Abstract
Objectives
The synergistic effect of resistance exercise and protein ingestion on muscle protein anabolism in young adults has been well described. However, it is unclear if this relationship is maintained in older adults who are at greater risk of sarcopenic muscle loss. To this end, we sought to determine if the synergistic response to a bout of resistance exercise and a protein-rich lean beef meal was altered by age.
Setting
The University of Texas Medical Branch, Clinical Research Center, Galveston, Texas. Participants: Healthy young (n=7, 29±3 y) and older (n=7, 67±2 y) adults.
Design
Mixed muscle fractional synthesis rate (FSR) was calculated during a 3 h post-absorptive/rest period and again during a 5 h period following ingestion of a protein-rich meal (340 g lean beef) and bout of resistance exercise (6 sets of 8 repetitions of isotonic knee extension exercise at 80% one repetition maximum).
Measurements
Venous blood samples and vastus lateralis muscle biopsy samples were obtained during a primed (2.0 μmol/kg) constant infusion (0.08 μmol·kg−1min−1) of L-[ring-13C6] phenylalanine.
Results
Mixed muscle FSR increased by approximately 108% in both young [pre: 0.073±0.008; post: 0.156±0.021(SE) %/h, p<0.001] and older adults (pre: 0.075±0.004; post: 0.152±0.017 %/h, p=0.003) following the meal and resistance exercise bout.
Conclusion
Aging does not diminish the increase in muscle protein synthesis following a high-quality protein rich meal and bout of resistance exercise.
Keywords: Nutrition, stable isotopes, beef, sarcopenia, diet
Introduction
The preservation of lean muscle mass and functional capacity are key components of healthy aging and the maintenance of independence (1, 2). Healthy lifestyle choices such as regular physical exercise and a balanced diet containing a moderate amount of high-quality protein are generally regarded as effective strategies to achieve this goal. Resistance exercise has a well described and potent effect on muscle protein anabolism across the age span (3–7). Similarly, the ingestion of free-form essential amino acids or protein (e.g., whey, soy, milk, beef) have also been shown to acutely increase muscle protein synthesis in young and older adults (8–12).
There is increasing evidence that older adults experience anabolic resistance or a blunted increase in muscle protein synthesis following meals containing what could be considered a “small or sub-optimal” amount of protein (i.e., less than approx. 15 g protein/meal) (13–15). However, it appears this is largely a dose-response phenomenon that may be overcome with the provision of slightly greater amounts of protein. For example, we recently reported that ingestion of a moderate (4 oz; 30 g protein) or large (12 oz; 90 g protein) serving of lean beef increased mixed muscle protein synthesis by approximately 50% in both young (34±3 y) and older (68±2 y) adults (10, 16).
The synergistic anabolic response to resistance exercise and hyperaminoacidemia has been well documented in young adults (9, 12, 17, 18). While we were confident that older adults would respond positively to the combination of resistance exercise and a protein rich meal, we did not know if this response would be as robust as a younger population. This is a key area of exploration as we continue to identify and refine practical and effective interventions to prevent sarcopenic muscle loss.
The goal of this study was to determine if the anabolic response of a bout of resistance exercise in combination with a high quality protein-rich meal was maintained in older adults compared to their younger counterparts.
Methods
Subjects and Experimental Design
Healthy young (n=7 {3 Caucasian, 3 Hispanic, 1 Africian American}, 29±3 y) and older (n=7 {4 Caucasian, 2 Hispanic, 1 Africian American}, 67±2 y) adults, participated in this study (Table 1). Participants were recruited through advertisements and the Sealy Center on Aging Volunteer Registry at The University of Texas Medical Branch (UTMB). This study was approved by the Institutional Review Board and Clinical Research Center Scientific Committee at UTMB and overseen by an independent data monitoring board.
Table 1.
Physical characteristics of study participants
| Young (n = 7) | Elderly (n = 7) | p | |
|---|---|---|---|
| Age, y | 29 ± 3 | 67 ± 2 | |
| Male (n) | 3 | 3 | |
| Female (n) | 4 | 4 | |
| Height, cm | 1.64 ± 0.04 | 1.67 ± 0.01 | 0.44 |
| Mass, kg | 79 ± 10 | 76 ± 5 | 0.80 |
| BMI, kg/cm2 | 28.9 ± 2.8 | 27.1 ± 1.5 | 0.58 |
| Body Fat, % | 31.9 ± 3.5 | 30.7 ± 3.7 | 0.82 |
| Lean Mass, kg | 49.6 ± 5.8 | 50.6 ± 3.8 | 0.89 |
Note: Values are means ± standard error of the mean; BMI = body mass index.
Written informed consent was obtained from all participants. Screening for study eligibility included a medical history, blood count, plasma electrolytes, blood glucose concentration and liver and renal function tests. Exclusion criteria included recent injury, abnormal dentition, metabolically unstable disease, low hematocrit or hemoglobin, vascular disease, hypertension, cardiac abnormality or other condition with the potential to confound the data or place volunteers at increased risk. All participants were physically active and independent but were not athletically trained.
For seventy-two hours prior to admission, participants were asked to maintain their normal diet and avoid strenuous activity. Participants stayed overnight in the Clinical Research Center and were studied the following morning after an overnight fast. Subjects remained largely physically inactive (i.e., rested in bed) for the duration of the study. On the morning of the study at approximately 0530, an 18-gauge polyethylene catheter (Insyte-W; Becton Dickinson, Sandy, UT) was inserted into a forearm vein for blood sampling. A second 18-gauge polyethylene catheter was inserted into a forearm vein of the contralateral limb for stable isotope tracer infusion. Background blood samples were drawn for the analysis of phenylalanine enrichments and concentrations (serum separator tubes; BD Vacutainer SST, Franklin Lakes, NJ). A primed (2 μmol/kg), constant infusion (0.08 μmol·kg−1min−1) of L-[ring-13C6] phenylalanine (Cambridge Isotope Laboratories, Andover, MA) was started and maintained for 11 hours.
During the post-absorptive period (0900–1200), venous blood samples were obtained hourly. Following the meal and exercise bout, venous blood samples were obtained every twenty minutes for the duration of the study (5 hours), (see Figure 1). Three muscle biopsy samples (100–150 mg) were taken under local anesthesia (2% lidocane) from the lateral portion of the vastus lateralis of the leg, 10 cm to 15 cm above the knee, using a 5 mm Bergstrom biopsy needle as previously described (19).
Figure 1.
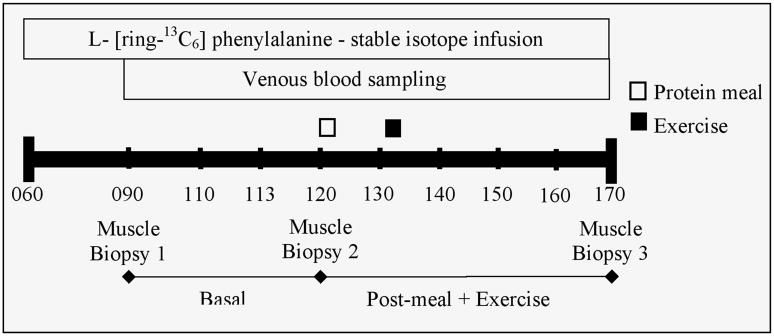
The infusion protocol. Venous blood samples and muscle biopsies were obtained before and after ingestion of aprotein-rich meal and bout of resistance exercise during an 11h infusion of L-[ring-13C6] phenylalanine
The pre-cooked, vacuumed sealed 90% lean ground beef meals were prepared by Texas Tech University. Each 340 g serving contained 660 kcal energy, 90 g protein and 33 g fat. The amino acid profile of the beef has been previously reported (10). We have also shown that plasma amino acid concentrations peak approximately 100 min following ingestion of a 113g serving of lean beef (10). The beef was gently warmed in a microwave oven and provided to the participant without condiments immediately following the second biopsy (1200). All volunteers were able to consume the meal within 10–15 minutes.
Sixty minutes after the meal, participants performed warm-up exercises followed by 6 sets of 8 repetitions of leg extension exercise at 80% of their one-repetition maximum (1RM). A two-minute rest period separated each set.
Analytical Methods
Plasma phenylalanine enrichment was calculated using gas chromatography mass spectroscopy (GC-MS; 6890 Plus GC, Agilent Technologies, Palo Alto, CA) as previously described (20, 21). Intracellular (mixed muscle) phenylalanine enrichments and concentrations were determined via a tertbutyldimethylsilyl derivative. Mixed muscle protein-bound L-[ring-13C6] phenylalanine enrichments were determined using GC-MS via the standard curve approach (22).
Calculations
Fractional synthesis rate (FSR) of mixed muscle protein was calculated by measuring the direct incorporation of L-[ring-13C6] phenylalanine into protein, via the precursor-product model:
where Ep1 and Ep2 are the enrichments of bound L-[ring-13C6] phenylalanine in two sequential biopsies, t is the time interval between two biopsies and Em is the mean L- [ring-13C6] phenylalanine enrichment in the muscle intracellular pool.
A pre-requisite for the determination of muscle intracellular phenylalanine enrichment is an isotopic steady state. While this assumption is met during the post-absorptive period, it is complicated by ingestion of a non-labeled phenylalanine source (e.g., lean beef) which transiently reduces the tracer/tracee ratio (i.e., decreased plasma L-[ring-13C6] phenylalanine enrichment) resulting in an underestimation of FSR. When free-form amino acids or rapidly digested proteins are ingested, an isotopic steady state may be maintained by adding a small quantity of tracer to the supplement (6, 11). However, the addition of L-[ring-13C6] phenylalanine to a meal of lean beef was clearly inappropriate. Therefore, as previously reported (10, 16), a correction factor (CF) was employed to account for the reduction in tracer/tracee ratio (i.e., decreased plasma L-[ring-13C6] phenylalanine enrichment) and underestimation of FSR following the ingestion of a non-labeled phenylalanine source:
where Ev(AUC) is the actual venous enrichment area under the curve between sequential biopsies (i.e., biopsy 2 and 3) and Ev(m2,m3) is the average venous enrichment at each biopsy time point (see Figure 2). This correction is based on the assumption that the depression in plasma phenylalanine enrichment following the unlabelled meal reflects the depression in muscle intracellular phenylalanine enrichment.
Figure 2.
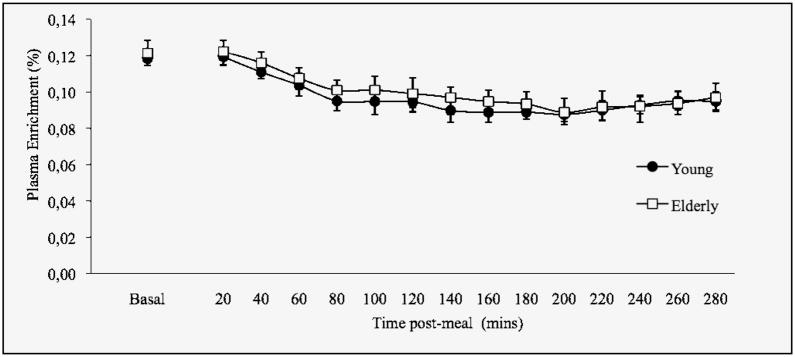
Mean (± SEM) postabsorptive and postprandial plasma L-[ring-13C6] phenylalanine enrichments
*denotes a significant difference from basal enrichment
Statistical Analysis
Data are presented as means ± standard error of the mean. Comparison of physical characteristics was carried out using two-tailed unpaired t-tests for equal variance. The change in FSR was analyzed using a two-way repeated measures ANOVA with one within-group factor (time: fasting vs. post-meal and resistance exercise) and one between-group factor (group: young vs. elderly). Secondary analysis was performed using pair-wise multiple comparison procedures with a Tukey correction. Analyses were performed using SigmaStat for Windows (version 3.5; Systat Software, Inc., San Jose, CA). Statistical significance for all analyses was accepted at α = 0.05.
Results
Physical Characteristics
The participants’ physical characteristics are presented in Table 1. There were no differences in height, body weight, body mass index, body fat or lean muscle mass. Although not directly excluded, older adults experiencing, or at greatest risk of sarcopenia were less likely to meet the conservative screening criteria put in place to protect participant safety. Consequently, age, and not health status, was the primary distinguishing variable in our volunteers.
Plasma Phenylalanine Enrichment
Post-absorptive plasma phenylalanine enrichments (tracer/tracee ratio) were similar in both age groups and there was a moderate dilution of labeled plasma phenylalanine following meal ingestion (Figure 2). The correction factor was consistent with previous studies (10, 16), and similar in young (avg., 0.78±0.06) and older (avg., 0.80±0.03) volunteers.
Mixed Muscle Protein Synthesis
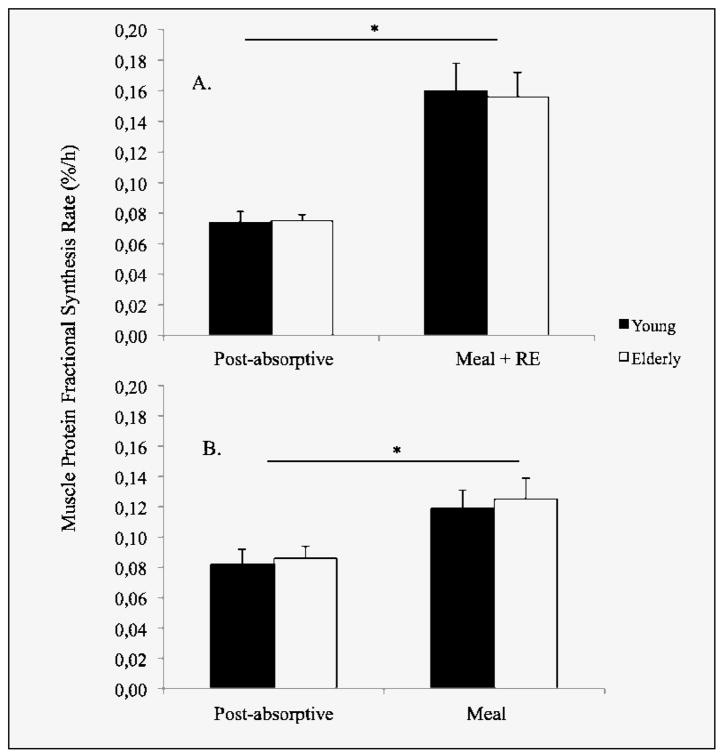
Post-absorptive mixed muscle FSR was similar in both age groups (Young: 0.073±0.008 %/h; Elderly: 0.075±0.004 %/h). During the 5 h period following the meal and bout of resistance exercise, FSR increased by approximately 108 % in both groups (Young: 0.156 ± 0.021 %/h, p < 0.00; Elderly: 0.152 ± 0.017 %/h, p=0.003), (Figure 3A). This corresponded to an additional 50% increase in FSR compared to previous cohorts receiving only the meal (10, 16) (Figure 3B).
Figure 3.
Mean (± SEM) mixed muscle fractional synthesis rate in young and older adults: A) before and after the protein-rich meal and bout of resistance exercise; B) following ingestion of the meal alone. Figure adapted from Symons et al., (16)
* denotes a significant increase from post-absorptive FSR
Plasma Insulin Response
During the post-absorptive period there were no age-related differences in plasma insulin concentrations. Following the meal and resistance exercise, plasma insulin concentrations increased in both the young (p < 0.001) and elderly (p < 0.001) and remained elevated for the duration of the study (3 hours: p < 0.001 and 4 hours: p < 0.001). There were no age-related differences detected (Figure 4).
Figure 4.
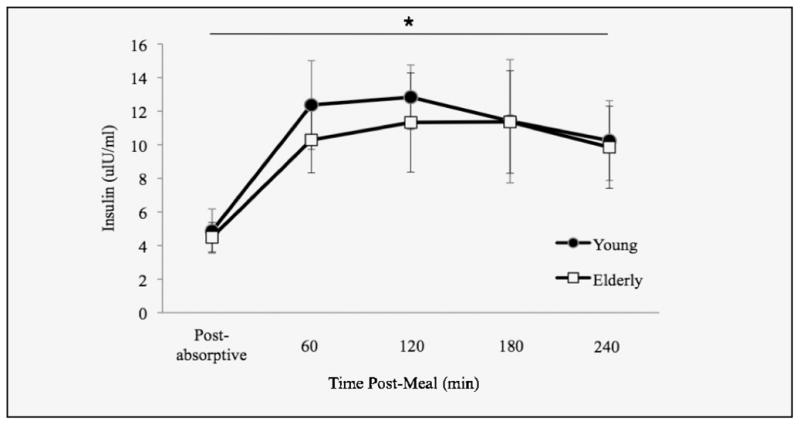
Mean (± SEM) postabsorptive and postprandial plasma insulin concentrations
* denotes a significant main effect for time.
Discussion
This study demonstrates that aging does not diminish the acute increase in muscle protein synthesis provided by a high-quality protein rich meal and bout of resistance exercise. These results are encouraging and are consistent with the message of promoting healthy aging through practical and sustainable lifestyle choices.
The ability of dietary protein and exercise to effectively stimulate skeletal muscle protein synthesis is central to the maintenance of muscle mass and function as we age. Our data are consistent with several earlier studies in young adults that explored changes in muscle protein anabolism in response to resistance exercise and free-form essential amino acids (EAA) or a variety of intact protein sources (i.e., whey, casein, milk, soy) (12, 17, 18, 23, 24). In one of the few studies to include both young and older subjects, Drummond et al. (25) noted a 100% (young) and 120% (elderly) increase in mixed muscle protein synthesis in response to a bout of leg extension exercise (8 sets of 10 repetitions at 70% 1RM) that was followed 60 minutes later by ingestion of 20 g of EAA. In our study, the EAA content of the more slowly digested lean beef was approximately 35 g, the meal was ingested 60 minutes prior to the exercise bout, and a total of 48 leg extension repetitions were performed. Nevertheless, despite these differences, the outcome of the two studies was similar, with our young and older volunteers experiencing a 108% increase in muscle protein synthesis.
The issue of optimizing muscle protein anabolism by manipulating the timing of protein/amino acid ingestion and resistance exercise continues to attract considerable academic and athletic attention (26, 27). In general, cell signaling and stable isotope studies suggest that an acute bout of resistance exercise temporarily inhibits protein synthesis via increased AMP-activated protein kinase (AMPK) activation and reduced phosphorylation of 4E-BP1 and other key regulators of translation initiation (12, 26, 28). However, approximately 60 minutes post-exercise, the capacity for muscle protein synthesis is restored and ultimately increased via the activation of protein kinase B, mTOR, S6K1 and eEF2 (29). Consequently, increasing plasma and intracellular amino acid availability during this rebound/recovery period (i.e., approximately 60 minutes following exercise) appears to offer the greatest anabolic advantage. However, care must be taken not to be misled by the nuances of controlled research studies. Specifically, it is important to consider that the majority of previous studies have characterized the protein/exercise timing response using rapidly digested protein or amino acids. Free-form amino acids and whey protein appear in the circulation within 15–30 minutes of ingestion (8, 9). In contrast, intact proteins, such as lean beef, may take up to 100 minutes to peak in the plasma (10). Consequently, in terms of the plasma amino acid precursor supply and ultimately the protein synthetic response, consuming a slowly digested protein-rich mixed meal 60 minutes prior to exercise, may be the physiological equivalent of ingesting a rapidly digested protein source 30–60 minutes post-exercise. Study design issues aside, thought should also be given to more practical considerations. In this instance, consuming a large protein rich meal prior to leg extension exercise was tolerated by all participants. However, recommendation on the most anabolic advantageous timing of meals and exercise must also be tempered by practical considerations such as satiety and gastric comfort.
We previously reported that a 113 g serving of 90% lean beef (30 g protein) produced the same ~50% increase in muscle protein synthesis as a much larger 340 g serving (90 g protein) (10, 16). It therefore appears likely that there is a ceiling effect for the stimulation of muscle protein synthesis by nutritional means alone (13, 30). However, it is also clear that the combination of a large protein rich meal and resistance exercise produces a robust acute synergistic effect, 2-fold greater than nutrition alone.
While it is tempting to project the results of this study and speculate on the longer-term benefits of exercise and a high-quality protein diet, a number of issues must be considered. Perhaps most importantly, there are several well controlled studies suggesting that while older individuals respond positively to resistance training, the concurrent provision of a diet higher in protein (>1.0 g protein/kg bodyweight) does not further enhance gains in muscle mass or strength as it does in younger adults (31, 32). This topic certainly warrants further investigation. Specifically, in longer duration feeding and exercise trials, we recommend that more attention be paid to the quantity and quality of protein ingested with each meal, rather than a more general recommendation for daily intake (e.g., 20–30 g protein/meal vs. 1.0 g protein/kg/day). For example, ingestion of more than approximately 30 g of protein in a single meal does not result in continued improvement in muscle protein synthesis (13, 16). Moreover, older adults experience anabolic resistance, or a blunted anabolic response when the protein/EAA content of a meal falls below a critical level (approximately 7–10 g EAA) (33). Thus, a diet in which the majority of an individual’s daily protein intake is consumed in a single meal is unlikely to optimize muscle protein anabolism over a 24 h period (30).
In the current study, volunteers were recruited and studied in concert with a previously published project (16). Consequently, we provided volunteers with a large serving of protein (340 g lean beef {12 oz}; 660 kcal; 90 g protein) to ensure a maximal nutrition-based response. In retrospect, this amount, while consistent with the overly generous portion sizes in many restaurants, it is clearly more than necessary to maximally stimulate muscle protein synthesis (13, 16, 30). We would hope that the combination of resistance exercise and a more moderate amount of protein (i.e., approximately 30 g) would produce a similar robust synergistic response.
The results of the current study should also be considered in the context of the elderly population studied. It is clear from Table 1 that our older volunteers were not sarcopenic or representative of a population with an obvious defect in protein metabolism. Rather, they should be considered to represent a best case aging-scenario, enabling us to conclude that, in the absence of accompanying disease or disability, aging per se does not alter the anabolic response to a protein-rich meal and bout of resistance exercise. It is unclear if sarcopenic older individuals would respond in a similar manner to their healthy counterparts. Certainly, a blunted response to these fundamental anabolic stimuli could contribute to the characteristic slow, progressive loss of muscle mass experienced by many older individuals (13, 34).
A final point that warrants comment is the fact that a number of similar and well performed studies from other research groups have identified an impairment in post-prandial protein metabolism in older individuals compared to their younger counterparts. While there are many procedural or location-specific factors that could contribute to this discrepancy, insightful commentary by Henderson et al., (35), noted that studies, such as ours, that do not demonstrate an age-specific difference in post-prandial protein metabolism, did not formally control or standardize the diets of participants during the days immediately preceding the metabolic study. The opposite appears to be true for those studies that have identified a blunted post-prandial anabolic response in elders. While valid arguments can be made for both designs, it is clear that we need to better understand the effects of habitual dietary practices on protein metabolism in elders.
In conclusion, the combination of a high-quality protein rich meal and resistance exercise produces a 2-fold greater increase in muscle protein synthesis than a meal alone. Further, aging does not necessarily impair the response to these two common and practical anabolic stimuli.
Acknowledgments
The authors would like to thank Shanon Casperson, David Chinkes, Tara Cocke, Christopher Danesi, Kate Randolph and Scott Schutzler for their assistance in data collection and analysis. DPJ and RRW contributed to the original experimental design. TBS, MSM, and DPJ were responsible for data acquisition and data analysis. TBS and MMM wrote the manuscript under the supervision of DPJ. All authors contributed to the interpretation of the results and take responsibility for the work.
Grants: This project was supported by the funding from the National Cattlemen’s Beef Association (NCBA) Checkoff Program (DPJ), the Beef Information Centre and the NIH/NIA Claude D. Pepper Older Americans Independence Center at the University of Texas Medical Branch, Grant #P30 AG1723. Studies were conducted in the Clinical Research Center at The University of Texas Medical Branch in Galveston and supported in part by grants MO1 RR-00073 and 1UL1RR029876-01 from the National Center for Research Resources, National Institutes of Health.
Footnotes
Disclosures: (a). This project was supported by the funding from the National Cattlemen’s Beef Association (NCBA) Checkoff Program (DPJ) and the NIH/NIA Claude D. Pepper Older Americans Independence Center at the University of Texas Medical Branch, Grant #P30 AG17231(J. Goodwin, PI). Studies were conducted in the General Clinical Research Center at The University of Texas Medical Branch in Galveston and funded by NIH Grant MO1 RR-00073. (b). DPJ and RRW have received compensation for speaking and consulting engagements with NCBA.
References
- 1.Evans W. Functional and metabolic consequences of sarcopenia. J Nutr. 1997;127:998S–1003S. doi: 10.1093/jn/127.5.998S. [DOI] [PubMed] [Google Scholar]
- 2.Taaffe DR. Sarcopenia--exercise as a treatment strategy. Aust Fam Physician. 2006;35:130–134. [PubMed] [Google Scholar]
- 3.Biolo G, Maggi SP, Williams BD, Tipton KD, Wolfe RR. Increased rates of muscle protein turnover and amino acid transport after resistance exercise in humans. Am J Physiol. 1995;268:E514–520. doi: 10.1152/ajpendo.1995.268.3.E514. [DOI] [PubMed] [Google Scholar]
- 4.Hikida RS, Staron RS, Hagerman FC, et al. Effects of high-intensity resistance training on untrained older men. II. Muscle fiber characteristics and nucleocytoplasmic relationships. J Gerontol A Biol Sci Med Sci. 2000;55:B347–354. doi: 10.1093/gerona/55.7.b347. [DOI] [PubMed] [Google Scholar]
- 5.Kosek DJ, Kim JS, Petrella JK, Cross JM, Bamman MM. Efficacy of 3 days/wk resistance training on myofiber hypertrophy and myogenic mechanisms in young vs. older adults. J Appl Physiol. 2006;101:531–544. doi: 10.1152/japplphysiol.01474.2005. [DOI] [PubMed] [Google Scholar]
- 6.Phillips SM, Tipton KD, Aarsland A, Wolf SE, Wolfe RR. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273:E99–107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 7.Phillips SM, Tipton KD, Ferrando AA, Wolfe RR. Resistance training reduces the acute exercise-induced increase in muscle protein turnover. Am J Physiol. 1999;276:E118–124. doi: 10.1152/ajpendo.1999.276.1.E118. [DOI] [PubMed] [Google Scholar]
- 8.Paddon-Jones D, Sheffield-Moore M, Katsanos CS, Zhang XJ, Wolfe RR. Differential stimulation of muscle protein synthesis in elderly humans following isocaloric ingestion of amino acids or whey protein. Exp Gerontol. 2006;41:215–219. doi: 10.1016/j.exger.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 9.Paddon-Jones D, Sheffield-Moore M, Zhang XJ, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 10.Symons TB, Schutzler SE, Cocke TL, Chinkes DL, Wolfe RR, Paddon-Jones D. Aging does not impair the anabolic response to a protein-rich meal. Am J Clin Nutr. 2007;86:451–456. doi: 10.1093/ajcn/86.2.451. [DOI] [PubMed] [Google Scholar]
- 11.Tang JE, Perco JG, Moore DR, Wilkinson SB, Phillips SM. Resistance training alters the response of fed state mixed muscle protein synthesis in young men. Am J Physiol Regul Integr Comp Physiol. 2008;294:R172–178. doi: 10.1152/ajpregu.00636.2007. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson SB, Tarnopolsky MA, Macdonald MJ, Macdonald JR, Armstrong D, Phillips SM. Consumption of fluid skim milk promotes greater muscle protein accretion after resistance exercise than does consumption of an isonitrogenous and isoenergetic soy-protein beverage. Am J Clin Nutr. 2007;85:1031–1040. doi: 10.1093/ajcn/85.4.1031. [DOI] [PubMed] [Google Scholar]
- 13.Cuthbertson D, Smith K, Babraj J, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. Faseb J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 14.Rennie MJ, Bohe J, Wolfe RR. Latency, duration and dose response relationships of amino acid effects on human muscle protein synthesis. J Nutr. 2002;132:3225S–3227S. doi: 10.1093/jn/131.10.3225S. [DOI] [PubMed] [Google Scholar]
- 15.Phillips SM. Physiologic and molecular bases of muscle hypertrophy and atrophy: impact of resistance exercise on human skeletal muscle (protein and exercise dose effects) Appl Physiol Nutr Metab. 2009;34:403–410. doi: 10.1139/H09-042. [DOI] [PubMed] [Google Scholar]
- 16.Symons TB, Sheffield-Moore M, Wolfe RR, Paddon-Jones D. A moderate serving of high-quality protein maximally stimulates skeletal muscle protein synthesis in young and elderly subjects. J Am Diet Assoc. 2009;109:1582–1586. doi: 10.1016/j.jada.2009.06.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rasmussen BB, Tipton KD, Miller SL, Wolf SE, Wolfe RR. An oral essential amino acid-carbohydrate supplement enhances muscle protein anabolism after resistance exercise. J Appl Physiol. 2000;88:386–392. doi: 10.1152/jappl.2000.88.2.386. [DOI] [PubMed] [Google Scholar]
- 18.Tang JE, Manolakos JJ, Kujbida GW, Lysecki PJ, Moore DR, Phillips SM. Minimal whey protein with carbohydrate stimulates muscle protein synthesis following resistance exercise in trained young men. Appl Physiol Nutr Metab. 2007;32:1132–1138. doi: 10.1139/H07-076. [DOI] [PubMed] [Google Scholar]
- 19.Bergstrom J. Percutaneous needle biopsy of skeletal muscle in physiological and clinical research. Scand J Clin Lab Invest. 1975;35:609–616. [PubMed] [Google Scholar]
- 20.Patterson BW, Zhang XJ, Chen Y, Klein S, Wolfe RR. Measurement of very low stable isotope enrichments by gas chromatography/mass spectrometry: application to measurement of muscle protein synthesis. Metabolism. 1997;46:943–948. doi: 10.1016/s0026-0495(97)90084-6. [DOI] [PubMed] [Google Scholar]
- 21.Wolfe RR, Chinkes DL. Isotope Tracers in Metabolic Research: Principles and Practice of Kinetic Analysis. 2. Hoboken, New Jersey: John Wiley & Sons, Inc; 2005. [Google Scholar]
- 22.Calder AG, Anderson SE, Grant I, Menurlan MA, Garlick PJ. The determination of low d5-phenylalanine enrichment (0.002–0. 09 atom percent excess), after conversion to phenylethylamine, in relation to protein turnover studies by gas chromatography/electron ionization mass spectrometry. Rapid Comm Mass Spec. 1992;6:421–424. doi: 10.1002/rcm.1290060704. [DOI] [PubMed] [Google Scholar]
- 23.Elliot TA, Cree MG, Sanford AP, Wolfe RR, Tipton KD. Milk ingestion stimulates net muscle protein synthesis following resistance exercise. Med Sci Sports Exerc. 2006;38:667–674. doi: 10.1249/01.mss.0000210190.64458.25. [DOI] [PubMed] [Google Scholar]
- 24.Tipton KD, Ferrando AA, Phillips SM, Doyle D, Jr, Wolfe RR. Postexercise net protein synthesis in human muscle from orally administered amino acids. Am J Physiol. 1999;276:E628–634. doi: 10.1152/ajpendo.1999.276.4.E628. [DOI] [PubMed] [Google Scholar]
- 25.Drummond MJ, Dreyer HC, Pennings B, et al. Skeletal muscle protein anabolic response to resistance exercise and essential amino acids is delayed with aging. J Appl Physiol. 2008;104:1452–1461. doi: 10.1152/japplphysiol.00021.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fujita S, Dreyer HC, Drummond MJ, Glynn EL, Volpi E, Rasmussen BB. Essential amino acid and carbohydrate ingestion before resistance exercise does not enhance postexercise muscle protein synthesis. J Appl Physiol. 2009;106:1730–1739. doi: 10.1152/japplphysiol.90395.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tipton KD, Rasmussen BB, Miller SL, et al. Timing of amino acid-carbohydrate ingestion alters anabolic response of muscle to resistance exercise. Am J Physiol Endocrinol Metab. 2001;281:E197–206. doi: 10.1152/ajpendo.2001.281.2.E197. [DOI] [PubMed] [Google Scholar]
- 28.Dreyer HC, Drummond MJ, Pennings B, et al. Leucine-enriched essential amino acid and carbohydrate ingestion following resistance exercise enhances mTOR signaling and protein synthesis in human muscle. Am J Physiol Endocrinol Metab. 2008;294:E392–400. doi: 10.1152/ajpendo.00582.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dreyer HC, Fujita S, Cadenas JG, Chinkes DL, Volpi E, Rasmussen BB. Resistance exercise increases AMPK activity and reduces 4E-BP1 phosphorylation and protein synthesis in human skeletal muscle. J Physiol. 2006;576:613–624. doi: 10.1113/jphysiol.2006.113175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paddon-Jones D, Rasmussen BB. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campbell WW. Synergistic use of higher-protein diets or nutritional supplements with resistance training to counter sarcopenia. Nutr Rev. 2007;65:416–422. doi: 10.1111/j.1753-4887.2007.tb00320.x. [DOI] [PubMed] [Google Scholar]
- 32.Verdijk LB, Jonkers RA, Gleeson BG, et al. Protein supplementation before and after exercise does not further augment skeletal muscle hypertrophy after resistance training in elderly men. Am J Clin Nutr. 2009;89:608–616. doi: 10.3945/ajcn.2008.26626. [DOI] [PubMed] [Google Scholar]
- 33.Katsanos CS, Kobayashi H, Sheffield-Moore M, Aarsland A, Wolfe RR. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;82:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 34.Kumar V, Selby A, Rankin D, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Henderson GC, Dhatariya K, Ford GC, et al. Higher muscle protein synthesis in women than men across the lifespan, and failure of androgen administration to amend age-related decrements. FASEB J. 2009;23:631–641. doi: 10.1096/fj.08-117200. [DOI] [PMC free article] [PubMed] [Google Scholar]