, Rodicio JL, Vanrenterghem Y. Calcium channel blockade and preservation of renal graft function in cyclosporine-treated recipients: a prospective randomized placebo-controlled 2-year study. Transplantation. 2004;78(8):1204–1211. doi: 10.1097/01.tp.0000137793.23371.42. [DOI] [PubMed] [Google Scholar]
- 150.Mangray M, Vella JP. Hypertension after kidney transplant. Am J Kidney Dis. 2011;57(2):331–341. doi: 10.1053/j.ajkd.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 151.Slawsky MT, Givertz MM. Rolofylline: a selective adenosine 1 receptor antagonist for the treatment of heart failure. Expert Opin Pharmacother. 2009;10(2):311–322. doi: 10.1517/14656560802682213. [DOI] [PubMed] [Google Scholar]
- 152.Massie BM, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, et al. Rolofylline, an adenosine A1-receptor antagonist, in acute heart failure. N Engl J Med. 2010;363(15):1419–1428. doi: 10.1056/NEJMoa0912613. [DOI] [PubMed] [Google Scholar]
- 153.Osama Gaber A, Mulgaonkar S, Kahan BD, Steve Woodle E, Alloway R, Bajjoka I, et al. YSPSL (rPSGL-Ig) for improvement of early renal allograftfunction: a double-blind, placebo-controlled, multi-center Phase IIa study. Clin Transplant. 2010 doi: 10.1111/j.1399-0012.2010.01295.x. [DOI] [PubMed] [Google Scholar]
- 154.Gaber A, Patel A, Polinsky M. Safety and Tolerability of Intravenous QPI-1002, a siRNA, for Prophylaxis of Delayed Graft Function (DGF) in Deceased Donor Renal Allograft Recipients at Increased Risk of DGF: Initial Results from a Double-Blind, Phase I First-in-Human Study. Oral presentation. Am J Transplant. 2010;10:132. [Google Scholar]
- 155.Martinez F, Kamar N, Pallet N, Lang P, Durrbach A, Lebranchu Y, et al. High dose epoetin beta in the first weeks following renal transplantation and delayed graft function: Results of the Neo-PDGF Study. Am J Transplant. 2010;10(7):1695–1700. doi: 10.1111/j.1600-6143.2010.03142.x. [DOI] [PubMed] [Google Scholar]
- 156.Cooper M, Kapur S, Stratta R, D’Alessandro A. Diannexin, a Novel Ischemia/Reperfusion Therapeutic Agent, Reduces Delayed Graft Function (DGF) in Renal Transplant Recipients from Marginal Donors. Oral presentation. Am J Transplant. 2010;10:83. [Google Scholar]
- 157.Neri F, Puviani L, Tsivian M, Prezzi D, Pacile V, Cavallari G, et al. Protective effect of an inhibitor of interleukin-8 (meraxin) from ischemia and reperfusion injury in a rat model of kidney transplantation. Transplant Proc. 2007;39(6):1771–1772. doi: 10.1016/j.transproceed.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 158.Cugini D, Azzollini N, Gagliardini E, Cassis P, Bertini R, Colotta F, et al. Inhibition of the chemokine receptor CXCR2 prevents kidney graft function deterioration due to ischemia/reperfusion. Kidney Int. 2005;67(5):1753–1761. doi: 10.1111/j.1523-1755.2005.00272.x. [DOI] [PubMed] [Google Scholar]
- 159.Bedke J, Nelson PJ, Kiss E, Muenchmeier N, Rek A, Behnes CL, et al. A novel CXCL8 protein-based antagonist in acute experimental renal allograft damage. Mol Immunol. 2010;47(5):1047–1057. doi: 10.1016/j.molimm.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 160.Knechtle SJ, Fernandez LA, Pirsch JD, Becker BN, Chin LT, Becker YT, et al. Campath-1H in renal transplantation: The University of Wisconsin experience. Surgery. 2004;136(4):754–760. doi: 10.1016/j.surg.2004.06.015. [DOI] [PubMed] [Google Scholar]
- 161.Farney AC, Doares W, Rogers J, Singh R, Hartmann E, Hart L, et al. A randomized trial of alemtuzumab versus antithymocyte globulin induction in renal and pancreas transplantation. Transplantation. 2009;88(6):810–819. doi: 10.1097/TP.0b013e3181b4acfb. [DOI] [PubMed] [Google Scholar]
- 162.Kandus A, Grego K, Bren AF. Prevention of early acute rejection with daclizumab and triple immunosuppression in cadaveric renal allograft recipients. Ther Apher Dial. 2005;9(3):262–264. doi: 10.1111/j.1774-9987.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 163.Pascual J, Bloom D, Torrealba J, Brahmbhatt R, Chang Z, Sollinger HW, et al. Calcineurin inhibitor withdrawal after renal transplantation with alemtuzumab: clinical outcomes and effect on T-regulatory cells. Am J Transplant. 2008;8(7):1529–1536. doi: 10.1111/j.1600-6143.2008.02260.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Vathsala A, Ona ET, Tan SY, Suresh S, Lou HX, Casasola CB, et al. Randomized trial of Alemtuzumab for prevention of graft rejection and preservation of renal function after kidney transplantation. Transplantation. 2005;80(6):765–774. doi: 10.1097/01.tp.0000166921.14670.33. [DOI] [PubMed] [Google Scholar]
- 165.Ciancio G, Burke GW, Gaynor JJ, Roth D, Sageshima J, Kupin W, et al. Randomized trial of mycophenolate mofetil versus enteric-coated mycophenolate sodium in primary renal transplant recipients given tacrolimus and daclizumab/thymoglobulin: one year follow-up. Transplantation. 2008;86(1):67–74. doi: 10.1097/TP.0b013e3181734b4a. [DOI] [PubMed] [Google Scholar]
- 166.Hernandez D, Miquel R, Porrini E, Fernandez A, Gonzalez-Posada JM, Hortal L, et al. Randomized controlled study comparing reduced calcineurin inhibitors exposure versus standard cyclosporine-based immunosuppression. Transplantation. 2007;84(6):706–714. doi: 10.1097/01.tp.0000282872.17024.b7. [DOI] [PubMed] [Google Scholar]
- 167.Ciancio G, Burke GW, Gaynor JJ, Carreno MR, Cirocco RE, Mathew JM, et al. A randomized trial of three renal transplant induction antibodies: early comparison of tacrolimus, mycophenolate mofetil, and steroid dosing, and newer immune-monitoring. Transplantation. 2005;80(4):457–465. doi: 10.1097/01.tp.0000165847.05787.08. [DOI] [PubMed] [Google Scholar]
- 168.Hardinger KL, Bohl DL, Schnitzler MA, Lockwood M, Storch GA, Brennan DC. A randomized, prospective, pharmacoeconomic trial of tacrolimus versus cyclosporine in combination with thymoglobulin in renal transplant recipients. Transplantation. 2005;80(1):41–46. doi: 10.1097/01.tp.0000162980.68628.5a. [DOI] [PubMed] [Google Scholar]
- 169.Hardinger KL, Schnitzler MA, Miller B, Lowell JA, Shenoy S, Koch MJ, et al. Five-year follow up of thymoglobulin versus ATGAM induction in adult renal transplantation. Transplantation. 2004;78(1):136–141. doi: 10.1097/01.tp.0000132329.67611.3f. [DOI] [PubMed] [Google Scholar]
- 170.Goggins WC, Pascual MA, Powelson JA, Magee C, Tolkoff-Rubin N, Farrell ML, et al. A prospective, randomized, clinical trial of intraoperative versus postoperative Thymoglobulin in adult cadaveric renal transplant recipients. Transplantation. 2003;76(5):798–802. doi: 10.1097/01.TP.0000081042.67285.91. [DOI] [PubMed] [Google Scholar]
- 171.Lebranchu Y, Bridoux F, Buchler M, Le Meur Y, Etienne I, Toupance O, et al. Immunoprophylaxis with basiliximab compared with antithymocyte globulin in renal transplant patients receiving MMF-containing triple therapy. Am J Transplant. 2002;2(1):48–56. doi: 10.1034/j.1600-6143.2002.020109.x. [DOI] [PubMed] [Google Scholar]
- 172.Brennan DC, Flavin K, Lowell JA, Howard TK, Shenoy S, Burgess S, et al. A randomized, double-blindedcomparison of Thymoglobulin versus Atgam for induction immunosuppressive therapy in adult renal transplant recipients. Transplantation. 1999;67(7):1011–1018. doi: 10.1097/00007890-199904150-00013. [DOI] [PubMed] [Google Scholar]
- 173.Malinow L, Walker J, Klassen D, Oldach D, Schweitzer E, Bartlett ST, et al. Antilymphocyte induction immunosuppression in the post-Minnesota anti-lymphocyte globulin era: incidence of renal dysfunction and delayed graft function. A single center experience. Clin Transplant. 1996;10(3):237–242. [PubMed] [Google Scholar]
- 174.Mourad G, Garrigue V, Squifflet JP, Besse T, Berthoux F, Alamartine E, et al. Induction versus noninduction in renal transplant recipients with tacrolimus-based immunosuppression. Transplantation. 2001;72(6):1050–1055. doi: 10.1097/00007890-200109270-00012. [DOI] [PubMed] [Google Scholar]
- 175.Vincenti F, Kirkman R, Light S, Bumgardner G, Pescovitz M, Halloran P, et al. Interleukin-2-receptor blockade with daclizumab to prevent acute rejection in renal transplantation. Daclizumab Triple Therapy Study Group. N Engl J Med. 1998;338(3):161–165. doi: 10.1056/NEJM199801153380304. [DOI] [PubMed] [Google Scholar]
- 176.Bumgardner GL, Hardie I, Johnson RW, Lin A, Nashan B, Pescovitz MD, et al. Results of 3-year phase III clinical trials with daclizumab prophylaxis for prevention of acute rejection after renal transplantation. Transplantation. 2001;72(5):839–845. doi: 10.1097/00007890-200109150-00017. [DOI] [PubMed] [Google Scholar]
- 177.Lacha J, Simova M, Noskova L, Teplan V, Vitko S. Zenapax versus OKT-3 prophylaxis in immunologically high-risk kidney transplant recipients. Transplant Proc. 2001;33(3):2273–2274. doi: 10.1016/s0041-1345(01)01988-1. [DOI] [PubMed] [Google Scholar]
- 178.Flechner SM, Goldfarb DA, Fairchild R, Modlin CS, Fisher R, Mastroianni B, et al. A randomized prospective trial of low-dose OKT3 induction therapy to prevent rejection and minimize side effects in recipients of kidney transplants. Transplantation. 2000;69(11):2374–2381. doi: 10.1097/00007890-200006150-00027. [DOI] [PubMed] [Google Scholar]
- 179.Cantarovich D, Le Mauff B, Hourmant M, Giral M, Denis M, Jacques Y, et al. Anti-IL2 receptor monoclonal antibody (33B3. 1) in prophylaxis of earlykidney rejection in humans: a randomized trial versus rabbit antithymocyte globulin. Transplant Proc. 1989;21(1 Pt 2):1769–1771. [PubMed] [Google Scholar]
- 180.Sollinger H, Kaplan B, Pescovitz MD, Philosophe B, Roza A, Brayman K, et al. Basiliximab versus antithymocyte globulin for prevention of acute renal allograft rejection. Transplantation. 2001;72(12):1915–1919. doi: 10.1097/00007890-200112270-00008. [DOI] [PubMed] [Google Scholar]
- 181.Liu Y, Zhou P, Han M, Xue CB, Hu XP, Li C. Basiliximab or antithymocyte globulin for induction therapy in kidney transplantation: a meta-analysis. Transplant Proc. 2010;42(5):1667–1670. doi: 10.1016/j.transproceed.2010.02.088. [DOI] [PubMed] [Google Scholar]
- 182.Brennan DC, Daller JA, Lake KD, Cibrik D, Del Castillo D. Rabbit antithymocyte globulin versus basiliximab in renal transplantation. N Engl J Med. 2006;355(19):1967–1977. doi: 10.1056/NEJMoa060068. [DOI] [PubMed] [Google Scholar]
- 183.Noel C, Abramowicz D, Durand D, Mourad G, Lang P, Kessler M, et al. Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol. 2009;20(6):1385–1392. doi: 10.1681/ASN.2008101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Andres A, Budde K, Clavien PA, Becker T, Kessler M, Pisarski P, et al. A randomized trial comparing renalfunction in older kidney transplant patients following delayed versus immediate tacrolimus administration. Transplantation. 2009;88(9):1101–1108. doi: 10.1097/TP.0b013e3181ba06ee. [DOI] [PubMed] [Google Scholar]
- 185.Novick AC, Hwei HH, Steinmuller D, Streem SB, Cunningham RJ, Steinhilber D, et al. Detrimental effect of cyclosporine on initial function of cadaver renal allografts following extended preservation. Results of a randomized prospective study. Transplantation. 1986;42(2):154–158. doi: 10.1097/00007890-198608000-00010. [DOI] [PubMed] [Google Scholar]
- 186.Kuypers DR, de Jonge H, Naesens M, Vanrenterghem Y. A prospective, open-label, observational clinical cohort study of the association between delayed renal allograft function, tacrolimus exposure, and CYP3A5 genotype in adult recipients. Clin Ther. 2010;32(12):2012–2023. doi: 10.1016/j.clinthera.2010.11.010. [DOI] [PubMed] [Google Scholar]
- 187.Borobia AM, Romero I, Jimenez C, Gil F, Ramirez E, De Gracia R, et al. Trough tacrolimus concentrations in the first week after kidney transplantation are related to acute rejection. Ther Drug Monit. 2009;31(4):436–442. doi: 10.1097/FTD.0b013e3181a8f02a. [DOI] [PubMed] [Google Scholar]
- 188.Kamar N, Garrigue V, Karras A, Mourad G, Lefrancois N, Charpentier B, et al. Impact of early or delayed cyclosporine on delayed graft function in renal transplant recipients: a randomized, multicenter study. Am J Transplant. 2006;6(5 Pt 1):1042–1048. doi: 10.1111/j.1600-6143.2006.01291.x. [DOI] [PubMed] [Google Scholar]
- 189.Van Loo AA, Vanholder RC, Bernaert PR, Vermassen FE, Van der Vennet M, Lameire NH. Pretransplantation hemodialysis strategy influences early renal graft function. J Am Soc Nephrol. 1998;9(3):473–481. doi: 10.1681/ASN.V93473. [DOI] [PubMed] [Google Scholar]
- 190.Van Biesen W, Veys N, Vanholder R, Lameire N. The impact of the pre-transplant renal replacement modality on outcome after cadaveric kidney transplantation: the ghent experience. Contrib Nephrol. 2006;150:254–258. doi: 10.1159/000093613. [DOI] [PubMed] [Google Scholar]
- 191.Kikic Z, Lorenz M, Sunder-Plassmann G, Schillinger M, Regele H, Gyori G, et al. Effect of hemodialysis before transplant surgery on renal allograft function--a pair of randomized controlled trials. Transplantation. 2009;88(12):1377–1385. doi: 10.1097/TP.0b013e3181bc03ab. [DOI] [PubMed] [Google Scholar]
- 192.Shoskes DA, Halloran PF. Delayed graft function in renal transplantation: etiology, management and long-term significance. J Urol. 1996;155(6):1831–1840. doi: 10.1016/s0022-5347(01)66023-3. [DOI] [PubMed] [Google Scholar]
- 193.Humar A, Johnson EM, Payne WD, Wrenshall L, Sutherland DE, Najarian JS, et al. Effect of initial slow graft function on renal allograft rejection and survival. Clin Transplant. 1997;11(6):623–627. [PubMed] [Google Scholar]
- 194.Verghese E, Ricardo SD, Weidenfeld R, Zhuang J, Hill PA, Langham RG, et al. Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol. 2009;20(10):2147–2153. doi: 10.1681/ASN.2008101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Faure JP, Baumert H, Han Z, Goujon JM, Favreau F, Dutheil D, et al. Evidence for a protective role of trimetazidine during cold ischemia: targeting inflammation and nephron mass. Biochem Pharmacol. 2003;66(11):2241–2250. doi: 10.1016/j.bcp.2003.07.011. [DOI] [PubMed] [Google Scholar]
- 196.He W, Dai C, Li Y, Zeng G, Monga SP, Liu Y. Wnt/beta-catenin signaling promotes renal interstitial fibrosis. J Am Soc Nephrol. 2009;20(4):765–776. doi: 10.1681/ASN.2008060566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.von Toerne C, Schmidt C, Adams J, Kiss E, Bedke J, Porubsky S, et al. Wnt pathway regulation in chronic renal allograft damage. Am J Transplant. 2009;9(10):2223–2239. doi: 10.1111/j.1600-6143.2009.02762.x. [DOI] [PubMed] [Google Scholar]
- 198.ClinicalTrials.gov, a service of the US National Institutes of Health. Available from: http://clinicaltrials.gov/ct2/results?term=delayed+graft+function.