Abstract
β-Thymosin (βT) and WH2 domains are widespread, intrinsically disordered actin-binding peptides that display significant sequence variability and different regulations of actin self-assembly in motile and morphogenetic processes. Here, we reveal the structural mechanisms by which, in their 1:1 stoichiometric complexes with actin, they either inhibit assembly by sequestering actin monomers like Thymosin-β4, or enhance motility by directing polarized filament assembly like Ciboulot βT. We combined mutational, functional or structural analysis by X-ray crystallography, SAXS (small angle X-ray scattering) and NMR on Thymosin-β4, Ciboulot, TetraThymosinβ and the long WH2 domain of WASP-interacting protein. The latter sequesters G-actin with the same molecular mechanisms as Thymosin-β4. Functionally different βT/WH2 domains differ by distinct dynamics of their C-terminal half interactions with G-actin pointed face. These C-terminal interaction dynamics are controlled by the strength of electrostatic interactions with G-actin. At physiological ionic strength, a single salt bridge with actin located next to their central LKKT/V motif induces G-actin sequestration in both isolated long βT and WH2 domains. The results open perspectives for elucidating the functions of βT/WH2 domains in other modular proteins.
Keywords: actin cytoskeleton, functional versatility, intrinsically disordered proteins, WH2 (Wiskott–Aldrich syndrome protein Homology domain 2) domains, β-thymosin domains
Introduction
The β-thymosin (βT) and WH2 (Wiskott–Aldrich syndrome protein Homology domain 2) domains are widespread, small actin-binding domains of 25–55 amino acids, inserted or repeated in many modular signalling proteins involved in actin-based motile and morphogenetic processes. βT/WH2 are mainly identified by a central consensus actin-binding motif LKKT/V flanked by variable N-terminal and C-terminal extensions (Carlier et al, 2007; Dominguez, 2007; Xue et al, 2007). βT shares a more extended and conserved C-terminal half than WH2. βT/WH2 domains are archetypes of intrinsically disordered proteins/domains (IDP) (Safer et al, 1997; Domanski et al, 2004; Tompa et al, 2009). IDPs exist in their unbound native, functional state as dynamic ensembles of interconverting structures and define a distinct new class of functional proteins or domains whose specific molecular mechanisms of regulations remain to be further characterized (Dyson and Wright, 2005; Tompa et al, 2005, 2009; Uversky and Dunker, 2010). Like other IDPs, βT/WH2 domains display large sequence variability and appear to display multifunctional activities. Single or repeated βT/WH2 domains in modular proteins induce for example actin sequestration, nucleation, filament barbed end regulation or processive assembly (Co et al, 2007; Breitsprecher et al, 2008; Renault et al, 2008; Dominguez, 2009; Qualmann and Kessels, 2009; Campellone and Welch, 2010; Husson et al, 2010).
The 15 highly homologous β-Thymosins of ∼5 kDa found in metazoans (referenced hereafter as βT) act in cells as major actin-sequestering peptides, that is, their complex with monomeric ATP-actin (G-ATP-actin) cannot polymerize at either filament (F-actin) end (Mannherz and Hannappel, 2009). Thymosin-β4 (Tβ4) is the most abundant and well-studied member and promotes cell migration. Tβ4 acts also extracellularly with less understood mechanisms to enhance embryonic endothelial cell migration, promote dermal and corneal wound healing or stimulate coronary vasculogenesis (Sosne et al, 2010). The three βT repeats of Drosophila Ciboulot or its isolated first βT domain (CibD1) make a complex with G-actin which, in contrast to sequestering β-Thymosins, participates in barbed end assembly like profilin:G-actin complex. This profilin-like activity accounts for Ciboulot in vivo function in axonal growth during metamorphosis (Hertzog et al, 2004). Similarly, individual WH2 domains characterized so far in vitro behave as functional homologues of profilin (Egile et al, 1999; Higgs and Pollard, 1999; Hertzog et al, 2002; Mattila et al, 2003).
Despite their significant sequence variability, individual βT and WH2 domains display a similar overall fold and binding path on G-actin with their N-terminal half but elusive interactions after their central LKKT/V motif (Safer et al, 1997; Hertzog et al, 2004; Irobi et al, 2004; Chereau et al, 2005; Aguda et al, 2006; Lee et al, 2007; Ducka et al, 2010; Rebowski et al, 2010). In all structural studies, an N-terminal amphipathic helix of βT/WH2 caps the barbed face of G-actin by binding into the hydrophobic cleft between its subdomains 1 and 3, thus preventing association with the slow-growing pointed ends of filaments. In contrast, the central and C-terminal portions of various βT and WH2 domains interact only partially with actin or not at all after their LKKT/V motif in crystal structures of 1:1 βT/WH2:G-actin complexes stabilized by different polymerization inhibiting methods (drug Latrunculin A/B, protein DNase1 or Gelsolin segment 1, cross-linking) (Hertzog et al, 2004; Chereau et al, 2005; Aguda et al, 2006; Lee et al, 2007; Ducka et al, 2010; Rebowski et al, 2010). Crystal structures from WH2 repeats capable to form multifunctional actin nuclei show similar disorders and incomplete interactions along the WH2 peptidic chains bound to actin monomers (Ducka et al, 2010; Rebowski et al, 2010). Significant interactions with the pointed face of actin were observed in a crystal structure only while using a chimera of a C-terminal segment of Tβ4 fused to gelsolin segment 1 bound to actin (Irobi et al, 2004). In this hybrid complex, Tβ4 C-terminal residues (30–39) fold into an α-helix that caps the pointed face of G-actin by binding between its subdomains 2 and 4. In contrast in solution without the stabilizing ligands or drugs used in crystal structures, NMR studies showed that central and C-terminal residues of both Tβ4 and CibD1 can interact with G-actin in non-polymerizing low ionic strength conditions (G-buffer), although more dynamically for Ciboulot at 35°C (Domanski et al, 2004; Hertzog et al, 2004). Overall, these data suggested that the switch of function of βT domains from inhibition to promotion of actin assembly might depend on different still ill-defined interactions of their C-terminal region with actin subdomains 2 and 4, mediated by sequence variations widely distributed within their C-terminal half. Mutations of residues at four critical positions in the C-terminal half of Tβ4 (Hertzog et al, 2004) were thus shown to convert Tβ4 into a profilin functional homologue. However, these critical residues in Tβ4 remain insufficient to explain the functional behaviours of many βT as they are not all conserved in all sequestering βT like in human Tβ15a.
Here, we determine the exact actin-binding mode and regulation of the various N- and C-terminal regions of β-Thymosins domains in native binary βT:actin complexes and in physiological conditions, and we identify precisely the general sequence elements that control the switch in function in several distantly related βT and WH2 sequences by combining complementary molecular, functional or structural approaches on Tβ4, CibD1 and TetraThymosinβ βT and the long WH2 of WASP-interacting protein (WIP).
Results
Design of chimeras of Tβ4 and the first βT repeat of Ciboulot (CibD1)
CibD1 recapitulates the actin binding and profilin-like activity of full-length Ciboulot (Hertzog et al, 2002, 2004). Sequence alignment of CibD1 and Tβ4 shows three main regions that may contribute differently in actin binding (Figure 1A). First, an N-terminal region including the amphipathic α-helix, longer in CibD1 (residues 4–24 in CibD1, 1–11 in Tβ4); second, a FxxxK linker region connecting the N-terminal helix to the LKKT/V motif (F25NQDK29 in CibD1, F12DKSK16 in Tβ4); third, a central and C-terminal region (residues 30–58 in CibD1, 17–43 in Tβ4). CibD1 and Tβ4 share 18, 60 and 55% sequence homology in the three regions, respectively.
Figure 1.
The function of CibD1/Tβ4 chimeras is controlled by different electrostatic interactions of a single residue of their FxxxK linker region with actin E334. (A) Structural organization of CibD1, Tβ4 and the derived chimeras CH1 to CH4, compared with WH2 domains. Side chains of residues highlighted in red are located below 5 Å from actin side chains bearing an opposite charge. Residues folding into helices upon G-actin binding in crystal structures or NMR studies are boxed. S: sequestering; P: profilin-like. (B) Overlay of the crystal structures of G-actin-bound CibD1 βT and WIP WH2. In WIP WH2:actin:DNase1 structure, the DNase1 protein is not shown for clarity. The actin electrostatic surface potential is colour-coded (basic: blue; acidic: red). CibD1 βT and WIP WH2 are in yellow and green worm representation, respectively. The side chains of Q27 in CibD1, R54 in WIP and the lysines of the LKKT/V motif are shown in ball-and-stick. (C) Rate of barbed end growth versus G-actin concentration in the absence (black) or in the presence of saturating concentrations of CibD1 (blue), CH1 (green), CH2 or CH3 (pink), Tβ4 (red). (D) Interactions provided by CibD1 and Tβ4 linkers in crystal structures of CH1:actin (left panel) and CH3:actin (right panel), respectively. Actin, CibD1 and Tβ4 residues are shown in green, yellow and grey, respectively. Side chain electrostatic interactions below 3.8 Å are shown. The weak H-bonds between CibD1 Q27 and actin E334 side chains are substituted in Tβ4 linker by a 2.8-Å salt bridge between its K14 and actin E334 side chains.
The linker of Tβ4 and CibD1 is longer by two residues than the linker in canonical WH2 domains, which bends the βT peptide towards the charged residue E334 of actin (Figure 1B). The fact that the linkers of Tβ4 and CibD1 contain different charged residues prompted us to examine the possible contribution of the linker in the function of these two peptides. Three chimeric peptides that all contained the N-terminal amphipathic helix of CibD1 followed by various sequences (Figure 1A) were designed. Chimera 1 (CH1) displayed the N-terminal and linker regions of CibD1 followed by Tβ4 sequence, chimera 2 (CH2) the full Tβ4 sequence from its linker to its C-terminus, and chimera 3 (CH3) the linker of Tβ4 followed by CibD1 sequence.
Both the N-terminal amphipathic helix and the linker region control the binding strength of Tβ4, CibD1 and derived βT chimeras to G-actin
The interaction of Tβ4 and the peptides CibD1, CH1, CH2 and CH3 was analysed using the fluorescence of AEDANS-labelled CaATP-G-actin as a probe (De La Cruz et al, 2000). The data (Supplementary Figure S1) are summarized in Table I. At low ionic strength (G-buffer), the peptides displayed a large range of affinities for G-actin. The substitution of the C-terminal half of CibD1 by the corresponding Tβ4 sequence in CH1 increased the affinity for G-actin by eight-fold. The equilibrium dissociation constant (Kd) of CH1 is thus similar to Tβ4 but remains in the μM range like CibD1 Kd. More drastic effects were seen upon introducing the linker of Tβ4 in a CH1 or CibD1 context. The resulting CH2 and CH3 bound CaATP-G-actin with two to three orders of magnitude higher affinity than CibD1 (Kd of 1–10 nM).
Table 1. Equilibrium dissociation constant (Kd) for the complexes of G-actin with various CibD1/Tβ4 and WIP WH2-derived peptides.
| Tβ4 | CibD1 | CH1 | CH2 | CH3 | CH4 | Tβ4 K14Q | CibD1 Q27K | wt WIP WH2 | R54N WIP WH2 mutant | |
|---|---|---|---|---|---|---|---|---|---|---|
| Kd in G-buffer (CaATP-G-actin) in μM | 0.2 μM | 2 μM | 0.25 μM | 0.001 μM | 0.01 μM | |||||
| Kd in F-buffera (MgATP-G-actin) in μM | 2.3 μM | 8.5 μM | 3.4 μM | 0.5 μM | 1.54 μM | |||||
| Kd in F-bufferb (MgATP-G-actin) in μM | 1.8 μM | 4.5 μM | 1.8 μM | 0.5 μM | 0.5 μM | 0.6 μM | 35 | 2.7 | 0.5 μM | 1.15 μM |
| Barbed end growth rate (k+ in μM−1 s−1) | 0 | 3 | 2 | 0 | 0 | 0 | 0.7 | 0 | 0 | 4 |
| Function in actin assembly, physiological ionic strength (⩾F-buffer) | S | P | P | S | S | S | P | S | S | P |
| Function in actin assembly, low ionic strength | S | S | S | S | S | S | S | S | ||
| Kd values were derived in G-buffer and in F-buffera from AEDANS fluorescence titration curves (Supplementary Figure S1), and in F-bufferb from functional F-actin assembly assays with filaments (steady-state measurements) and seeds (initial growth rate measurements) capped by gelsolin at their barbed ends (Supplementary Figure S2 for CibD1/Tβ4-derived peptides, Figure 5 for wt and mutated WIP WH2 peptides). The k+ of profilin:actin is 7 μM−1 s−1. | ||||||||||
The same measurements were performed at physiological ionic strength F-buffer with MgATP-G-actin (Supplementary Figure S1C). The affinities of all βT peptides were reduced by the increase in the ionic strength, suggesting that electrostatic interactions play an important role in the stability of βT:actin complexes. The affinities of Tβ4, CibD1, CH1 for G-actin were reduced by one order of magnitude, while the affinities of CH2 and CH3 decreased by two orders of magnitude, still remaining higher than those of Tβ4, CibD1 and CH1 (Table I). Pairwise comparison of the different βT peptides reveals that both the longer N-terminal amphipathic helix of CibD1 and the linker of Tβ4 have a major contribution in enhancing the affinity of full-length βT domains for actin while the central and C-terminal regions of Tβ4 and CibD1 can be interchanged with milder effect on affinity for actin, at both low and physiological ionic strength.
The linker region controls the function of CibD1, Tβ4 and derived chimeras in actin assembly
The activities of CibD1, Tβ4, CH1, CH2 and CH3 were compared in barbed and pointed end growth assays and in steady-state measurements of F-actin assembly, at physiological ionic strength (Supplementary Figure S2). All three chimeras caused linear concentration-dependent depolymerization of pre-assembled actin filaments capped at barbed ends by gelsolin, and complete inhibition of pointed end growth from gelsolin–actin seeds (data not shown). The Kd values derived from these two functional F-actin assembly assays (Table I) are in good agreement with each other and with those derived from AEDANS fluorescence titration experiments in F-buffer. On the other hand, when barbed ends are not capped, CH1 behaves like Ciboulot or profilin, that is, its complex with actin participates in barbed end assembly. The association rate constant (k+) of actin–CH1 to barbed end (2 μM−1 s−1) is lower than the k+ of profilin–actin (7 μM−1 s−1) (Gutsche-Perelroizen et al, 1999), or Ciboulot–actin (3 μM−1 s−1) (Figure 1C; Table I). In contrast, CH2 and CH3 both simply sequester G-actin like Tβ4, but more efficiently than Tβ4 due to their higher affinity.
In conclusion, remarkably CH1 keeps the function of CibD1 or profilin despite an affinity for G-actin similar to Tβ4, and CH2 sequesters actin like Tβ4, while it differs from CH1 only by harbouring the linker of Tβ4. Similarly, CH3 has lost the profilin function of CibD1 while it differs from CibD1 only by the three residues D13KS of Tβ4 linker (versus N26QD in CibD1). Overall, these data indicate that the stronger affinity brought by the longer amphipathic N-terminal helix in CibD1 than in Tβ4 enables a sequestering function even in the absence of the charged/polar central and C-terminal residues previously shown to be critical for this function in the case of Tβ4 (Domanski et al, 2004; Hertzog et al, 2004; Irobi et al, 2004; Aguda et al, 2006). In that case, the short linker by itself specifies the function of the entire βT peptides in actin assembly.
Crystal structures of βT:actin complexes provide functional insight into the linker region
To analyse the different interactions in profilin-like and sequestering βT:actin complexes, we solved the crystal structures of CH1:actin:Latrunculin A and of actin-bound CH1, CH2 and CH3 without the use of a drug or sequestering protein, at 1.75, 2.0, 2.5 and 2.3 Å resolution, respectively. Final crystallographic statistics and models are detailed in Supplementary Table SI. The drug Latrunculin A does not induce additional disorder in complexes, an issue that had been raised (Chereau et al, 2005; Aguda et al, 2006). In all structures, actin subdomain 2 was partially disordered as well as all βT chimera residues after the LKKT/V motif (Supplementary Figure S3), like in Ciboulot:ADP-actin:Latrunculin A structure (Hertzog et al, 2004). The different crystal structures show that the length of actin-bound CibD1 N-terminal α-helix (residues 16–24) is similar to the length of actin-bound WH2 domain N-terminal helices (see Supplementary data). The last three residues of the FxxxK linker in CibD1 and Tβ4 build a 310-helix in βT chimera:actin structures in which ATP is not hydrolyzed. The second, fourth and fifth residues of the linkers do not directly interact with actin but stabilize the bend of the linker by several hydrogen (H−) bonds (Figure 1D). Only the first and third residues of the FxxxK linkers make direct close contacts with actin residues. In both linkers, the Phe side chain anchors the segment on actin by similar hydrophobic interactions involving the neighbouring Leu of the central LKKT/V motif and Ile345 and Leu349 residues of actin (Figure 1D). The side chains of the third residue, Q27 in CibD1 and K14 in Tβ4, are engaged in different electrostatic interactions with the highly conserved E334 of actin: CibD1 Q27 side chain makes H-bonds with actin A144 main chain O and E334 side chain, while Tβ4 K14 supplies a stronger 2.8 Å long salt bridge with actin E334. Therefore, the nature of the third residue in the FxxxK linker controls the ionic strength dependence of the overall affinity of chimeras for actin and of their different functions in actin assembly. Point mutations Q27K in CibD1 and K14Q in Tβ4 at the linker critical position confirm the conclusions (Supplementary Figure S4). The mutation Q27K in CibD1 causes a mild (two-fold) increase in its affinity for G-actin, yet is sufficient to switch the profilin-like activity of wt CibD1 into a sequestering activity in steady-state measurements of actin assembly, comforted by barbed end growth assays showing a total inhibition of actin assembly at barbed ends by CibD1-Q27K (Table I). The mutation K14Q in Tβ4 leads to a large (17-fold) decrease of its affinity for G-actin. In steady-state measurements, the sequestering behaviours of Tβ4 K14Q at pointed and barbed ends correspond to an Kd of ∼35 and 58 μM, respectively, suggesting that the mutated Tβ4 has gained some ability to support barbed end assembly. Consistently, barbed end growth was not totally inhibited at saturation by the peptide and a value of k+ of 0.7 μM−1 s−1 was derived for the association rate constant of Tβ4-K14Q:actin to the barbed ends (Table I). However, the very low affinity and low value of k+ make it difficult to assess whether the sequestering function has been almost abolished or converted into a detectable profilin function. Our data suggest that the mutation K14Q causes a large destabilization of Tβ4 overall interaction with actin because it is adjacent to the N-terminal α-helix, which itself binds weakly to the barbed face of G-actin due to its short size (residues 5–11) (Figure 1A; Table I).
SAXS studies of the βT:actin complexes in solution distinguish overall conformational changes at different ionic strength in the CibD1:actin complex but not in CH3:actin
The different βT:actin complexes were next analysed at low resolution by small angle X-ray scattering (SAXS) at different ionic strengths to model an average conformation of all amino acids in solution, including flexible regions (Koch et al, 2003). Accurate SAXS measurements were conducted on-line on homogeneous G-actin or saturated 1:1 βT–actin complexes eluted from a size exclusion chromatography column (David and Pérez, 2009). The scattering patterns of ATP-G-actin-Lat.A in G- and F-buffers are identical up to a momentum transfer (Qmax) of 0.35 Å−1, testifying that no major conformational change of G-actin occurs upon increasing ionic strength. The SAXS patterns of the βT:actin complexes were measured for all chimeras in G-buffer to a Qmax of 0.3 Å−1. All of them could be fitted with similar sequestering conformations of the βT:ATP-actin complexes, in which the full structure of ATP-G-Actin-Lat.A was used and the βT peptides capped both its barbed and pointed faces (Figure 2A–C; Supplementary Figure S5).
Figure 2.
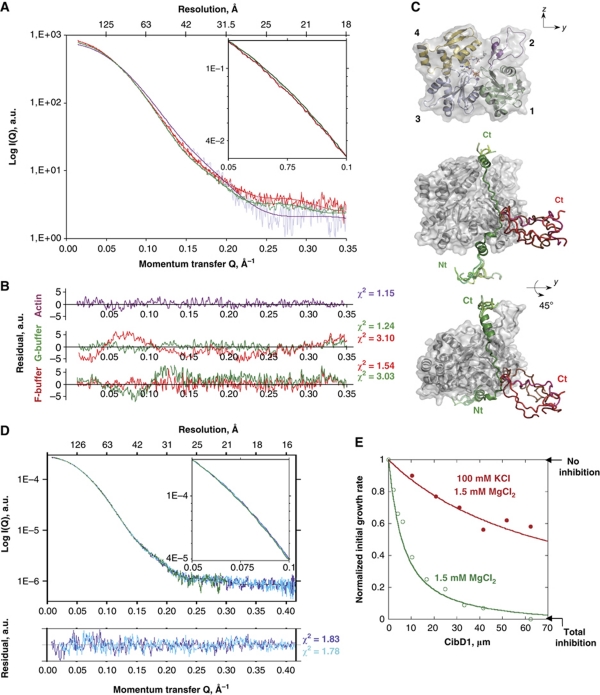
SAXS analysis of ionic strength-dependent structural changes of ATP-G-actin and its complexes with the profilin-like CibD1 or sequestering CH3. (A) Experimental X-ray scattering patterns at T∼12°C from G-actin-ATP alone (grey) in G- or F-buffer and of CibD1:Actin complex in G- (green) and F- (orange) buffer to a maximum momentum transfer Qmax= 4πsin(θ)/λ of 0.35 Å−1 (∼18 Å resolution). Computed scattering patterns in purple, green and red, respectively, derived from the models shown in (C). Inset: Zoom highlighting the divergences at small angles between 0.05 and 0.1 Å−1 between the SAXS patterns of CibD1:Actin in G- and F-buffers. (B) Reduced residuals of the fit of the computed scattering patterns of the models to the experimental scattering curves over the entire Q range, calculated as described in Materials and methods. (C) Models fitting the SAXS data for G-actin-ATP alone with its four subdomains shown in different colours (upper panel) and for CibD1:Actin-ATP complexes in G- or F-buffer in different orientations (middle and lower panels). The surface of actin is shown in grey and the ribbon representation of actin-bound CibD1 in green in G-buffer and red in F-buffer. Several averaged conformations of actin-bound CibD1 fitting equally well the SAXS data in F-buffer are shown. (D) The SAXS patterns for CH3:actin-ATP complex in G-buffer (cyan), F-buffer (blue) and CibD1:actin-ATP complex in G-buffer (green) all three superimpose up to a Qmax of 0.416 Å−1 (∼15 Å resolution). Inset: Here the three curves superimpose well between 0.05 and 0.1 Å−1. (E) The profilin-like activity of CibD1 in F-buffer reverses to a sequestering activity at low ionic strength. Initial rates of barbed end growth from spectrin–actin seeds were measured at 3 μM G-actin with increasing concentration of CibD1. The growth rate in the absence of CibD1 is set to 1. At saturating amounts of actin by CibD1 (70 μM), over 50% of barbed end growth occurs at physiological ionic strength (red) while 95% inhibition of barbed end growth was measured at low ionic strength (green).
The sequestering conformation of CibD1 on G-actin in G-buffer was unexpected given the profilin-like activity of this peptide at high ionic strength. Yet, actin assembly assays revealed the sequestering activity of CibD1 and CH1 at low ionic strength (1.5 mM MgCl2, no KCl), thus fully validating the SAXS data (Figure 2E). CH2 and CH3 on the other hand sequestered actin at low and high ionic strength (Table I).
The complexes of CibD1 and CH3 with G-actin, which differ only by their linker, were then analysed by SAXS at physiological ionic strength where they have the most divergent functions. The SAXS curve and model of CH3:actin showed the same sequestering conformation as in G-buffer up to a Qmax of 0.4 Å−1 (Figure 2D). In contrast, the SAXS curve of CibD1:actin in F-buffer does not superimpose on the scattering curve in G-buffer (Figure 2A). The curve could be fitted by conformations of actin-bound CibD1, where the interactions of the C-terminal half with the pointed face of G-actin are released (Figure 2C). This model appears unique, since the difference between the curves cannot be accounted for by a partial dissociation of the complex (free G-actin itself shows a very different scattering pattern), nor by an open conformation of G-actin in the complex (Chik et al, 1996), nor by any of the known conformations of actin subdomain 2 available in actin structures of the Protein Data Bank.
In conclusion, the SAXS analysis at different ionic strengths reveals conformational changes in βT:actin complexes that correlate with functional differences measured in biochemical assays. The release of CibD1 C-terminal half interactions with actin in SAXS models accounts for the disorder of this region in the crystal structure of Ciboulot:actin complex at similar high ionic strength (Hertzog et al, 2004). The C-terminal regions of sequestering CH2 and CH3 peptides were also disordered in their crystallized complex with actin, suggesting that they were more loosely bound to actin than their N-terminus in the non-physiological crystallization conditions. To test this possibility, the dynamics of all βT residues in solution was studied by NMR at different ionic strengths.
NMR provides atomic resolution evidence for the folding of βT peptides upon G-actin binding and for ionic strength-dependent dynamic behaviour
The folding and static and dynamic behaviour of the βT peptides were analysed by NMR spectroscopy in their free and actin-bound states at different ionic strengths. The amide bonds of the βT peptide residues were selectively observed, using 15N-labelling, with additional 2H and/or 13C-labelling for some experiments (see Materials and methods and Supplementary data). All chimeras are intrinsically disordered in their free state at 25°C, and undergo a complete folding upon binding to G-actin (data not shown) to adopt the secondary structure reported in Figure 3A. These results are consistent with previous NMR and crystallographic analysis of Tβ4 interactions with actin (Domanski et al, 2004; Irobi et al, 2004). In G-buffer, nOe correlations between protons of actin and amide backbone protons of chimera 2 confirm that interactions of βT peptides with actin extend beyond the N-terminal half segment that is ordered in chimera:actin crystal structures (Figure 3B; Supplementary Figure S6; Supplementary Table SII). The chemical shifts involved in these CH2:actin contacts are compatible with those expected for actin protons at a distance of <5 Å from the amide protons of both CibD1 N-terminal half in CH2:ATP-actin or Ciboulot:actin (Hertzog et al, 2004) crystal structures and Tβ4 central and C-terminal segments in Gelsolin-Tβ4:ATP-actin crystal structure (Irobi et al, 2004) (Figure 3B; Supplementary Figure S6).
Figure 3.
NMR analysis at low ionic strength (G-buffer): the whole sequence refolds and interacts with G-actin in all types of βT. (A) Secondary structures of bound CH1 (green linker) and CH2 (magenta linker), from amide protons, nitrogen and α-carbon chemical shifts, at T=30°C in G-buffer. (B) Agreement between actin–CH2 close contacts in solution and in crystal structures. NMR NOESY experiments are used to identify CH2 residues (highlighted in black in the sequence) whose amide protons are close to actin protons. These chemical shifts are all compatible with those expected for actin protons at a distance of <5 Å from equivalent amide protons of the N-terminal fragment of Ciboulot and of the C-terminal fragment of Tβ4 (red and blue sequence and structure elements, respectively) in the crystal structures 1SDK (red) and 1T44 (blue). Actin residues (in green) and black dashed lines schematize the distances below 5 Å in the crystal structures. Only one actin structure (1SDK) is represented in grey for clarity. (C) Superimposed (15N,1H) HSQC spectra of actin-bound CH1 (red) and CH2 (black). The non-overlapping peaks correspond only to the residues of the linker (green and magenta, as in panel (A)). Additional slight differences in the spectra arise from residues adjacent to the linker. (D) Diagram of the exchange between free and bound forms of a βT domain. Exchange peaks (magenta) between the free (F, cyan) and the bound (B, red) form appear if kex is fast compared with 15N longitudinal relaxation times, as observed for CH1, not for CH2. (E) Heteronuclear nOe (1H–>15N) of actin-bound CH2. High internal mobility of amide bonds in the 100-ps range is found for residues (32–51) (red dashed line) with nOes values <0.85 (fully restricted flexibility). (1H–>15N) nOes were obtained as the ratio of the intensities of peaks in (15N,1H) experiments with and without prior saturation of amide protons. Error bars account for the error in the peak intensities in each saturated (Isat) and unsaturated (Inosat) experiments (see Materials and methods).
In G-buffer, the overlay of (1H,15N) HSQC spectra of βT domains bound to actin shows that the regions that the chimeras share with CibD1 or Tβ4 are in identical chemical environment and thus adopt the same fold and localization on G-actin as in the native CibD1 or Tβ4 peptide, independently of the nature of the remaining sequence (Figure 3C and Supplementary Figure S7). The vicinity of amide protons of CH2 and protons of actin that was revealed by nOe data thus applies to homologous fragments of other βT peptides. Small but significant differences were detected only between Tβ4 and CH2 residues of the linker and central region, indicating that the N-terminal helix slightly influences either the arrangement of these two fragments on the surface of actin or the structure of actin. Overall, the structural analysis at atomic level validates the view that the different affinities and functions of the chimeras that are monitored at physiological ionic strength cannot be discriminated by a specified folding and positioning on actin in G-buffer. The similar positioning of all peptides on actin is consistent with their common sequestering activity in G-buffer.
In G-buffer, the differences between chimera affinities are emphasized: the DKS (Tβ4) to NQD (CibD1) mutations in βT linker decrease the affinity of CH2 for G-actin by two orders of magnitude in CH1 (Table I). The basis for this large difference in affinity was revealed by monitoring the exchange between the free and actin-bound states of CH1 and CH2 in Nzz exchange experiments (Farrow et al, 1994; Vialle-Printems et al, 2000). Exchange rate values fall in the 10–100s−1 range for CH1 and are lower than 1 s−1 for CH2 at low ionic strength (Figure 3D). The identical behaviour of all measurable residues in the Nzz experiments suggests that the exchange between the bound and free states of CH1 occurs in a one-step process in the 10-ms time scale. Since the measurements were performed at concentrations of chimeras much higher than the Kd values for both complexes, the exchange rate constant equals 2koff (see Materials and methods and Supplementary data). Thus, CH1 has a much faster off rate than CH2 from G-actin, accounting for its lower affinity for G-actin.
15N relaxation experiments provide detailed information on the dynamics of the interactions with actin all along the different regions of the chimeras. The overall correlation time of CH2 bound to G-actin has a value of 15.7±0.5 ns, fully compatible with the size of the complex (Supplementary Figure S8). However, lower values of steady-state (1H–>15N) nOes are measured in the C-terminal than in the N-terminal half of the peptide (Figure 3E). This result indicates that already in G-buffer the NH bonds of the C-terminal region of actin-bound CH2 have a higher flexibility, in the 100-ps time scale.
To understand how ionic strength differentially affects the function of CH1 and CH2, depending on the sequence of the linkers, HSQC spectra were recorded at increasing ionic strength from 0 to 150 mM KCl. CH1 and CH2 displayed different behaviours. Notably, the increase of signal characteristic of the free state of CH1 is more pronounced for C-terminal residues than for N-terminal residues, indicating that the interactions of the C-terminal region with actin are specifically released above 30–40 mM KCl (Figure 4; Supplementary Figure S10A). In contrast, the increase in signal characteristic of the free state of CH2 showed no residue-specific variations (Supplementary Figure S10A). However, at 150 mM KCl, the temperature dependence of the HSQC peaks of actin-bound CH2 changes and becomes identical to the CH1 pattern in G-buffer (Supplementary Figures S9 and S10B).
Figure 4.
NMR analysis at variable ionic strength: the interactions of CH1 C-terminal region with G-actin are specifically released above 30–40 mM KCl. Variations of the averaged intensities of NMR HSQC 1H–15N cross-peaks for the five N-terminal (V14, A15, L18, E23 and F25) or six C-terminal (E45, T46, I47, E48, Q49, K51) residues of CH1 in its free state, as a function of KCl concentration, in a sample containing 60 μM actin and 120 μM CH1 at T=30°C. The blue and red curves represent the averaged intensities for N-terminal and C-terminal residues, respectively. Intensities are normalized to those obtained without KCl. Error bars correspond to the standard deviations of the intensities over the five N-terminal or six C-terminal residues. The changes in intensities due to the increase in conductivity with KCl were corrected using a reference sample containing free CH1 (Supplementary Figure S10). For N-terminal residues, the global intensity increases are attributed to free CH1 arising from the formation of F-actin subsequent to the lowered affinity of CH1 for G-actin upon addition of KCl (Table I). The increase of signal characteristic of the free state of CH1 is significantly more pronounced for C-terminal residues than for N-terminal residues, indicating a prior specific release of the C-terminal segment from actin above 30–40 mM KCl.
In conclusion, NMR static and dynamic studies extend the SAXS analysis at atomic level on βT residues to show that the different function of the WH2/βT domain, linked to different dynamics of interaction of its central and C-terminal region with actin, depends on the strength of electrostatic interactions with actin of the FxxK/QxK linker of βT. Ionic strength has been instrumental in establishing this conclusion (Figure 6).
Figure 6.
Structural basis for the functional versatility of 1:1 βT/WH2:actin in actin assembly at different ionic strength. The N-terminal amphipathic helix (red cylinder) of all βT/WH2 domains caps the barbed face of G-actin by stable static hydrophobic interactions, which always locally inhibit association of their complexes with filament (F-actin) slow-growing pointed (−) ends. When a long basic residue in their sequence forms a salt bridge with actin next to their central consensus LKKT/V motif, the dynamic interactions of their central and C-terminal segments with actin are restrained (left panel). The presence of a long C-terminus in these βT/WH2 induces then an efficient capping of the pointed face of the bound G-actin, preventing βT/WH2:actin association at filament fast-growing barbed ends. In contrast, when the central region of βT/WH2 provides only weak electrostatic interactions (van der Waals/hydrogen (H) bonds), their central and C-terminal segments interact with G-actin pointed face with high dynamics at physiological ionic strength (right panel). In these conditions, long βT/WH2 behave like short βT/WH2: in their βT/WH2:actin complexes the free or transiently free pointed face of the bound G-actin can associate with filaments fast-growing barbed (+) ends. At low ionic strength, electrostatic interactions are less attenuated by solvent screening and van der Waals/H-bonds contribute more significantly overall to stabilize dynamic interactions. Consequently, all βT/long WH2 domains adopt a single main conformational and functional state corresponding to sequestration.
The more distantly related WIP WH2 and Tetrathymosinβ βT domains show a similar dependence of their functions with a single salt bridge close to their LKKT/V motif
The Caenorhabditis elegans Tetrathymosinβ (TT) protein displays both G-actin sequestering and filament binding capacity via its four βT repeats (Van Troys et al, 2004). Only the fourth βT domain of Tetrathymosinβ (TTD4) contains a FxKxx linker appropriate for a sequestering function (Figure 7). To see if a FxKxx linker could further control the function with other βT sequences than Tβ4 and CibD1–3, we analysed TTD4 with TTD1 and TTD3. The latters do not display the FxKxx linker signature and the three βT correspond in TT to the three most divergent βT sequences from Tβ4. In line with our view on the strong impact of the linker on the function of different βT sequences, TTD4 displayed a poor ability to support barbed end assembly despite its truncated C-terminal region compared with Tβ4 sequence. At physiological ionic strength, all three βT bound weakly G-actin (TTD4 and TTD1 Kd are ∼250 and ∼750 μM, respectively), which made difficult to assess their respective functions in F-buffer except for TTD4 (Supplementary Figure S12). Their differences in actin assembly were thus highlighted at 25 mM KCl where the affinities of the three βT for G-actin are increased like with CibD1 and TB4. TTD3 affinity remained however too low to impact significantly actin assembly as isolated domain. At low ionic strength, TTD1 and TTD4 totally inhibited pointed end growth like Tβ4 or CibD1 (Figure 5A). Barbed end growth was only modestly inhibited at saturation with TTD1, consistent with a profilin-like function, and a value of k+ of 4 μM−1 s−1 was derived for the association rate of TTD1:actin to the barbed end (Figure 5B). In contrast, the behaviour of TTD4 was consistent with a sequestering activity and a total inhibition.
Figure 7.
Structural insight into the functions of other βT/WH2 domains. Sequence alignments of βT and WH2 domains/repeats and known (in bold) or predicted sequestering (S) or profilin-like (P) function in actin assembly. Sequence accession numbers are in Supplementary data. The letters ‘h’ on top of Tβ4 sequence show the location of α-/310-helices in actin-bound Tβ4. Basic residues in βT FxK/Rxx linkers or WIP WH2 homologous central regions that can provide a short salt bridge with actin are shown in red. G-actin sequestration requires in addition a long C-terminus with residues other than Pro/Gly to provide specific interactions with actin.
Figure 5.
Analysis and control of the function of TetraThymosinβ βT and WIP WH2 domains by their linker or a single salt bridge located just after the LKKT/V motif. (A) At low ionic strength (25 mM KCl), TTD1 (squares) and TTD4 (circles) totally inhibit pointed end growth from gelsolin–actin seeds. TTD4 displays a higher affinity for G-actin than TTD1 and TTD3 (triangles) only a minor binding. The growth rates are measured with 2.5 μM MgATP-G-actin in (A, B) and set to 1 in the absence of the peptides. (B) At 25 mM KCl, TTD1 inhibits only modestly barbed end growth from spectrin–actin seeds, consistent with a profilin-like function (k+∼4 μM−1 s−1). The inhibition by TTD4 follows a sequestering behaviour with a total inhibition, while no significant impact on filament steady state is observed with TTD3 because of its too low affinity for G-actin. (C) WIP WH2 domain inhibits both pointed and barbed end growth by sequestering G-actin with an Kd of 0.7 μM. Barbed end or pointed end growth rates are measured in the presence of 2 μM MgATP-G-actin and WIP WH2 as indicated. Data obtained with Tβ4 as a control are shown. (D) The single mutation R54N, which abrogates the ionic bridge between WIP R54 and actin 93 side chains is sufficient to switch its function in actin assembly. The mutant inhibits pointed end growth by sequestering G-actin but fails to inhibit barbed end growth. Experimental conditions are as above. (E) Steady-state measurements of F-actin in the presence of WIP WH2 domain. Identical sequestering behaviour is observed at barbed and pointed ends. Total actin=2.25 μM. (F) Same measurements as in panel (C), with R54N-mutated WIP. When barbed ends are free, the measured amount of F-actin (solid line) is higher than expected if the peptide was sequestering actin (dashed line), indicating that the actin-mutated WIP complex supports monomer–polymer exchange at barbed ends.
WH2 domains share low sequence homology with βT and their linker region is too short to interact with actin E334 side chain (Figure 1A and B). We analysed if C-terminally extended WH2 domains could interact more or less transiently with the pointed face of G-actin and regulate different activities in actin assembly depending on a single residue change like in βT:actin complexes. We examined the long WH2 domain of the WIP protein (Figure 1A) whose crystal structure is known in complex with actin and the protein DNase1, which caps the pointed face of actin (Chereau et al, 2005). In this ternary structure, all the residues of the N-terminal and central regions of WIP WH2 domain that are not overlapping with the DNase1-binding site were stabilized on actin (WIP residues 29–60). In the light of our structural analysis of βT:actin complexes, we postulated that a 2.8-Å long salt bridge between R54 in the central region of WIP near its LKKT/V motif and E93 of actin (Figure 1A and B) could act like the K14–E334 salt bridge in Tβ4 linker. We analysed actin binding and function in assembly of the wild-type and R54N-mutated protein constructs (residues (18–76) of human WIP, including the WH2 domain). The Arg-to-Asn mutation disrupts the salt bridge but allows weaker electrostatic bonds with actin. In functional kinetic and thermodynamic actin assembly assays, the wild-type construct binds G-actin in F-buffer with an affinity about four-fold higher than Tβ4 (Kd∼0.5 μM; Figure 5C and F; Table I). The R54N mutant shows a modest approximately two-fold lower affinity. In spite of comparable binding parameters, the wild-type WIP WH2 domain sequesters actin (Figure 5C and E), whereas the R54N point mutant displays a profilin-like function, preventing assembly only at the pointed end (Figure 5D and F). In G-buffer, like CibD1, the R54N mutant recovers a sequestering function showing that the mutation does not prevent its C-terminal half to interact with G-actin.
Discussion
The natively unfolded character of βT/WH2 modules, their significant length and sequence variability, their repetition or association with other regulatory domains of actin assembly dynamics in proteins contribute to generate multiple functions in actin assembly. This context makes it difficult to identify their respective structure–function relationships. Here, we describe how the distribution of static and dynamic interactions in different 1:1 complexes with G-actin controls their ability to associate to each end of the filament in an ionic strength-dependent fashion. We highlight how small sequence elements in both βT and WH2 control these different interaction modes and the resulting functions in actin assembly.
Molecular determinants for the versatility of βT domains in actin assembly
The N-terminal amphipathic α-helix of βT/WH2 and the LKKT/V motif define the most stable part of the interactions with actin subdomains 1 and 3 (Van Troys et al, 1996; Safer et al, 1997; Hertzog et al, 2004; Chereau et al, 2005; Lee et al, 2007). The length and amphipathic character of the N-terminal α-helix control the affinity of these peptides for actin, anchoring them to the barbed face of the monomer. Replacing the short helix of Tβ4 by the long one of Ciboulot in CH2 increases the affinity for G-actin at physiological ionic strength approximately four-fold, making its sequestering function more efficient and potentially constitutive (Husson et al, 2010). In fact, the low sequestering efficiency of Tβ4 facilitates dissociation of actin monomers from the complex when free G-actin concentration is lowered by formation of new barbed ends in actin-based processes.
In contrast with the static nature of the interface of the N-terminal α-helix with actin, the central and C-terminal regions of the βT/WH2 domains undergo dynamic interactions with actin and its pointed face (Figure 3). At low ionic strength, electrostatic interactions are less attenuated by solvent screening. Therefore, even weak electrostatic interactions contribute in stabilizing the interaction of the peptide with subdomain 2 and induce a sequestering conformation (Table I; Figures 2 and 3B; Supplementary Figure S5 and S6). Increasing either the temperature (Hertzog et al, 2004) or the ionic strength to physiological values results in the selective release of the weaker electrostatic interactions from the pointed face of actin. Direct evidence for this selective dissociation is provided by our SAXS and NMR data.
We show that the functional relevance of the ionic strength relies on the charge of the third residue of the FxxxK linker region in βT peptides that interacts with E334 of actin. Thus, substituting of CibD1 linker (F25NQDK29) by the Tβ4 linker (F12DKSK17) or making the point mutation Q27K is sufficient to convert it into a G-actin sequesterer. While the F residue anchors the linkers via similar hydrophobic contacts with actin in both peptides, Q27 in CibD1 and K14 in Tβ4 supply, respectively, a hydrogen bond and a stronger 2.8 Å ionic bond with E334 of actin (Figure 1D). At high physiological ionic strength, the salt bridge is sufficient to reduce the dynamics of the central and C-terminal regions of both Tβ4 and CibD1, thus maintaining their sequestering activity. In contrast, the high physiological ionic strength destabilizes the weaker hydrogen bonds formed with a glutamine (in CibD1 and CH1) and promotes selective dissociation of these regions from G-actin pointed face and unidirectional assembly of the complexes at filament barbed ends (Figure 6).
In sequestering β-Thymosins, which are weakly anchored on G-actin barbed face via a shorter N-terminal helix, the salt bridge provided by their FxKxK linker appears crucial to stabilize also their overall binding with G-actin. Its mutation in Tβ4 (K14Q) impacts the sequestering efficiency of Tβ4 by decreasing ∼17-fold its affinity for G-actin (Supplementary Figure S4).
Other charged amino acids along Tβ4 sequence were reported to bring a minor contribution to its overall affinity for actin (Vancompernolle et al, 1992; Van Troys et al, 1996; Au et al, 2008). A profilin-like activity was elicited in Tβ4 by replacing four charged or polar residues in the central and C-terminal regions by the corresponding uncharged or non-polar residues of CibD1 (Hertzog et al, 2004). However, when the long N-terminal amphipathic helix of CibD1 was introduced in this mutated Tβ4, the resulting peptide (CH4 in Figure 1A) recovered a sequestering function (Supplementary Figure S11). Thus, the length and binding strength of the N-terminal helix have bearings on the effectiveness of mutations in the central/C-terminal region to control the function and counterbalance the stabilization brought by the favourable charged residue of the linker. With βT more tightly bound to G-actin via a longer N-terminal amphipatic helix like CH2, CH3 or CH4, the positions previously identified in Tβ4 can accommodate more sequence variations without much impact on the βT overall binding and function.
The proposal by Hertzog et al (2004), that the sequestering versus elongating function was controlled through the level of locking of the pointed face of G-actin by the C-terminal regions of βT/WH2 is confirmed here at physiological ionic strength. However, at that time, the mechanism by which this lock was achieved was not elucidated against many βT/WH2 sequence variations. Typically, the limited understanding of the structure–function relationship would not have allowed a correct prediction of the function of CH1, CH3 and CH4. We show here that the level of locking can be more generally controlled by small sequence variations located well outside the C-terminal regions of βT/WH2. The dynamics of the C-terminal region interactions over G-actin pointed face are primarily controlled by the strength of electrostatic interactions with G-actin, and, more strikingly, can be controlled by a single residue located in their linker region, in several distantly related βT sequences including βT with a long N-terminal helix (Figure 6).
Isolated long WH2 domains regulate either G-actin sequestration or unidirectional assembly using the same molecular mechanisms as βT
The linker of WH2 domains is often too short to interact with actin E334 side chain (Figure 1A and B). Short WH2 domains that do not contain a C-terminal extension, like in WASP/WAVE or MIM (missing in metastasis) proteins (Figure 1A) can only display a profilin-like function. Consistently, a C-terminally truncated variant of Tβ4 (residues 1–30), which does not extend to the cleft between actin subdomains 2 and 4, fails to completely inhibit salt-induced actin polymerization (Vancompernolle et al, 1992). In contrast, long WH2 domains can either inhibit or promote actin assembly like βT peptides. The sequence of the C-terminal extension of WH2 of WIP is unrelated to Tβ4 and is not predicted to fold into a C-terminal α-helix like Tβ4 residues 30–39 (Figure 1A). It nevertheless sequesters G-actin more efficiently than Tβ4, like CH2, due to its longer N-terminal amphipathic helix (Figure 5C). A C-terminal α-helix therefore is not required for the pointed face capping and sequestration of G-actin. Actin sequestration by WIP again relies on a single salt bridge between R54 in WIP and E93 in actin. Abolishing this salt bridge restores a profilin function in mutated WIP (Figure 5D and F). In conclusion, the long WH2 of WIP uses the same mechanism as the βT peptides to specify the function.
Perspectives for elucidating the multiple functions of other βT and WH2 domains
We have highlighted general sequence elements in βT and WH2 domain subfamilies of intrinsically disordered actin-binding peptides that impact their affinity for G-actin and control their function. The results fully support the correlation between the sequence of these peptides and either the sequestering activity of Tβ4, Tβ10 or Tβ15, or the profilin-like activity of the two WH2 domains of actobindin (Hertzog et al, 2002), which share with βT many charged residues in their central and C-terminal regions (Figure 7). Sequence alignments show that the WH2 domains of actobindin contain the typical short linkers of WH2 domains and extended C-termini (Figure 7). In their central region, which is otherwise highly homologous to the sequestering WIP WH2, a lysine stands in the position of R54 in WIP to face 93 of actin. A lysine occupies also this position in CibD1/D2/D3 or Tβ4. The lysine side chain, being shorter than an arginine, cannot make a strong electrostatic bond with E93 in a flexible region of actin close to subdomain 2. This one residue difference accounts for the failure of the extended C-terminal halves of actobindinD1/D2, CibD1 or CH1 (Figure 1A and C) to support G-actin sequestration like the corresponding region in WIP WH2.
Our work provides a general structural frame to dissect the structure–function relationship of βT/WH2 domains using limited site-directed mutagenesis or chimeric proteins in more complex multidomain proteins containing βT/WH2 inserted as single domain or in multifunctional WH2 repeats, or associated with other actin-binding motifs (Renault et al, 2008; Qualmann and Kessels, 2009; Dominguez, 2010; Husson et al, 2010). The βT/WH2 sequences of several proteins, including proteins from pathogens, appear also to exhibit the sequence signatures that we have revealed here to be critical for the binding strength and function of isolated single βT/WH2 with G-actin (Figure 7). For instance, the WH2 domain of Dictyostelium discoideum VASP (but not of human VASP, see Figure 7) also contains an extended C-terminus and a FxK/Rxx linker. Notably, in ddVASP, a C-terminally truncated version of its WH2 binds G-actin better than human VASP does, which correlates with the promotion of processive filament elongation by ddVASP (Breitsprecher et al, 2011). The third WH2 domain of the virulence actin-nucleating factors VopF and VopL of bacterial pathogens (Liverman et al, 2007; Tam et al, 2007) also has an extended C-terminus and a FxK/Rxx linker. Notably, a sequestering behaviour for VopL third WH2 domain has been recently pointed by observing its inhibitory effect on the nucleation activity of VopL C-terminal dimerization domain (Yu et al, 2011).
The present work finally open perspectives in the design of customized βT/WH2 domains inserted or repeated in modular proteins. The challenge for the near future will be to modify or replace at will WH2 domains of defined specificity in WH2-containing proteins, with the goal to elucidate and control the resulting biological function.
Materials and methods
Detailed protocols of protein purification, functional and structural analysis are provided in the Supplementary data. Briefly, skeletal α-actin from rabbit muscle, recombinant Drosophila CibD1 (residues A2 to E58) and Tβ4 were prepared as described (Domanski et al, 2004; Hertzog et al, 2004). Chimeras of CibD1 and Tβ4 and wt/mutant of human WIP (A18-G76) were cloned in pGEX-6P1 vector. Tβ4-K14Q and CibD1-Q27K mutants and TTD1 (K9-Q46), TTD3 (Q84-K121) and TTD4 (A118-Q151) were chemically synthesized by Proteogenix company.
Fluorescence measurements of the binding of Tβ4, CibD1 and derived CibD1/Tβ4 chimeras to G-actin
The binding of Tβ4 and the peptides CibD1, CH1, CH2 and CH3 to G-actin was analysed in low ionic strength G-buffer (5 mM Tris pH 7.8, 0.1 mM CaCl2, 0.2 mM ATP, 1 mM DTT) and at physiological ionic strength (1 mM MgCl2, 100 mM KCl) as described in the Supplementary data.
Functional analysis of actin-sequestering and assembly promoting activities
To discriminate actin-sequestering and profilin-like activities, two types of measurements were made, namely: measurements of assembled F-actin at steady state in ATP, and kinetic measurements of barbed and pointed end growth assays using spectrin–actin seeds or gelsolin–actin complexes as described (Domanski et al, 2004; Hertzog et al, 2004). The rate constant for filament barbed end growth from CA peptides (k'+) was directly derived from the slope of the linear increase in growth rate versus concentration of CA complex in the spectrin–actin seeded growth assay, called J(c) plot. Panels in Figures 1C, 2E and 5 show one experiment representative of at least two independent measurements.
Protein crystallization and crystallography
Complex concentrations for crystallization at 20°C were between 0.22 and 0.55 mM. All crystallization conditions contain higher ionic strength than the physiological ones (see Supplementary data).
SAXS measurements
Preliminary SAXS data were collected at SOLEIL synchrotron on Beamline SWING and at ESRF on Beamline ID14-3. All final SAXS data were measured on Beamline SWING (see Supplementary data).
NMR
NMR experiments were realized in solution with free and actin-bound labelled Tβ4, CibD1 and their chimeras ([U-15N], [U-15N13C], [U-15N13C2D]). Chemical shift assignment for CH1 and CH2 bound to actin were obtained from TROSY HNCA/HN(CO)CA experiments. Intermolecular nOes between amide protons of CH2 and protons of actin were obtained from 3D-NOESY-HSQC (mixing times equal to 150 and 200 ms) and (15N,13C) filtered, 15N edited 3D-NOESY-HSQC (mixing times equal to 150 and 500 ms) experiments. In Figure 3E, the error was taken equal to the noise in each experiment, yielding a relative error dnOe/nOe=dIsat/Isat+dInosat/Inosat.
Accession codes
Coordinates and structure factors have been deposited with the RCSB Protein Data Bank under accession numbers 3SJH (CH1:actin-ATP:Lat.A), 3U8X (CH1:actin-ATP), 3U9D (CH2:actin-ATP) and 39UZ (CH3:actin-ADP).
Supplementary Material
Acknowledgments
We thank SOLEIL and ESRF for providing synchrotron radiation facilities and the staff of beamlines SWING and ID14-EH3 for technical assistance during data collection, especially G David, P Pernot and A Round. We acknowledge financial support from the Agence Nationale de la Recherche (Grant ANR-06-JCJC-085 to LR, ANR-PCV 2006 to MFC), from HFSP (RGP00722003-C to MFC), from the European Research Council (advanced ERC Grant 249982 to MFC), from the French Ministry of Research (FXC PhD training fellowship), from the TGIR NMR, from the EU FP6 Design study SAXIER, Grant number RIDS 011934 to JP.
Author contributions: M-FC, LR, CvH and EG conceived the project; DD, AMEM and CH performed protein cloning, expression and purification; DD, AMEM, M-FC and LR performed biochemical characterizations; MH initiated chimera cloning; CLC initiated wt WIP analysis; LR performed the crystallographic studies; PR, LR and JP performed the SAXS studies; CvH conducted the NMR studies; F-XC produced isotopically labelled proteins and performed the NMR experiments; LR, CvH, M-FC and EG analysed the data; and LR, M-FC and CvH wrote the paper.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aguda AH, Xue B, Irobi E, Preat T, Robinson RC (2006) The structural basis of actin interaction with multiple WH2/beta-thymosin motif-containing proteins. Structure 14: 469–476 [DOI] [PubMed] [Google Scholar]
- Au JK, Olivares AO, Henn A, Cao W, Safer D, De La Cruz EM (2008) Widely distributed residues in thymosin beta4 are critical for actin binding. Biochemistry 47: 4181–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, Kiesewetter AK, Linkner J, Urbanke C, Resch GP, Small JV, Faix J (2008) Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation. EMBO J 27: 2943–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitsprecher D, Kiesewetter AK, Linkner J, Vinzenz M, Stradal TE, Small JV, Curth U, Dickinson RB, Faix J (2011) Molecular mechanism of Ena/VASP-mediated actin-filament elongation. EMBO J 30: 456–467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campellone KG, Welch MD (2010) A nucleator arms race: cellular control of actin assembly. Nat Rev Mol Cell Biol 11: 237–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Hertzog M, Didry D, Renault L, Cantrelle FX, van Heijenoort C, Knossow M, Guittet E (2007) Structure, function, and evolution of the beta-thymosin/WH2 (WASP-Homology2) actin-binding module. Ann NY Acad Sci 1112: 67–75 [DOI] [PubMed] [Google Scholar]
- Chereau D, Kerff F, Graceffa P, Grabarek Z, Langsetmo K, Dominguez R (2005) Actin-bound structures of Wiskott-Aldrich syndrome protein (WASP)-homology domain 2 and the implications for filament assembly. Proc Natl Acad Sci USA 102: 16644–16649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chik JK, Lindberg U, Schutt CE (1996) The structure of an open state of beta-actin at 2.65 A resolution. J Mol Biol 263: 607–623 [DOI] [PubMed] [Google Scholar]
- Co C, Wong DT, Gierke S, Chang V, Taunton J (2007) Mechanism of actin network attachment to moving membranes: barbed end capture by N-WASP WH2 domains. Cell 128: 901–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Pérez J (2009) Combined sampler robot and high-performance liquid chromatography: a fully automated system for biological small-angle X-ray scattering experiments at the Synchrotron SOLEIL SWING beamline. J Appl Crystallogr 42: 892–900 [Google Scholar]
- De La Cruz EM, Ostap EM, Brundage RA, Reddy KS, Sweeney HL, Safer D (2000) Thymosin-beta(4) changes the conformation and dynamics of actin monomers. Biophys J 78: 2516–2527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanski M, Hertzog M, Coutant J, Gutsche-Perelroizen I, Bontems F, Carlier MF, Guittet E, van Heijenoort C (2004) Coupling of folding and binding of thymosin beta4 upon interaction with monomeric actin monitored by nuclear magnetic resonance. J Biol Chem 279: 23637–23645 [DOI] [PubMed] [Google Scholar]
- Dominguez R (2007) The beta-thymosin/WH2 fold: multifunctionality and structure. Ann NY Acad Sci 1112: 86–94 [DOI] [PubMed] [Google Scholar]
- Dominguez R (2009) Actin filament nucleation and elongation factors--structure-function relationships. Crit Rev Biochem Mol Biol 44: 351–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez R (2010) Structural insights into de novo actin polymerization. Curr Opin Struct Biol 20: 217–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducka AM, Joel P, Popowicz GM, Trybus KM, Schleicher M, Noegel AA, Huber R, Holak TA, Sitar T (2010) Structures of actin-bound Wiskott-Aldrich syndrome protein homology 2 (WH2) domains of Spire and the implication for filament nucleation. Proc Natl Acad Sci USA 107: 11757–11762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson HJ, Wright PE (2005) Intrinsically unstructured proteins and their functions. Nat Rev Mol Cell Biol 6: 197–208 [DOI] [PubMed] [Google Scholar]
- Egile C, Loisel TP, Laurent V, Li R, Pantaloni D, Sansonetti PJ, Carlier MF (1999) Activation of the CDC42 effector N-WASP by the Shigella flexneri IcsA protein promotes actin nucleation by Arp2/3 complex and bacterial actin-based motility. J Cell Biol 146: 1319–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrow NA, Zhang O, Forman-Kay JD, Kay LE (1994) A heteronuclear correlation experiment for simultaneous determination of 15N longitudinal decay and chemical exchange rates of systems in slow equilibrium. J Biomol NMR 4: 727–734 [DOI] [PubMed] [Google Scholar]
- Gutsche-Perelroizen I, Lepault J, Ott A, Carlier MF (1999) Filament assembly from profilin-actin. J Biol Chem 274: 6234–6243 [DOI] [PubMed] [Google Scholar]
- Hertzog M, van Heijenoort C, Didry D, Gaudier M, Coutant J, Gigant B, Didelot G, Preat T, Knossow M, Guittet E, Carlier MF (2004) The beta-thymosin/WH2 domain; structural basis for the switch from inhibition to promotion of actin assembly. Cell 117: 611–623 [DOI] [PubMed] [Google Scholar]
- Hertzog M, Yarmola EG, Didry D, Bubb MR, Carlier MF (2002) Control of actin dynamics by proteins made of beta-thymosin repeats: the actobindin family. J Biol Chem 277: 14786–14792 [DOI] [PubMed] [Google Scholar]
- Higgs HN, Pollard TD (1999) Regulation of actin polymerization by Arp2/3 complex and WASp/Scar proteins. J Biol Chem 274: 32531–32534 [DOI] [PubMed] [Google Scholar]
- Husson C, Cantrelle FX, Roblin P, Didry D, Le KH, Perez J, Guittet E, Van Heijenoort C, Renault L, Carlier MF (2010) Multifunctionality of the beta-thymosin/WH2 module: G-actin sequestration, actin filament growth, nucleation, and severing. Ann NY Acad Sci 1194: 44–52 [DOI] [PubMed] [Google Scholar]
- Irobi E, Aguda AH, Larsson M, Guerin C, Yin HL, Burtnick LD, Blanchoin L, Robinson RC (2004) Structural basis of actin sequestration by thymosin-beta4: implications for WH2 proteins. EMBO J 23: 3599–3608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch MH, Vachette P, Svergun DI (2003) Small-angle scattering: a view on the properties, structures and structural changes of biological macromolecules in solution. Q Rev Biophys 36: 147–227 [DOI] [PubMed] [Google Scholar]
- Lee SH, Kerff F, Chereau D, Ferron F, Klug A, Dominguez R (2007) Structural basis for the actin-binding function of missing-in-metastasis. Structure 15: 145–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liverman AD, Cheng HC, Trosky JE, Leung DW, Yarbrough ML, Burdette DL, Rosen MK, Orth K (2007) Arp2/3-independent assembly of actin by Vibrio type III effector VopL. Proc Natl Acad Sci USA 104: 17117–17122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannherz HG, Hannappel E (2009) The beta-thymosins: intracellular and extracellular activities of a versatile actin binding protein family. Cell Motil Cytoskeleton 66: 839–851 [DOI] [PubMed] [Google Scholar]
- Mattila PK, Salminen M, Yamashiro T, Lappalainen P (2003) Mouse MIM, a tissue-specific regulator of cytoskeletal dynamics, interacts with ATP-actin monomers through its C-terminal WH2 domain. J Biol Chem 278: 8452–8459 [DOI] [PubMed] [Google Scholar]
- Qualmann B, Kessels MM (2009) New players in actin polymerization--WH2-domain-containing actin nucleators. Trends Cell Biol 19: 276–285 [DOI] [PubMed] [Google Scholar]
- Rebowski G, Namgoong S, Boczkowska M, Leavis PC, Navaza J, Dominguez R (2010) Structure of a longitudinal actin dimer assembled by tandem w domains: implications for actin filament nucleation. J Mol Biol 403: 11–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renault L, Bugyi B, Carlier MF (2008) Spire and Cordon-bleu: multifunctional regulators of actin dynamics. Trends Cell Biol 18: 494–504 [DOI] [PubMed] [Google Scholar]
- Safer D, Sosnick TR, Elzinga M (1997) Thymosin beta 4 binds actin in an extended conformation and contacts both the barbed and pointed ends. Biochemistry 36: 5806–5816 [DOI] [PubMed] [Google Scholar]
- Sosne G, Qiu P, Goldstein AL, Wheater M (2010) Biological activities of thymosin {beta}4 defined by active sites in short peptide sequences. FASEB J 24: 2144–2151 [DOI] [PubMed] [Google Scholar]
- Tam VC, Serruto D, Dziejman M, Brieher W, Mekalanos JJ (2007) A type III secretion system in Vibrio cholerae translocates a formin/spire hybrid-like actin nucleator to promote intestinal colonization. Cell Host Microbe 1: 95–107 [DOI] [PubMed] [Google Scholar]
- Tompa P, Fuxreiter M, Oldfield CJ, Simon I, Dunker AK, Uversky VN (2009) Close encounters of the third kind: disordered domains and the interactions of proteins. Bioessays 31: 328–335 [DOI] [PubMed] [Google Scholar]
- Tompa P, Szasz C, Buday L (2005) Structural disorder throws new light on moonlighting. Trends Biochem Sci 30: 484–489 [DOI] [PubMed] [Google Scholar]
- Uversky VN, Dunker AK (2010) Understanding protein non-folding. Biochim Biophys Acta 1804: 1231–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Dewitte D, Goethals M, Carlier MF, Vandekerckhove J, Ampe C (1996) The actin binding site of thymosin beta 4 mapped by mutational analysis. EMBO J 15: 201–210 [PMC free article] [PubMed] [Google Scholar]
- Van Troys M, Ono K, Dewitte D, Jonckheere V, De Ruyck N, Vandekerckhove J, Ono S, Ampe C (2004) TetraThymosinbeta is required for actin dynamics in Caenorhabditis elegans and acts via functionally different actin-binding repeats. Mol Biol Cell 15: 4735–4748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vancompernolle K, Goethals M, Huet C, Louvard D, Vandekerckhove J (1992) G- to F-actin modulation by a single amino acid substitution in the actin binding site of actobindin and thymosin beta 4. EMBO J 11: 4739–4746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vialle-Printems C, van Heijenoort C, Guittet E (2000) New (15)N NMR exchange experiments for the unambiguous assignment of (1)H(N)/(15)N resonances of proteins in complexes in slow chemical exchange with free form. J Magn Reson 142: 276–279 [DOI] [PubMed] [Google Scholar]
- Xue B, Aguda AH, Robinson RC (2007) Models of the actin-bound forms of the beta-thymosins. Ann NY Acad Sci 1112: 56–66 [DOI] [PubMed] [Google Scholar]
- Yu B, Cheng HC, Brautigam CA, Tomchick DR, Rosen MK (2011) Mechanism of actin filament nucleation by the bacterial effector VopL. Nat Struct Mol Biol 18: 1068–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.