ABSTRACT
Since its discovery in the early 2000s, methicillin-resistant Staphylococcus aureus (MRSA) clonal complex 398 (CC398) has become a rapidly emerging cause of human infections, most often associated with livestock exposure. We applied whole-genome sequence typing to characterize a diverse collection of CC398 isolates (n = 89), including MRSA and methicillin-susceptible S. aureus (MSSA) from animals and humans spanning 19 countries and four continents. We identified 4,238 single nucleotide polymorphisms (SNPs) among the 89 core genomes. Minimal homoplasy (consistency index = 0.9591) was detected among parsimony-informative SNPs, allowing for the generation of a highly accurate phylogenetic reconstruction of the CC398 clonal lineage. Phylogenetic analyses revealed that MSSA from humans formed the most ancestral clades. The most derived lineages were composed predominantly of livestock-associated MRSA possessing three different staphylococcal cassette chromosome mec element (SCCmec) types (IV, V, and VII-like) including nine subtypes. The human-associated isolates from the basal clades carried phages encoding human innate immune modulators that were largely missing among the livestock-associated isolates. Our results strongly suggest that livestock-associated MRSA CC398 originated in humans as MSSA. The lineage appears to have undergone a rapid radiation in conjunction with the jump from humans to livestock, where it subsequently acquired tetracycline and methicillin resistance. Further analyses are required to estimate the number of independent genetic events leading to the methicillin-resistant sublineages, but the diversity of SCCmec subtypes is suggestive of strong and diverse antimicrobial selection associated with food animal production.
IMPORTANCE
Modern food animal production is characterized by densely concentrated animals and routine antibiotic use, which may facilitate the emergence of novel antibiotic-resistant zoonotic pathogens. Our findings strongly support the idea that livestock-associated MRSA CC398 originated as MSSA in humans. The jump of CC398 from humans to livestock was accompanied by the loss of phage-carried human virulence genes, which likely attenuated its zoonotic potential, but it was also accompanied by the acquisition of tetracycline and methicillin resistance. Our findings exemplify a bidirectional zoonotic exchange and underscore the potential public health risks of widespread antibiotic use in food animal production.
Introduction
Methicillin-resistant Staphylococcus aureus (MRSA) has been described in animals since 1972 (1), but a new lineage, clonal complex 398 (CC398), has emerged among livestock and begun colonizing and infecting humans. Human MRSA infections have been categorized into three groups based on their putative sources: health care-associated MRSA, community-associated MRSA, and health care-associated MRSA with community onset. A fourth category has recently been added to describe human MRSA cases associated with exposure to livestock (livestock-associated MRSA [LA-MRSA]). Human colonization with LA-MRSA multilocus sequence type 398 (ST398) was first recognized among swine farmers in France and The Netherlands in the early 2000s (2, 3). Since those early reports, ST398 and closely related STs within CC398 have been reported in diverse livestock hosts in many countries around the world (4–8). Human cases of MRSA CC398 have also been increasing rapidly and now account for up to 25% of the total MRSA cases in some parts of The Netherlands (9). Given its rapid emergence and trajectory of increasing importance in humans, the evolutionary history of MRSA CC398 has relevance for the epidemiology of MRSA and global health.
Methicillin-susceptible S. aureus (MSSA) CC398 is prevalent among pigs in Europe (10), but the evolution and global dispersal of this group have yet to be clarified. A microarray-based study revealed that the core genomes of 6 CC398 isolates were distinct from those of more than 2,000 S. aureus isolates from humans (11), and it has been generally presumed that pigs or other animals are the natural hosts of CC398.
Attempts to better characterize the evolution and epidemiology of CC398 have been hampered by the limited resolution of conventional S. aureus typing methods, including multilocus sequence typing (MLST) and spa sequence typing. While MLST defines CC398 and is useful for placing CC398 in the context of other S. aureus clonal complexes, it is of no use for characterizing variation within the group. spa typing has revealed geographic clustering among CC398 isolates in Europe (12, 13); however, the limited number of common spa types among CC398 isolates and the potential for homoplasy in the spa gene data restrict its phylogenetic utility (14).
Whole-genome sequencing provides a superior genetic fingerprint, which can be used for source tracking and evolutionary studies. Thus far, whole-genome sequencing has been used to study the hospital transmission and spatiotemporal spread of ST239 (15). In this study, we applied whole-genome sequence typing (WGST) to a diverse collection of 89 CC398 isolates to study the origins and evolution of S. aureus CC398.
RESULTS
We sequenced the genomes of 88 S. aureus CC398 isolates (see Data set S1 in the supplemental material). Among the isolates, we sequenced an average of 2,651,848 bases (standard deviation [SD] = 80,311) at ≥10× coverage. Genomes were sequenced at an average depth of 104.36× (SD = 35.7, using the 2,872,582-base SO385 chromosome as a reference).
Rooting the CC398 tree using ST36 as the outgroup revealed that a cluster of four isolates, characterized by the t899 spa type, was the first lineage to diverge from the other CC398 isolates (see Fig. S1 in the supplemental material). Much of the similarity between the t899 cluster and the ST36 isolate was mapped to the same ~123,000-bp region surrounding the spa gene. However, this region was incongruent with the phylogenetic signal generated by the rest of the chromosome in the t899 isolates. Comparative genomic analysis with other STs suggested that this region was acquired horizontally from an ST9 donor (see Fig. S2 in the supplemental material). When single nucleotide polymorphisms (SNPs) from this region were excluded from the phylogenetic analysis, the t899 lineage clustered with a more derived clade of European isolates and a clade of human-associated MSSA isolates from France, French Guiana, and the United States was identified as the first divergent CC398 lineage (see Fig. S3 in the supplemental material). This lineage was used to root the final CC398 WGST tree (Fig. 1). With the ST36 genome removed and excluding the SNPs from the 123,000-bp putative horizontally transferred region, we identified 4,238 SNPs, including 1,102 parsimony-informative SNPs with a consistency index (CI) of 0.9591. Among the SNPs, 3,552 were from coding regions (1,071 synonymous and 2,481 nonsynonymous).
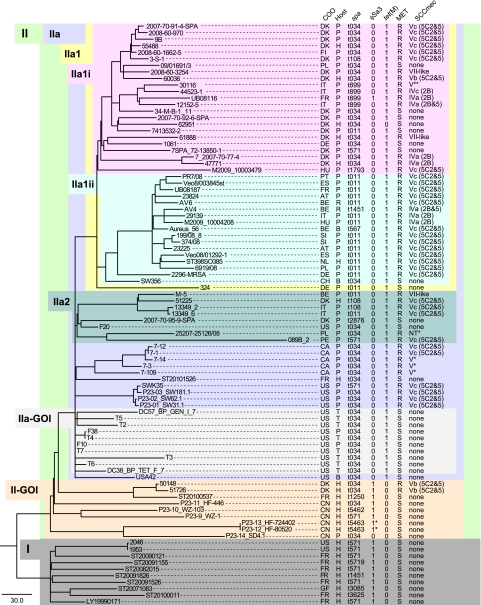
FIG 1 .
Maximum-parsimony tree of the 89 CC398 isolates (including ST398SO385) based on 4,238 total SNPs, including 1,102 parsimony-informative SNPs with a CI of 0.9591. Clades and groups of importance are labeled in a hierarchical fashion to facilitate description in the text. The tree was rooted with clade I based on an iterative selection process that identified this group as the most ancestral (see Materials and Methods). COO, country of origin; AT, Austria; BE, Belgium; CA, Canada; CH, Switzerland; CN, China; DE, Germany; DK, Denmark; ES, Spain; FI, Finland; FR, France; GF, French Guiana; HU, Hungary; IT, Italy; NL, The Netherlands; PE, Peru; PL, Poland; PT, Portugal; SI, Slovenia; US, United States; P, pig; H, human; R, horse; T, turkey; B, bovine; MET, methicillin susceptibility; R, resistant; S, susceptible.
The phylogenetic tree presented in Fig. 1 is a highly accurate depiction of the evolutionary relationships among the 89 CC398 strains included in this study (including the reference). The lack of homoplasy among informative SNPs (CI = 0.9591) obviated the need for additional measures of robustness such as bootstrapping (16). The most ancestral lineage (clade I) was composed entirely of MSSA strains from humans in North America, South America, and Europe. In addition, with the exception of one isolate (P23-14_SD4.1), the strains that accounted for the most ancestral lineages within clade II (II group of interest [II-GOI]) were human-associated S. aureus from China (including two MRSA strains isolated from Danish adoptees from China). All but one of the livestock-associated strains belonged to clade IIa, which was derived from the human-associated lineages. Clade IIa was composed of several lineages whose evolutionary relationships could not be determined due to poor hierarchical resolution. The lack of resolution was likely due to a rapid radiation following introduction into livestock, as homoplasy was exceedingly rare among the parsimony-informative SNPs. The isolates within clades IIa1 and IIa2 consisted almost entirely of European isolates, while the 10 remaining lineages consisted almost entirely of North American isolates. The IIa1i lineage was dominated by Danish isolates, one-third of which were MSSA, while the IIa1ii lineage consisted largely of MRSA isolates from several European countries, except Denmark. Interestingly, 6 of the 10 smaller lineages included MSSA strains isolated from turkey meat from the United States (IIa-GOI). In this report, we use the term “human associated” for isolates belonging to clade I and clade II-GOI (n = 19) and use the term “livestock associated” for isolates belonging to subclade IIa (n = 70).
Fifteen different spa types were identified among the 89 CC398 isolates, including t011, t034, t108, t567, t571, t899, t1250, t1451, t1793, t2876, t3085, t3625, t5462, t5463, and t5719 (Fig. 1). The two most common spa types, t011 and t034, represented 67% of the isolates. While some spa types were more common within individual clusters (e.g., t571 was disproportionately common among human-associated isolates), spa types were inconsistent with the overall CC398 phylogeny (Fig. 1).
Sixty-one percent (30/49) of the CC398 MRSA isolates harbored staphylococcal cassette chromosome mec element (SCCmec) subtype Vc (5C2&5) containing the cadmium-zinc resistance gene czrC. All of the SCCmec Vc (5C2&5) cassettes were present in LA-MRSA strains. Of note, the czrC gene was also found in two livestock-associated MSSA isolates. The remaining LA-MRSA isolates carried SCCmec (sub)types IVa (2B), IVa (2B&5), IVc (2B), Vb (5C2&5), V*, and V**; a novel VII-like SCCmec cassette; and a nontypeable (NT) SCCmec cassette. The mecA gene was detected in only two human-associated isolates; in both cases, the mecA gene was coded within SCCmec subtype Vb (5C2&5). The type V* and V** SCCmec cassettes contained structurally different J1 regions that did not match the J1 regions associated with subtypes a to c. The type VII-like SCCmec cassette contained ccr type 5 (ccrC) and a class C1-like mec gene complex element previously identified in SCCmec type X (7C1-like) (17). Accordingly, this novel SCCmec type was referred to as VII-like (5C1-like) to distinguish it from archetypal SCCmec type VII (5C1). The NT cassette contained a class C1-like mec gene complex without any previously described ccr gene complex. The tetracycline resistance gene tet(M) was present in 99% (69/70) of the livestock-associated isolates but absent from the human-associated isolates (Fig. 1; see Data set S1 in the supplemental material).
The prophage integrase gene Sa3int was detected in 29 CC398 isolates. Phylogenetic analysis of the Sa3int sequences showed that they belong to three separate clusters; one clade was typical of φSa3 prophages, one was typical of φAvβ prophages, and the third was suggestive of a novel φSa3 integrase variant (see Fig. S4 in the supplemental material). φSa3 prophages in association with one or more human innate immunomodulatory genes were detected in 95% (18/19) of the human-associated S. aureus isolates (Fig. 1; see Data set 1 in the supplemental material). All 18 positive isolates were from human samples, whereas the single isolate lacking φSa3 prophages originated from a pig farm. All 10 isolates belonging to clade I carried chp and scn (type C φSa3 prophages), whereas 6 of 8 isolates belonging to clade II-GOI carried sak, chp, and scn (type B φSa3 prophages) and 2 isolates carried scn only (an IEC type not previously described). In comparison, only 1 of 70 isolates belonging to clade II harbored a φSa3 prophage in association with sak, chp, and scn (type B). Interestingly, 10 livestock-associated isolates belonging to IIa-GOI were largely from turkey meat samples and carried a φAvβ prophage along with the associated genes SAAV_2008 and SAAV_2009 but lacked human innate immunomodulatory genes carried by φSa3 prophages. Sa2int and the lukF-lukS genes carried by φSa2 prophages were present in 6 of 19 human-associated isolates. Conversely, all livestock-associated S. aureus isolates lacked the lukF-lukS genes.
DISCUSSION
Since its discovery, MRSA CC398 has been perceived as a livestock-associated pathogen; however, the WGST-based phylogeny presented here strongly suggests that the CC398 lineage originated in humans as MSSA and then spread to livestock, where it subsequently acquired the SCCmec cassette and methicillin resistance. The isolates that formed the most basal clades (I and II-GOI) on the WGST-based phylogenetic trees were almost all human-associated MSSA strains, suggesting that these isolates were the most ancestral of those tested in this study (Fig. 1). Likewise, the clade structure observed in the livestock-dominated IIa clade supports a rapid radiation as CC398 moved from humans to animals (see Fig. S5 in the supplemental material). Thus, livestock-associated CC398 infections in humans may be seen as a reintroduction to the original host.
Epidemiological data suggest that livestock-associated CC398 strains have lower transfer rates, and may be less virulent, in humans than other well-known STs (18). In this study, we showed that the lukF-lukS genes encoding Panton-Valentine leukocidin (PVL) were present in only 6 of the 89 genomes, all of which were human associated (see Data set S1 in the supplemental material). Strikingly, we found that all of the human-associated MSSA strains from clade I and clade II-GOI carried φSa3 in association with human innate immunomodulatory genes, whereas φSa3 was identified in only one livestock-associated isolate (see Fig. S6 in the supplemental material). Instead, a φAvβ prophage and the associated genes SAAV_2008 and SAAV_2009 were identified among a group of mainly turkey meat isolates in the livestock-associated clade IIa-GOI. It therefore appears that φSa3 was lost prior to (or early in) the formation of clade II, while φAvβ was introduced into avian MSSA CC398 isolates thereafter (Fig. 1). The human innate immunomodulatory genes carried by φSa3 prophages play crucial roles in human niche adaptation (19, 20), whereas the φAvβ-carried SAAV_2008 and SAAV_2009 genes (encoding a putative ornithine cyclodeaminase and a putative membrane protease of the CAAX family, respectively) belong to the avian-niche-specific accessory gene pool for broiler chicken-associated S. aureus ST5 (21). The loss of human-niche-specific genes in livestock-associated isolates, including those from turkeys, may be a result of adaptation to nonhuman hosts. A similar natural history has been reconstructed for broiler chicken-associated S. aureus ST5, which appears to have been introduced from humans into the chicken-breeding system, transmitted vertically, and disseminated worldwide (21). The ST5 jump from humans to chickens also appears to have been followed by the acquisition of avian-niche-specific genes (including the SAAV_2008 and SAAV_2009 genes carried by φAvβ prophages) and partial loss of human-niche-specific genes (including human innate immunomodulatory genes carried by φSa3 prophages) (21).
The data presented here strongly suggest that CC398 acquired resistance to methicillin and tetracycline after the introduction to livestock from humans (see Fig. S7a and b in the supplemental material). The tetracycline resistance gene tet(M) was nearly universal among livestock-associated CC398 MRSA and MSSA isolates and completely missing from human-associated strains. Consequently, tetracycline use in food animal production is likely to select for livestock-associated S. aureus CC398 without differentially selecting for MRSA strains. MRSA can be selected for by a number of broad-spectrum cephalosporins that are used in food animal production in the United States and Europe. Likewise, zinc and other metals are frequently used in animal feed formulations and may coselect for MRSA CC398 strains that carry the czrC zinc resistance gene, as suggested previously (22). This hypothesis is supported by our findings that the vast majority of LA-MRSA strains carry SCCmec type Vc (5C2&5), which contains the czrC gene.
This study demonstrates the potential power of WGST for epidemiological investigations. For example, two of the Danish MRSA isolates came from infants adopted from China. Both isolates were spa type t034, which is consistent with the majority of Danish CC398 isolates from pigs and humans; however, WGST showed that the isolates shared a recent common ancestor with a French isolate and that this clade was derived from other clades within II-GOI that were strictly Chinese in origin (Fig. 1, II-GOI). Although the French isolate obscures these results, they are most consistent with a Chinese rather than Danish origin of the isolates. WGST revealed thousands of SNPs among the 89 CC398 strains. These mutations may provide robust phylogenetic signals for future epidemiological and epizootological investigations involving CC398 strains.
spa typing is routinely used for S. aureus epidemiology; however, in this study, homoplasy within the spa gene led to inconsistencies between the WGST CC398 phylogeny and spa types. Some spa types, such as t571 and t034, were observed in distant clades of the highly accurate WGST phylogenetic tree (Fig. 1). The t899 isolates exemplified the limitations of any single-locus typing method, as the spa gene was part of a ~123,000-bp region of DNA acquired from a distantly related S. aureus clone. A similar observation was made previously with S. aureus ST239, which originated as a hybrid between ST8-like and ST30-like chromosomes (23). Here, reliance on spa typing would have incorrectly placed these isolates outside of CC398. Interestingly, the large horizontally acquired region observed among the t899 CC398 strains also carries the SCCmec cassette, thus possibly presenting an alternative mechanism for SCCmec dissemination among S. aureus strains.
In this study, we provide strong evidence that CC398 originated in humans as MSSA and then spread to livestock, where it acquired resistance to methicillin and tetracycline. Genomic analyses presented here, in conjunction with previous epidemiological data, suggest that the jump from humans to animals was followed by a decreased capacity for human colonization, transmission, and virulence, yet livestock-associated CC398 has been linked to an increase in MRSA infections in northern Europe. Further research is required to characterize the full scope of the genetic changes associated with the shift from humans to livestock. Likewise, additional research and surveillance are required to predict the public health impact of MRSA CC398 in the future.
MATERIALS AND METHODS
Bacterial isolates.
This study included MRSA (n = 48) and MSSA (n = 40) CC398 isolates from 19 countries on four continents with strains from humans (n = 25) and livestock (n = 63, including strains from live animals, meat samples, and environmental contamination) (see Data set S1 in the supplemental material). A previously sequenced ST398 strain, SO385, from The Netherlands was used as the reference and included in all analyses (24).
MLST.
MLST was performed as described previously (http://saureus.mlst.net/misc/info.asp) (25). STs were assigned through the MLST database (http://www.mlst.net). The eBURST algorithm v3 was used to assign individual STs to specific CCs (http://eburst.mlst.net).
spa typing.
Amplification of the spa repeat region was performed using primers spa 1113f (5′ AAAGACGATCCTTCGGTGAGC 3′) and spa 1514r (5′ CAGCAGTAGTGCCGTTTGCTT 3′) and the conditions described previously (http://www.SeqNet.org). The spa types were determined based on the sequencing results using the spa plug-in included in the BioNumerics v4.6 software (Applied Math, Sint-Martens-Latem, Belgium).
Genome sequencing.
DNA samples were prepared for multiplexed, paired-end sequencing on an Illumina Genome Analyzer IIx (Illumina, Inc., San Diego, CA). For each isolate, 1 to 5 µg DNA in 200 µl was sheared in a 96-well plate with the SonicMAN (part no. SCM1000-3; Matrical BioScience, Spokane, WA) to a size range of 200 to 1,000 bp, with the majority of material at ca. 600 bp, using the following parameters: prechill, 0°C for 75 s; cycles, 20; sonication, 10 s; power, 100%; lid chill, 0°C for 75 s; plate chill, 0°C for 10 s; postchill, 0°C for 75 s. The sheared DNA was purified using the QIAquick PCR Purification kit (catalog no. 28106; Qiagen, Valencia, CA). The enzymatic processing (end repair, phosphorylation, A tailing, and adaptor ligation) of the DNA followed the guidelines described in the Illumina protocol (Preparing Samples for Multiplexed Paired-End Sequencing, catalog no. PE-930-1002, part no.1005361). The enzymes for processing were obtained from New England Biolabs (catalog no. E6000L; New England BioLabs, Ipswich, MA), and the oligonucleotides and adaptors were obtained from Illumina (catalog no. PE-400-1001). After ligation of the adaptors, the DNA was run on a 2% agarose gel for 2 h, after which a gel slice containing 500- to 600-bp fragments of each DNA sample was isolated and purified using the QIAquick Gel Extraction kit (catalog no. 28706; Qiagen, Valencia, CA). Individual libraries were quantified by quantitative PCR on an ABI 7900HT (part no. 4329001; Life Technologies Corporation, Carlsbad, CA) in triplicate at two concentrations, 1:1,000 and 1:2,000, using the Kapa Library Quantification kit (part no. KK4832 or KK4835; Kapa Biosystems, Woburn, MA). Based on the individual library concentrations, equimolar pools of no more than 12 indexed S. aureus libraries were prepared at a concentration of at least 1 nM using 10 mM Tris-HCl (pH 8.0)-0.05% Tween 20 as the diluent. To ensure accurate loading onto the flow cell, the same quantification method was used to quantify the final pools. The pooled paired-end libraries were sequenced on an Illumina Genome Analyzer IIx to a read length of at least 76 bp.
Identification of SNPs.
Illumina WGS data sets were aligned against the chromosome of the published ST398 reference genome (strain SO385; GenBank accession no. AM990992) (24) using the short-read alignment component of the Burrows-Wheeler Aligner. Each alignment was analyzed for SNPs using SolSNP (http://sourceforge.net/projects/solsnp/). In order to avoid false calls due to sequencing errors, SNP loci were excluded if they did not meet a minimum coverage of 10× and if the variant was present in less than 90% of the base calls for that position. SNP calls were combined for all of the sequenced genomes such that for the locus to be included in the final SNP matrix, it had to be present in all of the genomes. SNPs falling in the duplicated regions on the reference genome were discarded.
Phylogenetic analysis.
Phylogenetic trees were generated using the maximum-parsimony method in PAUP v4.0b10. For maximum-parsimony bootstrapping analysis, the analysis was constrained to build a maximum of 1,000 trees (100 replicates, 10 trees each). The root of the tree was determined through an iterative process as follows. A distance matrix and phylogenetic tree was generated comparing the chromosomes of ST398 (GenBank accession no. AM990992), ST36 (GenBank accession no. BX571856), ST8 (GenBank accession no. CP000255), ST1 (GenBank accession no. BA000033), and ST5 (GenBank accession no. BA000018). Through this process, ST36 was determined to be the most closely related non-CC398 STs. ST36 was used as an outgroup to root the CC398 WGST tree and identify the most ancient CC398 bifurcation point. CC398 descendants nearest to this bifurcation point were used to root subsequent trees.
SCCmec typing.
The presence of mecA and SCCmec types and subtypes was assessed in all 89 S. aureus CC398 isolates. The structural features unique to each of the type 1 to 5 ccr gene complexes; class A, B, and C2 mec gene complexes; and four J1 subtypes (a to d) of type IV SCCmec were determined by a PCR-based multiplex assay described by Kondo et al. (26). Structural features unique to the class C1 and C1-like mec gene complexes (17, 27) and the three subtypes of type V SCCmec (17) were determined by aligning the Illumina WGS data sets against reference sequences using CLC Genomics Workbench v4.7.2 (CLC bio, Aarhus, Denmark). The following reference sequences were used: mec class C1 (GenBank accession no. AB373032); mec class C1-like (GenBank accession no. AB505630); and SCCmec subtypes Va (5C2) (GenBank accession no. AB121219), Vb (5C2&5) (GenBank accession no. AB462393), and Vc (5C2&5) (GenBank accession no. AB505629).
SCCmec nomenclature was applied as proposed by the International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (28). For brevity, the type is indicated by roman numerals and the subtype is identified by a lowercase latin letter. The combination of ccr and mec gene complexes is indicated by an arabic number and a latin letter, respectively, in parentheses. When a composite of two SCC elements carrying distinct ccr gene complexes is identified, this is indicated by an ampersand and an arabic numeral designating the ccr type.
Detection of genes associated with antimicrobial resistance and host adaptation.
All 89 genomes were analyzed for the presence of the tetracycline resistance gene tet(M), the cadmium-zinc resistance gene czrC, the φSa3 and φSa2 prophages (identified by Sa3int and Sa2int integrase genes), five genes carried by φSa3 prophages (sea, sep, sak, chp, and scn), two putative avian-niche-specific genes carried by φAvβ (a φSa3-like prophage) (SAAV_2008 and SAAV_2009), and two PVL genes carried by φSa2 prophages (lukF-PV and lukS-PV). Local BLASTN searches were performed on de novo contigs assembled from the Illumina WGS data sets, as well as a reference assembly, using CLC Genomics Workbench v4.7.2. The presence or absence of genes was determined using thresholds of 90% nucleotide identity, 90% coverage of the query sequence length, and a sequence depth of >10×. The query sequences used were tet(M) and czrC (GenBank accession no. AM990992); Sa3int, sea, sak, chp, and scn (GenBank accession no. NC_009641); sep (GenBank accession no. BA000018); SAAV_2008 and SAAV_2009 (GenBank accession no. CP001781); and Sa2int, lukF-PV, and lukS-PV (GenBank accession no. AB006796).
All de novo contigs with BLASTN matches to Sa3int were selected, and the Sa3int genes were retrieved for phylogenetic reconstruction using Sa3int (GenBank accession no. NC_009641) and Avβint (GenBank accession no. CP001781) as reference sequences. Gene sequences were aligned using ClustalW v 2.0 (29), and the trees were generated using the maximum-parsimony method in PAUP v4.0b10.
The φSa3 prophages received letter designations to reflect unique combinations of the five prophage-carried genes that modulate human innate immune responses (sea, sep, sak, chp, and scn) as described elsewhere (30).
SUPPLEMENTAL MATERIAL
Isolates included in this study. Download Data set S1, XLSX file, 0.1 MB.
Maximum-parsimony tree based on 27,475 SNPs from 89 isolates (including ST398SO385) rooted with an ST36 isolate (USA200-MRSA252) as the outgroup. The CI for the parsimony-informative SNPs was 0.8969. The t899 group (12152-5, UB08116, 30116, and 44523-1) was identified as the most ancestral and is highlighted in gray. Download Figure S1, PDF file, 0.3 MB.
Graphic representation of the region flanking the spa gene of S. aureus ST398 strain SO385 (t011; MRSA), ST398 strain 12152-5 (t899; MRSA), and ST9 strain 2007-70-94-4 (t1334; MSSA). Light gray indicates areas with high homology to ST398 sequences, and dark gray indicates areas with high homology to ST9. The location of the spa gene is indicated in black. Approximate insertion nucleotides are reported based on the SO385 reference genome. Download Figure S2, PDF file, 0.7 MB.
Maximum-parsimony tree based on 26,324 SNPS from 89 isolates (including ST398SO385) rooted with ST36 (USA200-MRSA252) as the outgroup. The SNPs from the horizontally transferred region surrounding the spa gene were excluded (region in reference ST398SO385, 12252 to 135180). The CI for the parsimony-informative SNPs was 0.9089. The most ancestral isolates within the CC398 lineage are highlighted in gray. Download Figure S3, PDF file, 0.3 MB.
Phage tree. Shown is a maximum-parsimony tree of the Sa3int genes from 30 ST398 isolates, Sa3int (GenBank accession no. NC_009641), and Avβint (GenBank accession no. CP001781). Download Figure S4, PDF file, 0.7 MB.
Cladograms colored to show host species. Download Figure S5, PDF file, 0.2 MB.
Cladogram colored to show φSa3-positive isolates. Download Figure S6, PDF file, 0.2 MB.
Cladogram colored to show SCCmec types. Download Figure S7a, PDF file, 0.2 MB.
Cladogram colored to show tet(M)-positive isolates. Download Figure S7b, PDF file, 0.2 MB.
ACKNOWLEDGMENTS
This work was funded in part by the TGen Foundation, the Statens Serum Institut, and Center for Genomic Epidemiology (http://www.genomicepidemiology.org) grant 09-067103/DSF. D.A.R. was supported by a grant from the National Institutes of Health (GM080602).
We thank Xiaoling Ma for contributing isolates to this study.
Footnotes
Citation Price LB, et al. 2012. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio 3(1):e00305-11. doi:10.1128/mBio.00305-11.
REFERENCES
- 1. Devriese LA, Van Damme LR, Fameree L. 1972. Methicillin (cloxacillin)-resistant Staphylococcus aureus strains isolated from bovine mastitis cases. Zentralbl. Veterinaermed. B. 19:598–605 [DOI] [PubMed] [Google Scholar]
- 2. Armand-Lefevre L, Ruimy R, Andremont A. 2005. Clonal comparison of Staphylococcus aureus isolates from healthy pig farmers, human controls, and pigs. Emerg. Infect. Dis. 11:711–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Voss A, Loeffen F, Bakker J, Klaassen C, Wulf M. 2005. Methicillin-resistant Staphylococcus aureus in pig farming. Emerg. Infect. Dis. 11:1965–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Baba K, Ishihara K, Ozawa M, Tamura Y, Asai T. 2010. Isolation of meticillin-resistant Staphylococcus aureus (MRSA) from swine in Japan. Int. J. Antimicrob. Agents 36:352–354 [DOI] [PubMed] [Google Scholar]
- 5. Khanna T, Friendship R, Dewey C, Weese JS. 2008. Methicillin resistant Staphylococcus aureus colonization in pigs and pig farmers. Vet. Microbiol. 128:298–303 [DOI] [PubMed] [Google Scholar]
- 6. Lewis HC, et al. 2008. Pigs as source of methicillin-resistant Staphylococcus aureus CC398 infections in humans, Denmark. Emerg. Infect. Dis. 14:1383–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Smith TC, et al. 2009. Methicillin-resistant Staphylococcus aureus (MRSA) strain ST398 is present in midwestern U.S. swine and swine workers. PLoS One 4:e4258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wulf MW, Verduin CM, van Nes A, Huijsdens X, Voss A. 2011. Infection and colonization with methicillin resistant Staphylococcus aureus ST398 versus other MRSA in an area with a high density of pig farms. Eur. J. Clin. Microbiol. Infect. Dis. 31:61–65 [DOI] [PubMed] [Google Scholar]
- 9. van Cleef BA, et al. 2011. Livestock-associated methicillin-resistant Staphylococcus aureus in humans, Europe. Emerg. Infect. Dis. 17:502–505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hasman H, et al. 2010. Spa type distribution in Staphylococcus aureus originating from pigs, cattle and poultry. Vet. Microbiol. 141:326–331 [DOI] [PubMed] [Google Scholar]
- 11. van Belkum A, et al. 2008. Methicillin-resistant and -susceptible Staphylococcus aureus sequence type 398 in pigs and humans. Emerg. Infect. Dis. 14:479–483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gómez-Sanz E, et al. 2010. Detection, molecular characterization, and clonal diversity of methicillin-resistant Staphylococcus aureus CC398 and CC97 in Spanish slaughter pigs of different age groups. Foodborne Pathog. Dis. 7:1269–1277 [DOI] [PubMed] [Google Scholar]
- 13. Köck R, et al. 2009. Prevalence and molecular characteristics of methicillin-resistant Staphylococcus aureus (MRSA) among pigs on German farms and import of livestock-related MRSA into hospitals. Eur. J. Clin. Microbiol. Infect. Dis. 28:1375–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nübel U, et al. 2008. Frequent emergence and limited geographic dispersal of methicillin-resistant Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 105:14130–14135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Harris SR, et al. 2010. Evolution of MRSA during hospital transmission and intercontinental spread. Science 327:469–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pearson T, Okinaka RT, Foster JT, Keim P. 2009. Phylogenetic understanding of clonal populations in an era of whole genome sequencing. Infect. Genet. Evol. J. Mol. Epidemiol. Evol. Genet. Infect. Dis. 9:1010–1019 [DOI] [PubMed] [Google Scholar]
- 17. Li S, et al. 2011. Novel types of staphylococcal cassette chromosome mec elements identified in clonal complex 398 methicillin-resistant Staphylococcus aureus strains. Antimicrob. Agents Chemother. 55:3046–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Graveland H, Wagenaar JA, Bergs K, Heesterbeek H, Heederik D. 2011. Persistence of livestock associated MRSA CC398 in humans is dependent on intensity of animal contact. PLoS One 6:e16830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Foster TJ. 2005. Immune evasion by staphylococci. Nat. Rev. Microbiol. 3:948–958 [DOI] [PubMed] [Google Scholar]
- 20. Rooijakkers SH, et al. 2005. Immune evasion by a staphylococcal complement inhibitor that acts on C3 convertases. Nat. Immunol. 6:920–927 [DOI] [PubMed] [Google Scholar]
- 21. Lowder BV, et al. 2009. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc. Natl. Acad. Sci. U. S. A. 106:19545–19550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cavaco LM, Hasman H, Aarestrup FM. 2011. Zinc resistance of Staphylococcus aureus of animal origin is strongly associated with methicillin resistance. Vet. Microbiol. 150:344–348 [DOI] [PubMed] [Google Scholar]
- 23. Robinson DA, Enright MC. 2004. Evolution of Staphylococcus aureus by large chromosomal replacements. J. Bacteriol. 186:1060–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schijffelen MJ, Boel CH, van Strijp JA, Fluit AC. 2010. Whole genome analysis of a livestock-associated methicillin-resistant Staphylococcus aureus ST398 isolate from a case of human endocarditis. BMC Genomics 11:376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Enright MC, Day NP, Davies CE, Peacock SJ, Spratt BG. 2000. Multilocus sequence typing for characterization of methicillin-resistant and methicillin-susceptible clones of Staphylococcus aureus. J. Clin. Microbiol. 38:1008–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kondo Y, et al. 2007. Combination of multiplex PCRs for staphylococcal cassette chromosome mec type assignment: rapid identification system for mec, ccr, and major differences in junkyard regions. Antimicrob. Agents Chemother. 51:264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Berglund C, et al. 2008. Novel type of staphylococcal cassette chromosome mec in a methicillin-resistant Staphylococcus aureus strain isolated in Sweden. Antimicrob. Agents Chemother. 52:3512–3516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Iwg SCC. 2009. Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob. Agents Chemother. 53:4961–4967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Larkin MA, et al. 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23:2947–2948 [DOI] [PubMed] [Google Scholar]
- 30. van Wamel WJ, Rooijakkers SH, Ruyken M, van Kessel KP, van Strijp JA. 2006. The innate immune modulators staphylococcal complement inhibitor and chemotaxis inhibitory protein of Staphylococcus aureus are located on beta-hemolysin-converting bacteriophages. J. Bacteriol. 188:1310–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Isolates included in this study. Download Data set S1, XLSX file, 0.1 MB.
Maximum-parsimony tree based on 27,475 SNPs from 89 isolates (including ST398SO385) rooted with an ST36 isolate (USA200-MRSA252) as the outgroup. The CI for the parsimony-informative SNPs was 0.8969. The t899 group (12152-5, UB08116, 30116, and 44523-1) was identified as the most ancestral and is highlighted in gray. Download Figure S1, PDF file, 0.3 MB.
Graphic representation of the region flanking the spa gene of S. aureus ST398 strain SO385 (t011; MRSA), ST398 strain 12152-5 (t899; MRSA), and ST9 strain 2007-70-94-4 (t1334; MSSA). Light gray indicates areas with high homology to ST398 sequences, and dark gray indicates areas with high homology to ST9. The location of the spa gene is indicated in black. Approximate insertion nucleotides are reported based on the SO385 reference genome. Download Figure S2, PDF file, 0.7 MB.
Maximum-parsimony tree based on 26,324 SNPS from 89 isolates (including ST398SO385) rooted with ST36 (USA200-MRSA252) as the outgroup. The SNPs from the horizontally transferred region surrounding the spa gene were excluded (region in reference ST398SO385, 12252 to 135180). The CI for the parsimony-informative SNPs was 0.9089. The most ancestral isolates within the CC398 lineage are highlighted in gray. Download Figure S3, PDF file, 0.3 MB.
Phage tree. Shown is a maximum-parsimony tree of the Sa3int genes from 30 ST398 isolates, Sa3int (GenBank accession no. NC_009641), and Avβint (GenBank accession no. CP001781). Download Figure S4, PDF file, 0.7 MB.
Cladograms colored to show host species. Download Figure S5, PDF file, 0.2 MB.
Cladogram colored to show φSa3-positive isolates. Download Figure S6, PDF file, 0.2 MB.
Cladogram colored to show SCCmec types. Download Figure S7a, PDF file, 0.2 MB.
Cladogram colored to show tet(M)-positive isolates. Download Figure S7b, PDF file, 0.2 MB.