Abstract
Tropomyosins are widespread actin-binding proteins that influence numerous cellular functions including actin dynamics, cell migration, tumour suppression, and Drosophila oocyte development. Synaptopodin is another actin-binding protein with a more restricted expression pattern in highly dynamic cell compartments such as kidney podocyte foot processes, where it promotes RhoA signalling by blocking the Smurf1-mediated ubiquitination of RhoA. Here, we show that synaptopodin has a shorter half-life but shares functional properties with the highly stable tropomyosin. Transgenic expression of synaptopodin restores oskar mRNA localization in Drosophila oocytes mutant for TmII, thereby rescuing germline differentiation and fertility. Synaptopodin restores stress fibres in tropomyosin-deficient human MDA-MB 231 breast cancer cells and TPMα-depleted fibroblasts. Gene silencing of TPMα but not TPMβ causes loss of stress fibres by promoting Smurf1-mediated ubiquitination and proteasomal degradation of RhoA. Functionally, overexpression of synaptopodin or RhoA(K6,7R) significantly reduces MDA-MB 231 cell migration. Our findings elucidate RhoA stabilization by structurally unrelated actin-binding proteins as a conserved mechanism for regulation of stress fibre dynamics and cell motility in a cell type-specific fashion.
Keywords: actin filaments, cell migration, oskar mRNA, RhoA, Smurf1
Introduction
Tropomyosins form a family of actin-binding proteins that influence numerous intracellular functions including actin cytoskeleton dynamics, cell migration, and tumour suppression (Cooper, 2002; Hitchcock-DeGregori et al, 2007; O’Neill et al, 2008).
Tropomyosin (Tm) has a well-defined function in Drosophila. During oogenesis, the assembly of the posterior pole plasm requires the polarized microtubule-mediated transport of oskar (osk) mRNA to the posterior pole (Clark et al, 1994; Shulman et al, 2000; Tomancak et al, 2000; Doerflinger et al, 2006; Zimyanin et al, 2008). Osk mediates the nucleation of the pole plasm, which is required for germ cell and abdomen development (Ephrussi et al, 1991; Kim-Ha et al, 1991). Failure to localize and enrich the pole plasm results in offspring lacking germ cells, referred to as grandchildlessness. Besides mutations in the factors that are required to assemble the pole plasm (e.g., Osk), TmII loss of function also causes grandchildlessness (Erdelyi et al, 1995). In the developing oocytes of TmII mutant females, the germline determinants do not get localized to the posterior pole plasm because osk mRNA is not properly transported to and localized to the posterior pole, subsequently giving rise to very few germ cells (Erdelyi et al, 1995). More recent data suggest that TmII is required for osk mRNA trafficking and affects the speed and reversal of the directional bias of particle movement (Singer, 2008; Zimyanin et al, 2008). Of note, similar to TmII deficiency, mutations in the Drosophila moesin gene that encodes for another actin-binding protein also result in loss of osk mRNA and protein from the posterior subcortical region and failure of oocyte development (Jankovics et al, 2002). The importance of the actin cytoskeleton for proper targeting and function of Oskar during oocyte development is underscored by the observation that long filamentous actin projections at the oocyte posterior pole are induced by and intermingled with Oskar protein. These data suggest that Oskar maintains its localization to the posterior pole through dual functions in regulating endocytosis and F-actin dynamics (Vanzo et al, 2007).
The downregulation of high molecular weight Tm isoforms is a common feature observed in many transformed cell lines (Hendricks and Weintraub, 1981; Cooper et al, 1985) and in human tumours (Bhattacharya et al, 1990; Wang et al, 1996; Hughes et al, 2003; Pawlak et al, 2004). The loss of Tm contributes to the neoplastic phenotype of altered cell shape, loss of stress fibres, and anchorage-independent growth (Boyd et al, 1995). The transformed phenotype in v-ki-ras transformed NIH3T3 cells and human MDA-MB 231 breast cancer cells can be rescued by reintroduction of Tm1, an isoform of TPMβ (Prasad et al, 1993; Bharadwaj et al, 2005), implying that the loss of Tm is a crucial event in oncogenic transformation. Tm2, an isoform of TPMα, is necessary for transforming growth factor-β-induced stress fibre formation in NMuMG mouse mammary epithelial cells and SiHa human cervical carcinoma cells (Bakin et al, 2004). Thus, in non-muscle cells Tm acts as a tumour suppressor and is necessary to maintain actin filament integrity, but the underlying molecular mechanisms remain elusive (Gunning et al, 2005; Hitchcock-DeGregori et al, 2007; O’Neill et al, 2008).
Rho GTPases can switch between an inactive GDP-bound and an active GTP-bound form (Jaffe and Hall, 2005). The E3 ubiquitin ligase Smurf1 can bind to nucleotide-free RhoA and promote RhoA ubiquitination, leading to its proteasomal degradation (Wang et al, 2003; Ozdamar et al, 2005). Synaptopodin is a proline-rich scaffolding protein that is strongly expressed in highly dynamic cell compartments such as dendritic spines in the brain and podocyte foot processes (FPs) in the kidney (Mundel et al, 1997a; Faul et al, 2007). Similarly to Tm, synaptopodin binds to actin and α-actinin and regulates the actin-bundling activity of α-actinin (Asanuma et al, 2005). The clinical importance of synaptopodin is underscored by the observation that it is a direct target of the anti-proteinuric effect of the calcineurin inhibitor cyclosporine A (Faul et al, 2008). Synaptopodin is not a target of proteasomal degradation but instead is degraded by cathepsin L-mediated cleavage (Faul et al, 2008). Synaptopodin induces stress fibres in podocytes by blocking the Smurf1-mediated ubiquitination of RhoA, thereby preventing the targeting of RhoA for proteasomal degradation (Asanuma et al, 2006; Faul et al, 2007). As it stands, the general relevance of this mechanism for the regulation of RhoA signalling beyond highly specialized podocytes is unclear.
Tm has long been known to regulate actin dynamics, but the precise mechanisms remained elusive (Gunning et al, 2005; Hitchcock-DeGregori et al, 2007; O’Neill et al, 2008). Here, we describe the important observation that synaptopodin is sufficient to rescue TmII deficiency in Drosophila by regulating osk mRNA localization and function, which suggests that these two structurally distinct proteins share functional properties. We confirmed this by showing that the mammalian orthologue TPMα regulates stress fibre formation through inhibition of Smurf1-mediated ubiquitination of RhoA in fibroblasts and human cancer cells. Our findings unveil the stabilization of RhoA protein abundance as a mechanism by which TPMα but not TPMβ can modulate stress fibre dynamics and cell motility.
Results
Synaptopodin rescues Drosophila TmII loss of function
During Drosophila development, TmII is required for germ cell formation because mutations in TmII (TmIIeg9) virtually abolish osk mRNA localization to the posterior pole of the oocyte (Erdelyi et al, 1995). Both Tm and synaptopodin induce actin filaments. To test whether these two structurally distinct actin-binding proteins are interchangeable in vivo, we attempted to rescue the grandchildless phenotype of Drosophila TmII mutants (Erdelyi et al, 1995) by introducing synaptopodin via the Gal4/UAS system (Brand and Perrimon, 1993). Synaptopodin was expressed in the developing oocyte via the maternal nanos-Gal4 driver (nosGal4, UAS-synpo) and crossed into the TmIIeg9 mutant background (Supplementary Figure S1). The nosGal4, UAS-synpo, TmIIeg9 genotype was analysed for osk mRNA localization by in-situ hybridization and quantification of F2 progeny (grandchildren; see Supplementary Figure S1 for details). In both assays, the expression of synaptopodin induced a rescue to wild-type conditions. Osk mRNA localized tightly to the posterior egg pole in rescue flies in a pattern indistinguishable from wild-type flies (Figure 1A). The rescue of osk mRNA targeting was functionally relevant, as it led to the production of normal numbers of germ cells deduced by the number of F2 progeny/grandchildren (Figure 1B). Of note, the eggs laid by nos-synpo, TmIIeg9 developed and hatched normally. We thus conclude that vertebrate synaptopodin can fully substitute for the function of TmII in this in-vivo assay in Drosophila.
Figure 1.
Synaptopodin rescues the grandchildless phenotype of Drosophila TmII mutants. (A) In-situ localization of osk mRNA in stage 10 egg chambers of indicated genotypes. Transgenic expression of synaptopodin restores proper osk mRNA localization to the posterior pole in oocytes of TmIIeg9 mutants. The average normal osk mRNA localization in wild type was 82±10% (n=325), nos-synpo 80±7% (n=478), nos-synpo, TmIIeg9 59±14 (n=393), and TmIIeg9 35±6% (n=302). Data were obtained from three independent experiments. (B) F2 egg count confirms the rescue of TmIIeg9 grandchildless phenotype by synaptopodin. Two separate experiments were counted twice and averaged. TmIIeg9 mutant, nos-synpo transgenic, and nos-synpo/TmIIeg9 transgenic flies were analysed. The average egg count was 110±16% (n=1253) for wild-type (WT) flies and 90±16% (n=1085) for synaptopodin transgenic flies versus 10±6% (n=146) for the TmIIeg9 genotype. The nosGal4, UAS-synpo, TmIIeg9 flies displayed WT levels: 94±15% (n=1008). *P<0.0001, Student's t-test.
Overexpression of synaptopodin induces stress fibres and reduces motility in cancer cells
To test whether synaptopodin and Tm share overlapping functions at the cellular level, we analysed MDA-MB 231 cells that lack stress fibres (Bharadwaj et al, 2005) and express a Tm isoform not found in NIH3T3 fibroblasts (Supplementary Figure S2A). The transformed phenotype of MDA-MD 231 cells including the loss of stress fibres can be rescued by introduction of Tm1 (Bharadwaj et al, 2005). To determine whether the ectopic expression of synaptopodin would have a similar effect, we infected MDA-MB 231 cells, which do not express endogenous synaptopodin, with synaptopodin or empty vector (Figure 2A). We then visualized the actin cytoskeleton by phalloidin staining (Figure 2B). Similar to non-infected MDA-MB 231 cells, only a few cells infected with the empty control vector contained stress fibres. In contrast, synaptopodin overexpression induced stress fibres (Figure 2B). Quantitative analysis showed a significant increase in the percentage of stress fibre containing cells after infection with synaptopodin (Figure 2C). Functionally, the restoration of stress fibres was associated with the reduction of cell migration as determined by a wound-healing assay (Figure 2D and E).
Figure 2.
Synaptopodin rescues Tm deficiency in human cancer cells. (A) Western blot analysis for synaptopodin after infection of MDA-MB 231 cells with synaptopodin (synpo) or empty vector. Wild-type MDA-MB 231 cells do not express synaptopodin. Protein extracts from isolated mouse kidney glomeruli (Glom) serve as positive control. GAPDH serves as loading control. (B) Overexpression of synaptopodin, but not the empty vector control, induces stress fibres in MDA-MB 231 cells as shown by phalloidin labelling. Scale bar: 50 μm. (C) Synaptopodin significantly increases the percentage of stress fibre containing MDA-MB 231 cells: wild-type cells: 24.36±5.13% stress fibre containing cells; empty vector: 19.01±3.08%; synaptopodin: 60.06±4.32%. P<0.005, Student's t-test. (D) Synaptopodin but not the empty vector control reduces migration of MDA-MB 231 cells. Scale bar: 125 μm. (E) Quantitative analysis shows a significant delay of wound closure at 12 h for MDA-MB 231 cells infected with synaptopodin: wild type: 80.39±8.07%; empty vector: 60.55±3.95%; synaptopodin 35.39±4.73%. P<0.005, Student's t-test.
Synaptopodin restores stress fibres in TPMα-depleted fibroblasts
Similar to synaptopodin in podocytes (Mundel et al, 1997a), Tm is found along actin filaments in fibroblasts (Gunning et al, 2005; Hitchcock-DeGregori et al, 2007; O’Neill et al, 2008; Figure 3A). Given that synaptopodin can functionally replace TmII deficiency in Drosophila and human cancer cells, we hypothesized that it may also rescue TPMα depletion in fibroblasts. To directly test this hypothesis, we used a gene silencing approach to reduce the expression of TPMα isoforms in NIH3T3 cells, which do not express endogenous synaptopodin (Figure 3B). Gene silencing with a Tm shRNA that recognizes a sequence in TPMα transcript variants 2, 3, 4, 6, and 7, but not with a control shRNA caused the loss of stress fibres (Figure 3A), reduction of focal contacts (Supplementary Figure S2B), and downregulation of Tm protein abundance (Figure 3B). Of note, the lentiviral overexpression of synaptopodin (Figure 3B) was sufficient to restore stress fibres in TPMα-depleted cells (Figure 3C, lower panels). Quantitative analysis revealed that synaptopodin significantly increased the percentage of stress fibre containing TPMα knockdown fibroblasts (Figure 3D). In order to determine if isoform specificity is important in the loss of stress fibres, we silenced TPMβ expression using three independent TPMβ-specific shRNAs (Supplementary Figure S3). Because the Tm311 antibody detects many Tm isoforms (Tm6, 1, 2, 3, α, β, γ muscle Tm) (Nicholson-Flynn et al, 1996), we performed a semi-quantitative RT–PCR to monitor RNA knockdown. The TPMβ shRNAs specifically depleted TPMβ isoforms including Tm1 but not TPMα isoforms (Supplementary Figure S3A). By western blot analysis, we found a reduction of TPMβ (upper band) but not TPMα (lower band) protein abundance (Supplementary Figure S3B). In contrast to the depletion of TPMα (Figure 3A), the depletion of TPMβ did not cause the loss of stress fibres (Supplementary Figure S3C and D). Collectively, these results support the conclusion that TPMα and synaptopodin but not TPMβ have overlapping cellular functions in spite of their structural differences.
Figure 3.
Synaptopodin restores stress fibres in TPMα-depleted fibroblasts. (A) Tm is localized along stress fibres in NIH3T3 wild-type and control shRNA fibroblasts. Gene silencing of TPMα (TPMα shRNA) causes the loss of stress fibres. Scale bar: 25 μm. (B) Tm protein levels are decreased in TPMα shRNA cells. Synaptopodin is detected in NIH3T3 cells after infection with synaptopodin (Synpo) but not in wild-type (wt) cells or in cells infected with the empty vector control. Con: non-silencing control shRNA. (C) Synaptopodin restores stress fibres in TPMα knockdown cells. Synaptopodin staining is found along stress fibres. The secondary antibody alone also stains non-specifically the nuclei of all cells. Scale bar: 25 μm. (D) Quantitative analysis shows a significant decrease in the percentage of stress fibre containing TPMα knockdown cells, which can be rescued by ectopic expression of synaptopodin (Synpo) but not by the empty vector control: wild-type cells: 72.74±1.11% stress fibre containing cells; control shRNA+empty vector: 70.03±6.31%, TPMα shRNA+empty vector: 33.41±5.61%; TPMα shRNA+synaptopodin: 68.28±4.78%; TPMα shRNA+empty vector versus TPMα shRNA+synaptopodin; P<0.005, Student's t-test. Wild type: non-infected wild-type cells; control: non-silencing control shRNA.
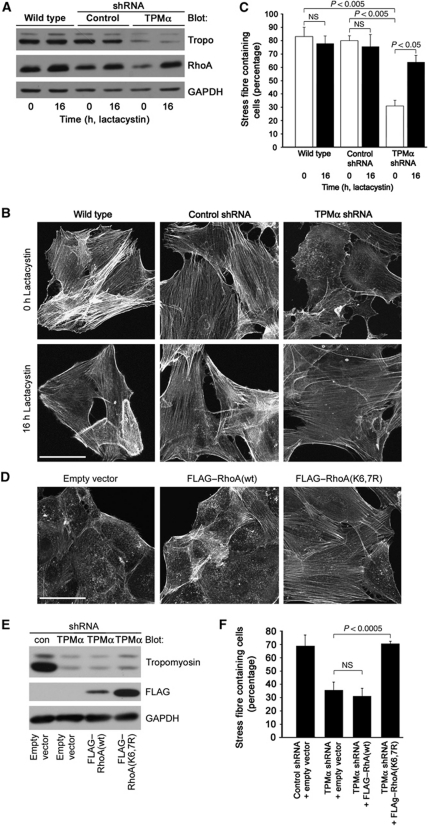
Lactacystin restores stress fibres in TPMα-depleted fibroblasts
NIH3T3 cells, MDA-MB 231 cancer cells, and podocytes, serving as a positive control (Asanuma et al, 2006), express the E3 ubiquitin protein ligase Smurf1 (Supplementary Figure S2C), which can induce the proteasomal degradation of RhoA (Wang et al, 2003; Asanuma et al, 2006; Sahai et al, 2007). In fibroblasts, gene silencing of TPMα reduced RhoA steady-state protein levels (Figure 4A). This raised the possibility that TPMα depletion, by analogy to synaptopodin depletion (Asanuma et al, 2006), may result in Smurf1-induced degradation of RhoA. In keeping with this hypothesis, the proteasome inhibitor lactacystin increased RhoA protein abundance (Figure 4A) and the percentage of stress fibre containing TPMα knockdown fibroblasts (Figure 4B and C). The upregulation of stress fibres by lactacystin was independent of Tm, because Tm protein abundance remained unchanged (Figure 4A). To confirm these results with an independent approach, we infected TPMα knockdown fibroblasts with wild-type RhoA or RhoA(K6,7R) that is resistant to Smurf1-mediated ubiquitination and proteasomal degradation (Wang et al, 2003; Ozdamar et al, 2005). Infection with FLAG–RhoA(K6,7R) but not with wild-type FLAG–RhoA or empty vector restored stress fibres in TPMα knockdown fibroblasts (Figure 4D). Overexpression of FLAG–RhoA was confirmed by western blot analysis (Figure 4E). Quantitative analysis showed a significant increase in the percentage of stress fibre containing Tm knockdown cells infected with Smurf1-resistant FLAG–RhoA(K6,7R) but not with wild-type FLAG–RhoA (Figure 4F). Collectively, these data establish a competitive relationship between Smurf1-mediated ubiquitination of RhoA and Tm activity.
Figure 4.
TPMα induces stress fibres by blocking the proteasomal degradation of RhoA. (A) Western blot analysis shows decreased baseline (0 h) RhoA steady-state protein levels in TPMα knockdown fibroblasts (TPMα) compared with non-infected wild-type cells or cells expressing a non-silencing control shRNA (control). Lactacystin (16 h) causes the upregulation of RhoA steady-state protein levels in TPMα knockdown cells but does not alter Tm protein abundance. GAPDH serves as loading control. (B) Phalloidin labelling shows that lactacystin restores stress fibres in TPMα knockdown cells. Scale bar: 25 μm. (C) Lactacystin does not change the percentage of stress fibre containing wild-type and control shRNA infected fibroblasts but significantly increases the percentage of stress fibre containing TPMα knockdown cells: 0 h lactacystin: 30.96±4.33% stress fibre containing cells versus 16 h lactacystin: 63.86±5.15%; P<0.05; Student's t-test. (D) Smurf1-resistant FLAG–RhoA(K6,7R), but not wild-type FLAG–RhoA (WT) or empty vector induces stress fibres in TPMα knockdown fibroblasts. Scale bar: 25 μm. (E) Western blot analysis of FLAG–RhoA levels after infection with empty vector, wild-type FLAG–RhoA or FLAG–RhoA(K6,7R). The first lane shows co-infection with the non-silencing control shRNA (con) and the empty pLentix control vector. The second lane shows co-infection with the TPMα shRNA and the empty pLentix control vector. The third lane shows co-infection with the TPMα shRNA and FLAG-tagged wild-type RhoA. The last lane show co-infection with the Tm shRNA and FLAG-tagged Smurf1-resistant RhoA(K6,7R). GAPDH serves as loading control. Con: non-silencing shRNA. (F) Quantitative analysis shows a significant increase in the percentage of stress fibre containing TPMα knockdown cells infected with Smurf1-resistant RhoA(K6,7R): control shRNA+empty vector: 68.63±8.18% stress fibre containing cells; TPMα shRNA+empty vector: 35.73±6.05%; Tm shRNA+FLAG–RhoA(wt): 31.15±5.93%; TPMα shRNA+FLAG–RhoA(K6,7R): 70.30±1.59%; TPMα shRNA+empty vector versus TPMα shRNA+FLAG–RhoA(K6,7R): P<0.0005, Student’s t-test.
Rho kinase inhibition abrogates synaptopodin- or lactacystin-induced stress fibres in TPMα-depleted fibroblasts
RhoA and its downstream effector Rho kinase are principal mediators of stress fibre formation through their effects on actin and myosin (Etienne-Manneville and Hall, 2002). Synaptopodin induces stress fibres in podocytes by increasing RhoA abundance and activation (Asanuma et al, 2006). Now, we find that ectopic synaptopodin expression also induces stress fibres in TPMα-depleted fibroblasts (Figure 3). To demonstrate that this effect is dependent on an increase of RhoA, we used the Rho kinase inhibitor Y-27632 to block synaptopodin-induced stress fibres in TPMα-depleted fibroblasts (Figure 5A and B). Similarly, Y-27632 blocked lactacystin-induced stress fibres (Figure 5A and C). These results confirm that synaptopodin- or lactacystin-induced stress fibre formation depends on RhoA and Rho kinase signalling.
Figure 5.
Rho kinase inhibition suppresses synaptopodin- or lactacystin-induced stress fibres in TPMα knockdown fibroblasts. (A) Synaptopodin (synpo)-induced stress fibre formation in TPMα-depleted fibroblasts is blocked by the Rho kinase inhibitor Y-27632 but not the vehicle control. Similarly, Y-27632, but not the vehicle control, abrogates lactacystin-induced stress fibres. Scale bar: 25 μm. (B) Quantitative analysis shows a significant loss of synaptopodin-induced stress fibre formation in TPMα-depleted fibroblasts after Y-27632 treatment: Vehicle control: 60.30±0.99% stress fibre containing cells; Y-27632: 0.0±0.0%; P<0.0005, Student’s t-test. (C) Quantitative analysis shows a significant loss of lactacystin-induced stress fibre formation in TPMα-depleted fibroblasts after Y-27632 treatment. Vehicle control: 63.84±9.25% stress fibre containing cells; Y-27632: 0.0±0.0%; P<0.0005, Student’s t-test.
Tm regulates stress fibre formation and cell migration in MDA-MB 231 breast cancer cells through inhibition of RhoA ubiquitination
Given that lactacystin restores stress fibres in TPMα-depleted fibroblast, we asked if lactacystin also affected RhoA protein abundance and stress fibre formation in MDA-MB 231 cells. Similarly to podocytes (Asanuma et al, 2006) and TPMα knockdown fibroblasts (Figure 4A), lactacystin increased RhoA steady-state protein levels in MDA-MB 231 cells (Figure 6A). As in TPMα knockdown cells (Figure 4B and C), lactacystin induced stress fibres in MDA-MB 231 cancer cells (Figure 6B and C). The induction of stress fibres was independent of Tm because it did not change Tm protein abundance (Figure 6A). To determine whether the ectopic expression of Smurf1-resistant RhoA(K6,7R) (Wang et al, 2003; Ozdamar et al, 2005) would have a similar effect as the overexpression of synaptopodin or lactacystin treatment, we infected MDA-MB 231 cells with empty vector, FLAG–RhoA or Smurf1-resistant FLAG–RhoA(K6,7R) (Figure 6D). Similarly to synaptopodin (Figure 2B) or lactacystin (Figure 6B), Smurf1-resistant RhoA(K6,7R), but not wild-type RhoA induced stress fibres (Figure 6E and F). As in fibroblasts (Figure 5), the Rho kinase inhibitor Y-27632 suppressed synaptopodin- or lactacystin-induced stress fibres (Supplementary Figure S4A–C), thereby confirming that RhoA is a downstream mediator of synaptopodin- or lactacystin-induced stress fibre formation in MDA-MB 231 cells. Functionally, RhoA(K6,7R) reduced the migration of MDA-MB 231 cells (Figure 6G and H). Thus, the lack of Tm in MDA-MB 231 cells leads to the loss of stress fibres and increased cell migration through proteasomal degradation of RhoA.
Figure 6.
Synaptopodin induces stress fibres and reduces cell migration in human MDA-MB 231 breast cancer cells. (A) Lactacystin causes an upregulation of RhoA steady-state protein levels in MDA-MB 231 breast cancer cells without affecting Tm protein abundance. GAPDH serves as loading control. (B) Lactacystin induces stress fibres in MDA-MB 231 breast cancer cells. Scale bar: 25 μm. (C) Quantitative analysis shows a significant increase in the percentage of stress fibre containing MDA-MB 231 after lactacystin treatment: 0 h lactacystin: 16.09±5.00% stress fibre containing cells versus 8 h lactacystin: 43±2.20%; P<0.05, Student’s t-test. (D) Western blot analysis shows FLAG–RhoA protein abundance after infection of MDA-MB 231 cells with empty vector, wild-type FLAG–RhoA or Smurf1-resistant FLAG–RhoA(K6,7R). GAPDH serves as loading control. Non-infected wild-type cells serve as baseline control. (E) Overexpression of FLAG–RhoA(K6,7R) but not wild-type FLAG–RhoA or the empty vector control induces stress fibres in MDA-MB 231 cells. Scale bar: 25 μm. (F) FLAG–RhoA(K6,7R) significantly increases the percentage of stress fibre containing MDA-MB 231 cells: wild-type cells: 20.99±9.38% stress fibre containing cells; empty vector: 10.29±2.94%; wild-type RhoA: 16.47±5.20%; RhoA(K6,7R): 45.87±7.21%; P<0.005, Student's t-test. (G) FLAG–RhoA(K6,7R) but not wild-type FLAG–RhoA reduces MDA-MB 231 cell migration. Scale bar: 125 μm. (H) Quantitative analysis shows a significant delay of wound closure at 12 h for MDA-MB231 cells infected with RhoA(K6,7R): wild type: 78.17±11.13%; empty vector: 76.37±2.63%; wild-type RhoA: 64.65±9.06%; RhoA(K6,7R): 36.91±4.60; P<0.0005, Student's t-test.
Tm2, an isoform encoded by the TPMα gene, blocks the Smurf1-mediated ubiquitination of RhoA
Synaptopodin can bind to RhoA, thereby blocking the binding of Smurf1 to RhoA (Asanuma et al, 2006). Therefore, we tested whether Tm can also bind to RhoA. We found that both GFP-tagged Tm1 (encoded by the TPMα gene) and GFP-tagged Tm2 (encoded by the TPMβ gene) but not the GFP control (GFP–sui) could bind to dominant-active FLAG–RhoA(G14V) and dominant-negative FLAG–RhoA(T19N) in co-transfected HEK293 cells (Figure 7A). GFP–Tm1 and GFP–Tm2 did not bind to the FLAG control (FLAG–Cdc42) (Figure 7A), confirming the specificity of the interaction. Synaptopodin can displace Smurf1 from GDP-bound RhoA, thereby suppressing the Smurf1-mediated ubiquitination of GDP–RhoA (Asanuma et al, 2006). To test whether Tm2 can compete with Smurf1 for RhoA binding, we conducted in vitro reconstitution assays according to our published protocols (Faul et al, 2005; Asanuma et al, 2006; Yanagida-Asanuma et al, 2007). In the absence of Tm2, purified Smurf1 bound to GST–RhoA(T19N), but not to the GST control. However, with the addition of increasing amounts of purified FLAG–Tm2 protein, the binding of FLAG–Smurf1 to GST–RhoA(T19N) progressively decreased, with a concomitant increase in Tm2 binding to GST–RhoA (Figure 7B). To test whether the observed inhibition of Smurf1 binding to RhoA by Tm2 is functionally relevant, we performed co-transfection studies in HEK293 cells using FLAG–RhoA(T19N), Myc–Smurf1 (wild-type or catalytically inactive, CA) and HA–ubiquitin in the presence of GFP–Tm1, GFP–Tm2, or GFP alone (Figure 7C). In cells expressing the GFP control, RhoA was strongly ubiquitinated by Smurf1, as expected from previous studies (Wang et al, 2003; Asanuma et al, 2006). In keeping with previous studies (Asanuma et al, 2006), the ubiquitination of RhoA was markedly reduced in cells co-transfected with synaptopodin. Similarly, the ubiquitination of RhoA was markedly reduced in cells coexpressing Tm2 but not in cells coexpressing Tm1 or α-actinin-4, another actin-binding protein serving as a negative control (Asanuma et al, 2006; Figure 7C). Reduced ubiquitination of RhoA was also found in cells expressing catalytically inactive Smurf1(CA) (Figure 7C). Although we did not conduct a two step IP experiment to explore the effects of Tm on RhoA ubiquitination, collectively our data suggest that at least one isoform of TMPα, Tm2, but not the TPMβ isoform Tm1, can block the Smurf1-mediated ubiquitination and proteasomal degradation of RhoA.
Figure 7.
Tm2 blocks the ubiquitination of RhoA by Smurf1. (A) GFP–Tm1 and GFP–Tm2 interact with dominant-active (G14V) and more strongly with dominant-negative (T19N) RhoA but not with the negative control GFP–sui (GFP control). GFP–Tm1 and GFP–Tm2 do not interact with FLAG–Cdc42 (FLAG control) also serving as negative control. (B) Immobilized GST–RhoA(T19N) but not GST alone directly binds to purified FLAG–Smurf1. In the presence of increasing amounts of FLAG–Tm2, the binding of RhoA to Smurf1 is gradually lost, and concomitantly increased binding of Tm2 to RhoA is detected. In the presence of 1 μg of FLAG–Tm2, only Tm2 binds to RhoA. (C) FLAG–RhoA is ubiquitinated by wild-type (WT) but not by catalytically inactive (CA) Myc–Smurf1 in transfected HEK293 cells expressing GFP as revealed by anti-HA immunoblotting. GFP–Tm2 and GFP–Synaptopodin (Synpo) but not GFP–Tm1 or GFP–α-actinin-4 (Act-4) markedly reduces the ubiquitination of RhoA. No RhoA ubiquitination is detected in the absence of HA–ubiquitin. (D) Synaptopodin and Tm differ in their protein half-life. Treatment of podocytes with cycloheximide results in the rapid degradation of endogenous synaptopodin with a calculated half-life of 17.1±2.4 h. In contrast, Tm protein abundance remains stable over the entire 24-h observation period. (E) An extended model for the regulation of RhoA signalling. In the canonical model of the Rho GTPase cycle, the activity of Rho GTPases is regulated by a switch between an inactive GDP-bound and an active GTP-bound form. The cycle is tightly regulated mainly by guanine exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine dissociation inhibitors (GDIs). Tm and synaptopodin promote stress fibre formation through the inhibition of Smurf1-mediated ubiquitination of RhoA. Thus, repression of Smurf1-induced proteasomal degradation of RhoA by structurally unrelated actin-binding proteins is a conserved mechanism for the regulation of RhoA signalling, in addition to the GDP/GTP switch.
The half-life of synaptopodin is markedly shorter than that of Tm
In podocytes, Tm is localized to the cell body (Drenckhahn et al, 1990), whereas synaptopodin is found in FPs (Mundel et al, 1997a; Faul et al, 2007). FPs are highly dynamics structures, so rapid changes in FP morphology require dynamic control of their actin cytoskeleton, a function thought to be subserved by synaptopodin (Faul et al, 2007). Synaptopodin is readily degraded by TRPC5/Ca2+-induced (Tian et al, 2010) and calcineurin/cathepsin L-mediated proteolysis (Faul et al, 2008). On the other hand, Tm in cardiac myocytes is highly stable (Martin, 1981), and therefore an unlikely candidate for dynamic, rapid actin cytoskeleton remodelling. To compare the half-life of synaptopodin with that of Tm, we overexpressed FLAG–synaptopodin or FLAG–Tm2 in HEK293 cells. The cells were treated with cycloheximide to block de-novo protein synthesis and the levels of both proteins were followed over time. Synaptopodin was rapidly degraded (Supplementary Figure S3D) with a calculated half-life of 14.6±4.5 h. In contrast, Tm2 remained stable over the entire course of the experiment (24 h), in keeping with its previously reported long half-life of about 5.5 days in myocytes (Martin, 1981). To validate the results in the context of endogenous proteins, we examined the turnover of synaptopodin and Tm in podocytes. The estimated half-life of endogenous synaptopodin in podocytes was 17.1±2.4 h (Figure 7D), similarly to exogenous synaptopodin in HEK293 cells. As in HEK293 cells, endogenous Tm in podocytes remained stable over the course of the experiment (Figure 7D).
Discussion
The results of this study revealed that synaptopodin can rescue Tm deficiency by functional mimicry in vitro in cancer cells and fibroblasts and in vivo in Drosophila. The stabilization of RhoA abundance offers a molecular mechanism by which Tm can regulate stress fibre dynamics. Our results show further that Tm can induce stress fibres by blocking the Smurf1-mediated ubiquitination of RhoA, thus protecting RhoA against proteasomal degradation. Our findings support the notion that, in addition to the regulation by guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide-dissociation inhibitors (GDIs) (Jaffe and Hall, 2005), the proteasomal degradation of RhoA is a conserved mechanism for the control of RhoA signalling, stress fibre dynamics, and cell motility (Figure 7E). The level of RhoA protein can be regulated by the ubiquitin-proteasome system through multiple mechanisms. Besides Smurf1, the Cullin3–BACURD complex can also regulate RhoA degradation (Chen et al, 2009). This study showed that Smurf1-resistant RhoA(K6,7R) is sufficient to rescue stress fibres in TPMα depleted fibroblast and MDA-MB 231 cancer cells. Thus, Tm probably does not affect Cullin3–BACURD-mediated degradation of RhoA. Future studies are needed to confirm or refute this idea.
A further interesting outcome of this study is the identification of RhoA as Tm-binding protein. In muscle cells, Tm interacts with troponin T, caldesmon, and tropomodulin as part of the contractile apparatus (Perry, 2001). In non-muscle cells, various Tm isoforms bind to calponin (Takahashi et al, 1986), pEL98 (Takenaga et al, 1994), and glutamic dehydrogenase (Akutsu and Miyazaki, 2002) although the physiological significance of these interactions is largely unknown. By linking Tm directly to RhoA signalling, we establish a new paradigm in which structurally unrelated actin-binding proteins such as Tm and synaptopodin not only serve as passive structural components of actin filaments, but also can actively regulate stress fibre dynamics (Figure 7E).
Our results demonstrate that Tm and synaptopodin employ similar molecular mechanisms by which they regulate actin dynamics, cell migration, and oocyte differentiation. The functional similarities between both proteins are underscored by the observation that synaptopodin is not only sufficient to rescue RhoA signalling in TPMα-depleted fibroblasts and human cancer cells in vitro, but also osk mRNA localization and fertility in TmIIeg9 mutant flies in vivo. Synaptopodin not only blocks the proteasomal degradation of RhoA (Asanuma et al, 2006), but also elongates α-actinin-induced actin filaments (Asanuma et al, 2005), another function that is shared by Tm (Hitchcock-DeGregori et al, 2007). Synaptopodin and Tm2 can prevent ubiquitination of RhoA and thereby cause its stabilization, thus allowing the formation of actin stress fibres necessary for proper regulation of cell migration and possibly osk mRNA localization. Mammalian Tm2 is not the same as TmII in Drosophila. Therefore, future studies will be necessary to establish more precisely the molecular link between TmII in Drosophila and Tm2 in mammals and to determine whether synaptopodin rescues TPMα deficiency by virtue of restoring RhoA signalling, through the restoration of Oskar-induced long action projections (Vanzo et al, 2007) or via regulation of osk mRNA trafficking or anchoring (Singer, 2008; Zimyanin et al, 2008).
The outcome of the half-life studies suggests that synaptopodin is better poised to mediate rapid temporal changes in actin dynamics, while Tm seems better suited for maintaining a more static actin cytoskeleton. This notion is further supported by the observation that synaptopodin is a target of rapid TRPC5/Ca2+ and calcineurin-dependent degradation (Faul et al, 2008; Tian et al, 2010). Therefore, our results suggest another mechanism for spatial and temporal regulation of the actin cytoskeleton in highly dynamics polarized cells such as podocytes or neurons.
To start exploring the question of isoform specificity, we established that in contrast to TPMα depletion, gene silencing of TPMβ is not sufficient to cause a loss of stress fibres in fibroblasts. Together with our finding that Tm1, which is encoded by the TPMβ gene, is not sufficient to block the ubiquitination of RhoA, these findings merit further investigation in future studies to determine the underlying molecular basis of isoform specificity. For example, Tm1 or a subset of other Tm isoforms may be proven to control stress fibre dynamics via other mechanisms such as regulation of RhoA GEFs or GAPs (Jaffe and Hall, 2005).
In summary, this work provides novel insights into Tm signalling. Our results suggest that the regulation of stress fibre dynamics by Smurf1-mediated degradation of RhoA is not only essential in highly specialized cell such as podocytes (Asanuma et al, 2006), but also represents a conserved mechanism for the regulation of stress fibre dynamics and cell motility by the structurally distinct but functionally similar actin-binding proteins Tm2 and synaptopodin.
Materials and methods
Plasmid constructs
Mouse Tm2 cDNA was obtained from ATCC (GenBank ID BC026720). Rat Tm1 cDNA was provided by Peter W Gunning, GST–RhoA(T19N) by Tadaomi Takenawa, Smurf1-resistant RhoA(K6,7R) by Jeffrey L Wrana, wild-type and CA Smurf1 by Takeshi Imamura. cDNAs were subcloned by PCR amplification into pEGFP-C1 (Clontech), pFLAG-6c or pFLAG-5a (Sigma). All constructs were verified by DNA sequencing. FLAG–synaptopodin long and FLAG–α-actinin-4 constructs have been described previously (Asanuma et al, 2005, 2006). Synaptopodin was amplified by PCR from GFP–synaptopodin long (Asanuma et al, 2005) and cloned into the LXSN vector (Clontech). Synaptopodin, wild-type FLAG-RhoA, and FLAG–RhoA(K6,7R) were also cloned into the pLentix (Clontech) vector. The GIPZ TPMα shRNA and GIPZ non-silencing control shRNAs were acquired from Open Biosystems. The GIPZ TPMα shRNA (Open Biosystems, Catalog Number RMM4431-98754654, Clone ID V2LMM_25293shRNA) is targeted to the following sequence: CACTACATATGTAATTGGT. When blasted, the shRNA matches TPM1 transcript variants 2, 3, 4, 6, and 7. The GIPZ TPMβ shRNAs (Open Biosystems) target the following sequences: AAATACGAAGAAGAGATCA (#1), CGGACAAGTATTCCACCAA (#2), and CCGAGCAGAGTTTGCTGAA (#3). The Open Biosystems TPMα shRNA targets a sequence in exon 9D of TPMα that is predicted to knockdown isoforms Tm6, Tm2, Tm3, Tm5a, and Tm5b. This is not predicted to knockdown any TPMβ isoforms, including Tm1.
RNA isolation and RT–PCR
NIH3T3 cells were lysed from a 10-cm dish and homogenized by passing through a QIAshredder (Qiagen). RNA was isolated using the RNeasy Mini Kit according to the manufacturer's protocol. cDNA was synthesized from 200 to 350 ng total RNA using oligo(dT)20 and SuperScript III RT (Invitrogen) according to the manufacturer's protocol. In all, 1 μl cDNA was amplified by PCR (30 cycles at 94°C for 30 s, 58°C for 30 s, 72°C for 30 s) using Choice-Taq DNA Polymerase (Denville Scientific). The primer sets used were GAPDH (5′-CCAATGTGTCCGTCGTGGATCT-3′; 5′-GTTGAAGTCGCAGGAGACAACC-3′), TPMβ (5′-TGGCTGAGAGTAAATGTGGGGACC-3′; 5′-CAGGCTGGCTGTGCAACGTG-3′), and TPMα (5′-AGGAGGCTGAAACTCGGGCTGA-3′; 5′-TGGCCGCAGCTAAGGAGGGT-3′). TPMα amplifies TPMα transcript variants 2, 3, 4, 6, and 7.
Cell culture and transient transfection
NIH3T3 cell, which express Tm1, Tm2, Tm3, Tm6, Tm5a, Tm5b, Tm5NM1, and Tm5NM2 (Percival et al, 2000; Schevzov et al, 2005), was cultured in DMEM (Gibco) with 10% newborn calf serum (Invitrogen or Sigma) and 100 units/ml of penicillin–streptomycin (Invitrogen). Podocytes and HEK293 cells were cultured as described previously (Mundel et al, 1997a; Asanuma et al, 2005, 2006). MDA-MB 231 cells were obtained from ATCC and cultured as recommended. Transient transfection of HEK293 cells was performed as described before (Asanuma et al, 2005). For proteasome inhibition, NIH3T3 was treated for 16 h with 5–10 μM lactacystin (Santa Cruz) and MDA-MB 231 for 8 h with 2.5 μM lactacystin (Asanuma et al, 2006). For the Rho kinase inhibitor studies, MDA-MB 231 cells were treated with 10 μM Rho kinase inhibitor (Y-27632, Sigma) or the vehicle control (DMSO) for 1 h prior to fixation and stress fibre quantification. In experiments where NIH3T3 cells were treated with both lactacystin and Y-27632, fibroblasts were first treated with 5 μM lactacystin for 15 h before 5 μM Y-27632 was added to the media for another hour. GFP fusion proteins were analysed by direct fluorescence microscopy in living cells or after fixation and double labelling confocal microscopy (Asanuma et al, 2005, 2006). Stress fibres were counted manually in each individual cell in independent images. A stress fibre was defined as a phalloidin-positive structure that was represented by a line spanning across the length of the cell. In all, 200 TPMα-depleted NIH3T3 cells and 100 MDA-MB 231 cells from three independent experiments were analysed for the presence of stress fibres. In addition, for each TPMβ shRNA 100 cells were counted. A P-value <0.05 with the Student's t-test was considered statistically significant.
Lentivirus production
The GIPZ plasmids (TPMα, TPMβ shRNA, or control shRNA) and the pLentix plasmids (FLAG–RhoA, FLAG–RhoA(K6,7R), and synaptopodin), along with the helper plasmids psPAX2 and pMD2.G were transfected into HEK293T cells at 70% confluence using Lipofectamine 2000 (Invitrogen) or Fugene 6 (Roche). LXSN vector (LXSN vector only and LXSN–synaptopodin)-based retroviruses were prepared by transient transfection of the retroviral vectors in Phoenix cells, which stably express the packaging plasmids. Media was replaced 16–18 h after transfection. In all, 36 and 60 h after transfection, virus-containing supernatants were harvested and centrifuged at 3000 r.p.m. for 5 min. Viral particles were then passed through a 0.45-μm filter (Corning) and supernatants were used for infection of target cells in the presence of 4 μg/ml polybrene (Sigma). NIH3T3 cells were infected for 24 h and then selected. Non-infected cells were removed by selection in either 2 μg/ml puromycin (Sigma) for GIPZ vectors or 0.7 μg/ml G418 (Gibco) for LXSN and pLentix constructs, respectively. Non-infected MDA-MB 231 cells were selected with 3 mg/ml G418.
Drosophila histology and in-situ hybridization
Nos-Gal4 is Gal4∷VP16-nos.UTR. Females of wild type, TmIIeg9 mutant, nos-Gal4, UAS-synaptopodin/+ and nos-Gal4, UAS-synaptopodin/+; TmIIeg9 genotypes were selected from the intercross of nos-Gal4/w FM6; TmIIeg9/TM6b and UAS-synaptopodin; Tm2eg9/TM6 Ubx. Expression of synaptopodin in the germline during oogenesis was confirmed by immunofluorescence microscopy. In-situ hybridization was performed on all four genotypes according to Tautz and Pfeifle (1989) with modifications for RNA probes and ovaries. A digoxigenin-labelled antisense osk mRNA probe was generated with T7 RNA polymerase (Roche). Hybridization was performed at 55°C. Ovaries of 10–15 females per genotype were separated into ovarioles and fixed in 4% paraformaldehyde (PFA) in PBS containing 0.1% Tween-20/DMSO/heptane in a 20:2:60 mixture at room temperature for 20 min with agitation. The heptane phase was removed and replaced by 50 parts 4% PFA in PBS containing 0.1% Tween-20 followed by further incubation for 5 min with agitation. Ovaries were rehydrated in methanol/4% PFA in PBS containing 0.1% Tween-20 in a 1:1 mixture for 5 min and processed as described by others (Tautz and Pfeifle, 1989). Immunolabelling of Drosophila ovaries was done according to protocols described by the Cooley laboratory (http://info.med.yale.edu/cooley/Protocol%20Ovaries.html).
Immunocytochemistry
Cultured cells were plated on coverslips and fixed with 2% PFA and 4% sucrose in PBS for 10 min followed by permeabilization with 0.3% Triton X-100 in PBS for 5 min at room temperature. After three 5 min washes with PBS, non-specific binding sites were blocked with 2% FCS, 2% BSA, and 0.2% fish gelatin in PBS for at least 30 min. Primary antibodies (prediluted in blocking solution) were applied for 60 min at room temperature. Antigen–antibody complexes were visualized using Alexa Fluor 488 or 647 conjugated secondary antibodies at 1:500 to 1:1000 (Molecular Probes). Stress fibres were visualized by staining with rhodamine-labelled phalloidin (Molecular Probes) at 1:750 for 30 min along with the secondary antibodies. Cells were washed with PBS, rinsed with ddH2O, and mounted in 15% Mowiol and 50% glycerol in PBS. Monoclonal anti-Tm (Tm311, Sigma) and anti-vinculin (Sigma) were used at 1:400. Confocal images were captured on a Leica TC5 SP5 or a Zeiss LSM 510 upright confocal microscope. ImageJ and Photoshop softwares were used for image analysis.
Western blotting and immunoprecipitation
SDS–PAGE, western blotting, and co-immunoprecipitation of FLAG- and GFP-tagged fusion proteins from transfected HEK293 cells were performed as described previously (Asanuma et al, 2005, 2006). It was previously found by others that an N-terminally tagged Tm1 is unable to induce stress fibres in transformed cells (Bharadwaj et al, 2004). Therefore, we cloned the tropomyosin constructs used in the present study into pFLAG–CMV-5 vectors, thereby placing the tags on the C-terminus and avoiding interfere with the critical function of the N-terminal domain (Bharadwaj et al, 2004). This is in keeping with other studies that have also successfully used C-terminal FLAG–Tm constructs for localization and functional studies (Michele et al, 1999; Hillberg et al, 2006). Protein extraction from isolated mouse glomeruli has been described before (Mundel et al, 1997b; Asanuma et al, 2005). Antibodies were used as follow: Synaptopodin NT (Mundel et al, 1997a) at 1:1000, FLAG (Sigma) at 1:10 000, GFP (BD Transduction Laboratories) at 1:1000, GAPDH (Calbiochem) at 1:3000, RhoA (Cell Signaling) at 1:1000, and Tm (Tm311, Sigma) at 1:1000. The Tm311 antibody recognizes a common epitope in the long Tm isoforms (Nicholson-Flynn et al, 1996). HRP-conjugated secondary antibodies (Promega) were used at 1:10 000.
GST binding assays
To study the competitive binding of Tm2 and Smurf1 to RhoA, GST pull-down studies with purified recombinant proteins were carried out as described previously for synaptopodin (Asanuma et al, 2006). Recombinant proteins were bound to GST or GST–RhoA(T19N) in Triton buffer (50 mM Tris–HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100) and washed five times in Triton buffer. We immobilized 1 μg purified GST–RhoA(T19N) on GSH beads and incubated it with 0.5 μg of purified FLAG-tagged catalytically inactive Smurf1(CA) (Wang et al, 2003) and increasing amounts (0, 0.25, 0.5, or 1 μg) of FLAG–Tm2.
Wound-healing assays
Cell migration of wild-type MDA-MB 231 cells and MDA-MB 231 cells infected with empty pLentix vector, pLentix-FLAG–RhoA(wt), pLentix-FLAG–RhoA(K6,7R) or pLentix–synaptopodin was assessed using a wound-healing assay. The cells were plated in 24-well plates and allowed to proliferate to form a confluent monolayer. A 200-ml pipette tip was used to scratch a single wound through the middle of the cell monolayer. Cells were fixed with methanol and stained with crystal violet at 0 and 12 h post wounding. Stained cells were imaged by use of a light microscope equipped with an Olympus digital camera. The percent wound closure at the 12-h time point was compared with determine differences in the rate of cell migration. In each experiment, we analysed a total n=6.
Protein half-life assays
To determine the turnover of exogenous synaptopodin and Tm2, we transfected HEK293 cells with FLAG–synaptopodin or FLAG–Tm2. Forty-eight hours later, the cells were treated with 10 μg/ml cycloheximide (Sigma-Aldrich) for indicated times (0, 1, 2, 4, 8, 12, and 24 h). Protein extracts were prepared in CHAPS buffer (20 mM Tris pH 7.5, 500 mM NaCl, 0.5% CHAPS) and synaptopodin and tropomyosin levels were quantified using ImageJ software. GAPDH levels were used as an internal loading control and the protein levels at the beginning of the experiment (0 h) as 100%. Using the best-fit curve, half-life of each protein was determined and reported as average±standard deviation of four independent experiments. To analyse the half-life of endogenous synaptopodin and Tm in podocytes, we differentiated podocytes for 10 days as described before (Mundel et al, 1997b). The podocytes were then treated with 10 μg/ml cycloheximide for indicated time points (0, 4, 8, 12, 16, and 24 h). Protein extracts were prepared in CHAPS buffer and the half-life of synaptopodin and tropomyosin was determined from three independent experiments as described above. Tropomyosin was detected with Tm311 antibody as described above.
Supplementary Material
Acknowledgments
We thank Miklos Erdelyi, Szeged, Hungary for providing oskar plasmid and TmII mutant flies; the Drosophila stock center, Bloomington, IN, USA for fly strains; and Sophy Okello, New York, NY, USA for transgenic injections. We thank Anna Greka, Boston, MA, USA for help with confocal microscopy. We also thank Peter Gunning Westmead, Australia for Tm1 cDNA; Tadaomi Takenawa, Tokyo, Japan for GST–RhoA(T19N) cDNA; Jeffrey Wrana, Toronto, Canada for RhoA(K6,7R) cDNA; Keiji Tanaka, Tokyo, Japan for HA–ubiquitin cDNA; and Takeshi Imamura (The JFCR Cancer Institute, Tokyo, Japan) for wild-type Smurf1 and catalytically inactive Smurf1(C710A) cDNAs. MNR was supported by a Child Health Research Center grant HD052890. This work was supported by NIH grants DK57683 and DK062472 to PM and EY14597 to MM. This manuscript has been used by JSW to fulfil the requirements for obtaining a PhD degree from the Graduate School at the Mount Sinai School of Medicine, New York, NY, USA.
Footnotes
The authors declare that they have no conflict of interest.
References
- Akutsu S, Miyazaki J (2002) Biochemical and immunohistochemical studies on tropomyosin and glutamate dehydrogenase in the chicken liver. Zoolog Sci 19: 275–286 [DOI] [PubMed] [Google Scholar]
- Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P (2005) Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest 115: 1188–1198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asanuma K, Yanagida-Asanuma E, Faul C, Tomino Y, Kim K, Mundel P (2006) Synaptopodin orchestrates actin organization and cell motility via regulation of RhoA signalling. Nat Cell Biol 8: 485–491 [DOI] [PubMed] [Google Scholar]
- Bakin AV, Safina A, Rinehart C, Daroqui C, Darbary H, Helfman DM (2004) A critical role of tropomyosins in TGF-beta regulation of the actin cytoskeleton and cell motility in epithelial cells. Mol Biol Cell 15: 4682–4694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bharadwaj S, Hitchcock-DeGregori S, Thorburn A, Prasad GL (2004) N terminus is essential for tropomyosin functions: N-terminal modification disrupts stress fiber organization and abolishes anti-oncogenic effects of tropomyosin-1. J Biol Chem 279: 14039–14048 [DOI] [PubMed] [Google Scholar]
- Bharadwaj S, Thanawala R, Bon G, Falcioni R, Prasad GL (2005) Resensitization of breast cancer cells to anoikis by tropomyosin-1: role of Rho kinase-dependent cytoskeleton and adhesion. Oncogene 24: 8291–8303 [DOI] [PubMed] [Google Scholar]
- Bhattacharya B, Prasad GL, Valverius EM, Salomon DS, Cooper HL (1990) Tropomyosins of human mammary epithelial cells: consistent defects of expression in mammary carcinoma cell lines. Cancer Res 50: 2105–2112 [PubMed] [Google Scholar]
- Boyd J, Risinger JI, Wiseman RW, Merrick BA, Selkirk JK, Barrett JC (1995) Regulation of microfilament organization and anchorage-independent growth by tropomyosin 1. Proc Natl Acad Sci USA 92: 11534–11538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand AH, Perrimon N (1993) Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development 118: 401–415 [DOI] [PubMed] [Google Scholar]
- Chen Y, Yang Z, Meng M, Zhao Y, Dong N, Yan H, Liu L, Ding M, Peng HB, Shao F (2009) Cullin mediates degradation of RhoA through evolutionarily conserved BTB adaptors to control actin cytoskeleton structure and cell movement. Mol Cell 35: 841–855 [DOI] [PubMed] [Google Scholar]
- Clark I, Giniger E, Ruohola-Baker H, Jan LY, Jan YN (1994) Transient posterior localization of a kinesin fusion protein reflects anteroposterior polarity of the Drosophila oocyte. Curr Biol 4: 289–300 [DOI] [PubMed] [Google Scholar]
- Cooper HL, Feuerstein N, Noda M, Bassin RH (1985) Suppression of tropomyosin synthesis, a common biochemical feature of oncogenesis by structurally diverse retroviral oncogenes. Mol Cell Biol 5: 972–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JA (2002) Actin dynamics: tropomyosin provides stability. Curr Biol 12: R523–R525 [DOI] [PubMed] [Google Scholar]
- Doerflinger H, Benton R, Torres IL, Zwart MF, St Johnston D (2006) Drosophila anterior-posterior polarity requires actin-dependent PAR-1 recruitment to the oocyte posterior. Curr Biol 16: 1090–1095 [DOI] [PubMed] [Google Scholar]
- Drenckhahn D, Schnittler H, Nobiling R, Kriz W (1990) Ultrastructural organization of contractile proteins in rat glomerular mesangial cells. Am J Pathol 137: 1343–1351 [PMC free article] [PubMed] [Google Scholar]
- Ephrussi A, Dickinson LK, Lehmann R (1991) Oskar organizes the germ plasm and directs localization of the posterior determinant nanos. Cell 66: 37–50 [DOI] [PubMed] [Google Scholar]
- Erdelyi M, Michon AM, Guichet A, Glotzer JB, Ephrussi A (1995) Requirement for Drosophila cytoplasmic tropomyosin in oskar mRNA localization. Nature 377: 524–527 [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A (2002) Rho GTPases in cell biology. Nature 420: 629–635 [DOI] [PubMed] [Google Scholar]
- Faul C, Asanuma K, Yanagida-Asanuma E, Kim K, Mundel P (2007) Actin up: regulation of podocyte structure and function by components of the actin cytoskeleton. Trends Cell Biol 17: 428–437 [DOI] [PubMed] [Google Scholar]
- Faul C, Donnelly M, Merscher-Gomez S, Chang YH, Franz S, Delfgaauw J, Chang JM, Choi HY, Campbell KN, Kim K, Reiser J, Mundel P (2008) The actin cytoskeleton of kidney podocytes is a direct target of the antiproteinuric effect of cyclosporine A. Nat Med 14: 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul C, Huttelmaier S, Oh J, Hachet V, Singer RH, Mundel P (2005) Promotion of importin {alpha}-mediated nuclear import by the phosphorylation-dependent binding of cargo protein to 14-3-3. J Cell Biol 169: 415–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunning PW, Schevzov G, Kee AJ, Hardeman EC (2005) Tropomyosin isoforms: divining rods for actin cytoskeleton function. Trends Cell Biol 15: 333–341 [DOI] [PubMed] [Google Scholar]
- Hendricks M, Weintraub H (1981) Tropomyosin is decreased in transformed cells. Proc Natl Acad Sci USA 78: 5633–5637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillberg L, Zhao Rathje LS, Nyakern-Meazza M, Helfand B, Goldman RD, Schutt CE, Lindberg U (2006) Tropomyosins are present in lamellipodia of motile cells. Eur J Cell Biol 85: 399–409 [DOI] [PubMed] [Google Scholar]
- Hitchcock-DeGregori SE, Greenfield NJ, Singh A (2007) Tropomyosin: regulator of actin filaments. Adv Exp Med Biol 592: 87–97 [DOI] [PubMed] [Google Scholar]
- Hughes JA, Cooke-Yarborough CM, Chadwick NC, Schevzov G, Arbuckle SM, Gunning P, Weinberger RP (2003) High-molecular-weight tropomyosins localize to the contractile rings of dividing CNS cells but are absent from malignant pediatric and adult CNS tumors. Glia 42: 25–35 [DOI] [PubMed] [Google Scholar]
- Jaffe AB, Hall A (2005) RHO GTPases: biochemistry and biology. Annu Rev Cell Dev Biol 21: 247–269 [DOI] [PubMed] [Google Scholar]
- Jankovics F, Sinka R, Lukacsovich T, Erdelyi M (2002) MOESIN crosslinks actin and cell membrane in Drosophila oocytes and is required for OSKAR anchoring. Curr Biol 12: 2060–2065 [DOI] [PubMed] [Google Scholar]
- Kim-Ha J, Smith JL, Macdonald PM (1991) oskar mRNA is localized to the posterior pole of the Drosophila oocyte. Cell 66: 23–35 [DOI] [PubMed] [Google Scholar]
- Martin AF (1981) Turnover of cardiac troponin subunits. Kinetic evidence for a precursor pool of troponin-I. J Biol Chem 256: 964–968 [PubMed] [Google Scholar]
- Michele DE, Albayya FP, Metzger JM (1999) Thin filament protein dynamics in fully differentiated adult cardiac myocytes: toward a model of sarcomere maintenance. J Cell Biol 145: 1483–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundel P, Heid HW, Mundel TM, Kruger M, Reiser J, Kriz W (1997a) Synaptopodin: an actin-associated protein in telencephalic dendrites and renal podocytes. J Cell Biol 139: 193–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundel P, Reiser J, Borja AZ, Pavenstadt H, Davidson GR, Kriz W, Zeller R (1997b) Rearrangements of the cytoskeleton and cell contacts induce process formation during differentiation of conditionally immortalized mouse podocyte cell lines. Exp Cell Res 236: 248–258 [DOI] [PubMed] [Google Scholar]
- Nicholson-Flynn K, Hitchcock-DeGregori SE, Levitt P (1996) Restricted expression of the actin-regulatory protein, tropomyosin, defines distinct boundaries, evaginating neuroepithelium, and choroid plexus forerunners during early CNS development. J Neurosci 16: 6853–6863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill GM, Stehn J, Gunning PW (2008) Tropomyosins as interpreters of the signalling environment to regulate the local cytoskeleton. Semin Cancer Biol 18: 35–44 [DOI] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL (2005) Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science 307: 1603–1609 [DOI] [PubMed] [Google Scholar]
- Pawlak G, McGarvey TW, Nguyen TB, Tomaszewski JE, Puthiyaveettil R, Malkowicz SB, Helfman DM (2004) Alterations in tropomyosin isoform expression in human transitional cell carcinoma of the urinary bladder. Int J Cancer 110: 368–373 [DOI] [PubMed] [Google Scholar]
- Percival JM, Thomas G, Cock TA, Gardiner EM, Jeffrey PL, Lin JJ, Weinberger RP, Gunning P (2000) Sorting of tropomyosin isoforms in synchronised NIH 3T3 fibroblasts: evidence for distinct microfilament populations. Cell Motil Cytoskeleton 47: 189–208 [DOI] [PubMed] [Google Scholar]
- Perry SV (2001) Vertebrate tropomyosin: distribution, properties and function. J Muscle Res Cell Motil 22: 5–49 [DOI] [PubMed] [Google Scholar]
- Prasad GL, Fuldner RA, Cooper HL (1993) Expression of transduced tropomyosin 1 cDNA suppresses neoplastic growth of cells transformed by the ras oncogene. Proc Natl Acad Sci USA 90: 7039–7043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai E, Garcia-Medina R, Pouyssegur J, Vial E (2007) Smurf1 regulates tumor cell plasticity and motility through degradation of RhoA leading to localized inhibition of contractility. J Cell Biol 176: 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schevzov G, Vrhovski B, Bryce NS, Elmir S, Qiu MR, O’Neill GM, Yang N, Verrills NM, Kavallaris M, Gunning PW (2005) Tissue-specific tropomyosin isoform composition. J Histochem Cytochem 53: 557–570 [DOI] [PubMed] [Google Scholar]
- Shulman JM, Benton R, St Johnston D (2000) The Drosophila homolog of C. elegans PAR-1 organizes the oocyte cytoskeleton and directs oskar mRNA localization to the posterior pole. Cell 101: 377–388 [DOI] [PubMed] [Google Scholar]
- Singer RH (2008) Highways for mRNA transport. Cell 134: 722–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Hiwada K, Kokubu T (1986) Isolation and characterization of a 34,000-dalton calmodulin- and F-actin-binding protein from chicken gizzard smooth muscle. Biochem Biophys Res Commun 141: 20–26 [DOI] [PubMed] [Google Scholar]
- Takenaga K, Nakamura Y, Sakiyama S, Hasegawa Y, Sato K, Endo H (1994) Binding of pEL98 protein, an S100-related calcium-binding protein, to nonmuscle tropomyosin. J Cell Biol 124: 757–768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tautz D, Pfeifle C (1989) A non-radioactive in situ hybridization method for the localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma 98: 81–85 [DOI] [PubMed] [Google Scholar]
- Tian D, Jacobo SM, Billing D, Rozkalne A, Gage SD, Anagnostou T, Pavenstaedt H, Hsu HH, Schlondorff J, Ramos A, Greka A (2010) Antagonistic regulation of actin dynamics and cell motility by TRPC5 and TRPC6 channels. Sci Signal 3: ra77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomancak P, Piano F, Riechmann V, Gunsalus KC, Kemphues KJ, Ephrussi A (2000) A Drosophila melanogaster homologue of Caenorhabditis elegans par-1 acts at an early step in embryonic-axis formation. Nat Cell Biol 2: 458–460 [DOI] [PubMed] [Google Scholar]
- Vanzo N, Oprins A, Xanthakis D, Ephrussi A, Rabouille C (2007) Stimulation of endocytosis and actin dynamics by Oskar polarizes the Drosophila oocyte. Dev Cell 12: 543–555 [DOI] [PubMed] [Google Scholar]
- Wang FL, Wang Y, Wong WK, Liu Y, Addivinola FJ, Liang P, Chen LB, Kantoff PW, Pardee AB (1996) Two differentially expressed genes in normal human prostate tissue and in carcinoma. Cancer Res 56: 3634–3637 [PubMed] [Google Scholar]
- Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, Wrana JL (2003) Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science 302: 1775–1779 [DOI] [PubMed] [Google Scholar]
- Yanagida-Asanuma E, Asanuma K, Kim K, Donnelly M, Young Choi H, Hyung Chang J, Suetsugu S, Tomino Y, Takenawa T, Faul C, Mundel P (2007) Synaptopodin protects against proteinuria by disrupting Cdc42:IRSp53:Mena signaling complexes in kidney podocytes. Am J Pathol 171: 415–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimyanin VL, Belaya K, Pecreaux J, Gilchrist MJ, Clark A, Davis I, St Johnston D (2008) In vivo imaging of oskar mRNA transport reveals the mechanism of posterior localization. Cell 134: 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.