Abstract
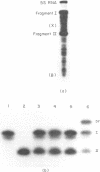
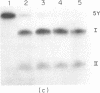
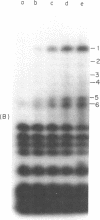
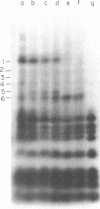
Aminomethyltrioxsalen (AMT), a psoralen, is known to cause interstrand crosslinks in double stranded nucleic acids. We have demonstrated the photochemical reversal of this reaction, and have used this result to develop a method for identification of specific sequences which are adjacent because of RNA secondary structure formation. E. coli 5S rRNA is used as a model system. We isolated and characterized a product that is derived from the stem region of 5S RNA.
Full text
PDF




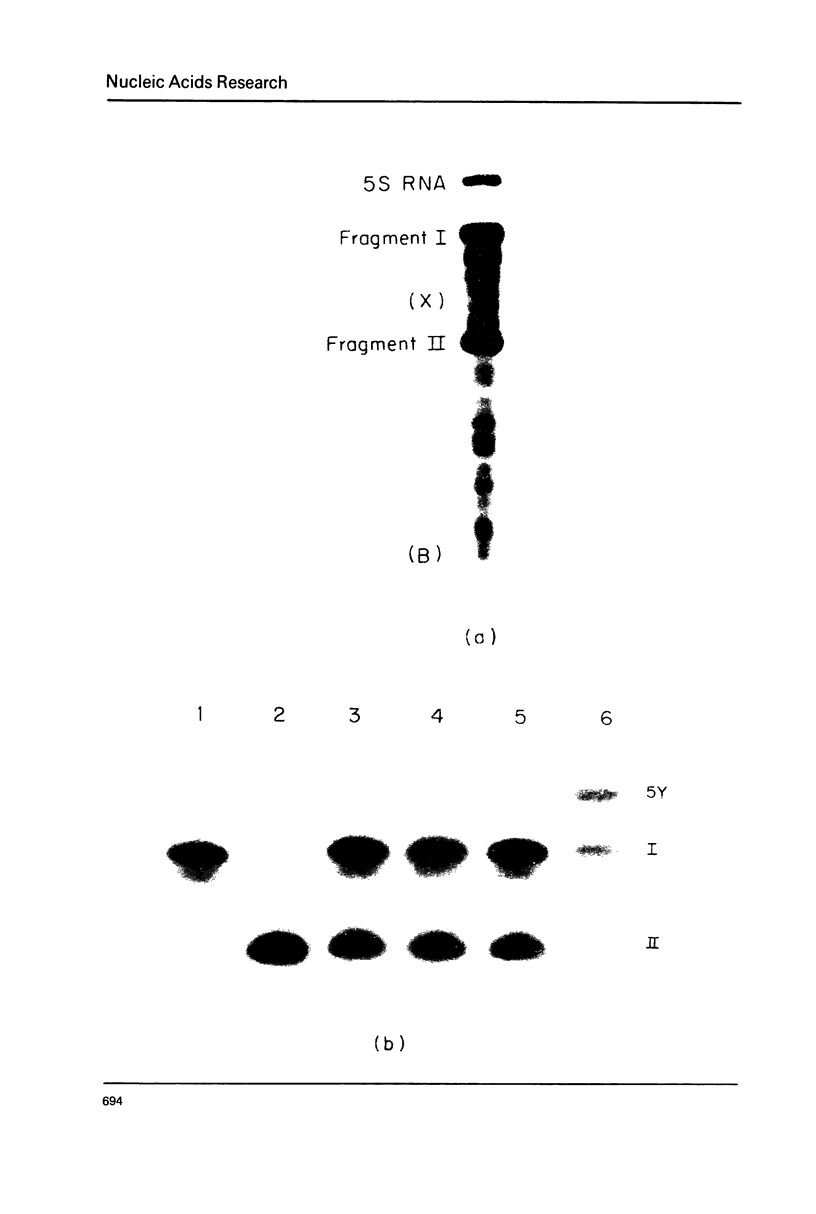

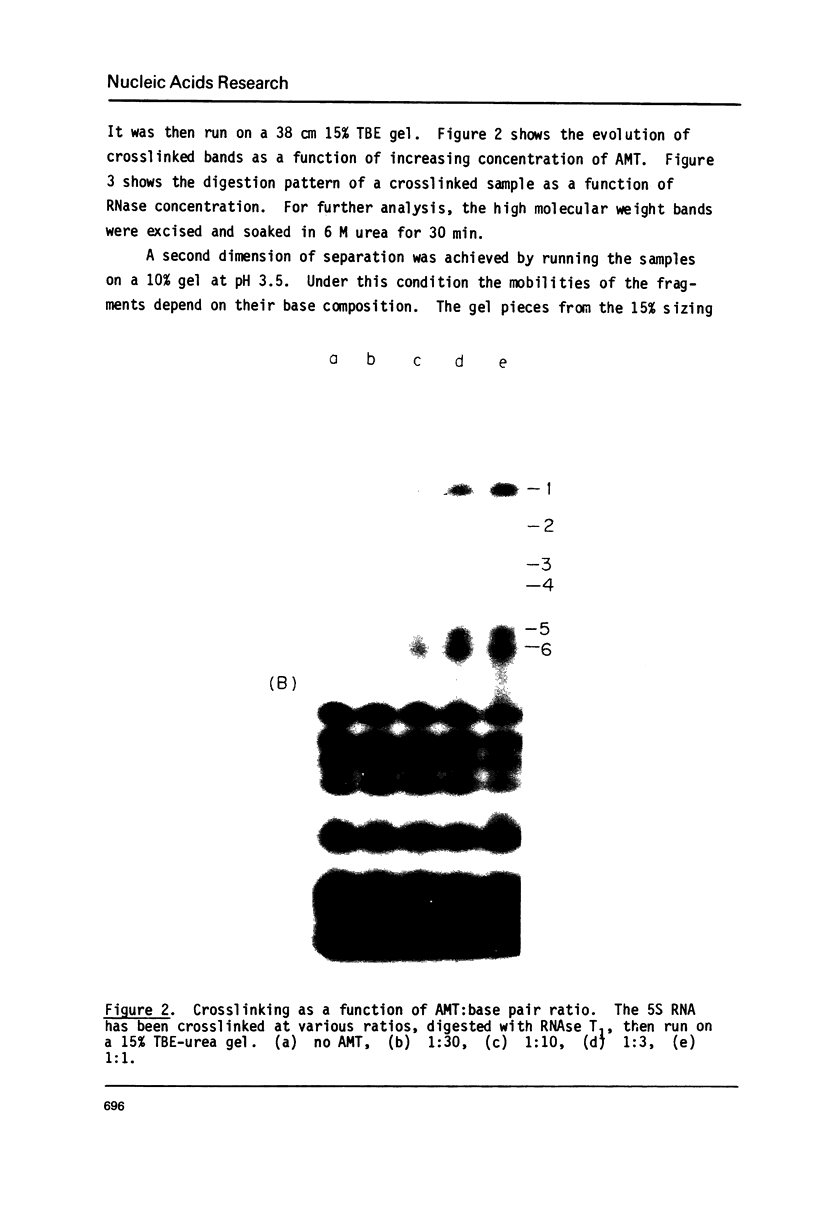
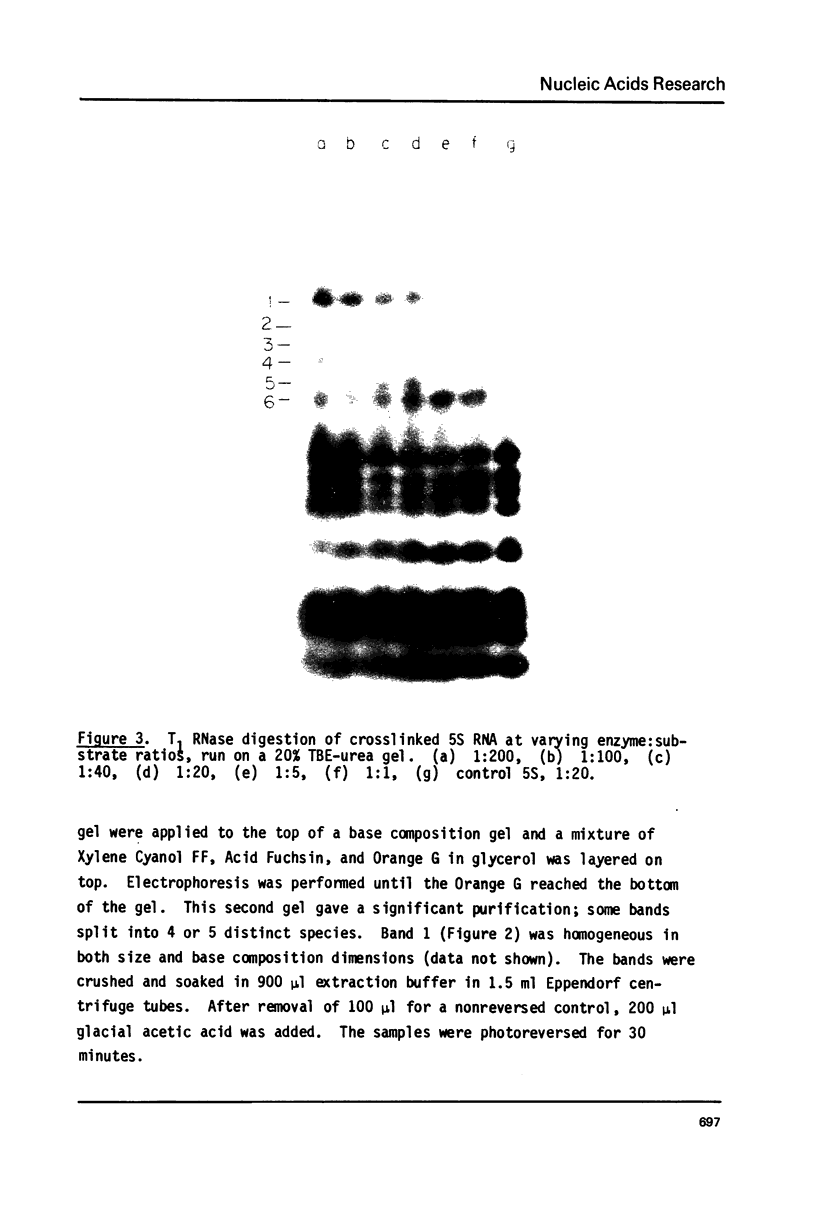

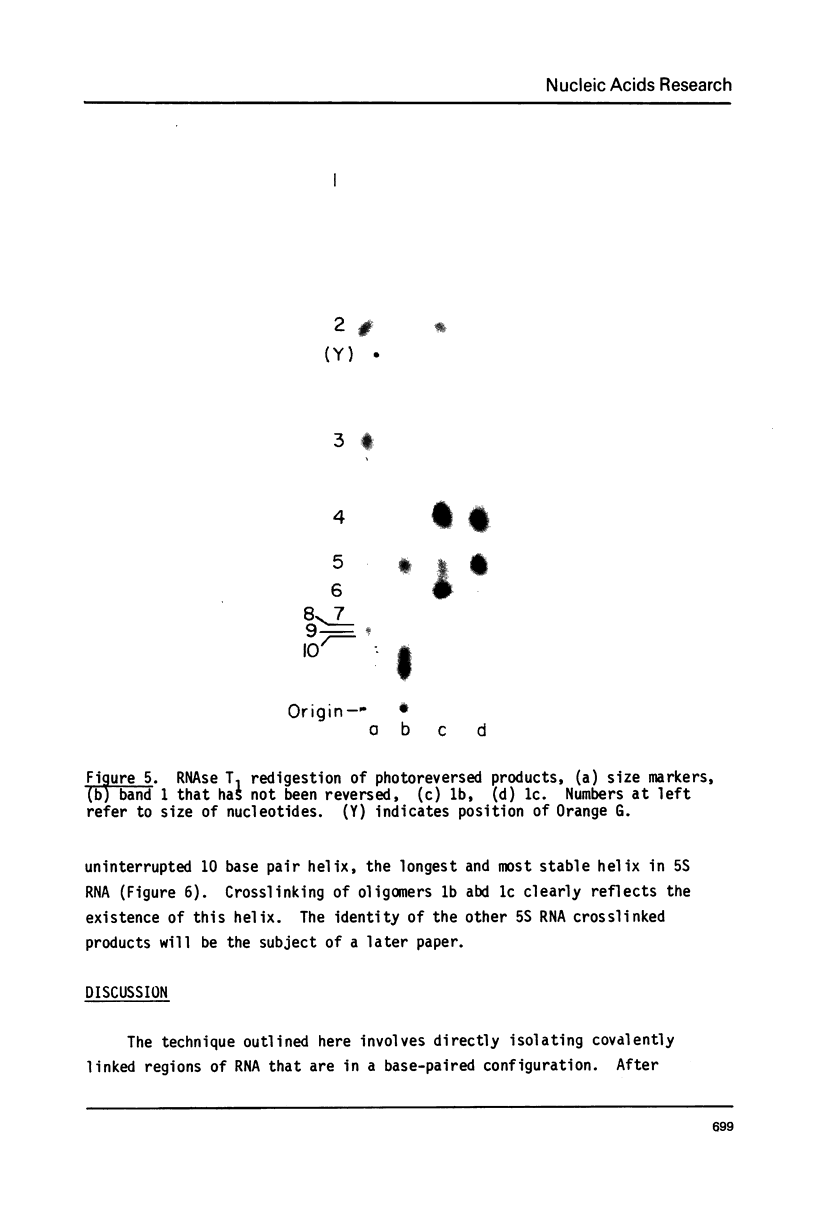
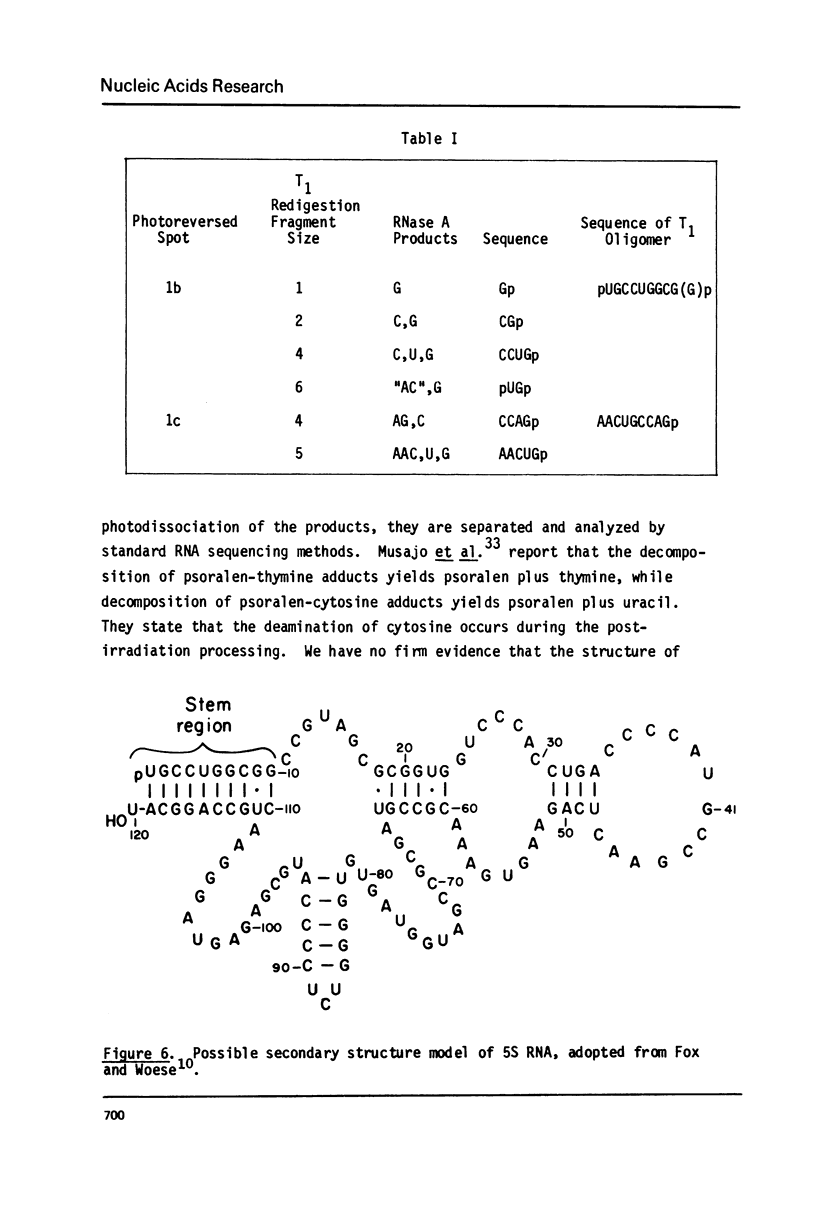



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod V. D., Vartikyan R. M., Aivazashvili V. A., Beabealashvili R. S. Specific termination of RNA polymerase synthesis as a method of RNA and DNA sequencing. Nucleic Acids Res. 1978 Oct;5(10):3549–3563. doi: 10.1093/nar/5.10.3549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellemare G., Jordan B. R., Rocca-Serra J., Monier R. Accessibility of Escherichia coli 5S RNA base residues to chemical reagents. Influence of chemical alterations on the affinity of 5S RNA for the 50S subunit structure. Biochimie. 1972;54(11):1453–1466. doi: 10.1016/s0300-9084(72)80087-7. [DOI] [PubMed] [Google Scholar]
- Borer P. N., Dengler B., Tinoco I., Jr, Uhlenbeck O. C. Stability of ribonucleic acid double-stranded helices. J Mol Biol. 1974 Jul 15;86(4):843–853. doi: 10.1016/0022-2836(74)90357-x. [DOI] [PubMed] [Google Scholar]
- Bresler S. E., Kalinin V. L., Perumov D. A. Inactivation and mutagenesis on isolated DNA. V. The importance of repairing enzymes for the inactivation of transforming DNA in vitro. Mutat Res. 1970 Jan;9(1):1–19. doi: 10.1016/0027-5107(70)90066-7. [DOI] [PubMed] [Google Scholar]
- Brosius J., Palmer M. L., Kennedy P. J., Noller H. F. Complete nucleotide sequence of a 16S ribosomal RNA gene from Escherichia coli. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4801–4805. doi: 10.1073/pnas.75.10.4801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet J. P., Pederson T. Heterogeneous nuclear RNA double-stranded regions probed in living HeLa cells by crosslinking with the psoralen derivative aminomethyltrioxsalen. Proc Natl Acad Sci U S A. 1979 Feb;76(2):755–759. doi: 10.1073/pnas.76.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra P., Kraft S., Wacker A., Rodighiero S., Dall'Acqua F., Marciani S. Studies on the reactivation of bacteria photodamaged by psoralen. Biophysik. 1971;7(4):251–258. doi: 10.1007/BF01190236. [DOI] [PubMed] [Google Scholar]
- Dall'Acqua F., Marciani S., Bordin F., Bevilacqua R. Studies on the photoreaction (365 nm) between psoralen and thymine. Ric Sci. 1968 Nov;38(11):1094–1099. [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdmann V. A. Structure and function of 5S and 5.8 S RNA. Prog Nucleic Acid Res Mol Biol. 1976;18:45–90. [PubMed] [Google Scholar]
- Fox G. E., Woese C. R. 5S RNA secondary structure. Nature. 1975 Aug 7;256(5517):505–507. doi: 10.1038/256505a0. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. 3. Small internal loops resulting from mismatches. J Mol Biol. 1973 Aug 5;78(2):301–319. doi: 10.1016/0022-2836(73)90118-6. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. II. Small hairpin loops. J Mol Biol. 1973 Feb 5;73(4):497–511. doi: 10.1016/0022-2836(73)90096-x. [DOI] [PubMed] [Google Scholar]
- Igali S., Bridges B. A., Ashwood-Smith M. J., Scott B. R. Mutagenesis in Escherichia coli. IV. Photosensitization to near ultraviolet light by 8-methoxypsoralen. Mutat Res. 1970 Jan;9(1):21–30. doi: 10.1016/0027-5107(70)90067-9. [DOI] [PubMed] [Google Scholar]
- Isaacs S. T., Shen C. K., Hearst J. E., Rapoport H. Synthesis and characterization of new psoralen derivatives with superior photoreactivity with DNA and RNA. Biochemistry. 1977 Mar 22;16(6):1058–1064. doi: 10.1021/bi00625a005. [DOI] [PubMed] [Google Scholar]
- Jack A., Ladner J. E., Klug A. Crystallographic refinement of yeast phenylalanine transfer RNA at 2-5A resolution. J Mol Biol. 1976 Dec 25;108(4):619–649. doi: 10.1016/s0022-2836(76)80109-x. [DOI] [PubMed] [Google Scholar]
- Jordan B. R., Monier R. 5 s RNA molecules formed by reassociation of separated fragments. J Mol Biol. 1971 Jul 14;59(1):219–222. doi: 10.1016/0022-2836(71)90425-6. [DOI] [PubMed] [Google Scholar]
- Jordan B. R. Studies on 5 s RNA conformation by partial ribonuclease hydrolysis. J Mol Biol. 1971 Feb 14;55(3):423–439. doi: 10.1016/0022-2836(71)90327-5. [DOI] [PubMed] [Google Scholar]
- Kramer F. R., Mills D. R. RNA sequencing with radioactive chain-terminating ribonucleotides. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5334–5338. doi: 10.1073/pnas.75.11.5334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musajo L., Bordin F., Caporale G., Marciani S., Rigatti G. Photoreactions at 3655 Angstrom between pyrimidine bases and skin-photosensitizing furocoumarins. Photochem Photobiol. 1967 Oct;6(10):711–719. doi: 10.1111/j.1751-1097.1967.tb08736.x. [DOI] [PubMed] [Google Scholar]
- Quigley G. J., Wang A. H., Seeman N. C., Suddath F. L., Rich A., Sussman J. L., Kim S. H. Hydrogen bonding in yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4866–4870. doi: 10.1073/pnas.72.12.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Schibler U., Wyler T., Hagenbüchle O. Changes in size and secondary structure of the ribosomal transcription unit during vertebrate evolution. J Mol Biol. 1975 May 25;94(3):503–517. doi: 10.1016/0022-2836(75)90217-x. [DOI] [PubMed] [Google Scholar]
- Scott B. R., Pathak M. A., Mohn G. R. Molecular and genetic basis of furocoumarin reactions. Mutat Res. 1976;39(1):29–74. doi: 10.1016/0165-1110(76)90012-9. [DOI] [PubMed] [Google Scholar]
- Simoncsits A., Brownlee G. G., Brown R. S., Rubin J. R., Guilley H. New rapid gel sequencing method for RNA. Nature. 1977 Oct 27;269(5631):833–836. doi: 10.1038/269833a0. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Borer P. N., Dengler B., Levin M. D., Uhlenbeck O. C., Crothers D. M., Bralla J. Improved estimation of secondary structure in ribonucleic acids. Nat New Biol. 1973 Nov 14;246(150):40–41. doi: 10.1038/newbio246040a0. [DOI] [PubMed] [Google Scholar]
- Wagner R., Garrett R. A. A new RNA-RNA crosslinking reagent and its application to ribosomal 5S RNA. Nucleic Acids Res. 1978 Nov;5(11):4065–4075. doi: 10.1093/nar/5.11.4065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellauer P. K., Dawid I. B. Secondary structure maps of ribosomal RNA and DNA. I. Processing of Xenopus laevis ribosomal RNA and structure of single-stranded ribosomal DNA. J Mol Biol. 1974 Oct 25;89(2):379–395. doi: 10.1016/0022-2836(74)90526-9. [DOI] [PubMed] [Google Scholar]
- Wollenzien P. L., Youvan D. C., Hearst J. E. Structure of psoralen-crosslinked ribosomal RNA from Drosophila melanogaster. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1642–1646. doi: 10.1073/pnas.75.4.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]