Abstract
The regulation of muscarinic acetyicholine receptor (MAChR) subtypes in rat striatum, bladder and heart was examined following a 14-day administration of neuroleptics (clozapine or fluphenazine), anticholinergics (atropine) or a combination of anticholinergics and neuroleptics. Levels of MAChRs were ascertained by the use of immunoprecipitation and radioligand binding. The combined treatment of fluphenazine and atropine produced an increase in all MAChR subtype levels in striatum with m1 receptor levels having the largest increase (270%) from control. A significant increase (105%) was also seen striatal in m2 receptor levels. Residual muscarinic receptor levels, representing the m3 and m4 subtypes, were increased (72%) to a lesser degree above control. Fluphenazine treatment alone increased levels of the m2 MAChR, whereas clozapine administration had no significant effect on levels of any MAChR subtype in this tissue. Administration of the cholinergic antagonist, atropine, showed a significant increase (89%) in the striatal m1 MAChR subtype. Of the MAChRs found in rat bladder and rat heart, the m2 subtype has been shown to be the most abundant. Results from the rat bladders indicated a reduction (50%) in muscarinic antagonist binding that was limited to the fluphenazine treatment group. In heart, atropine treatment alone produce a slight increase (ca. 10%) in receptor binding. No significant effect on muscarinic receptor levels was seen with the other treatment groups. These data demonstrate that there are differences in muscarinic receptor level modulation between central and peripheral tissues.
Psychosis is a general term denoting any mental disorder in which an individual’s capability to distinguish reality and communicate with others is impaired. These impairments are usually serious enough to disrupt the everyday lives of these individuals. Since the 1950s pharmacological agents known as neuroleptics have been used to treat psychotic patients. The action and success of neuroleptics in the treatment of psychosis is thought to be related, in part, to their ability to act as dopamine receptor antagonists in the mesocortical and mesolimbic areas of the brain (Davis and Mostert, 1986; Miklos et al., 1987). Blockade of dopamine binding sites in the basal ganglia via the prolonged use of typical neuroleptics may result in severe EPS (Matthyse, 1973; Snyder et al., 1974a; Meltzer and Stahl, 1976). These side effects may be manifested as acute and subacute movement disorders as well as the most severe side effect: tardive dyskinesia (Jeste and Wyatt, 1982; Jenner and Marsden, 1983; Levinson, 1991). Side effects associated with neuroleptic treatment may be reduced by co-administration of neuroleptics with anticholinergic agents (Baldessarini, 1985). Some atypical neuroleptics, which have a high potency to inhibit muscarinic receptor activity in binding studies, have been described (Snyder et al., 1974b). These neuroleptics, such as clozapine and thioridazine, tend to produce much less severe EPS than typical neuroleptics (Miller and Hiley, 1974; Snyder et al., 1974a; Baldessarini and Tarsy, 1979). Previous studies have been performed in which effects of chronic typical and atypical neuroleptic treatment on brain dopamine and MAChR levels were compared (Boyson et al., 1988). These data showed that changes in MAChR levels in all treatment groups were quite small. Since those experiments were performed, at least five subtypes of the MAChR have been shown to exist in brain (Bonner et al., 1988; Bonner, 1989). It therefore becomes possible that the rather small changes in receptor binding found in the previous study was due to an inability to measure subtype levels. In this case, a large increase in levels of one subtype may have been overlooked due to no increase or even a decrease in the levels of another subtype. Several groups have since developed antibodies specific for subtypes of the MAChR, that may be used to address these possibilities (Luetje et al., 1987; Luthin et al., 1988; Levey et al., 1991).
We studied the levels of the m1 and m2 subtypes of MAChR in rat corpus striatum after treatment with typical and atypical neuroleptics or anticholinergic drugs. We also assessed the effect of the different drug treatments on two peripheral organs which are quite rich in MAChRS, the heart and the bladder. These organs were not only chosen because they are dense in MAChRS, but also for the fact that the major (~90%) MAChR subtype in both organs is of the m2 subtype (Subers et al., 1988; Wall et al., 1991; Li et al., 1991; P. Wang and co-workers, unpublished data, 1993). These studies were designed to compare the specific receptor responses across the different tissues studied.
Methods
Materials
Tris, cholate, EDTA, anti-mouse immunoglobulin G1 agarose beads, atropine sulfate and cholic acid (sodium salt) were obtained from Sigma Chemical Co. (St Louis, MO). Male, Sprague-Dawley rats (200–250 g) were purchased from Charles River. QNB was from New England Nuclear (Boston, MA). Pansorbin was purchased from Cal-Biochem (La Jolla, CA). The anti-m2 antibody, 31-1D1 was obtained as a gift from Dr. Neil M. Nathanson. An anti-MAChR antibody (m1 subtype) was generated as previously described (Luthin et al., 1988).
Neuroleptic/anticholinergic drug treatment of animals
Animals (male, Sprague-Dawley rats) were treated as described elsewhere (Boyson et al., 1988). Animals were housed three per cage in a climate-controlled facility with 12-hr light/dark cycles and were fed ad libitum. Animals were acclimatized to cages for 3 days and then were treated as follows: clozapine (20 mg/kg i.p.) (Chido and Bunny, 1983); atrophine sulfate (20 mg/kg i.p.) (Westlind et al., 1981); fluphenazine (in corn oil) (12.5 mg/kg s.c.) between the scapulas (Boyson et al., 1984); fluphenazine/atropine co-administration (see above) or saline (0.5 ml i.p.). All injections were daily for 14 days, except for fluphenazine which was injected on day 1 only. After day 1, fluphenazine-treated animals received distilled water, 0.5 ml i.p., daily thereafter. Weights were determined on odd days starting with day 1 and drug dosages were adjusted accordingly.
On day 15, animals were anesthetized with Metofane and decapitated. The brains (dissected to yield striatum), hearts and bladders were removed and all tissues were immediately frozen on dry ice.
Membrane preparation
Membrane preparation methods have been described previously (Luthin et al., 1988). All procedures were performed at 4°C unless otherwise noted. Striate were thawed and homogenized in 10mM Tris/1 mM EDTA, pH 7.5, using a BIOSPEC homogenizer (setting high, 10 sec). Hearts and bladders were first minced, then homogenized using a homogenizer (heart: setting high, 10 sec; bladder setting high, 15 sec.). Tissues were centrifuged at 20,000 × g for 15 min. Pellets were resuspended in the Tris/EDTA buffer and used immediately or stored at −80°C until use. Typical tissue weights were as follows: heart, 1 g; bladder, 100 to 130 mg; corpus striatum (halved), 60 to 85 mg. Membrane preparation volumes were as follows: 1 heart in 10 ml, 1 bladder in 1 ml, 1 hemisphere of corpus striatum in 0.5 ml.
Receptor solubilization
Receptor solubilization methods have been described elsewhere (Luthin et al., 1988) and were used in a modified form here. Membrane preparations were used fresh or thawed. Membranes were centrifuged at 20,000 × g for 15 min and resuspended in 10 mM Tris/1 mM EDTA, 0.1% digitonin, 0.02% cholate. Samples were centrifuged at 20,000 × g for 25 min. Pellets were resuspended in 10mM Tris/1 mM EDTA, 1.0% digitonin, 0.2% cholate and incubated at 4°C for 50 mm. The homogenates were then centrifuged at 100,000 × g for 40 mm. The supernatants (solubilized receptor) thus obtained were used immediately in immunoprecipitation assays. It has been determined that 40% of the striatal receptors present in the membranes can be recovered after solubilization as described above.
Immunoprecipitation
Immunoprecipitation assays have been described elsewhere (Luthin et al., 1988). Solubilized receptors were incubated with [3H]QNB (2.5 nM) and MAChR antibody (m1, 0.3 mg/ml or m2, 1:3000 dilution) for 30 min at 25°C. Incubations were continued overnight at 4°C. Samples volumes were brought to 0.5 ml using 10 mM Tris (pH 7.5), 1 mM EDTA, 0.1% digitonin, 0.02% cholate and 0.9% NaCl and then were desalted over 3-ml columns of Sephadex G-50 to a final volume of 1 ml. Anti-m1 MAChR antibodies were precipitated with Pansorbin. Anti-m2 MAChR antibodies were precipitated with 100 µl of a 1:1 slurry of anti-mouse agarose beads. Samples containing m1 antisera were incubated for 45 min at 4°C whereas samples containing the m2 antibody (31-1D1) were incubated for 2 hr at 4°C. Precipitates were collected by microcentrifugation. Pellets were washed with 0.5 ml of 10 mM Tris (pH 7.5), 1 mM EDTA, 0.1% digitonin, 0.02% cholate and 0.9% NaCl, then were suspended in 50 µl of 3% NaOH/3% deoxycholate. Samples were transferred to counting vials after the addition of 0.5 ml of H2O. It should be noted that residual muscarinic binding is defined as: (total muscarinic receptor) – (m1 subtype + m2 subtype).
Radioligand binding
[3H]QNB binding to membrane fractions was performed using conditions as described previously (Luthin and Wolfe, 1984). Six concentrations of [3H]QNB ranging from 10 to 500 pM were used to determine Kd and Bmax values. Various concentrations of [3H]QNB and 50 to 100 µg of protein were incubated in triplicate in a final volume of 1 ml of buffer containing 10 mM Tris and 1 mM EDTA. Atrophic (1 µM) was used to define nonspecific binding at each radioligand concentration. Assays were terminated after 1 hr of incubation at 32°C with 3 ml of an ice-cold buffer containing 10 mM Tris/1 mM EDTA/150 mM NaCl, filtered via vacuum filtration over glass fiber filters (#30, Schleicher & Schuell, Keene, New Hampshire) and washed twice with 3 ml of dilution buffer. Saturation isotherms were transformed using the method of Hanes (Segel, 1975, 1976). Kd, and Bmax values were estimated using unweighted linear regression analysis of the transformed data.
Protein analysis
Protein values were determined on solubilized (striatum) or membrane (heart, bladder) fractions using the method of Lowry et al. (1951).
Results
Animals were treated as described under “Methods.” Animal body weights were recorded on odd days and did not differ significantly among experimental groups throughout the treatment regimen. Likewise, wet weights and protein content of dissected tissue did not differ significantly among treatment groups.
Corpus striatum
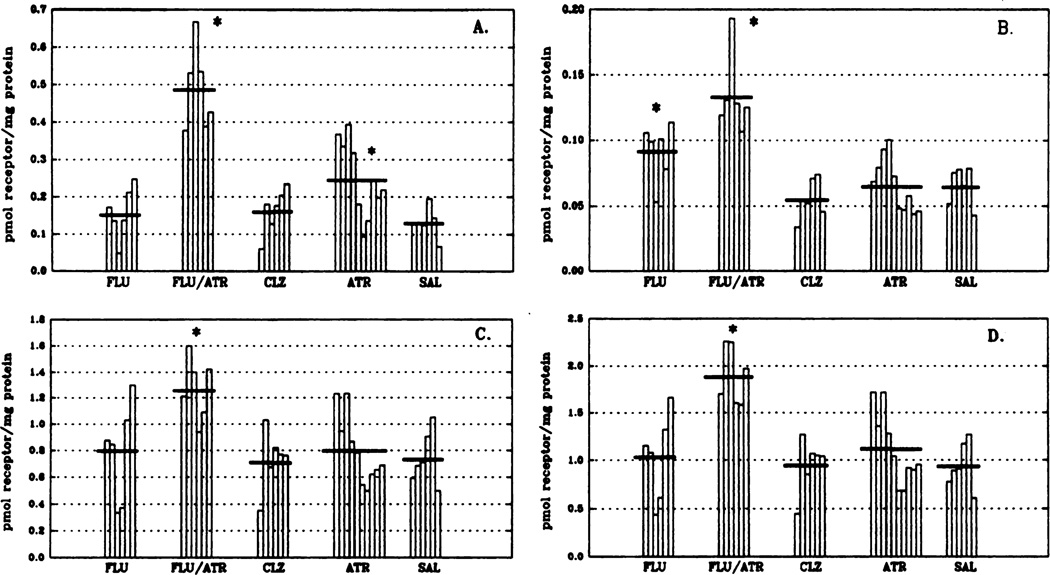
Immunoprecipitation of MAChR subtypes was used to determine levels of m1 and m2 receptor subtypes after the 14-day drug regimen (fig. 1). The combined treatment of fluphenazine and atropine produced a significant increase (102% above control values; P < .05) in total muscarinic receptor binding to receptors solubilized from rat corpus striatum (fig. 1, panel D). This is attributed to an increase in all MAChR subtype levels with m1 receptor levels (fig. 1, panel A) having, by far, the largest increase (270%; P < .05) from control. The m2 receptor levels (fig. 1, panel B) doubled after the combined fluphenazine/atropine treatment (105% above control values; P < .05). Residual muscarinic receptor levels (fig. 1, panel C), representing ms and m4 subtypes (Levey et al., 1991) were elevated 72% (P < .05) over control levels in the fluphenazine/atropine treatment group. Fluphenazine treatment alone significantly elevated m2 (fig. 1, panel B) receptor levels (65 ± 5–92 ± 9 fmol receptor/mg of protein; P < .05), whereas clozapine administration had no significant effect on the levels of any MAChR subtype in corpus striatum. Administration of the cholinergic antagonist, atropine, resulted in an increase of <20% in total MAChRs. This is due primarily to a significant elevation (89%; P > .05) in the m1 (fig. 1, panel A) receptor subtype. Overall, chronic typical (fluphenazine) neuroleptic treatment increased M2 receptor levels, chronic atypical (clozapine) neuroleptic treatment had no effect on receptor subtypes, whereas fluphenazine and atropine given in combination increased all receptor subtypes in striatum.
Fig. 1.
Levels of specific MAChR subtypes in rat corpus striatum after chronic drug treatment as determined by immunoprecipitation. Data are expressed as picomoles of receptor/milligram of protein and have been replotted so that each vertical bar represents an individual animal within the respective treatment groups. Horizontal bars indicate the mean for each group. Under these conditions, the m1 antibody is 95% efficient in precipitating m1 receptors from cells expressing the cloned m1 cDNA (Mei et al., 1989) whereas the m2 antibody is approximately 75% efficient in precipitating m2 receptors from heart. m2 values herein have been corrected to reflect 75% efficiency. It is thought that the residual binding levels are indicative of the m3 and m4 MAChR subtypes (Levey et al., 1991). Total muscarinic binding is defined by using the nonselective antagonist [3H] QNB in the presence and absence of atropine FLU, n = 6; FLU/ATR, n = 6; CLZ, n = 6; ATR, n = 10; SAL, n = 6. Analysis of variance was used to identify initially statistical differences between groups. Significance of differences between means of control and experimental groups and significance of difference among experimental groups was confirmed by the Least Significant Difference using SPSS-PC for the personal computer. *P < .05 (denotes statistical difference from all other treatment groups within each receptor subtype). Abbreviations used are: FLU, fluphenazine; ATR, atropine; CLZ, clozapine; SAL, saline. Panel A, m1 receptors; panel B, m2 receptors; panel C, residual muscarinic binding; panel D, total muscarinic receptors.
Peripheral tissues (heart/bladder)
Because it has been shown that approximately 90% of the MAChRs in rat heart and bladder are the m2 subtype, radioligand binding studies were performed on the tissues in question to generate receptor levels (N.B.: animals were the same as those used in the brain studies). Receptor levels were estimated by the use of saturation isotherms and Hanes-Woolf plots. Representative graphs for control groups are shown in figures 2 and 3 (heart) and figures 4 and 5 (bladder). Data obtained from bladder revealed that a reduction from control levels was seen in the fluphenazine treatment group (51% reduction; P < .05) (table 1). Immunoprecipitation of the m2 MAChR receptor subtype with the monoclonal antibody 31-1D1 was performed in both bladder and heart to confirm the results from the binding studies (data not shown). Binding experiments in rat heart demonstrated that no statistically significant effect on MAChR binding levels was seen after any of the treatment paradigms.
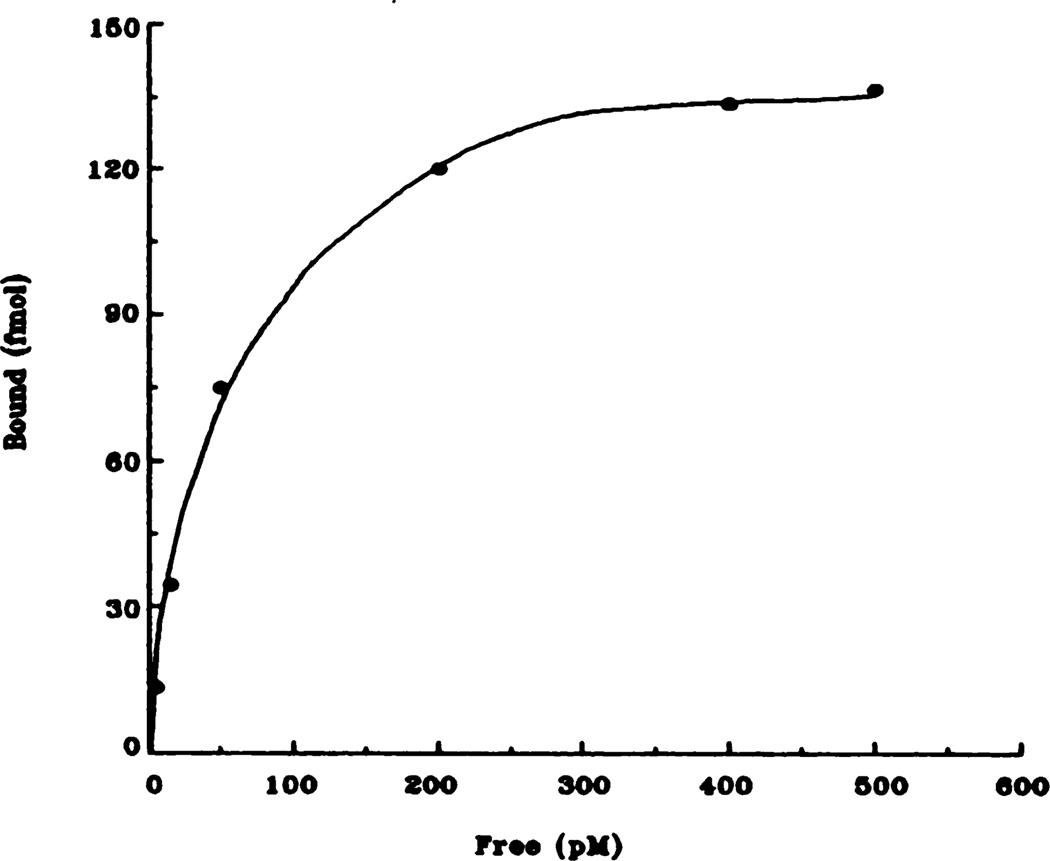
Fig. 2.
Representative saturation isotherm of specific [3H]QNB binding to rat heart membranes. Data shown here are taken from the control (saline) animals and are the result of at least five independent experiments.
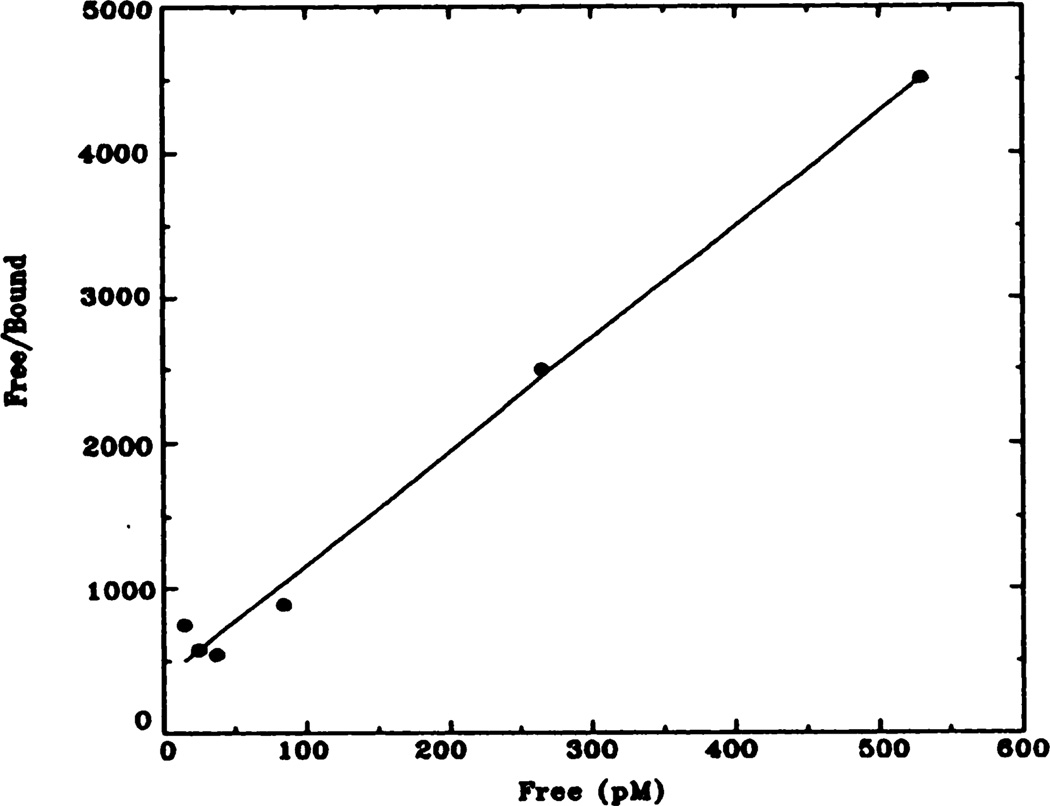
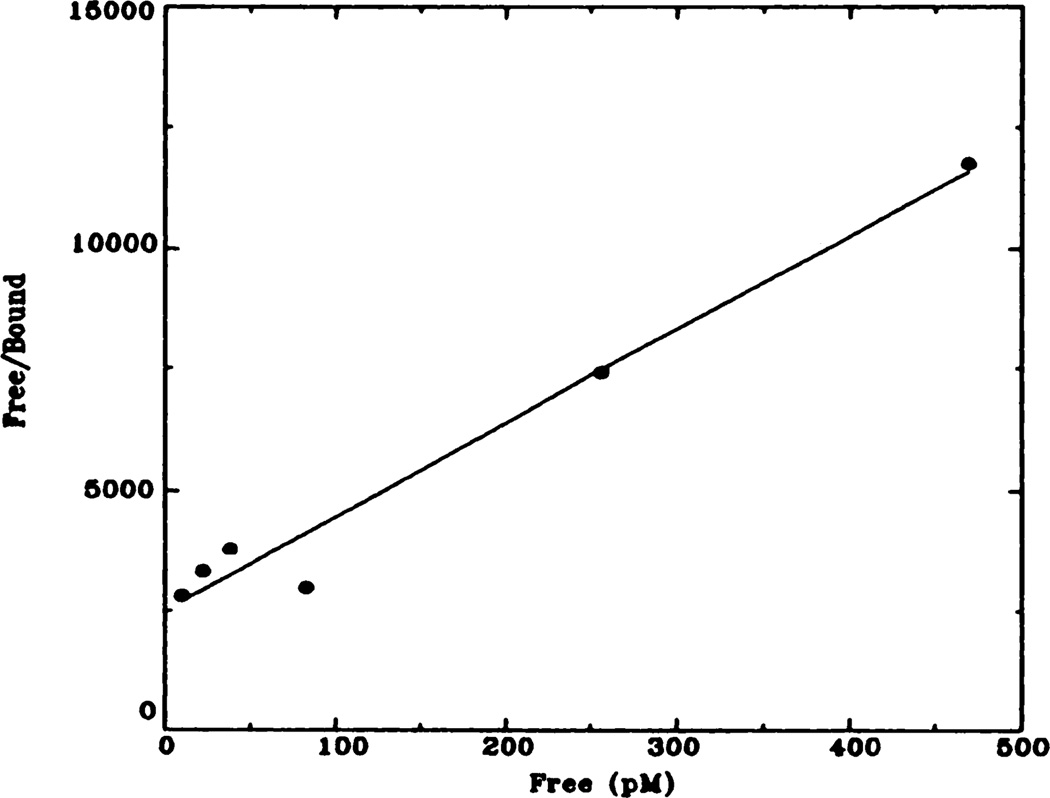
Fig. 3.
Representative Hanes-Woolf plot of [3H]QNB binding to rat heart membranes. Data shown here have been transformed from data shown in figure 2 (control animals). Kd = 72 pM; Bmax = 137 fmol.
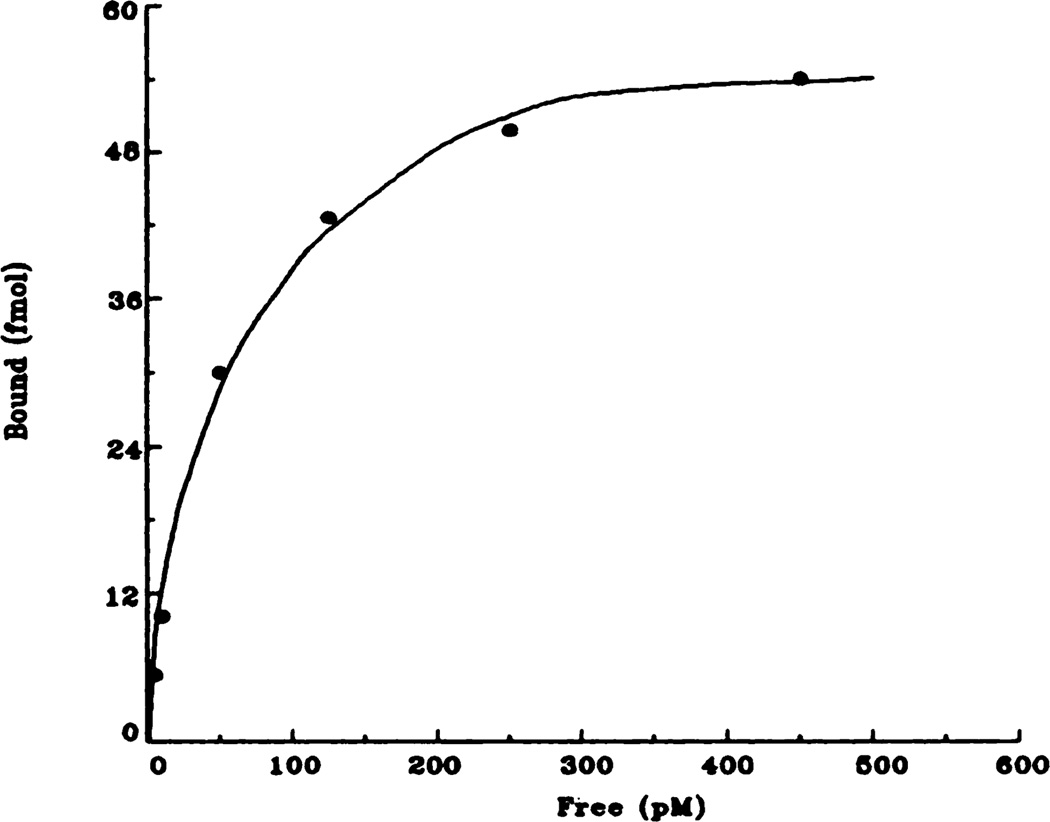
Fig. 4.
Representative saturation isotherm of specific [3H]QNB binding to rat bladder membranes. Data shown here are taken from the control (saline) animals and are the result of at least five independent experiments.
Fig. 5.
Representative Hanes-Woolf plot of [3H]QNB binding to rat bladder membranes. Data shown here have been transformed from data shown in figure 4 (control animals). Kd = 136 pM; Bmax = 53 fmol.
TABLE 1. Comparison of Bmax and apparent Kd and in rat bladder membranes and rat heart membranes after chronic drug treatment.
Comparison of Kd and Bmax in rat bladder membranes and rat heart membranes after chronic drug treatment. Data are expressed in terms of Bmax (fmol/mg of protein) or Kd (pM) for each drug treatment group and are the average of five independent experiments ± S.E.M. Hill analysis carried out in these tissues revealed slopes ranges from .94 though 1.00, thus denoting a single binding site.
| Treatment Group | Bmax (bladder) | Bmax (heart) | Kd (bladder) | Kd (heart) |
|---|---|---|---|---|
| fmol/mg protein | pM | |||
| Saline | 432 (±25) | 121 (±12) | 146 (±20) | 57 (±21) |
| Fluphenazine | 210 (±16)* | 105 (±16) | 165 (±15) | 52 (±10) |
| Fluphenazine/Atropine | 407 (±26) | 164 (±34) | 171 (±10) | 56 (±12) |
| Clozapine | 404 (±12) | 158 (±34) | 188 (±12) | 56 (±11) |
| Atropine | 385 (±6) | 145 (±5) | 151 (±24) | 64 (±7) |
Denotes a statistically significant difference from all other treatment groups at P < .05.
Discussion
Motor control has been shown to be an important function of the corpus striatum (Divac et al., 1979; Delong and Georgopoulos, 1982). Movement disorders caused by such disease states as Parkinsonism, Huntington’s disease and tardive dyskinesia have been linked to the dopamine system within the basal ganglia (Hornykiewicz, 1966; Caine and Sandler, 1970; Reisine et al., 1979; Ansell, 1981; Marsden, 1982). It should also be noted that chronic treatment with typical neuroleptics (fluphenazine) may cause severe side effects that may be manifested as EPS, Parkinson-like disorders or even tardive dyskinesia (Baldessarini, 1985). These drug-induced disorders have therefore been linked to dopamine receptor blockade within the basal ganglia (Snyder et al., 1974a; Matthyse, 1973; Meltzer and Stahl, 1976). Much is now known about the striatal dopamine system and the effects of chronic neuroleptic treatment on this system. In contrast, less is known about the cholinergic interneuron system within the striatum, and these interneurons have been shown to be under an inhibitory influence from nigrostriatal dopaminergic projections (Lehmann and Langer, 1983). Within the striatum, the m1 MAChR subtype has been localized to cell bodies and neurites (Levey et al., 1991). Because of this it is thought to be a major postsynaptic muscarinic receptor in striatum. There is some evidence to suggest that m1 MAChRs may regulate dopamine release via presynaptic receptors. On the other hand, the m2 MAChR has a localization similar to that of an autoreceptor (Levey et al., 1991) and may regulate the release of ACh. Levey et al. (1991) and others (Raiteri et al., 1990) have provided evidence that the m2 MAChR may presynaptically modulate the release of other neurotransmitters as well as ACh. It should be noted that to date, no comprehensive and unambiguous cellular localization of both receptor proteins and receptor mRNA has been per formed in a single species, including rat. Similarly, colocalization of receptor markers with cellular markers for dopaminergic and cholinergic neurons has not been rigorously analyzed. The following interpretations in the current study thus await these clarifications, but are based on the evidence available at this time.
In our experiments a <20% increase in total MAChR binding within the atropine treatment group was attributable to a significant increase in levels of the m1 receptor subtype. Others (Raiteri et al., 1990) have shown that chronic treatment with scopolamine produces an increase in MAChRs that mediate dopamine release in striatum. This receptor subtype is believed to correspond to the m1 subtype of receptor, as activity is inhibited potently by the drug pirenzepine (Raiteri et al., 1990). Levels of high-affinity pirenzepine binding were shown to decrease after 6-hydroxydopamine lesioning in striatum (Joyce, 1991a,b), in spite of the fact that levels of m1 MAChR mRNA were not detectable in rat substantia nigra, whereas m5 MAChR mRNA was found in this brain region (Weiner et al., 1990). The m5 MAChR has low affinity for pirenzepine (Liao et al., 1989), so the physiological and anatomical studies cited do not reconcile with each other. Our experiments support the contention that chronic antagonist treatment increases levels of the m1 receptor subtype, and these may reside on dopamine-containing neurons. However, as discussed below, they could as well be present on neurons postsynaptic to intrinsic cholinergic neurons.
Fluphenazine treatment alone resulted in an increase in striatal MAChR levels that was restricted to the m2 subtype of receptor. Based on pharmacological and immunohistochemical evidence, these receptors are likely to be localized on cholinergic neurons in the striatum, and regulate ACh release from these neurons (Joyce, 1991a,b). If the m2 receptors represent autoreceptors that modulate ACh release, one might predict an increase in receptor levels after removal of dopamine inhibition by fluphenazine. Because the m2 receptor is a very low-abundance receptor in striatum, any previous studies (e.g., Boyson et al., 1988) may not have seen alterations in receptor levels after fluphenazine treatments. In fact prior studies using somewhat different drug delivery paradigms have shown decreases in total muscarinic receptor levels after neuroleptic administration (Friedman et al., 1983). However, there were no decreases seen in the levels of any striatal MAChR subtype in this study, and we cannot, therefore fully account for this.
To confirm that the effects seen with fluphenazine were attributable to a neuronal connectivity unique to the striatum, and not due to a direct interaction with cholinergic receptors, the m2 receptor levels were also measured in two peripheral tissues expressing high levels and essentially homogeneous (~90%) populations of this receptor subtype. Even so, each responded differently from striatum to fluphenazine, and in fact differently from each other. The effect seen in rat bladder was not a direct effect of fluphenazine on bladder D2 receptors, as we could find no detectable D2 receptors in rat bladder (G. Luthin, unpublished). The effect of fluphenazine could have arisen from a central locus, such as the brainstem micturition center. Some of the urinary side effects seen with fluphenazine treatment, such as incontinence, may therefore be partially explained by its effects on bladder cholinergic receptors. If centrally mediated, the mechanism cannot be the same as that associated with regulation of central cardiac centers. In any event, the central effect seen with fluphenazine must therefore be explained by the connectivity of the striatum itself.
The combined treatment with fluphenazine plus atropine produced an increase in the levels of m1, m2 and combined m3 plus m4 receptors in striatum. It is likely that the latter increase reflects an increase in m4 receptor levels, as several groups have shown extremely low levels of the m3 subtype in any brain region, including striatum (Wall et al., 1991). Conversely, the m4 receptor is expressed at quite high levels in striatum (Levey et al., 1991). Our previous studies (Boyson et al., 1988) demonstrated that this treatment produced an increase in D2 dopamine receptor levels in striatum, in addition to an increase in muscarinic receptor levels. The increase reported in that study was smaller to that shown in the current study, and presumably reflects the differences in the age of the rats and the tissue preparation between that study and this one. In any case, the increases observed in this study were large (overall, an increase of ca. 100%), and must invoke a complex interaction between the cholinergic and dopaminergic systems. If chronic blockade of the presynaptic (m1) receptors that increase dopamine release, are combined with a blockade of the presynaptic receptors that inhibit ACh release and blockade of postsynaptic ACh and dopamine D2 receptors, a variety of scenarios becomes possible. These combined effects may be sufficient to produce reflexive upregulation of receptors from different pre- or post-synaptic sites, that were insufficiently provoked by atropine blockade alone. These in turn may arise from intrinsic or extrinsic terminals, and may compensate for observed effects of fluphenazine on the presynaptic, m2 receptor levels.
In regarding the outcomes of the above experiments, and given the fact that the atypical neuroleptic clozapine did not produce changes in MAChR levels, we come to several conclusions. The EPS associated with chronic neuroleptic treatment may be the product of striatal dysfunction, and this could relate to the absolute or relative levels of the relevant dopaminergic and cholinergic receptors. However, fluphenazine, but not clozapine, increases levels of striatal D2 dopamine receptors. Fluphenazine also increases levels of a single MAChR subtype, that may be presynaptic on cholinergic neurons. Fluphenazine, which produces EPS, therefore can disrupt both dopaminergic and cholinergic systems, whereas clozapine apparently cannot. Clozapine has been shown to have higher affinity for other subtypes of dopamine receptor, and its lack of activity in striatum is suggestive of activity in other brain regions. On the other hand, co-administration of neuroleptics with anticholinergics has been shown to present reduced EPS. We now show that in all relevant cases (saline-, clozapine- or fluphenazine plus atropine-treated animals) where EPS would be expected to be reduced, the ratio of striatal muscarinic receptors is maintained. In spite of this, it is clear that the mechanism of action of clozapine cannot be attributed to a combination of anticholinergic and antidopaminergic activity with a striatal locus.
In conclusion, the effects of a typical and an atypical neuroleptic were shown to be different in the striatal cholinergic system. Coadministration of an anticholinergic and neuroleptic produced changes that were not simple additive effects of the two drugs, and were not the same as those produced by clozapine. The effects of the atypical neuroleptic clozapine therefore probably do not reflect its anticholinergic properties on striatal tissue. The effects of fluphenazine alone were not attributable to a direct effect on cholinergic receptors, and are presumably the result of a feedback interaction between dopaminergic and cholinergic neurons. These results highlight the complexity of the striatal dopaminergic and cholinergic interactions, and add some insight into this already intricate association. Further more, they support the concept that subtypes of muscarinic receptor do not coregulate in a single direction, even in response to a nonselective muscarinic drug.
Acknowledgments
Supported by National Institutes of Health Grant NSAG-19069 and a Scottish Rite Schizophrenia Research Program Grant to G.R.L and by National Institutes of Health Grant RO1-DK43333 to G.R.L and M.R.R.
ABBREVIATIONS
- EPS
extrapyramidal slide effects
- MAChR
muscarinic acetylcholine receptor
- QNB
quinuclidinylbenzilate
- ACh
acetylcholine
References
- Ansell GB. The biochemical background to tardive dyskinesia. Neuropharmacology. 1981;20:311–317. doi: 10.1016/0028-3908(81)90001-0. [DOI] [PubMed] [Google Scholar]
- Baldessarini RU. Drugs and the treatment of psychiatric disorders. In: Gilman A, Goodman L, Rall T, Murad F, editors. Goodman and Gilman’s, The Pharmacological Basis of Therapeutics. 7th ed. New York: MacMillan; 1985. pp. 387–445. [Google Scholar]
- Baldessarini P, Tarsy D. Relationship of the actions of neuroleptic drugs to the pathophysiology of tardive dyskinesia. Int Rev. Neurobiology. 1979;21:1–45. doi: 10.1016/s0074-7742(08)60636-4. [DOI] [PubMed] [Google Scholar]
- Bonner TI. The molecular basis of muscarinic receptor diversity. Trends Neurosci. 1989;12:148–151. doi: 10.1016/0166-2236(89)90054-4. [DOI] [PubMed] [Google Scholar]
- Bonner TI, Young AC, Brann MR, Buckley NJ. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988;1:403–419. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Luthin GR, Wolfe BB, Molinoff PB. Effects of chronic neuroleptic and anticholinergic therapy on dopainine-2 and muscarinic cholinergic receptors in rat striatum. Soc. Neurosci. Abstr. 1984;10:538. [PubMed] [Google Scholar]
- Boyson SJ, McGonigle P, Luthin GR, Wolfe BB, Molinoff PB. Effects of chronic administration of neuroleptic and anticholinergic agents on densities of D2 dopamine and muscarinic cholinergic receptors in rat striatum. J. Pharmacol. Exp. Ther. 1988;244:987–993. [PubMed] [Google Scholar]
- Caine DB, Sandler M. l-Dopa and Parkinsonism. Nature. 1970;226:21–24. doi: 10.1038/226021a0. [DOI] [PubMed] [Google Scholar]
- Chido LA, Bunny BS. Typical and atypical neuroleptics: Differential effects of chronic administration on the activity of A9 and A10 midbrain dopaminergic neurons. J. Neurosci. 1983;3:1607–1619. doi: 10.1523/JNEUROSCI.03-08-01607.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis JM, Mostert MA. Biological treatment of schizophrenic disorders. In: Berger PA, Brodie HKH, editors. American Handbook of Psychiatry. vol. VIII. New York: Basic Books; 1986. pp. 466–512. [Google Scholar]
- Delong MR, Georgopoulos AP. Motor Functions of the basal ganglia. In: Brooks VB, editor. Handbook of Physiology. The Nervous System II. Washington, DC: American Physiological Society; 1982. pp. 1017–1061. [Google Scholar]
- Divac I, Gunilla R, Oberg E. The Neostriatum. Oxford: Pergamon Press; 1979. [Google Scholar]
- Friedman E, Giantutsos G, Kuster J. Chronic fluphenazine and clozapine elicit opposite changes in brain muscarinic receptor binding: Implications for understanding tardive dyskinesia. J. Pharmacol. Exp. Ther. 1983;226:7–12. [PubMed] [Google Scholar]
- Hornykiewicz O. Dopamine (3-hydroxytryamine) and brain function. Pharmacol. Rev. 1966;18:924–964. [PubMed] [Google Scholar]
- Jenner P, Marsden CD. Neuroleptics and tardive dyskinesia. In: Coyle JT, Enna SJ, editors. Neuroleptics: Neurochemical, Behavioral and Clinical Perspectives. New York: Raven Press; 1983. pp. 223–253. [Google Scholar]
- Jeste DV, Wyatt RJW. Therapeutic strategies against tardive dyskinesia: Two decades of experience. Arch. Gen. Psychiat. 1982;39:803–816. doi: 10.1001/archpsyc.1982.04290070037008. [DOI] [PubMed] [Google Scholar]
- Joyce JN. Differential response to striatal dopamine and muscarinic cholinergic receptor subtypes to the loss of dopamine. I. Effects of intranigral or intracerebroventricular 6-hydroxdopamine lesions of the mesostriatal dopamine system. Exp. Neurol. 1991a;113:261–276. doi: 10.1016/0014-4886(91)90016-6. [DOI] [PubMed] [Google Scholar]
- Joyce JN. Differential response to striatal dopamine and muscarinic cholinergic receptor subtypes to the loss of dopamine. II. Effects of 6-hydroxdopamine or colchicine micro-injections into the VTA or reserpine treatment. Exp. Neurol. 1991b;113:277–290. doi: 10.1016/0014-4886(91)90017-7. [DOI] [PubMed] [Google Scholar]
- Lehmann J, Langer SZ. The striatal cholinergic interneuron: Synaptic target and dopaminergic terminals? Neuroscience. 1983;10:1105–1120. doi: 10.1016/0306-4522(83)90102-1. [DOI] [PubMed] [Google Scholar]
- Levey AI, Kitt CA, Simonds WF, Price DL, Brann MR. Identification and localization of muscarinic acetylcholine receptor proteins in brain with subtype-specific antibodies. J. Neurosci. 1991;11:3218–3226. doi: 10.1523/JNEUROSCI.11-10-03218.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levinson DF. Pharmacologic treatment of schizophrenia. Clin. Ther. 1991;13:326–352. [PubMed] [Google Scholar]
- Li M, Yasuda RP, Wall SJ, Wellstein A, Wolfe BB. Distribution of m2 muscarinic receptors in rat brain using antisera selective for m2 receptors. Mol. Pharmacol. 1991;40:28–35. [PubMed] [Google Scholar]
- Liao CF, Themmen APN, Jobo R, Barberis C, Birnbaumer M, Birnbaumer L. Molecular Cloning and expression of a fifth muscarinic acetylcholine receptor. J. Biol. Chem. 1989;264:7328–7337. [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Luetje CW, Brumwell KC, Norman MG, Peterson GL, Schimerlik MI, Nathanson NM. Isolation and characterization of monoclonal antibodies specific for the cardiac muscarinic acetylcholine receptor. Biochemistry. 1987;26:6892–6896. doi: 10.1021/bi00396a003. [DOI] [PubMed] [Google Scholar]
- Luthin GR, Harkness J, Artymyshyn RP, Wolfe BB. Antibodies to a synthetic peptide can be used to distinguish between muscarinic acetylcholine receptor binding sites in brain and heart. Mol. Pharmacol. 1988;34:327–333. [PubMed] [Google Scholar]
- Luthin GR, Wolfe BB. Comparison of [3H] Pirenzepine and [3H] quinuclidinylbenzilate binding to muscarinic cholinergic receptors in rat brain. J. Pharmacol. Exp. Ther. 1984;228:648–655. [PubMed] [Google Scholar]
- Marsden CD. Neurotransmitters and CNS disease: Basal ganglia disease. Lancet. 1982;2:1141–1146. [Google Scholar]
- Matthyse S. Antipsychotic drug actions: A clue to the neuropathology of schizophrenia? Fed. Proc. 1973;32:200–205. [PubMed] [Google Scholar]
- Mei L, Lai J, Roeske WR, Fraser CM, Venter JC, Yamamura HI. Pharmacological characterization of the M1 muscarinic receptors expressed in murine fibroblast B82 cells. J. Pharmacol. Exp. Ther. 1989;248:661–670. [PubMed] [Google Scholar]
- Meltzer HY, Stahl SM. The dopamine hypothesis of schizophrenia: A review. Schizophr. Bull. 1976;2:19–76. doi: 10.1093/schbul/2.1.19. [DOI] [PubMed] [Google Scholar]
- Miklos F, Losonczy NMD, Davis K. The dopamine hypothesis of schizophrenia. In: Meltzer HY, editor. Psychopharmacology: The Third Generation of Progress. New York: Raven Press; 1987. pp. 715–726. [Google Scholar]
- Miller RJ, Hiley CR. Antimuscarinic properties of neuroleptics and drug-induced Parkinsonism. Nature (Lond.) 1974;248:596–597. doi: 10.1038/248596a0. [DOI] [PubMed] [Google Scholar]
- Raiteri M, Marchi P, Paudice P. Presynaptic muscarinic receptors in the central nervous system. Ann. N.Y. Acad. Sci. 1990;604:113–129. doi: 10.1111/j.1749-6632.1990.tb31987.x. [DOI] [PubMed] [Google Scholar]
- Reisine TD, Beaumont K, Bird ED, Spokes E, Yamamura HI. Huntington’s disease: Alterations in neurotransmitter receptor binding in the human brain. In: Chase TN, Wexler NS, Barbeau A, editors. Advances in Neurology. vol. 23. New York: Raven Press; 1979. pp. 717–726. [Google Scholar]
- Segal IH. Enzyme Kinetics, Behavior and Analysis of Rapid Equilibrium and Steady-State Systems. New York: John Wiley & Sons; 1975. [Google Scholar]
- Segal IH. Biochemical Calculations. 2nd ed. New York: John Wiley & Sons; 1976. [Google Scholar]
- Snyder SH, Banjeree SP, Yamamura HI, Greenberg D. Drugs, neurotransmitters and schizophrenia. Science. 1974a;184:1243–1263. doi: 10.1126/science.184.4143.1243. [DOI] [PubMed] [Google Scholar]
- Snyder SH, Greenberg D, Yamamura HI. Antischizophrenic drugs affinity for muscarinic cholinergic receptor sites in the brain predicts extrapyramidal effects. Arch. Gen. Psychiat. 1974b;31:58–61. doi: 10.1001/archpsyc.1974.01760130040006. [DOI] [PubMed] [Google Scholar]
- Subers EM, Liles WC, Luetje CW, Nathanson NM. Biochemical and immunological studies on the regulation of cardia and neuronal muacarinic acetylcholine receptor number and function. Trends Pharmacol. Sci. 1988 Suppl:25–28. [PubMed] [Google Scholar]
- Wall SJ, Yasuda RP, Li M, Wolfe BB. Development of an antisera against m3 muscarinic receptor Distribution of m3 receptors in rat tissues and clonal cell lines. Mol. Pharmacol. 1991;40:783–789. [PubMed] [Google Scholar]
- Weiner DM, Levey AI, Brann MR. Expression of muscarinic acetylcholine and dopamine receptor mRNAS in rat basal ganglia. Pro. Natl. Acad. Sci. U.S.A. 1990;87:7050–7054. doi: 10.1073/pnas.87.18.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westlind A, Grynfarb M, Hedlund B, Bartfai T, Fuxe K. Muscarinic supersensitivity induced by septal lesions or chronic atropine treatment. Brain Res. 1981;225:131–141. doi: 10.1016/0006-8993(81)90323-1. [DOI] [PubMed] [Google Scholar]