Abstract
We previously reported the presence of MUC2, MUC5AC and, for the first time, MUC5B in a 58-year-old male with pseudomyxoma peritonei (PMP). This is a report on the biochemical and immunohistochemical characterization of mucin in a 50-year-old female with the same rare illness. A right oophorectomy and appendicectomy and a resection of the involved omentum were performed. Approximately a litre of crude material in the sol and gel phases was obtained from the patient during laparotomy. This was briefly homogenized in 6 M guanidinium hydrochloride and proteolytic inhibitors and purified by density gradient centrifugation in caesium chloride. At laparotomy it was noted that the patient had appendiceal and ovarian masses as well as extensive mucinous deposits in the omentum and peritoneum. A mucinous adenocarcinoma of the appendix and ovary was confirmed on histology. The cells expressed both sulphated and non-sulphated acidic mucins. The presence of MUC2, MUC5AC, MUC5B and a-1-acid glycoprotein was shown by Western blotting and MUC4 by immunohistochemical staining. MUC1 and MUC6 were not detectable in the tissue. The study confirms that MUC2, MUC5AC and MUC5B are produced in the mucus of patients with PMP. The expression of MUC4 in this disease has not been previously reported.
Key Words: MUC, Mucin, Mucus, Pseudomyxoma peritonei, Ovary
Introduction
Mucins are high-molecular-weight, heavily O-glycosylated glycoproteins that either form crude visco-elastic gels on the epithelial surfaces of the internal tracts of the body [1], or like MUC7 (a salivary mucin) are of the secreted and non-gel-forming kind [2]. Furthermore a host of transmembrane mucins, for example MUC1 and MUC4, have been described [3], and some of these have been found to function as tumour cell modulators affecting tumour cell phenotype, making them potentially useful clinically [4]. Overexpression and underglycosylation of mucins are found in certain cancers, for example MUC1, which is found to be elevated in carcinoma of the breast and associated with a poor prognosis [5].
Mucin genes are highly polymorphic due to the presence of long stretches of a variable number of tandem repeats that are heavily glycosylated. Mucin gene expression is relatively organ-specific and an extensive loss of normal mucin gene regulation may occur with intestinal metaplasia [6, 7] or malignancy [7, 8]. There has been an increasing interest in mucins in the diagnosis and immunotherapeutic treatment of carcinomas in general [9, 10].
It has previously been reported that pseudomyxoma peritonei (PMP), which was first described in 1842, is a disease of MUC2-expressing goblet cells, although the copious intraperitoneal mucinous ascites containing huge globules of extracellular mucin [11, 12] may also contain MUC5AC [13]. The accumulation of large amounts of extracellular mucus in the abdominal cavity is probably the main cause of the disease's morbidity and mortality, irrespective of the site of origin [12]. The first and only report, to our knowledge, of the presence of MUC5B in tissue and gelatinous material of a 58-year-old male diagnosed with PMP was from our laboratory [13]. The expression of MUC2 in PMP accounts for both the voluminous deposits of extracellular mucus and in women would help distinguish the appendix rather than the ovary as the site of origin of the disease [14].
Our laboratory previously reported the presence of MUC2, MUC5AC and, for the first time, MUC5B in the crude mucus in the peritoneal cavity of a 58-year-old male with PMP [13]. Herein we report the biochemical and immunohistochemical characteristics of mucus obtained from a 50-year-old female with PMP following mucinous adenocarcinoma of the appendix and ovary. Again, we have shown the presence of MUC2, MUC5AC and MUC5B in the soluble phase of the viscous material in the abdominal cavity of the patient. More interestingly the immunohistochemistry of tissue, besides showing a positive expression for MUC2, MUC5AC and MUC5B, also showed the presence of MUC4, a transmembrane mucin, which to our knowledge has not been reported previously.
Patient and Methods
Case Report
A 50-year-old woman presented with a six-month history of lower abdominal pain, weight loss and nausea. On examination she was found to have a large lower abdominal mass arising from the pelvis. A CT scan of the abdomen and pelvis revealed a large, multiseptated mass originating in the pelvis, with solid and cystic components. An omental cake was noted. On this basis, a clinical diagnosis of mucinous ovarian malignancy was made. She was taken to the operating theatre for a laparotomy, at which a large ovarian mass was noted as well as extensive mucinous deposits on the omentum and peritoneum and an appendix mass. A right oophorectomy and appendicectomy were performed, as well as resection of the involved omentum. Histology confirmed a mucinous adenocarcinoma of the appendix with involvement of the ovary. The appendicular tumour had ruptured, and the omental and peritoneal deposits were consistent with mucinous deposits from the ruptured appendix (PMP).
Preparation and Analysis of Mucin
One litre of viscous fluid from the patient's abdomen, containing sol and gel phases, was provided on ice to the laboratory by the colorectal surgeons. Extraction of mucus and isolation of purified mucin were performed as described by Mall et al. [13]. Briefly, soluble mucus was treated with 10 mM dithiotreitol (DTT) in 6 M guanidinium hydrochloride (GuHCl), 5 mM EDTA, 0.1 M Tris-HCl buffer pH 8.0, for 5 h at 37.0°C or 0.2 M sodium dihydrogen phosphate buffer, pH 8.0, for reduction of mucins and subsequently alkylated with 25 mM iodoacetamide (IAA) for 15 h at room temperature in the dark [15, 16, 17, 18]. Mucin subunits were separated by gel electrophoresis in 1% (w/v) agarose gels and Western blotting performed as described by Mall et al. [13], for MUC2, MUC5AC and MUC5B [19, 20]. An aliquot of mucin from the sol phase was subjected to a density gradient in 3.5 M caesium chloride (CsCl)/4 M GuHCl, twice for 48 h at 105,000 g with a starting density of 1.39-1.42 g/ml [15, 16, 17, 18]. The mucin-rich fractions were pooled, dialyzed against three changes of distilled water and lyophilized. Polyacrylamide gel electrophoresis, Western blotting and amino acid analysis have also been described in detail by Mall et al. [13]. The polyclonal antibody for a-1-acid glycoprotein was raised in our laboratory and the second antibody was a goat anti-rabbit antibody (Dako, Cape Town).
Analytical Determinations
Glycoprotein was estimated using the periodic acid Schiff (PAS) procedure [21] and protein according to the method of Lowry et al. [22].
Histochemistry. Formalin-fixed paraffin wax-embedded (FFPE) tissue blocks were retrieved from the archives of the Division of Anatomical Pathology, National Health Laboratory Service (NHLS), Groote Schuur Hospital, Cape Town, South Africa. The FFPE blocks were cut and the 2 μm tissue sections were routinely stained for haematoxylin and eosin. Tissue sections were also stained with the PAS/AB (alcian blue) and HID (high iron diamine)/AB.
Immunohistochemistry. Primary mouse monoclonal antibodies MUC1, MUC1core (MUC1c), MUC2, MUC5AC and MUC6 were obtained from NovoCastra Laboratories, Newcastle upon Tyne (UK) and MUC4 was kindly donated by S. Batra, University of Omaha, USA. MUC5B was a gift from Prof. Dallas Swallow, University College, London, UK. The Primary Armenian Hamster antibody MUC1 (CT2) was kindly donated by Dr. Sandra Gendler, Mayo Clinic, Scottsdale, Arizona, USA (table 1). Breast cancer tissue was used as a positive control for MUC1, MUC1 (CT2) and MUC1c, normal colon for MUC2, MUC4 and MUC5B, and normal stomach for MUC5AC and MUC6. The APES-coated slides were incubated overnight at 50°C to fix the tissue onto the slide. The tissue sections were dewaxed in xylene, rehydrated through graded alcohols to water and treated with 1% hydrogen peroxide/methanol to quench endogenous peroxidase activity. Antigen retrieval was achieved using 0.01 M citrate buffer pH 6 in a pressure cooker at full pressure. After cooling in tap water, blocking was done with normal goat serum for non-specific binding. MUC5B required reduction with 10 mM DTT and alkylation with 25 mM IAA for 30 min each at room temperature prior to blocking. Primary antibody incubation times were as follows: 30 min for MUC1, MUC1 core and MUC5AC, 1 h for MUC2 and MUC5B, 2 h for MUC1 (CT2) and MUC6, all at room temperature. MUC4 was incubated overnight at 4°C. Phosphate-buffered saline Tween pH 7.4 was used for the washes. The specific secondary antibody (Envision Mouse from DakoCytomation for MUC1, MUC1c, MUC2, MUC4, MUC5AC, MUC5B and MUC6) or biotinylated goat anti-Armenian from SantaCruz for MUC1 (CT2) was applied for 30 min at room temperature. The MUC1 (CT2) required another detection step using avidin/HRP (DakoCytomation). The substrate-chromogen DAB (3,3-diaminobenzidine tetrahydrochloride) was applied to develop the color intensity, enhanced with 1% copper sulphate, counterstained with haematoxylin, rehydrated through graded alcohols to xylene and coverslipped using entellan. The primary antibody was omitted in the negative control. Staining of the tissue was graded as follows: <5% = 0, 5-25% = 1, 26-50% = 2, 51-75% = 3, >75% = 4.
Table 1.
Antibodies used for immunohistochemistry in this study and their sources
| Antibody | Clonality mAbs | Clone | Dilution | Manufacturer |
|---|---|---|---|---|
| MUC1 | mouse | Ma695 | 1:100 | NovoCastra |
| MUC1 | hamster | CT2 | 1:100 | Mayo Clinic, USA |
| MUC1c | mouse | Ma552 | 1:100 | NovoCastra |
| MUC2 | mouse | Cep58 | 1:100 | NovoCastra |
| MUC4 | mouse | 8G7 | 1:500 | University of Omaha, USA |
| MUC5AC | mouse | CLH2 | 1:100 | NovoCastra |
| MUC5B | mouse | 5Bb2-2 | 1:100 | E.U. Consortium |
| MUC6 | mouse | CLH5 | 1:100 | NovoCastra |
EU. = European Union; mAb = monoclonal antibody.
Ethics
The University of Cape Town Research Ethics Committee approved the study (ethics number REC REF: 302/2005).
Results
Macroscopy
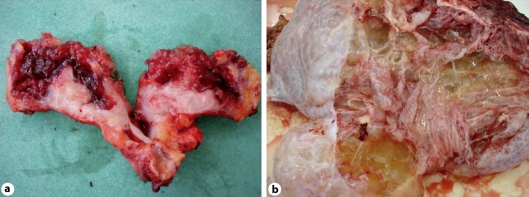
One specimen consisted of a hyperaemic appendix measuring 75 × 25 × 18 mm, the distal segment of which showed marked globular enlargement with mural perforation. On cut section, it was noted that the appendiceal wall was thickened and the appendiceal lumen contained abundant glistening, highly viscous mucus which also clung to the serosal surface of the organ (fig. 1a). The right ovary consisted of a ruptured, multiloculated, cystic ovarian mass measuring 240 × 220 × 70 mm and containing massive quantities of highly viscous, yellowish, glistening mucus similar to that seen within the appendix. A 520-mm-long sheet of omentum contained multiple mucinous deposits (fig. 1b).
Fig. 1.
a Cut section of the appendix showing distal cystic enlargement with abundant thick mucus. b A multiloculated cystic ovarian mass with abundant viscous mucus.
Microscopy
Sections of the appendix showed focal superficial papillary projections lined by atypical mucinous epithelium including numerous goblet cells. The tumour consisted of irregular mucinous glands and clusters of epithelial cells lying in large pools of extracellular mucus. There was invasion through the muscularis propria with abundant production of extracellular mucus which dissected through the wall. The wall of the appendix was perforated in areas, with spilling of mucus onto the serosal surface. The appendiceal resection margin was clear of invasive tumour and epithelial dysplasia. The ovary showed dissection of ovarian stroma by large pools of mucus with focal papillary architecture. The epithelium consisted of numerous goblet cells with basally oriented nuclei and apical mucin. Also noted were widespread stromal and surface peritoneal deposits of mucinous epithelium lying within large pools of mucin. Extensive omental deposits of tumour, essentially identical to that seen in the ovary and the appendix, were present. Based on the above findings a mucinous adenocarcinoma of the appendix with involvement of the right ovary and PMP was diagnosed.
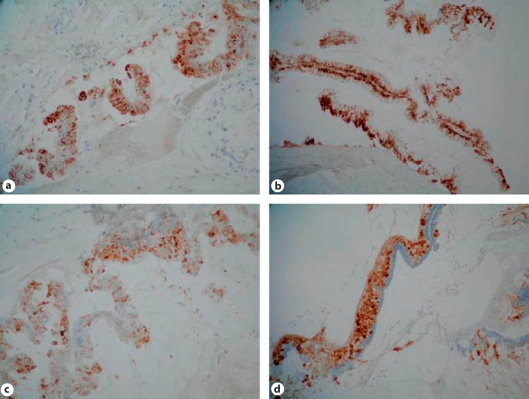
The histochemistry results (table 2) were similar for both the appendiceal and ovarian tumours. The tumour cells secreted predominantly acidic mucins (AB-positive) consisting of a mixture of sulphated and non-sulphated acidic mucin (HID/AB stain). More than 75% of tumour cells showed cytoplasmic staining for MUC2 (fig. 2a) and MUC5AC (fig. 2b). Between 50 and 75% of tumour cells expressed MUC4 (fig. 2c) in the cytoplasm. MUC5B (fig. 2d) expression was higher in the goblet cells of the appendix tumour than in the ovary. MUC1, MUC1 (CT2), MUC1c and MUC6 were negative. MUC2, MUC4, MUC5AC and MUC5B were co-expressed by neoplastic cells.
Table 2.
Histochemical and immunohistochemical data
| H&E | PAS/AB | HID | MUC1 | MUC1c | MUC1 (CT2) | MUC2 | MUC4 | MUC5AC | MUC5Bb2-2 | MUC6 |
|---|---|---|---|---|---|---|---|---|---|---|
| Appendix Mucinous cystadeno-carcinoma | Goblet cells AB pos Secreted mucin pred AB | Mixed AB & HID | neg | neg | neg | 4+ cytoplasmic | 3+ cytoplasmic | 4+ cytoplasmic | 3+ mainly goblet | neg |
| Ovary Metastatic mucinous cystadeno-carcinoma | Goblet cells AB pos Secreted mucin pred AB | Mixed AB & HID | neg | neg | neg | 4+ cytoplasmic | 3+ cytoplasmic | 4+ cytoplasmic | 2+ | neg |
Fig. 2.
Cytoplasmic staining for MUC2 (a), MUC5AC (b), MUC4 (c) and MUC5B (d).
Purification of Mucins by CsCl Density Gradient Ultra-Centrifugation
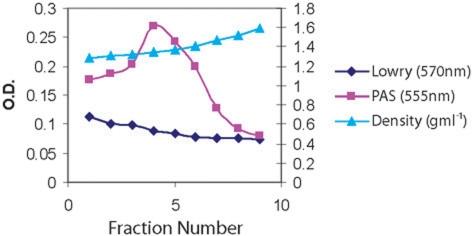
Mucins extracted from the viscous crude PMP material were purified by density gradient centrifugation, twice in CsCl and 4 M GuHCl with a buoyant density between 1.39 and 1.40 g/ml to remove proteins and nucleic acids. After the second spin, there was a clear separation of the proteins from the mucins (fig. 3) [13]. The mucin-rich fractions were pooled, dialyzed against three changes of distilled water and lyophilized.
Fig. 3.
Second CsCl density gradient centrifugation of human mucus from a patient with PMP. Solid CsCl was added to semi-purified mucins obtained from the first CsCl density centrifugation spin to give a starting density of 1.39–1.40 g/ml. After centrifugation (40,000 r.p.m. for 48 h) the tubes were fractionated into 9 equal fractions and the density, mucin and protein of each fraction.
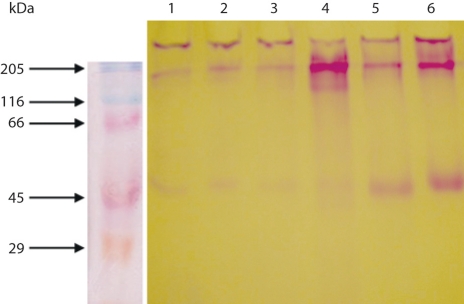
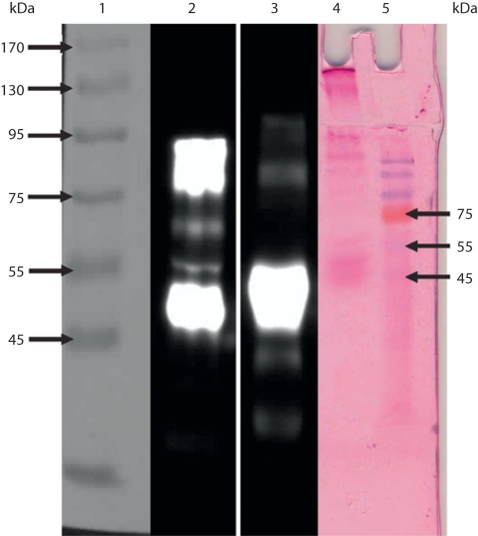
SDS-PAGE and Western Blotting. Equal loadings of purified mucin samples on a 10% gel stained for mucin with PAS (fig. 4) showed PAS-positive material on the top of the stacking gel (fig. 4, lanes 1–6), with varying amounts of material in the stacking gel, moving into the running gel. There seemed to be no difference between untreated crude material (fig. 4, lane 1), that which was reduced with 10 mM DTT and alkylated with 25 mM IAA (fig. 4, lane 2) and that which was digested with papain (fig. 4, lane 3), which showed material at the top of the stacking gel and the beginning of the running gel, just below molecular size 205 kDa. When crude material was digested and reduced, more material was released and appeared as a smear in the stacking gel and into the running gel (fig. 4, lane 4). Purified material (fig. 4, lane 5) released more PAS-positive material when reduced with 0.2 M 2-mercaptoethanol (fig. 4, lane 6), quite similar to crude material when reduced and digested (fig. 4, lane 4). A distinct smaller-sized species of mucin of higher electrophoretic mobility and varying intensity was seen at a molecular weight of approximately 40-50 kDa (fig. 4, lanes 1–6), which was more prominent in the purified samples (lanes 5 and 6) than in the crude samples. This 40-50 kDa glycoprotein was also seen in the serum of a patient with carcinoma of the stomach upon staining with PAS [23] and reacted strongly in a Western blot using the polyclonal antibody to a-1-acid glycoprotein for serum from the same patient (fig. 5, lane 3). An even stronger reaction was seen for the purified mucin for PMP (fig. 5, lane 2), which gave two very intense reactions. This mucin stains with PAS in SDS-PAGE (fig. 5, lane 5).
Fig. 4.
10% SDS-PAGE, stained with PAS, of crude and purified mucin from a patient with PMP. Lane 1: crude material; lane 2: crude material reduced with DTT; lane 3: crude material digested with papain; lane 4: crude material digested and reduced; lane 5: purified mucin; lane 6: purified mucin reduced with DTT.
Fig. 5.
A composite of a 10% SDS-PAGE gel stained with PAS (lanes 4 and 5) and a Western blot of material stained with the antibody against a-1-acid glycoprotein (lanes 2–3). Lane 1: molecular weight marker; lane 2: purified mucin from a patient (in this study) with PMP; lane 3: serum from a patient diagnosed with carcinoma of the stomach; lane 4: purified mucin from a patient (in this study) with PMP (PAS stain); lane 5: molecular weight marker.
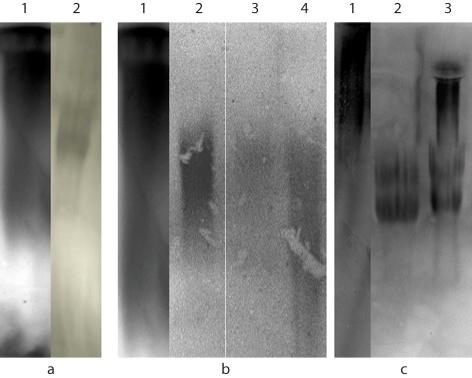
Agarose Gel Electrophoresis and Western Blotting. Western blotting showed the presence of MUC5AC (fig. 6a, lane 2), MUC5B (fig. 6b, lanes 2–4) and MUC2 (fig. 6c, lanes 2 and 3). Each experiment had a positive and negative control, confirming the specificity of the antibody.
Fig. 6.
Western blot following agarose gel electrophoresis identifying mucins in PMP. a MUC5AC expression. Lane 1: gastric cancer (positive control); lane 2: crude PMP material. b MUC5B expression. Lane 1: saliva (positive control); lane 2: crude PMP material; lane 3: crude reduced PMP material; lane 4: purified PMP. c MUC2 expression. Lane 1: normal colon (positive control); lane 2: purified PMP; lane 3: purified reduced PMP.
Discussion
It has been reported that PMP originates in the appendix rather than the ovary [24] and is associated with a high MUC2 expression which confirms the intestinal rather than an ovarian origin for the disease [12]. Whilst MUC2 appears to be a reliable molecular marker for PMP [12, 13, 14], the expression of MUC5AC, albeit in lesser amounts, has also been reported in this disease [13, 14]. MUC2 is the dominant mucin in the colon [25] and MUC5AC is found in the stomach [26] and respiratory tract [27].
In a previous study of a 58-year-old male patient [13] we showed the expression of not only MUC2 and MUC5AC, but also, for the first time, the presence of MUC5B [13], in the very viscous mucus in PMP [27]. These mucins together with another gastric mucin, MUC6 [28], are secreted gel-forming mucins, which could explain the highly viscous nature of the material obtained from such patients, the occurrence of which is associated with the morbidity and mortality of the disease [12]. Sheehan et al. [29] reported that respiratory mucus of a patient who died in status asthmaticus presented with tenacious plugs of mucus [30] that ‘tether’ to the airway epithelium [31], presumably because of the presence of two glycoforms of MUC5B. The difficulties of the extraction of mucus from human small intestine which expresses largely MUC2, even in chaotropic agents such as guanidinium chloride, producing an insoluble glycoprotein complex, have been previously reported [25].
In this study we obtained mucus from a 50-year-old female with PMP that originated in the gastrointestinal tract and spread to the ovary. Biochemical analysis of the soluble phase of the visco-elastic material in the abdominal cavity of the patient again showed the presence of MUC2, MUC5AC and MUC5B (to our knowledge MUC5B has not been previously shown in this material in a female patient), and a-1-acid glycoprotein. This particular PAS-positive glycoprotein has previously been reported in crude mucus scrapings of patients with carcinoma of the stomach, which was later identified as a-1-acid glycoprotein, a marker for inflammation [23, 32, 33]. Interestingly a-1-acid glycoprotein was expressed in the parietal cells of the gastric mucosa rather than the mucin-producing cells [33]. We now report it to be present in purified material obtained from PMP.
Immunohistochemical studies showed, in addition, the presence of MUC4, a transmembrane mucin, which again to our knowledge has not been previously reported. MUC4 has been reported to be a significant clinical marker in the diagnosis of pancreatic cancer [4, 34], an aggressive cancer that is increasing worldwide with a survival rate ranging from 4 to 6 months for patients diagnosed with metastatic disease [35]. MUC4 is not expressed in normal pancreas or chronic pancreatitis, but is highly expressed in human pancreatic carcinomas [36, 37, 38]. The expression of MUC4 in this case in both the primary appendiceal tumour and in the ovary, at a level >50%, warrants a closer investigation of the potential role of MUC4 in the diagnosis and prognosis of PMP. The limited range of antibodies in our possession allowed for the staining of only those mucins reported in this study. It is possible that the tissue and secreted material obtained from this patient expresse mucins besides those reported here.
As in our previous study on the biochemistry of PMP, the high viscosity of the material obtained from such patients makes it extremely difficult to solubilize despite reduction with DTT during the extraction process, resulting in a low yield of soluble material available for further purification of mucins for analysis. Different methods of removing the protein which could be contributing to the viscosity of the material are being considered. Thus far we have found preparative gel filtration to be unsuccessful due to blockages in the Sepharose 2B column even after rather extensive dilution of the sample [13]. In this study analysis by SDS-PAGE showed that mucus had to be both reduced with DTT and digested with enzyme/papain prior to electrophoresis to enable the entry of mucin into the gel, albeit still at the top of the gel. This could well be through the presence of MUC5B [13], a lowly charged glycoform which is thought to be responsible for the tenacity of respiratory mucin secretions in asthma [29]. Also the huge amounts of protein associated with material in samples obtained from patients with PMP could contribute to the viscous nature of the material [13].
We have found gel-forming mucins MUC2, MUC5AC, MUC5B and a transmembrane mucin MUC4 in the abdominal fluid and neoplastic tissue of a patient with PMP. The appearance of MUC5B in this study and previously [13] and MUC4 in this case are, to our knowledge, new findings which require further investigation with respect to the morbidity and mortality of the disease and the diagnostic and prognostic potential of these mucins in this disease.
Acknowledgements
This work was supported by the Medical Research Council of South Africa and the University of Cape Town Research Grant. Thank you to Sandra Gendler of the Mayo Clinic College of Medicine, Scottsdale, Arizona, USA and to Surinder Batra of the Nebraska Medical Center, Omaha, Nebraska, USA for CT2 and MUC4 antibodies, respectively.
Footnotes
A.S.M. conceived of and designed the study, participated in its design and coordination and both drafted and finalised the manuscript. N.C. used the material as his project towards a BSc (Hons) degree. D.G. was the pathologist who analysed the slides and helped with writing the histology, Z.L. participated in the biochemical studies and M.T. did all the histology. D.K. and P.G. contributed ideas to the design and coordination of the study and P.G. operated on the patient and helped with the first draft of the paper. J.R. is our amino acid analyst. All authors read and approved the final manuscript.
This is an Open Access article licensed under the terms of the Creative Commons Attribution-NonCommercial-NoDerivs 3.0 License (www.karger.com/OA-license), applicable to the online version of the article only. Distribution for non-commercial purposes only.
References
- 1.Allen A. Structure and function of gastric mucus. In: Johnson LR, editor. Physiology of the Gastro-Intestinal Tract. New York: Raven Press; 1981. pp. 617–639. [Google Scholar]
- 2.Bobek LA, Liu J, Sait SN, Shows TB, Bobek YA, Levine MJ. Structure and chromosomal localization of the human salivary mucin gene, MUC7. Genomics. 1996;31:277–282. doi: 10.1006/geno.1996.0049. [DOI] [PubMed] [Google Scholar]
- 3.Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- 4.Mall AS. Analysis of mucins: role in laboratory diagnosis. J Clin Pathol. 2008;61:1018–1024. doi: 10.1136/jcp.2008.058057. [DOI] [PubMed] [Google Scholar]
- 5.Taylor-Papadimitriou J, Burchell J, Miles DW, Dalziel M. MUC1 and cancer. Biochim Biophys Acta. 1999;1455:301–313. doi: 10.1016/s0925-4439(99)00055-1. [DOI] [PubMed] [Google Scholar]
- 6.Reis CA, David L, Correa P, Carneiro F, de Bolos C, Garcia E, Mandel U, Clausen H, Sobrinho-Simoes M. Intestinal metaplasia of human stomach displays distinct patterns of mucin (MUC1, MUC2, MUC5AC, and MUC6) expression. Cancer Res. 1999;59:1003–1007. [PubMed] [Google Scholar]
- 7.Taylor KL, Mall AS, Barnard RA, Ho SB, Cruse JP. Immunohistochemical detection of gastric mucin in normal and disease states. Oncol Res. 1998;10:465–473. [PubMed] [Google Scholar]
- 8.Ho SB, Shekels LL, Toribara NW, Kim YS, Lyftogt C, Cherwitz DL, Niehans GA. Mucin gene expression in normal, preneoplastic, and neoplastic human gastric epithelium. Cancer Res. 1995;55:2681–2690. [PubMed] [Google Scholar]
- 9.Graham RA, Burchell JM, Taylor-Papadimitriou J. The polymorphic epithelial mucin: potential as an immunogen for a cancer vaccine. Cancer Immunol Immunother. 1996;42:71–80. doi: 10.1007/s002620050254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Devine PL, McKenzie IF. Mucins: structure, function, and associations with malignancy. Bioessays. 1992;14:619–625. doi: 10.1002/bies.950140909. [DOI] [PubMed] [Google Scholar]
- 11.Weaver CH. Mucocele of the appendix with pseudomucinous degeneration. Am J Surg. 1937;16:523–526. [Google Scholar]
- 12.O'Connell JT, Tomlinson JS, Roberts AA, McGonigle KF, Barsky SH. Pseudomyxoma peritonei is a disease of MUC2-expressing goblet cells. Am J Pathol. 2002;161:551–564. doi: 10.1016/S0002-9440(10)64211-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mall AS, Chirwa N, Govender D, Lotz Z, Tyler M, Rodrigues J, Kahn D, Goldberg P. MUC2, MUC5AC and MUC5B in the mucus of a patient with pseudomyxoma peritonei: biochemical and immunohistochemical study. Pathol Int. 2007;57:537–547. doi: 10.1111/j.1440-1827.2007.02137.x. [DOI] [PubMed] [Google Scholar]
- 14.O'Connell JT, Hacker CM, Barsky SH. MUC2 is a molecular marker for pseudomyxoma peritonei. Mod Pathol. 2002;15:958–972. doi: 10.1097/01.MP.0000026617.52466.9F. [DOI] [PubMed] [Google Scholar]
- 15.Mall AS, Sellars LA, Allen A. Purification of pig duodenal mucus glycoprotein from protein and nucleic acid. Biochem Soc Trans. 1987;15:1047–1048. [Google Scholar]
- 16.Mall AS, Hutton DA, Coan RA, et al. Gastric mucins: different size of subunit according to reducing conditions. Biochem Soc Trans. 1988;16:585–586. [Google Scholar]
- 17.Carlstedt I, Lindgren H, Sheehan JK. The macromolecular structure of human cervical-mucus glycoproteins. Studies on fragments obtained after reduction of disulphide bridges and after subsequent trypsin digestion. Biochem J. 1983;213:427–435. doi: 10.1042/bj2130427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carlstedt I, Lindgren H, Sheehan JK, Ulmsten U, Wingerup L. Isolation and characterization of human cervical-mucus glycoproteins. Biochem J. 1983;211:13–22. doi: 10.1042/bj2110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thornton DJ, Carlstedt I, Howard M, Devine PL, Price MR, Sheehan JK. Respiratory mucins: identification of core proteins and glycoforms. Biochem J. 1996;316(Pt 3):967–975. doi: 10.1042/bj3160967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thornton J. Characterization of mucins for cultured normal human tracheobronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2000;278:1118–1128. doi: 10.1152/ajplung.2000.278.6.L1118. [DOI] [PubMed] [Google Scholar]
- 21.Mantle M, Allen A. A colorimetric assay for glycoproteins based on the periodic acid/Schiff stain [proceedings] Biochem Soc Trans. 1978;6:607–609. doi: 10.1042/bst0060607. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Mall AS, McLeod HA, Hickman R, Kahn D, Dent DM. Fragmentation pattern of mucins in normal and diseased gastric mucosae: a glycoprotein fractionates with gastric mucins purified from mucosal scrapings of cancer and peptic ulcer patients. Digestion. 1999;60:216–226. doi: 10.1159/000007662. [DOI] [PubMed] [Google Scholar]
- 24.Ronnett BM, Kurman RJ, Zahn CM, Shmookler BM, Jablonski KA, Kass ME, Sugarbaker PH. Pseudomyxoma peritonei in women: a clinicopathologic analysis of 30 cases with emphasis on site of origin, prognosis, and relationship to ovarian mucinous tumors of low malignant potential. Hum Pathol. 1995;26:509–524. doi: 10.1016/0046-8177(95)90247-3. [DOI] [PubMed] [Google Scholar]
- 25.Herrmann A, Davies, Lindell G, Martensson S, Packer NH, Swallow DM, Carlstedt I. Studies on the ‘insoluble’ glycoprotein complex from human colon. Identification of reduction-insensitive MUC2 oligomers and C-terminal cleavage. J Biol Chem. 1999;274:15828–15836. doi: 10.1074/jbc.274.22.15828. [DOI] [PubMed] [Google Scholar]
- 26.Ho SB, Roberton AM, Shekels LL, Lyftogt CT, Niehans GA, Toribara NW. Expression cloning of gastric mucin complementary DNA and localization of mucin gene expression. Gastroenterology. 1995;109:735–747. doi: 10.1016/0016-5085(95)90380-1. [DOI] [PubMed] [Google Scholar]
- 27.Davies, Svitacheva N, Lannefors L, Kornfalt R, Carlstedt I. Identification of MUC5B, MUC5AC and small amounts of MUC2 mucins in cystic fibrosis airway secretions. Biochem J. 1999;344(Pt 2):321–330. [PMC free article] [PubMed] [Google Scholar]
- 28.De Bolos C, Garrido M, Real FX. MUC6 apomucin shows a distinct normal tissue distribution that correlates with Lewis antigen expression in the human stomach. Gastroenterology. 1995;109:723–734. doi: 10.1016/0016-5085(95)90379-8. [DOI] [PubMed] [Google Scholar]
- 29.Sheehan JK, Howard M, Richardson PS, Longwill T, Thornton DJ. Physical characterization of a low-charge glycoform of the MUC5B mucin comprising the gel-phase of an asthmatic respiratory mucous plug. Biochem J. 1999;338(Pt 2):507–513. [PMC free article] [PubMed] [Google Scholar]
- 30.Jeffery PK. Differences and similarities between chronic obstructive pulmonary disease and asthma. Clin Exp Allergy. 1999;29(suppl 2):14–26. [PubMed] [Google Scholar]
- 31.Rogers DF. Mucus pathophysiology in COPD: differences to asthma, and pharmacotherapy. Monaldi Arch Chest Dis. 2000;55:324–332. [PubMed] [Google Scholar]
- 32.Mall AS, McConney Z, Lotz Z, Tyler M, Mcleod H, Hickman R, Dent DM, Kahn D. Increased fragmentation of MUC5AC mucins in gastric juice of patients with ulceration and carcinoma. S Afr J Sci. 2000;96:39–43. [Google Scholar]
- 33.Chirwa N: The isolation, purification, tissue localization and identification of a glycoprotein found in the crude mucus gel of patients with carcinoma of the stomach. Thesis for the Degree of Doctor of Philosophy, Department of Surgery, University of Cape Town, 2008.
- 34.Singh AP, Chaturvedi P, Batra SK. Emerging roles of MUC4 in cancer: a novel target for diagnosis and therapy. Cancer Res. 2007;67:433–436. doi: 10.1158/0008-5472.CAN-06-3114. [DOI] [PubMed] [Google Scholar]
- 35.Moniaux N, Andrianifahanana M, Brand RE, Batra SK. Multiple roles of mucins in pancreatic cancer, a lethal and challenging malignancy. Br J Cancer. 2004;91:1633–1638. doi: 10.1038/sj.bjc.6602163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Andrianifahanana M, Moniaux N, Schmied BM, Ringel J, Friess H, Hollingsworth MA, Buchler MW, Aubert JP, Batra SK. Mucin (MUC) gene expression in human pancreatic adenocarcinoma and chronic pancreatitis: a potential role of MUC4 as a tumor marker of diagnostic significance. Clin Cancer Res. 2001;7:4033–4040. [PubMed] [Google Scholar]
- 37.Balague C, Gambus G, Carrato C, Porchet N, Aubert JP, Kim YS, Real FX. Altered expression of MUC2, MUC4, and MUC5 mucin genes in pancreas tissues and cancer cell lines. Gastroenterology. 1994;106:1054–1061. doi: 10.1016/0016-5085(94)90767-6. [DOI] [PubMed] [Google Scholar]
- 38.Choudhury A, Moniaux N, Winpenny JP, Hollingsworth MA, Aubert JP, Batra SK. Human MUC4 mucin cDNA and its variants in pancreatic carcinoma. J Biochem. 2000;128:233–243. doi: 10.1093/oxfordjournals.jbchem.a022746. [DOI] [PubMed] [Google Scholar]