Abstract
The level of proteolysis within phagosomes of dendritic cells (DCs) is thought to be tightly regulated, as it directly impacts the cell's efficiency to process antigen. Activity of the antimicrobial effector NADPH oxidase (NOX2) has been shown to reduce levels of proteolysis within phagosomes of both macrophages and DCs. However, the proposed mechanisms underlying these observations in these two myeloid cell lineages are dissimilar. Using real-time analysis of lumenal microenvironmental parameters within phagosomes in live bone marrow-derived DCs, we show that the levels of phagosomal proteolysis are diminished in the presence of NOX2 activity, but in contrast to previous reports, the acidification of the phagosome is largely unaffected. As found in macrophages, we show that NOX2 controls phagosomal proteolysis in DCs through redox modulation of local cysteine cathepsins. Aspartic cathepsins were unaffected by redox conditions, indicating that NOX2 skews the relative protease activities in these antigen processing compartments. The ability of DC phagosomes to reduce disulphides was also compromised by NOX2 activity, implicating this oxidase in the control of an additional antigen processing chemistry of DCs.
Keywords: antigen processing, cysteine cathepsin, dendritic cell, NOX2, redox
Introduction
Although the complexities of dendritic cell (DC) heterogeneity continue to provoke controversy, it is generally accepted that conventional myeloid DCs and macrophages are cell lineages derived from a common precursor, and exist within a functional and phenotypic continuum (Geissmann et al, 2010). Cells that lie on the DC side of this spectrum are more efficient at activating naïve T cells through antigen presentation than those that are skewed towards the macrophage phenotype, which tend to possess greater microbicidal potency (Hume, 2008). Hence, it is reasonable to extrapolate that many cellular processes of macrophages and conventional DCs are similar, or at least share common mechanisms. Since the organization and activity of proteolytic machinery within the phagosomes of DCs are of particular importance to antigen processing, we investigated the control of proteolysis within the phagosome by NADPH oxidase (NOX2) in bone marrow derived-DCs (BMDCs). NOX2 activity has been shown to decrease levels of phagosomal proteolysis in both macrophages and DCs but the proposed mechanisms attributed to this control are dissimilar.
Amigorena and co-workers have shown that the efficiency of phagosomal proteolysis in BMDCs is decreased by NOX2 production of reactive oxygen species (ROS) (Savina et al, 2006, 2009; Savina and Amigorena, 2007). Through FACS-based analysis, they presented evidence that NOX2 activity prevents acidification of the phagosome, which, they reasoned, is mediated by superoxide (O2−·) consumption of lumenal protons. Based on these data, they hypothesized that, since lysosomal proteases generally require an acidic pH for optimal activity, the NOX2-induced alkalinization of the phagosomal lumen is responsible for the observed inhibition of phagosomal proteolysis. Similarly, our group has observed a relationship between NOX2 activity and proteolytic efficiency of phagosomes in bone marrow-derived macrophages (BMMØs). However, we demonstrated that this relationship is independent of changes to phagosomal pH (Rybicka et al, 2010). The mechanism through which NOX2 modulates phagosomal proteolysis in these cells was found to be redox-mediated, affecting local cysteine cathepsins through perturbation of the reductive capacity of the phagosome.
Since changes to proteolytic efficiency significantly affect antigen processing, and DCs are the dominant antigen-presenting cells, we thought it was prudent to investigate whether NOX2-mediated redox control of phagosomal proteolysis through modification of cysteine cathepsin activity occurs in conventional DCs, as it does in macrophages. Consistent with the findings of Amigorena and colleagues (Savina et al, 2006), we found that NOX2 activity was inversely correlated with phagosomal proteolytic efficiency in BMDCs as well as in the DC-like cell line DC2.4. In contrast to these earlier studies, using highly resolved real-time measurements of phagosomal acidification, we were unable to detect any significant changes in lumenal pH resulting from NOX2 activity. We further demonstrated that NOX2 activity in DCs modifies the redox microenvironment within the phagosomal lumen, leading to compromised disulphide reduction. This is consistent with the oxidative inhibition of phagosomal cysteine cathepsins by NOX2, which could be restored by the addition of exogenous reducing reagents. Collectively, these data support a redox-mediated mechanism of phagosomal protease control by NOX2 in DCs.
Results
Phagosomal NOX2 activity decreases proteolysis in DCs
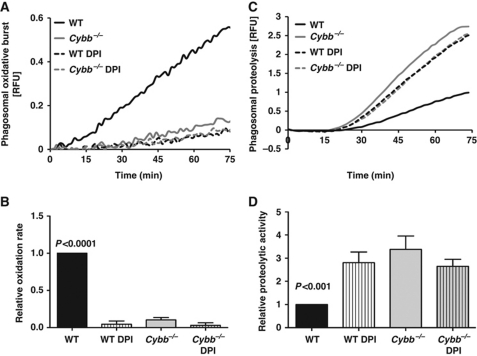
We first sought to determine the extent and pattern of the NOX2-mediated respiratory burst in BMDCs. These cells were derived from bone marrow of C57Bl/6 mice (WT) using a widely utilized method, and displayed hallmark characteristics of immature DCs (Supplementary Figure S1) (Savina et al, 2006; Geissmann et al, 2010). Using real-time fluorometry, the oxidation of a particle-restricted fluorogenic substrate was measured following coordinated phagocytosis of 3 μm experimental particles. Evidence of the respiratory burst was seen starting at 10 min after internalization and, unlike the pattern observed in macrophages, was sustained beyond 1 h, consistent with prolonged NOX2 association with the phagosome in DCs (Amigorena and Savina, 2010). No detectable substrate oxidation was observed in BMDCs derived from mice deficient in the gp91 subunit of the NOX2 complex (Cybb−/−), or in WT BMDCs treated with the NOX2 inhibitor diphenyleneiodonium (DPI) (Figure 1A and B). To establish whether ROS production by NOX2 in DC phagosomes decreases their proteolytic efficiencies, the hydrolysis of a particle-restricted general protease substrate (DQ-green Bodipy albumin) was measured following its phagocytosis by WT and Cybb−/− BMDCs in the presence or absence of DPI. Consistent with previous findings, the absence of NOX2 activity significantly increased the rate of bulk phagosomal proteolysis (2.38±0.29 fold higher in Cybb−/− BMDCs when compared with untreated WT controls) (Figure 1C and D). The observed differences did not result from differential particle uptake or oxidation of the substrate's fluorophore (Supplementary Figures S2 and S3). Additionally, no differences in protease expression or the proteolytic activity of whole-cell lysates were observed between WT and Cybb−/− BMDCs, indicating that NOX2 specifically impacts phagosomal proteolytic efficiency rather than total cellular proteolytic capacity (Supplementary Figure S4A–C).
Figure 1.
Generation of ROS by phagosomal NOX2 in BMDCs negatively affects proteolytic efficiency. The generation of ROS (respiratory burst) and general proteolytic activity in phagosomes were evaluated following coordinated phagocytosis of IgG-conjugated 3 μm experimental particles in BMDCs derived from C57Bl/6 (WT) or NOX2-deficient (Cybb−/−) mice. Where indicated, BMDCs were pre-treated with the NOX2 inhibitor DPI (0.5 μM) for 10 min at 37°C prior to phagocytosis. (A, B) Production of ROS in BMDC phagosomes was evaluated by measuring fluorescence released during oxidation of particle-conjugated H2HFF-OxyBURST substrate (λex485 nm; λem520 nm) relative to a calibration fluor Alexa Fluor 594 (λex594 nm; λem620 nm). (C, D) Proteolytic activity was assessed by measuring the amount of fluorescence released during hydrolysis of particle-associated DQ-green Bodipy albumin (λex485 nm; λem520 nm) relative to fluorescence of the calibration fluorophore Alexa Fluor 594 (λex544 nm; λem620 nm). (A, C) Representative real-time traces. (B, D) Average rates of substrate oxidation/hydrolysis over four independent experiments relative to untreated controls. Rates were determined by calculation of the slope of the linear portion of the real-time traces (as described by y=mx+c, where y is the relative fluorescence, m is the slope and x is time) and expressed relative to DMSO-treated WT samples. Error bars represent s.e.m. P-values were calculated using repeated measures one-way ANOVA.
NOX2 control of phagosomal proteolysis is locally mediated
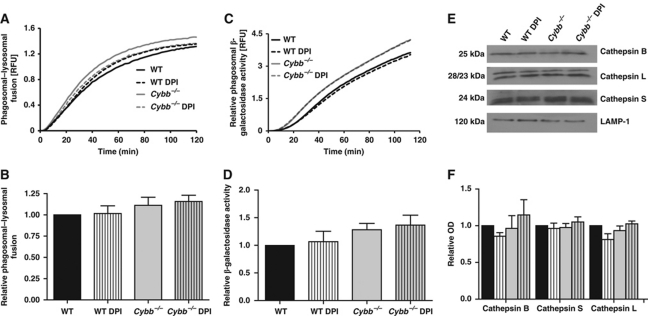
Control of the delivery of lysosomal constituents to the phagosome through the regulation of the fusion of these compartments is a major regulator of phagosomal physiology (Vieira et al, 2002). To investigate whether NOX2 exerts its inhibitory effect on proteolysis by limiting lysosomal contribution to the phagosome in DCs, we utilized a FRET-based assay that quantifies the accumulation of preformed lysosomal components within the maturing phagosome in real time (Yates et al, 2005; Yates and Russell, 2008). Although we found that the timing and extent of the FRET efficiency between the particle-conjugated phagosomal donor fluor and the lysosomal fluid-phase acceptor fluor was influenced by V-ATPase and calmodulin inhibition (Supplementary Figure S5), no modification of the assay's profile was associated with NOX2 function (Figure 2A and B). This indicates that phagosome–lysosome communication was not influenced by NOX2 activity. To further discount the possibility that NOX2 perturbs phagosomal–lysosomal fusion and thus contributes to differences in phagosomal proteolysis, we followed the phagosomal acquisition of β-galactosidase, a representative lysosomal hydrolase. Consistent with FRET-fusion assay data, we found no significant differences in recruitment of the lysosomal β-galactosidase activity to NOX2-proficient or -compromised phagosomes (Figure 2C and D). Furthermore, phagosomes showed similar relative recruitment of the active forms of cathepsin B, S and L in the presence or absence of NOX2 function, as evidenced by western blot analysis of isolated BMDC phagosomes (Figure 2E and F). These data demonstrate that the decreased proteolytic activity in NOX2-competent phagosomes is not mediated by differential recruitment of lysosomal proteases, indicating that NOX2 affects the activity, rather than the recruitment, of phagosomal proteases.
Figure 2.
NOX2 activity does not affect phagosome–lysosome communication in BMDCs. (A, B) Lysosomal contribution to the phagosome was measured by evaluating FRET efficiency between a particle-conjugated donor fluor Alexa Fluor 488 (λex485 nm; λem520 nm) and a fluid-phase lysosomal acceptor fluor Alexa Fluor 594 hydrazide (λex485 nm; λem620 nm) relative to the donor fluorescence. RFUs are indicative of the concentration of lysosomal constituents within the phagosome at any given point in time. (C, D) Acquisition of β-galactosidase activity to BMDC phagosomes was measured by following the hydrolysis of the particle restricted fluorogenic β-galactosidase substrate 5-dodecanoylaminofluorescein di-β-D-galactopyranoside (λex485 nm; λem520 nm) relative to a calibration fluor Rhodamine B C10 (λex544 nm; λem620 nm). (A, C) Representative real-time traces. (B, D) Average rates of the acquisition of FRET efficiency or substrate hydrolysis over three independent experiments. Rates were determined by calculation of the slope of the linear portion of the real-time traces (as described by y=mx+c, where y is the relative fluorescence, m is the slope and x is time) and expressed relative to DMSO-treated WT samples. (E, F) Representative western blot images and relative band densities of active forms of cathepsin B (25 kDa), L (28 and 23 kDa) and S (24 kDa) in BMDC phagosomes isolated 1 h following phagocytosis. Volumes of pixels were determined using Quantity One 1-D analysis software and relative densities were calculated relative to DMSO-treated WT BMDC samples over three independent experiments. LAMP-1 was used as a loading control. (B, D, F) Error bars denote s.e.m. No statistical differences between samples were found by ANOVA.
Phagosomal NOX2 activity does not significantly affect phagosomal acidification in DCs
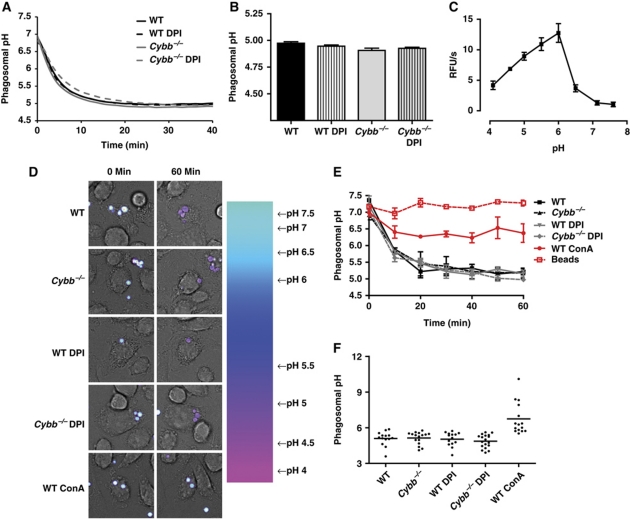
Utilizing FACS-based measurements of phagosomal pH in BMDCs, Amigorena and colleagues generated data to suggest that NOX2 activity ablated the acidification of phagosomes (Savina et al, 2006). Since phagosomal acidification is largely unaffected by NOX2 activity in BMMØs, we thought it was important to revisit these experiments using real-time acidification assays. These assays dynamically measure the pH of phagosomes in live, undisturbed BMDCs at physiological temperature by single-fluorophore excitation ratio fluorometry. This approach enables measurement of phagosomal pH in a highly resolved, robust and extensively validated manner (Yates and Russell, 2005, 2008; VanderVen et al, 2009). Initially, we utilized the pH-sensitive fluorophore carboxyfluorescein succinimidyl ester (CFSE) covalently bound to IgG-conjugated 3 μm experimental particles in a population-based format. Consistent with the findings in BMMØs, we found no statistical difference between the final pH of phagosomes with or without NOX2 function in BMDCs (Figure 3A and B). Similar results were generated using non-opsonized experimental particles targeted to the mannose receptor (Supplementary Figure S6) and pH measurements performed using an alternative pH-sensitive fluorophore Oregon Green succinimidyl ester (OGSE) (Supplementary Figure S7). In the original series of experiments, however, there was a statistically insignificant trend towards slightly more acidified phagosomes (0.17±0.056 units) in Cybb−/− BMDCs (Figure 3B). To calculate the theoretical impact of this small difference in pH on phagosomal proteolysis, phagosomal pH measurements were regressed against a curve describing the proteolytic efficiency of BMDC lysosomal contents at different pH values (Figure 3C). It was found that, even if significant, the small NOX2-mediated alkalinization would theoretically increase, not decrease, the proteolytic efficiency of the phagosome of BMDCs by ∼8%. Indeed, the phagosomal pH would have to increase to above a value of 6.0 before the total proteolytic efficiency would be adversely affected (Figure 3C; Supplementary Figure S8). Presumably, in this scenario, lysosome fusion with these largely unacidified phagosomes would also be compromised, which was not evidenced by phagosome–lysosome fusion profiles (Figure 2A and B) (Supplementary Figure S5) (Clague et al, 1994; van Weert et al, 1995; Yates et al, 2005).
Figure 3.
NOX2 activity does not significantly compromise phagosomal acidification in BMDCs. Phagosomal pH was measured by excitation ratio fluorometry using the pH-sensitive fluor CFSE (λex1485 nm, λex2450 nm; λem520 nm) conjugated to IgG-coupled experimental particles followed by ratio regression to a standard curve. (A) Representative real-time acidification profile in the phagosomes of WT and Cybb−/− BMDCs in the presence or absence of 0.5 μM DPI. (B) Average phagosomal pH at 45 min following phagocytosis from four independent experiments. (C) Curve describing the effect of pH on the proteolytic efficiency of total lysosomal extract from BMDCs. The curve was generated by measuring proteolytic efficiency of magnetically isolated lysosomal extract of BMDCs using the fluorogenic substrate DQ-green Bodipy albumin (λex485 nm; λem520 nm) in buffers of known pH; n=3. (D) Representative pseudo-colour ratio images (λex1488 nm; λem520 nm/λex2458 nm; λem520 nm) of BMDCs at time 0 and 60 min. Ratio/overlay images were generated using Leica Application Suite Advanced Fluorescence software. (E) Average real-time phagosomal acidification profile of WT and Cybb−/− BMDCs measured using confocal microscopy in the presence or absence of 0.5 μM DPI or 100 nM concanamycin A (ConA) (V-ATPase inhibitor) from three independent experiments. Extracellular beads were included as an additional control (beads). Error bars denote s.e.m. (F) Final pH of individual phagosomes from three independent experiments.
Since the methods of BMDC differentiation could potentially explain phenotypic differences between the current study and that of Savina et al (2006), we repeated these experiments in alternative, widely used, conventional DC types. Consistent with our earlier findings, NOX2 activity negatively impacted phagosomal proteolysis, but not acidification, in the immortalized DC line DC2.4 (Shen et al, 1997) and BMDCs derived using standard protocols with recombinant GM-CSF (Supplementary Figures 9A–F and S10A–G).
As fluorophore peroxidation and chlorination has previously led to inaccurate pH measurement in the presence of oxidative radicals and their products (Segal et al, 1981; Hurst et al, 1984), we tested the fluorescent stability of both CFSE and OGSE within BMDC phagosomes. Firstly, CFSE-generated acidification profiles were not perturbed in the presence of the peroxidase inhibitor sodium azide (Supplementary Figure S11) (Jankowski et al, 2002). Secondly, CFSE- and OGSE-coupled experimental particles displayed identical pH-sensitive fluorescent properties following recovery from either WT or Cybb−/− BMDC phagosomes (Supplementary Figure S12A and B). Together, these data indicate that the fluorescent properties of the pH-sensitive fluorophores used in this study were not affected by NOX2 products generated in BMDC phagosomes. It is interesting to note however, that in contrast to CFSE and OGSE, the fluorescent stability of pH-sensitive fluorophores pHrodo (Invitrogen) and cypHer (GE Healthcare) were found to be significantly affected by NOX2 activity. These two newer-generation fluorophores showed vastly disparate fluorescent properties following recovery from WT and Cybb−/− BMDC phagosomes, suggesting that they are inappropriate for quantitative measurement of phagosomal pH in the presence of ROS (Supplementary Figure S12C and D).
In addition to the measurement of lumenal pH across populations of synchronized BMDC phagosomes, we measured the pH of individual BMDC phagosomes containing CFSE-coupled, IgG-opsonized experimental particles under physiological conditions using real-time fluorometric confocal microscopy (Figure 3D and F). Consistent with previous data, we found that NOX2 activity did not influence the rate or extent of the acidification of individual BMDC phagosomes, and little heterogeneity of pH between phagosomes was observed (Figure 3F). To further test the effect of NOX2 on the heterogeneity of phagosomal acidification in BMDCs, we measured the accumulation of the acidotropic probe LysoTracker® Green (LTG) in mature phagosomes. LTG is an acid-selective organellar probe, which accumulates in acidified, but not unacidified, lysosomes and phagosomes. Consistent with previous results, mature (90-min) phagosomes containing IgG-opsonized or mannosylated silica experimental particles accumulated similar amounts of pulsed LTG in WT or Cybb−/− BMDCs (Supplementary Figure S13). Similar observations were made in phagosomes containing 3 μm latex beads used by Savina et al (2006). Interestingly, WT BMDC phagosomes containing zymosan displayed increased heterogeneity of phagosomal pH, with a greater proportion of unacidified phagosomes when compared with Cybb−/− BMDCs. The mechanism underlying the observed heterogeneity in this particular case is undetermined. Nevertheless, the vast majority of phagosomes containing zymosan, latex, IgG-coupled and mannose-coupled experimental particles acidified in NOX2-competent BMDCs.
Phagosomal NOX2 activity is dependent on charge compensation provided by translocation of protons into the phagosome
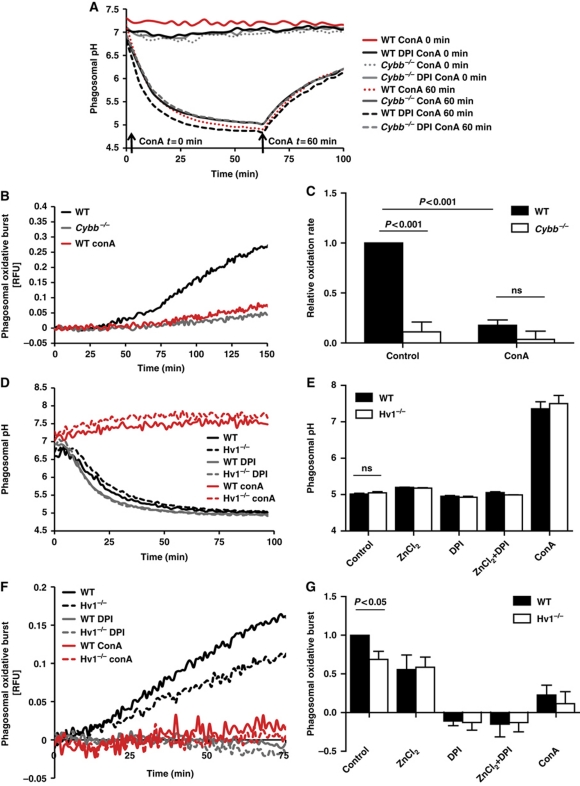
To further explore the relationship between NOX2 activity and phagosomal acidification in BMDCs from a stoichiometric perspective, we investigated the dependence of NOX2 activity on the translocation of protons into the phagosomal lumen by vacuolar ATPase (V-ATPase) and through the voltage-gated proton channel 1 (Hv1). Since NOX2 translocates electrons from cytosolic NADPH to O2 within the phagosome, compensatory movement of counter-ions across the phagosomal membrane is required to sustain NOX2 activity (Henderson et al, 1987; DeCoursey et al, 2003). The movement of protons across the plasma membrane provide the majority of charge compensation during the extracellular respiratory burst in neutrophils (Gabig et al, 1984; Takanaka and O'Brien, 1988), and the same mechanism has been proposed to support the phagosomal NOX2 activity in these cells (Morgan et al, 2009; DeCoursey, 2010). Should this be the case in DC phagosomes, then proton scavenging by superoxide and the proton counter-ion influx needed to sustain superoxide generation would occur at a 1:1 ratio and thus, theoretically, not influence lumenal pH. We first investigated the relationship between NOX2 and V-ATPase in BMDC phagosomes. If NOX2 activity is independent of charge compensation provided by V-ATPase activity, NOX2 would still function after inhibition of V-ATPase. In this scenario, there would be no active acidification of the phagosome but unimpeded superoxide production, which would result in the rapid depletion of existing lumenal protons and dramatic alkalinization of the phagosome. To test this, we used the specific V-ATPase inhibitor concanamycin A to temporally inhibit active phagosomal acidification in BMDCs in the presence or absence of the NOX2 inhibitor DPI. When the V-ATPase complex was inhibited before phagocytosis, NOX2-competent phagosomes only displayed marginal lumenal alkalinization at this neutral pH (0.30±0.090 increase in pH) (Figure 4A). When the V-ATPase was inhibited in fully acidified phagosomes, NOX2 had no observable influence on the rates of the subsequent alkalinization of the phagosome, which could be entirely attributed to passive proton leak (Lukacs et al, 1991). To further investigate the relationship of V-ATPase and NOX2 activity, we evaluated the magnitude of the respiratory burst in the presence of concanamycin A by following the oxidation of a particle-restricted fluorogenic substrate. We found that inhibition of V-ATPases profoundly decreased the NOX2-associated substrate oxidation within the phagosome (Figure 4B and C). Although the rates of substrate oxidation are possibly affected by pH directly, these findings, in conjunction with the acidification data, strongly suggest that NOX2 activity is compromised in the absence of the compensating electrogenic activity provided by phagosomal V-ATPase. In addition to V-ATPase, another potential source of charge compensation for NOX2 activity is the voltage-gated proton channel Hv1 (Ramsey et al, 2006). Selective movement of protons across the plasma and phagosomal membranes through this divalent cation (Zn2+, Cd2+)-sensitive proton channel has been proposed to provide the majority of charge compensation during NOX2 activity in neutrophils (Morgan et al, 2009; Ramsey et al, 2009; DeCoursey, 2010). We thus investigated the possible role of Hv1 during the phagosomal respiratory burst in BMDCs. Both WT and Cybb−/− BMDCs expressed similar amounts of Hv1 mRNA as determined by RT–PCR and quantitative PCR (Supplementary Figure S14). BMDCs derived from WT and Hv1−/− mice showed comparable rates and extents of phagosomal acidification in the presence and absence of NOX2 activity (Figure 4D and E). The respiratory burst, however, was found to be significantly compromised in Hv1−/− phagosomes or in the presence of the Hv1 inhibitor ZnCl2 (31.18%±5.26 and 44.47%±9.44 reduction, respectively) and completely compromised in the absence of Hv1 and V-ATPase activity (Figure 4F and G). These data suggest that a major proportion of phagosomal NOX2 activity in BMDCs is dependent on the charge compensation provided by proton translocation into the phagosomal lumen by V-ATPase and Hv1. Overall, these findings are consistent with maintained phagosomal acidification during NOX2 activity, as evidenced by direct measurement of pH (Figure 3), and support the existence of a pH-independent control mechanism of phagosomal proteolysis in DCs.
Figure 4.
Dependence of NOX2 activity on charge compensation provided by V-ATPase and Hv1 in BMDC phagosomes. (A) Representative real-time acidification profiles of phagosomes containing IgG-, CFSE-coupled experimental particles in WT and Cybb−/− BMDCs (± 0.5 μM DPI) with the addition of the V-ATPase inhibitor concanamycin A (100 nM) (ConA) prior to, or after, acidification of the phagosome. (B, C, F, G) NOX2 activity in BMDC phagosomes was evaluated by measuring fluorescence released during oxidation of particle-conjugated H2HFF-OxyBURST substrate (λex485 nm; λem520 nm) relative to a calibration fluor Alexa Fluor 594 (λex594 nm; λem620 nm) in the presence of V-ATPase inhibitor concanamycin A (100 nM) (ConA), Hv1 inhibitor ZnCl2 (50 μM) and/or DPI (0.5 μM DPI). (B, F) Representative real-time traces. (C, G) Average rates of substrate oxidation over three independent experiments relative to untreated controls. Error bars represent s.e.m. P-values were calculated using ANOVA. (D, E) Acidification of phagosomes containing IgG-, CFSE-coupled experimental particles in WT and Hv1−/− BMDCs (±0.5 μM DPI, ±100 nM ConA). (D) Representative real-time acidification profiles. (E) Average of final phagosomal pH at 70 min after phagocytosis from three independent experiments. Error bars represent s.e.m. P-values were calculated using ANOVA.
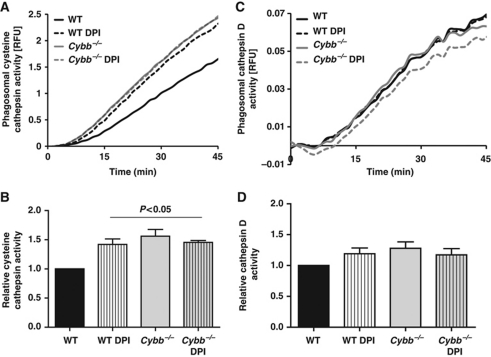
Phagosomal NOX2 activity inhibits the activities of local cysteine, but not aspartic, cathepsins
To explore whether NOX2 activity differentially affects different classes of phagosomal proteases, we followed the phagosomal hydrolysis of fluorogenic substrates for the cysteine cathepsins B/S/L and the aspartic cathepsins D/E in NOX2-proficient and NOX2-compromised BMDCs. Similar to BMMØs, NOX2 negatively impacted phagosomal cysteine cathepsin activity while aspartic cathepsin activity was unaffected (Figure 5). Since cysteine cathepsins require a reducing environment for activity (Kirschke et al, 1998; Jordans et al, 2009) and aspartic cathepsins are generally unaffected by redox conditions, these data indicate that NOX2 activity perturbs phagosomal proteolysis through redox means in BMDCs. Moreover, since cathepsins D and E have particularly acidic optima, the inability of NOX2 to affect their activities further supports a non-pH mechanism of inhibition of phagosomal proteolysis in these cells (Cunningham and Tang, 1976; Yasuda et al, 1999).
Figure 5.
NOX2 activity negatively affects activity of cysteine but not aspartic cathepsins in BMDC phagosomes. Relative phagosomal activities of cysteine (A, B) and aspartic (C, D) cathepsins were measured using cathepsin B/L and D/E peptide-based fluorogenic substrates [(biotin-LC-Phe-Arg)2-rhodamine 110: λex485 nm; λem520 nm and Mca-GKPILFFRLK(Dnp)-r-NH2: λex320 nm; λem405 nm] bound to IgG-opsonized particles, following phagocytosis by WT and Cybb−/− BMDCs, in the presence or absence of 0.5 μM DPI. (A, C) Representative traces of cathepsin B/L (A) and D/E (C) substrate hydrolysis relative to a calibration fluor Alexa Fluor 594 (λex544 nm; λem620 nm). (B, D) Average rates of substrate hydrolysis over five independent experiments relative to untreated controls. Rates were determined by calculation of the slope of the linear portion of the real-time traces (as described by y=mx+c, where y is the relative fluorescence, m is the slope and x is time) and expressed relative to DMSO-treated WT samples. Error bars represent s.e.m. P-values were calculated by ANOVA.
NOX2-generated ROS negatively affects the reductive capacity of the phagosome and cysteine cathepsin activities in DCs
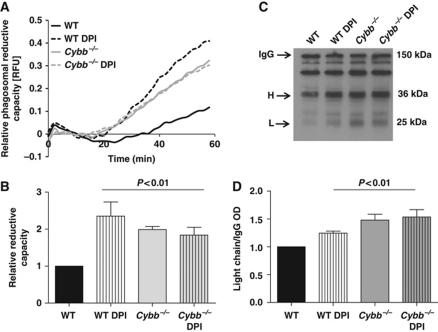
To determine whether NOX2 activity in BMDCs affects the redox microenvironment within the maturing phagosomal lumen, we measured the ability of the phagosomal compartment to reduce disulphides in the presence and absence of NOX2 function. We first employed a dynamic fluorometric assay that records the disulphide reduction of a cystine-based substrate covalently coupled to phagocytosed experimental particles (Rybicka et al, 2010). This assay revealed that NOX2-proficient phagosomes within BMDCs were significantly less efficient at reducing disulphides (Figure 6A and B). Consistent with these data, NOX2-proficient BMDC phagosomes were less efficient at reducing intermolecular disulphide bridges between the IgG subunits used to opsonize experimental particles (Figure 6C and D). Together, these data support the hypothesis that NOX2 influences phagosomal proteolysis of BMDCs through the perturbation of the phagosome's reductive capacity required for the optimal activity of the recruited cysteine cathepsins. Furthermore, since the reduction of protein disulphides is an important feature of antigen processing in DCs, it implicates NOX2 as a regulator of an additional antigen processing chemistry (Collins et al, 1991).
Figure 6.
NOX2 activity compromises the reductive capacity of phagosomes in BMDCs. Phagosomal reductive capacity was assessed by monitoring the ability of BMDC phagosomes to reduce mixed disulphides. (A, B) Reduction of a modified cystine-based fluorogenic substrate conjugated to an IgG-opsonized experimental particle following phagocytosis by WT and Cybb−/− BMDCs, in the presence or absence of 0.5 μM DPI. (A) Representative real-time traces of Bodipy FL L-cystine substrate reduction (λex485 nm; λem520 nm) relative to a calibration fluor Alexa Fluor 594 (λex544 nm; λem620 nm). (B) Average rates of substrate reduction over four independent experiments relative to untreated controls. Rates were determined by calculation of the slope of the linear portion of the real-time traces (as described by y=mx+c, where y is the relative fluorescence, m is the slope and x is time) and expressed relative to untreated WT samples. (C, D) Reduction of intermolecular disulphide bridges of IgG in BMDC phagosomes was determined by the degree of dissociation of light (L) and heavy (H) chains at 1 h following phagocytosis. BMDC phagosomes were allowed to phagocytose biotinylated IgG molecules used to opsonize BSA-conjugated experimental particles in the presence of protease inhibitors. Subunits were resolved by SDS–PAGE under non-reducing conditions and detected by western blotting. (C) Representative western blot image indicating relative dissociation of heavy (H) and light (L) chains from holo-IgG (IgG). (D) Average dissociation of the light chain relative to untreated controls over three independent experiments. Relative light chain dissociation was calculated by the density of the light chain band over the density of the holo-IgG band and expressed relative to DMSO-treated WT samples (B, D) Error bars denote s.e.m. P-values were calculated using ANOVA.
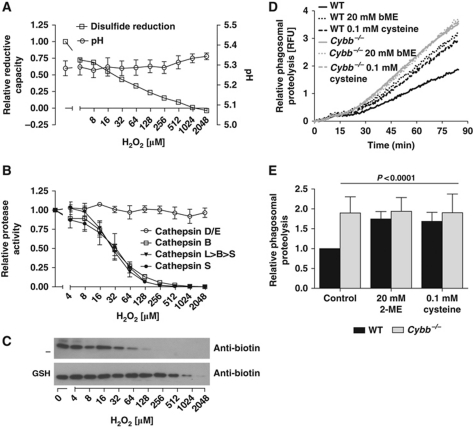
To further investigate the relationship between NOX2-generated products and the antigen processing chemistries of disulphide reduction and proteolysis, we followed these chemistries in a reconstituted system using freshly isolated BMDC lysosomal extracts. The rates of disulphide reduction and hydrolysis of substrates for specific cathepsins were recorded in the presence of an increasing concentration of hydrogen peroxide (H2O2). As anticipated, H2O2 decreased the ability of the lysosomal extract to reduce disulphides (Figure 7A), and also profoundly inhibited the hydrolytic efficiencies of the cysteine cathepsins (B, S and L) (Figure 7B). In contrast, the activity of the aspartic cathepsins (D/E) was unaffected by H2O2. In conjunction with fluorometric analysis of the cysteine cathepsin activities, the activity of cathepsin B from isolated BMDC lysosomes was measured using an activity-based biotinylated probe (biotin-FA-FMK) and western blotting. Here, we showed that the inhibition of cathepsin B by H2O2 could be reversed by the addition of reduced glutathione (GSH) (Figure 7C). Collectively, these data demonstrate that H2O2, a downstream product of NOX2, decreases the ability of lysosomal machinery to reduce disulphides and oxidatively inactivates lysosomal cysteine cathepsins. Consistent with the oxidative inactivation of lysosomal proteases in this reconstituted system, we found that the NOX2-mediated inhibition of phagosomal proteolysis could be reversed in live BMDCs by the addition of the reducing agents 2-mercaptoethanol or L-cysteine (Figure 7D and E). Similarly, the addition of H2O2 decreased the rates of phagosomal proteolysis in both WT and Cybb−/−BMDCs without overtly affecting pH (Supplementary Figure S15). Together, these findings strongly support a redox-based, rather than a pH-based, mechanism connecting NOX2 activity and phagosomal proteolysis in DCs.
Figure 7.
Phagosomal disulphide reduction and cysteine cathepsin activities are controlled by NOX2 through redox means. Specific reductive and cathepsin activities of BMDC lysosomal extracts were determined in a reconstituted system with varying concentrations of H2O2. (A) Effect of H2O2 concentration on rates of reduction of Bodipy FL L-cystine substrate (λex485 nm; λem520 nm) and pH of the reconstituted system. (B) Effect of H2O2 concentration on the rate of hydrolysis of substrates specific for aspartic (D/E) (Mca-GKPILFFRLK(Dnp)-r-NH2 λex320 nm; λem405 nm) and cysteine (B, L>B>S and S) cathepsins (Z-RR-MNA: λex360 nm; λem460 nm; (biotin-LC-Phe-Arg)2-rhodamine 110: λex485 nm; λem520 nm; Ac-KQKLR-AMC: λex360 nm; λem460 nm) by BMDC lysosomal extract. (A, B) Rates of substrate reduction/hydrolysis were determined fluorometrically and expressed as averages over four independent experiments relative to untreated samples. Error bars denote s.e.m. (C) Effect of H2O2 concentration on the activity of cathepsin B from BMDC lysosomal extract with or without 30 mM reduced glutathione (GSH). Images of representative western blots depicting cathepsin B activity as determined by the degree of active site reaction with the activity-based probe biotin-FA-FMK in the reconstituted lysosomal system with varying concentrations of H2O2. (D, E) Addition of exogenous reducing agents reverses NOX2-mediated inhibition of phagosomal proteolysis in BMDCs. General proteolytic activity of phagosomes of live WT and Cybb−/− BMDCs with or without pre-treatment with 2-mercaptoethanol (20 mM) or L-cysteine (0.1 mM). Proteolytic activity was assessed by measuring the amount of fluorescence released during hydrolysis of particle-associated DQ-green Bodipy albumin (λex485 nm; λem520 nm) relative to Alexa Fluor 594 calibration fluorescence (λex544 nm; λem620 nm). (D) Representative real-time traces. (E) Average rates of substrate hydrolysis over four independent experiments relative to untreated controls. Rates were determined by calculation of the slope of the linear portion of the real-time traces (as described by y=mx+c, where y is the relative fluorescence, m is the slope and x is time) and expressed relative to untreated WT samples. Error bars denote s.e.m. P-values were calculated using ANOVA.
Discussion
The manner by which DC phagosomes process protein antigen directly influences the efficiency of antigen presentation to T cells (Delamarre et al, 2005). The antimicrobial effector NOX2 has been shown to inhibit proteolytic activity in DC phagosomes, which was proposed to be mediated through the alkalinization of the phagosomal lumen (Savina et al, 2006, 2009; Mantegazza et al, 2008; Amigorena and Savina, 2010). Here, using real-time analysis of phagosomal parameters in conventional myeloid DCs under physiological conditions, we show that NOX2 activity does not significantly affect phagosomal acidification, yet still impacts phagosomal proteolysis. We further demonstrate that NOX2 significantly compromises the reductive capacity of the phagosome, which, in turn, affects the activity of local cysteine cathepsins.
This study describes an alternative, or at least an additional, mechanism by which NOX2 controls proteolysis in DCs. The underlying determinants that led to discrepancies in the effect of NOX2 activity on phagosomal pH between this study and previous reports are difficult to discern, but likely stem from either experimentally or biologically derived differences between the studies. Experimentally, previous reports utilized a FACS-based methodology, where chilled populations of BMDCs containing experimental particles were subject to flow cytometric measurement of pH-sensitive fluorochrome intensities at defined time points (Savina et al, 2006). In the present study, phagosomal pH was determined using similar fluorometric principles, but performed in real time in BMDCs maintained at physiological temperature and in the absence of physical disturbance or shear forces inherent to flow cytometry. Indirect indicators of phagosomal pH examined in this study (such as the activity of cathepsin D/E and rates of phagosome–lysosome fusion) provided additional evidence that phagosomal acidification was maintained in the presence of NOX2 activity. Another potential difference between this and the previous studies is the biological variation in the model of myeloid DCs used. Although identical protocols were utilized to derive and validate BMDCs both in the current study and in Savina et al (2006), subtle undetermined differences in derivation could have led to altered NOX2, V-ATPase or counter-ion channel expression levels or activities. Interestingly, the kinetics and extent of phagosomal acidification of BMDC phagosomes, even in the absence of NOX2, varied significantly between the two studies. In the current study, BMDCs were able to fully acidify their phagosomes to a pH below 5.0 within 30 min, whereas Savina et al (2006) reported that even NOX2-deficient BMDCs could only achieve a phagosomal pH of ∼6.5 after 1–2 h. Another possible explanation could be derived from the nature of the phagocytic cargo, which may cause modified membrane dynamics or differential V-ATPase and NOX2 recruitment/assembly. While there were no NOX2-mediated differences in the acidification of phagosomes containing IgG-opsonized or mannosylated 3 μm experimental particles or even the uncoated latex beads used by Savina et al (2006), we did notice an increased number of unacidified phagosomes containing zymosan in WT when compared with Cybb−/− BMDCs. It is possible that the lack of acidification in these phagosomes resulted from superoxide proton scavenging. However, if this were to be the case, it remains to be determined why this effect was observed only in a small proportion of the total phagosome population and only found with zymosan particles.
In addition to the assessment of NOX2's impact on phagosomal acidification by direct fluorometric measurement of lumenal pH, we explored the relationship between NOX2 activity and the charge compensation provided by counter-ion movement of protons. Production of O2−· by NOX2 relies on the movement of electrons from cytosolic NADPH into the phagosome. For every electron translocated by NOX2, approximately one proton is generated in the cytosol (DeCoursey, 2010). In an uncompensated system, the charge imbalance across the membrane, as well as acidification of the cytosol, would quickly inhibit further NOX2 activity (Jankowski and Grinstein, 2002; Lamb et al, 2009). Since we found that NOX2 activity was greatly dependent on V-ATPase function, it is likely that the majority of charge compensation for NOX2 activity is generated by active translocation of protons into the phagosome, providing a reverse current of similar magnitude. We also explored the potential role of the proton channel Hv1 in sustaining NOX2 activity through passive charge compensation across the DC phagosomal membrane. Hv1, encoded by the Hvcn1 gene, has been recently identified as a voltage-gated proton channel required for NOX2 function at the plasma membrane of neutrophils and eosinophils (Murphy and DeCoursey, 2006; Ramsey et al, 2006, 2009; El Chemaly et al, 2010). Here, Hv1 is thought to provide the majority of charge compensation for NOX2 activity, as well as maintain intracellular pH during a respiratory burst by facilitating proton movement out of the cell. More recently, a role for Hv1 in supporting the respiratory burst in the phagosome of neutrophils has been proposed (Morgan et al, 2009; Okochi et al, 2009; DeCoursey, 2010). In this study, we show that BMDCs express Hv1, at least at the mRNA level, and that phagosomal NOX2 activity is moderately attenuated in the absence Hv1 function. In the absence of both Hv1 and V-ATPase function, phagosomal NOX2 activity could not be detected. These findings suggest that the translocation of protons into the phagosome by Hv1 and V-ATPase provides the majority of charge compensation needed to sustain the electrogenic activity of NOX2 in DCs. Indeed, should the production of O2−· in the DC phagosome be dependent on an equimolar influx of protons, no net loss of phagosomal protons would occur in the production of H2O2. This would further support our observations that phagosomal pH is not significantly altered by NOX2 activity in BMDCs.
In the presence of the unchanged acidification of phagosomes in the three conventional myeloid DC models used in this study, NOX2 activity still significantly impacted the rates of phagosomal proteolysis. This directly implicates at least one additional mechanism of NOX2 control of phagosomal proteolysis that is independent of pH. A large portion of lysosomal proteolytic activity is derived from the papain-like cysteine proteases, many of which perform essential functions in antigen and invariant chain (Ii) processing (Medd and Chain, 2000; Hsing and Rudensky, 2005). These hydrolases include the exopeptidases cathepsins B and Z and the endopeptidases cathepsins S and L. Although substrate specificity and even pH optima differ between these enzymes, they all require reducing conditions to maintain the catalytic Cys25 (papain numbering) in its thiol state for peptidase activity (Godat et al, 2008). Thus, the redox microenvironment regulates the local activity of these lysosomal proteinases in addition to the regulation by pH, prodomain removal and the interaction with endogenous inhibitors such as cystatin (Kirschke et al, 1998). While the redox regulation of papain-like cysteine proteases in the extracellular milieu is well considered (Lockwood, 2000; Jordans et al, 2009), the redox regulation of these important hydrolases within the endolysosomal system is often overlooked. This probably stems from the ambiguities and difficulties in assessing the redox conditions within these vesicles (Austin et al, 2005). Using rates of disulphide reduction as a redox indicator, we have shown that, as in BMMØs (Rybicka et al, 2010), the reductive nature of the lumenal microenvironment of phagosomes in BMDCs was compromised in the presence of NOX2 activity. This, in turn, correlated with the selective inhibition of cysteine, but not aspartic, cathepsin activities in BMDC phagosomes (Figure 5). This inhibition of proteolysis was reversed by the addition of free thiols in a reconstituted system, or by the addition of reducing agents to live BMDCs during phagocytosis (Figure 7C, D and E). Together with the evidence previously generated in BMMØs, these data support a redox-based mechanism of NOX2-mediated regulation of proteolysis in BMDC phagosomes.
The relationship between phagosomal proteolytic activity and antigen processing efficiency has been well documented (Delamarre et al, 2005, 2006; McCurley and Mellman, 2010). While intralumenal proteolysis within antigen processing compartments is a pre-requisite for antigen presentation, excessive proteolysis is detrimental, as it leads to the destruction of the oligopeptides required for presentation. Thus, it has become increasingly apparent that the level of proteolysis within these compartments requires stringent control in order to generate, but also preserve, the antigenic oligopeptides necessary for productive antigen presentation (Delamarre et al, 2006; Savina et al, 2006). In addition to the limitation of total proteolytic capacity in BMDC phagosomes, we show that NOX2 modulation of the redox microenvironment specifically inhibits cysteine proteases, while other protease classes (such as the aspartic proteases) are unaffected. As yet, the consequences of this NOX2-mediated bias in protease activity on the processing of phagocytosed antigen are unknown. Numerous studies on protease-directed epitope selection, however, have provided strong evidence that the modulation of expression or activities of various lysosomal proteases within antigen processing compartments can influence MHC-II-restricted repertoires, and, in some cases, result in the presentation of otherwise cryptic epitopes (Drakesmith et al, 1998; Moudgil et al, 1998; Honey et al, 2002; Burster et al, 2005; Hsing and Rudensky, 2005; Zou et al, 2007). Hence, in addition to its general limitation of excessive antigen degradation, the mechanism by which NOX2 influences phagosomal proteolysis in DCs becomes increasingly relevant. A pH-based mechanism would favour proteases with a neutral pH optimum, such as cathepsin S, whereas a redox-based mechanism, as proposed here, would favour the serine and aspartic cathepsins including cathepsin D. Thus, the dominance of either mechanism would skew relative protease activities, leading to altered repertoires and consequences beyond the nuances of DC cell biology.
The efficiency and manner by which antigens are processed within antigen processing compartments of DCs are fundamentally important to the initiation and maintenance of many T-cell functions. Here, we report that, as found in macrophages, NOX2 regulates two key antigen processing chemistries in DCs: phagosomal proteolysis and disulphide reduction. In contrast to previous reports, we found little evidence of NOX2-mediated modulation of phagosomal acidification in BMDCs. Given the heterogeneity of the DC lineages, pH modification by NOX2 in all DC subtypes cannot be discounted, and it may indeed play an important role in the DC subsets that possess less robust phagosomal acidification. However, within the limitations of this study, it is clear that in the widely utilized conventional myeloid DCs models used here, redox control of cysteine cathepsin activity is the dominant mechanism by which NOX2 influences phagosomal proteolysis.
Materials and methods
Mice, cells and chemicals
C57Bl/6 mice were purchased from Charles River Laboratories. The congenic B6.129S6-Cybb−/− mice were purchased from Jackson Laboratories. Hv1−/− mice were a generous gift from Dr David E Clapham, Howard Hughes Medical Institute at Harvard University. All animal experiments were conducted according to the protocols approved by the University of Calgary Animal Use and Care Committee. BMDCs were derived from bone marrow with either commercially acquired recombinant GM-CSF (Peprotech), or conditioned media derived from the supernatant of Ag8653 melanoma cells transfected with murine GM-CSF cDNA, as previously described (Inaba et al, 1992; Zal et al, 1994; Savina et al, 2006). BMDCs were evaluated for expression of CD11c, CD11b, F4/80, Gr-1 and CD86 by flow cytometry using standard procedures in comparison to BMMØs generated as previously described (Yates et al, 2005). Prior to evaluation of CD86 expression, where indicated, BMDCs were pre-treated with 100 ng/ml of LPS (Sigma) for 18 h. All antibodies used for flow cytometry were purchased from eBioscience, with the exception of CD11b (BD Pharmingen). The data were acquired with a Beckman Coulter Cell Lab Quanta™ SC/MPL and analysed using Flowjo software v8.6. The DC-like cell line DC2.4 was kindly provided by Dr Yan Shi, University of Calgary and has been previously characterized (Shen et al, 1997). Fully differentiated BMDCs or DC2.4 cells were incubated in μ-clear 96-well plates (Greiner Bio-One) or appropriate culture plasticware 18 h prior to use. Where indicated, cells were incubated with 0.5 μM of DPI (Calbiochem), 100 nM of concanamycin A (Alexis Biochemicals) or 5 mM sodium azide (Sigma) for 10 min, or 20 mM 2-mercaptoethanol or 0.1 mM cysteine (Sigma) for 20 min, prior to phagocytosis of experimental particles. In the experiments indicated, H2O2 (0.5 mM) was added 30 min after the addition of experimental particles.
Real-time fluorometric phagosomal analysis in populations of live cells
IgG- or mannose-conjugated experimental particles used to evaluate phagosomal pH, phagosomal–lysosomal fusion, disulphide reduction, proteolysis, cysteine and aspartic cathepsin activities, oxidative burst and β-galactosidase activity, were prepared as previously described (Yates and Russell, 2005, 2008; Yates et al, 2005, 2009; VanderVen et al, 2009; Rybicka et al, 2010). Relative fluorescent units (RFUs) are defined by the equation: RFU=SFRT/CF, where SFRT is substrate florescence in real time, while CF represents average calibration fluorescence and was represented relative to time expressed in minutes. Hydrolytic capacities were evaluated by plotting the slopes (as described by the equation y=mx+c, where y=RFU, m=slope and x=time) of the linear portion of the relative substrate fluorescence against time, and were calculated relative to an appropriate internal control indicated. Experimental groups were compared by one-way analysis of variance (ANOVA) with Bonferroni's multiple comparisons post-hoc test using GraphPad Prism software.
For measurements of phagosomal pH, fatty acid-free bovine serum albumin (BSA) (Calbiochem) and human IgG (Sigma) were covalently coupled to 3 μm carboxylated silica experimental particles (Kisker Biotech), followed by fluorescent labelling with CFSE (Molecular Probes) or OGSE (Molecular Probes), as previously described (Yates et al, 2005; Yates and Russell, 2008). Mannosylated particles were generated by covalent coupling of BSA to the carboxylated experimental particles, followed by reaction with CFSE and α-D-mannopyranosyl-phenyl isothiocyanate (Sigma). Quenched and washed experimental particles were added to BMDC monolayers at an MOI of 1–2 particles per cell prior to fluorescent measurement. Fluorescence emission at 520 nm was measured using FluorStar Optima plate reader (BMG Technologies) every 60 s with two alternating excitation wavelengths of 450 and 485 nm. Phagosomal pH was determined using the calculated excitation ratio between the pH-insensitive excitation at 450 nm and the pH-sensitive excitation at 485 nm. Conversion of excitation ratios to phagosomal pH was achieved through third-order polynomial regression to a standard curve generated using excitation ratios of beads in buffers of known pH. Excitation values generated in this way were equivalent to those of generated using particle-containing BMDCs in standard buffers containing the ionophore nigericin (10 μg/ml).
Real-time measurement of phagosomal pH by confocal microscopy
Images of WT and Cybb−/− BMDCs were taken in the presence or absence of DPI (0.5 μM) or concanamycin A (100 nM) upon phagocytosis of CFSE-conjugated experimental particles using a Leica SP5 scanning confocal microscope equipped with a × 40 (air), NA=0.75 objective. CFSE emission, following 488 and 458 nm argon laser excitation (emission bandwidth of 498–593 nm and 478–593 nm, respectively), was sequentially captured every 10 min from section thickness of 5 μm. A minimum of four phagosomes was monitored over a period of 90 min from each field of view. All microscope parameters including PMT gain and focal planes were kept constant between experiments. Calculation of the average intensity of phagosomal regions was performed using MetaMorph® software (version 1.3.0) and plotted as an excitation ratio (488/458 nm) over time. For conversion of the excitation ratio to phagosomal pH, a standard curve was generated using an adapted nigericin/high K+ technique (Thomas et al, 1979). Briefly, cells were washed and incubated at 37°C in 30 mM sodium acetate (pH 4–5.5) or PIPES (pH 6–7.5) containing 130 mM KCl, 1 mM MgCl2 and 10 μg/ml nigericin. Images were captured following phagosomal equilibration with the exogenous buffers (30 min). Standard curves were generated following calculation of average excitation ratios at each pH value and used for conversion of excitation ratio of experimental samples to phagosomal pH.
Measurement of cathepsin activities from isolated lysosomes
For measurement of cathepsin activity in a reconstituted system, lysosomal extracts were diluted in 0.2 M potassium acetate buffer containing 1 mM cysteine/cystine redox buffer 600:1 (−221 to −236 mV) at pH 5.0 (Pillay et al, 2002). For measurement of enzymatic activity, the following fluorogenic substrates were used: cathepsin B- Z-Arg-Arg-7-amido-4-methylcoumarin hydrochloride (Sigma), cathepsin D/E- Mca-Gly-Lys-Pro-Ile-Leu-Phe-Phe-Arg-Leu-Lys(Dnp)-D-Arg-NH2 and cathepsin S- Ac-Lys-Gln-Lys-Leu-Arg-AMC (Anaspec). Cysteine cathepsin activity (L>B>S) was measured using (biotin-LC-Phe-Arg)2-rhodamine 110 substrate (kindly donated by Dr David Russell, Cornell University, Ithaca, NY). Measurement of disulphide reduction was achieved by following the fluorescence dequenched through reduction of Bodipy FL L-cystine (Invitrogen). The fluorescent traces were generated using a FLUOstar Optima plate reader (BMG Labtech) at 37°C. Slopes of initial reaction rates were determined by curve-fitting applications in Excel® and expressed relative to untreated controls. Semi-quantitative assessment of cathepsin B activity using the activity-based cathepsin B probe biotin-Phe-Ala-FMK (SM Biochemicals) was performed in the buffer system described above. The samples were incubated for 10 min at 37°C with agitation and the reaction was terminated by addition of 2 × SDS sample buffer. The proportion of biotinylated (active) cathepsin B was determined by western blotting using standard procedures.
Supplementary Material
Acknowledgments
We thank Dr David Russell, Cornell University, for his critical reading of the manuscript and Dr Mi-Jeong Kim, Harvard University, for her logistical support. We also thank Dr David Clapham and Long-Jun Wu, Harvard University, for providing us with Hv1−/− bone marrow. This work was supported by the Canadian Institutes of Health Research and Alberta Innovates.
Author contributions: JMR, DRB and RMY conceived, designed and performed the experiments as well as analysed the data. SC and EROA performed the experiments and analysed the data. JMR and RMY wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Amigorena S, Savina A (2010) Intracellular mechanisms of antigen cross presentation in dendritic cells. Curr Opin Immunol 22: 109–117 [DOI] [PubMed] [Google Scholar]
- Austin CD, Wen X, Gazzard L, Nelson C, Scheller RH, Scales SJ (2005) Oxidizing potential of endosomes and lysosomes limits intracellular cleavage of disulfide-based antibody-drug conjugates. Proc Natl Acad Sci USA 102: 17987–17992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burster T, Beck A, Tolosa E, Schnorrer P, Weissert R, Reich M, Kraus M, Kalbacher H, Haring HU, Weber E, Overkleeft H, Driessen C (2005) Differential processing of autoantigens in lysosomes from human monocyte-derived and peripheral blood dendritic cells. J Immunol 175: 5940–5949 [DOI] [PubMed] [Google Scholar]
- Clague MJ, Urbe S, Aniento F, Gruenberg J (1994) Vacuolar ATPase activity is required for endosomal carrier vesicle formation. J Biol Chem 269: 21–24 [PubMed] [Google Scholar]
- Collins DS, Unanue ER, Harding CV (1991) Reduction of disulfide bonds within lysosomes is a key step in antigen processing. J Immunol 147: 4054–4059 [PubMed] [Google Scholar]
- Cunningham M, Tang J (1976) Purification and properties of cathepsin D from porcine spleen. J Biol Chem 251: 4528–4536 [PubMed] [Google Scholar]
- DeCoursey TE (2010) Voltage-gated proton channels find their dream job managing the respiratory burst in phagocytes. Physiology (Bethesda) 25: 27–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey TE, Morgan D, Cherny VV (2003) The voltage dependence of NADPH oxidase reveals why phagocytes need proton channels. Nature 422: 531–534 [DOI] [PubMed] [Google Scholar]
- Delamarre L, Couture R, Mellman I, Trombetta ES (2006) Enhancing immunogenicity by limiting susceptibility to lysosomal proteolysis. J Exp Med 203: 2049–2055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delamarre L, Pack M, Chang H, Mellman I, Trombetta ES (2005) Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307: 1630–1634 [DOI] [PubMed] [Google Scholar]
- Drakesmith H, O′Neil D, Schneider SC, Binks M, Medd P, Sercarz E, Beverley P, Chain B (1998) In vivo priming of T cells against cryptic determinants by dendritic cells exposed to interleukin 6 and native antigen. Proc Natl Acad Sci USA 95: 14903–14908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Chemaly A, Okochi Y, Sasaki M, Arnaudeau S, Okamura Y, Demaurex N (2010) VSOP/Hv1 proton channels sustain calcium entry, neutrophil migration, and superoxide production by limiting cell depolarization and acidification. J Exp Med 207: 129–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabig TG, Lefker BA, Ossanna PJ, Weiss SJ (1984) Proton stoichiometry associated with human neutrophil respiratory-burst reactions. J Biol Chem 259: 13166–13171 [PubMed] [Google Scholar]
- Geissmann F, Gordon S, Hume DA, Mowat AM, Randolph GJ (2010) Unravelling mononuclear phagocyte heterogeneity. Nat Rev Immunol 10: 453–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godat E, Herve-Grvepinet V, Veillard F, Lecaille F, Belghazi M, Bromme D, Lalmanach G (2008) Regulation of cathepsin K activity by hydrogen peroxide. Biol Chem 389: 1123–1126 [DOI] [PubMed] [Google Scholar]
- Henderson LM, Chappell JB, Jones OT (1987) The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem J 246: 325–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honey K, Nakagawa T, Peters C, Rudensky A (2002) Cathepsin L regulates CD4+ T cell selection independently of its effect on invariant chain: a role in the generation of positively selecting peptide ligands. J Exp Med 195: 1349–1358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsing LC, Rudensky AY (2005) The lysosomal cysteine proteases in MHC class II antigen presentation. Immunol Rev 207: 229–241 [DOI] [PubMed] [Google Scholar]
- Hume DA (2008) Macrophages as APC and the dendritic cell myth. J Immunol 181: 5829–5835 [DOI] [PubMed] [Google Scholar]
- Hurst JK, Albrich JM, Green TR, Rosen H, Klebanoff S (1984) Myeloperoxidase-dependent fluorescein chlorination by stimulated neutrophils. J Biol Chem 259: 4812–4821 [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman RM (1992) Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med 176: 1693–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski A, Grinstein S (2002) Modulation of the cytosolic and phagosomal pH by the NADPH oxidase. Antioxid Redox Signal 4: 61–68 [DOI] [PubMed] [Google Scholar]
- Jankowski A, Scott CC, Grinstein S (2002) Determinants of the phagosomal pH in neutrophils. J Biol Chem 277: 6059–6066 [DOI] [PubMed] [Google Scholar]
- Jordans S, Jenko-Kokalj S, Kuhl NM, Tedelind S, Sendt W, Bromme D, Turk D, Brix K (2009) Monitoring compartment-specific substrate cleavage by cathepsins B, K, L, and S at physiological pH and redox conditions. BMC Biochem 10: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschke H, Barrett A, Rawlings N (1998) Lysosomal Cysteine Proteases, 2nd edn Oxford University Press: New York [Google Scholar]
- Lamb FS, Moreland JG, Miller FJ Jr (2009) Electrophysiology of reactive oxygen production in signaling endosomes. Antioxid Redox Signal 11: 1335–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lockwood TD (2000) Redox control of protein degradation. Antioxid Redox Signal 2: 851–878 [DOI] [PubMed] [Google Scholar]
- Lukacs GL, Rotstein OD, Grinstein S (1991) Determinants of the phagosomal pH in macrophages. In situ assessment of vacuolar H(+)-ATPase activity, counterion conductance, and H+ ″leak″. J Biol Chem 266: 24540–24548 [PubMed] [Google Scholar]
- Mantegazza AR, Savina A, Vermeulen M, Perez L, Geffner J, Hermine O, Rosenzweig SD, Faure F, Amigorena S (2008) NADPH oxidase controls phagosomal pH and antigen cross-presentation in human dendritic cells. Blood 112: 4712–4722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurley N, Mellman I (2010) Monocyte-derived dendritic cells exhibit increased levels of lysosomal proteolysis as compared to other human dendritic cell populations. PLoS One 5: e11949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medd PG, Chain BM (2000) Protein degradation in MHC class II antigen presentation: opportunities for immunomodulation. Semin Cell Dev Biol 11: 203–210 [DOI] [PubMed] [Google Scholar]
- Morgan D, Capasso M, Musset B, Cherny VV, Rios E, Dyer MJ, DeCoursey TE (2009) Voltage-gated proton channels maintain pH in human neutrophils during phagocytosis. Proc Natl Acad Sci USA 106: 18022–18027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moudgil KD, Sercarz EE, Grewal IS (1998) Modulation of the immunogenicity of antigenic determinants by their flanking residues. Immunol Today 19: 217–220 [DOI] [PubMed] [Google Scholar]
- Murphy R, DeCoursey TE (2006) Charge compensation during the phagocyte respiratory burst. Biochim Biophys Acta 1757: 996–1011 [DOI] [PubMed] [Google Scholar]
- Okochi Y, Sasaki M, Iwasaki H, Okamura Y (2009) Voltage-gated proton channel is expressed on phagosomes. Biochem Biophys Res Commun 382: 274–279 [DOI] [PubMed] [Google Scholar]
- Pillay CS, Elliott E, Dennison C (2002) Endolysosomal proteolysis and its regulation. Biochem J 363: 417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Moran MM, Chong JA, Clapham DE (2006) A voltage-gated proton-selective channel lacking the pore domain. Nature 440: 1213–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey IS, Ruchti E, Kaczmarek JS, Clapham DE (2009) Hv1 proton channels are required for high-level NADPH oxidase-dependent superoxide production during the phagocyte respiratory burst. Proc Natl Acad Sci USA 106: 7642–7647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicka JM, Balce DR, Khan MF, Krohn RM, Yates RM (2010) NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes. Proc Natl Acad Sci USA 107: 10496–10501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savina A, Amigorena S (2007) Phagocytosis and antigen presentation in dendritic cells. Immunol Rev 219: 143–156 [DOI] [PubMed] [Google Scholar]
- Savina A, Jancic C, Hugues S, Guermonprez P, Vargas P, Moura IC, Lennon-Dumenil AM, Seabra MC, Raposo G, Amigorena S (2006) NOX2 controls phagosomal pH to regulate antigen processing during crosspresentation by dendritic cells. Cell 126: 205–218 [DOI] [PubMed] [Google Scholar]
- Savina A, Peres A, Cebrian I, Carmo N, Moita C, Hacohen N, Moita LF, Amigorena S (2009) The small GTPase Rac2 controls phagosomal alkalinization and antigen crosspresentation selectively in CD8(+) dendritic cells. Immunity 30: 544–555 [DOI] [PubMed] [Google Scholar]
- Segal AW, Geisow M, Garcia R, Harper A, Miller R (1981) The respiratory burst of phagocytic cells is associated with a rise in vacuolar pH. Nature 290: 406–409 [DOI] [PubMed] [Google Scholar]
- Shen Z, Reznikoff G, Dranoff G, Rock KL (1997) Cloned dendritic cells can present exogenous antigens on both MHC class I and class II molecules. J Immunol 158: 2723–2730 [PubMed] [Google Scholar]
- Takanaka K, O'Brien PJ (1988) Proton release associated with respiratory burst of polymorphonuclear leukocytes. J Biochem 103: 656–660 [DOI] [PubMed] [Google Scholar]
- Thomas JA, Buchsbaum RN, Zimniak A, Racker E (1979) Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry 18: 2210–2218 [DOI] [PubMed] [Google Scholar]
- van Weert AW, Dunn KW, Gueze HJ, Maxfield FR, Stoorvogel W (1995) Transport from late endosomes to lysosomes, but not sorting of integral membrane proteins in endosomes, depends on the vacuolar proton pump. J Cell Biol 130: 821–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderVen BC, Yates RM, Russell DG (2009) Intraphagosomal measurement of the magnitude and duration of the oxidative burst. Traffic 10: 372–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira OV, Botelho RJ, Grinstein S (2002) Phagosome maturation: aging gracefully. Biochem J 366: 689–704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda Y, Kageyama T, Akamine A, Shibata M, Kominami E, Uchiyama Y, Yamamoto K (1999) Characterization of new fluorogenic substrates for the rapid and sensitive assay of cathepsin E and cathepsin D. J Biochem 125: 1137–1143 [DOI] [PubMed] [Google Scholar]
- Yates RM, Hermetter A, Russell DG (2005) The kinetics of phagosome maturation as a function of phagosome/lysosome fusion and acquisition of hydrolytic activity. Traffic 6: 413–420 [DOI] [PubMed] [Google Scholar]
- Yates RM, Hermetter A, Russell DG (2009) Recording phagosome maturation through the real-time, spectrofluorometric measurement of hydrolytic activities. Methods Mol Biol 531: 157–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates RM, Russell DG (2005) Phagosome maturation proceeds independently of stimulation of toll-like receptors 2 and 4. Immunity 23: 409–417 [DOI] [PubMed] [Google Scholar]
- Yates RM, Russell DG (2008) Real-time spectrofluorometric assays for the lumenal environment of the maturing phagosome. Methods Mol Biol 445: 311–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zal T, Volkmann A, Stockinger B (1994) Mechanisms of tolerance induction in major histocompatibility complex class II-restricted T cells specific for a blood-borne self-antigen. J Exp Med 180: 2089–2099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Henderson L, Thomas V, Swan P, Turner AN, Phelps RG (2007) Presentation of the Goodpasture autoantigen requires proteolytic unlocking steps that destroy prominent T cell epitopes. J Am Soc Nephrol 18: 771–779 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.